The Role of Glial Cells in Different Phases of Migraine: Lessons from Preclinical Studies
Abstract
1. Introduction
1.1. Migraine
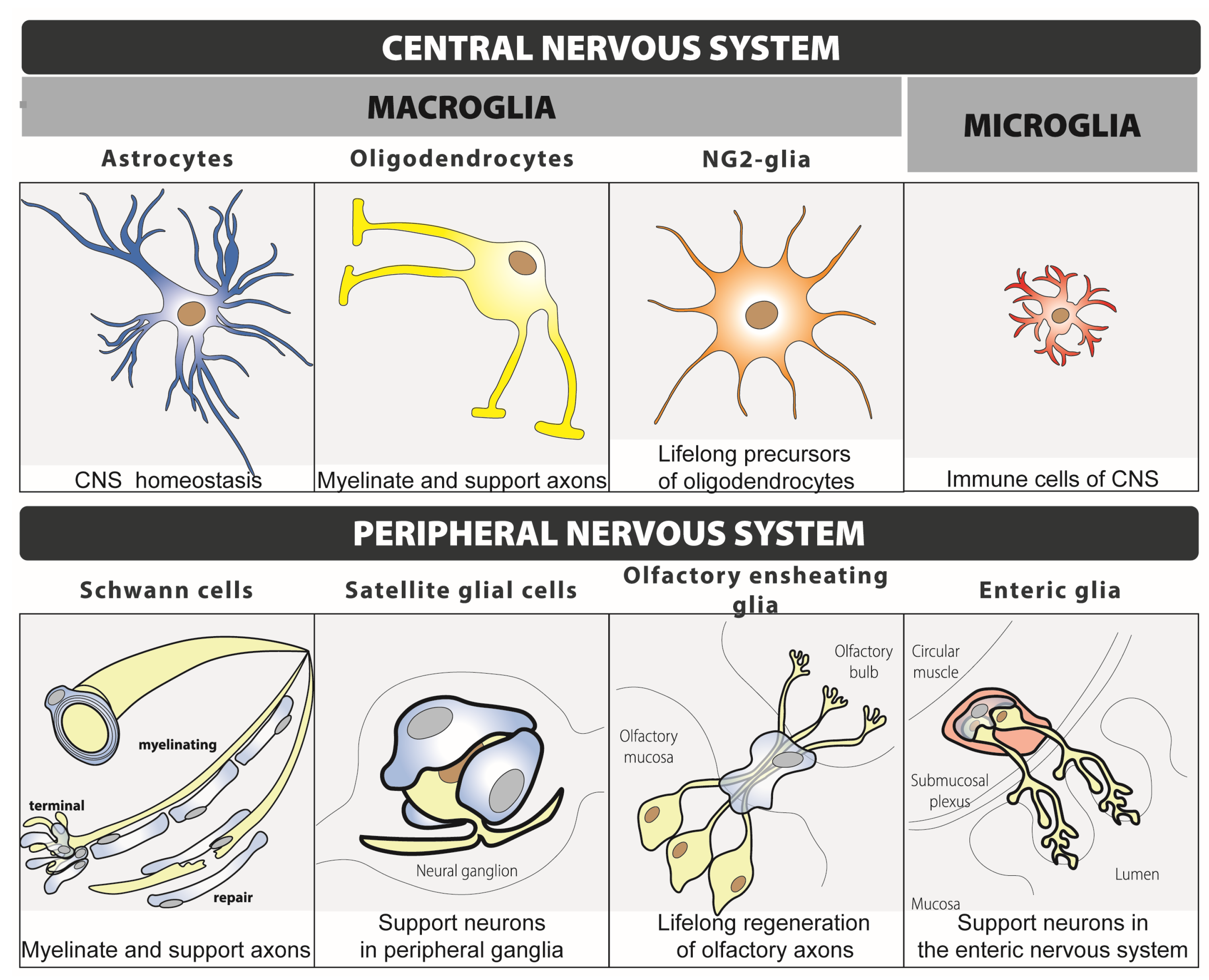
1.2. Glial Cells
1.3. Overview of the Role of Glia in Neurological Diseases
2. Role of Glia in Cortical Spreading Depolarization: Implications for the Migraine Aura Phase
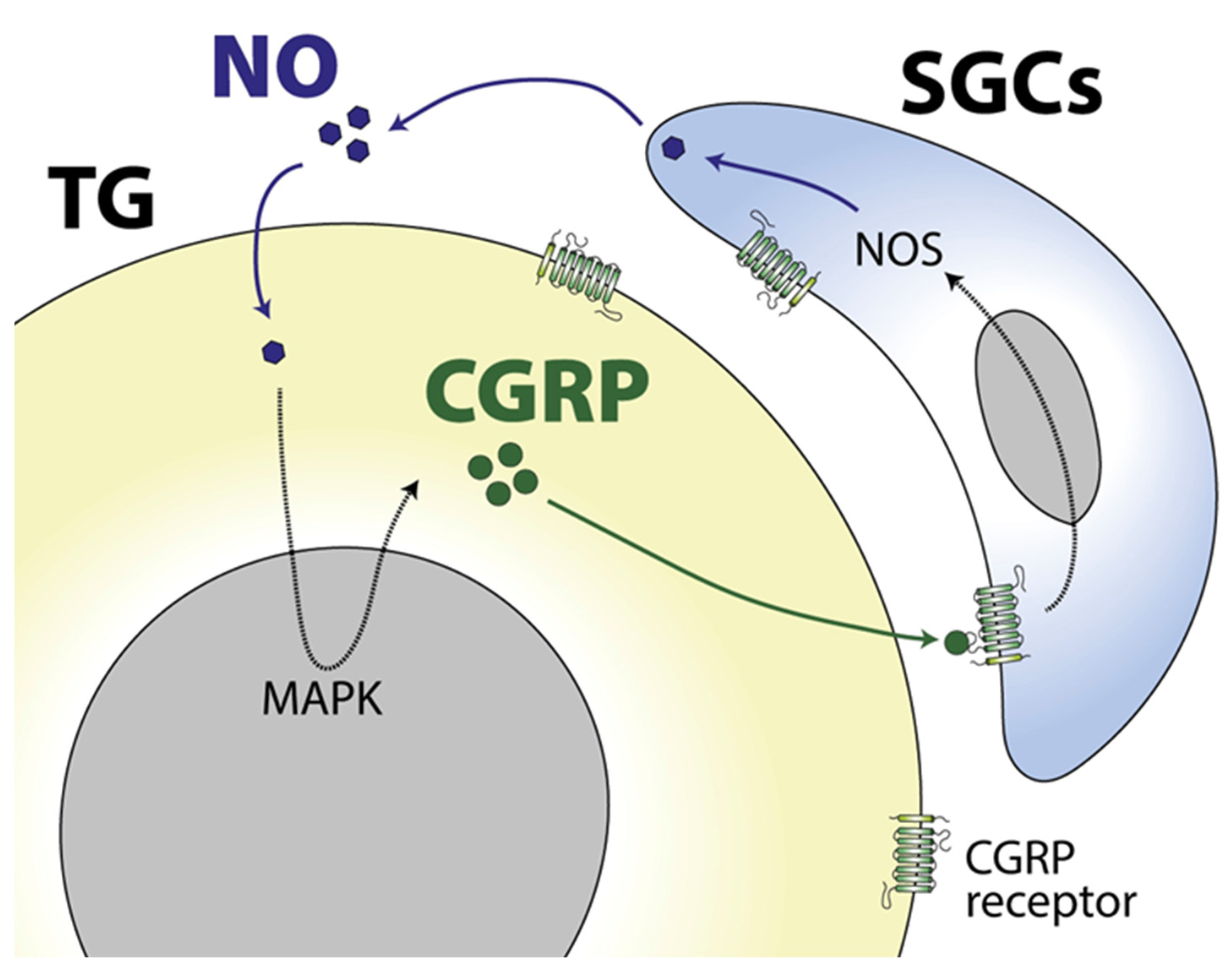
3. Role of Glia in Orofacial Pain: Implications for the Migraine Headache Phase
4. Role of Glia in Chronic Pain: Implications for Migraine Chronification
5. Targeting Glia: Potential Future Therapeutic and Diagnostic Opportunities for Migraine
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Goadsby, P.J.; Holland, P.R. An Update: Pathophysiology of Migraine. Neurol. Clin. 2019, 37, 651–671. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.H. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar]
- Lanteri-Minet, M. Economic burden and costs of chronic migraine. Curr. Pain Headache Rep. 2014, 18, 385. [Google Scholar] [CrossRef]
- Ashina, M.; Hansen, J.M.; Do, T.P.; Melo-Carrillo, A.; Burstein, R.; Moskowitz, M.A. Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol. 2019, 18, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.; Burstein, R. Sensitization of the trigeminovascular pathway: Perspective and implications to migraine pathophysiology. J. Clin. Neurol. 2012, 8, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.; Holland, P.R.; Goadsby, P.J. Diencephalic and brainstem mechanisms in migraine. Nat. Rev. Neurosci. 2011, 12, 570–584. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Vila-Pueyo, M.; Hoffmann, J.; Romero-Reyes, M.; Akerman, S. Brain structure and function related to headache: Brainstem structure and function in headache. Cephalalgia 2019, 39, 1635–1660. [Google Scholar] [CrossRef]
- Vila-Pueyo, M.; Strother, L.C.; Kefel, M.; Goadsby, P.J.; Holland, P.R. Divergent influences of the locus coeruleus on migraine pathophysiology. Pain 2019, 160, 385–394. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990, 28, 183–187. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L. The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann. Neurol. 1993, 33, 48–56. [Google Scholar] [CrossRef]
- Edvinsson, L.; Petersen, K.A. CGRP-receptor antagonism in migraine treatment. CNS Neurol. Disord. Drug Targets 2007, 6, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Lassen, L.H.; Haderslev, P.A.; Jacobsen, V.B.; Iversen, H.K.; Sperling, B.; Olesen, J. CGRP may play a causative role in migraine. Cephalalgia 2002, 22, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies-successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef]
- Holland, P.R.; Saengjaroentham, C.; Vila-Pueyo, M. The role of the brainstem in migraine: Potential brainstem effects of CGRP and CGRP receptor activation in animal models. Cephalalgia 2019, 39, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ferrús, M.; Ursitti, F.; Alpuente, A.; Brunello, F.; Chiappino, D.; de Vries, T.; Di Marco, S.; Ferlisi, S.; Guerritore, L.; Gonzalez-Garcia, N.; et al. From transformation to chronification of migraine: Pathophysiological and clinical aspects. J. Headache Pain 2020, 21, 42. [Google Scholar] [CrossRef]
- Haydon, P.G.; Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006, 86, 1009–1031. [Google Scholar] [CrossRef]
- Butt, A.; Verkhratsky, A. Neuroglia: Realising their true potential. Brain Neurosci. Adv. 2018, 2, 2398212818817495. [Google Scholar] [CrossRef] [PubMed]
- Jakel, S.; Dimou, L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell Neurosci. 2017, 11, 24. [Google Scholar] [CrossRef]
- Vezzani, A.; Ravizza, T.; Bedner, P.; Aronica, E.; Steinhauser, C.; Boison, D. Astrocytes in the initiation and progression of epilepsy. Nat. Rev. Neurol. 2022, 18, 707–722. [Google Scholar] [CrossRef]
- Henning, L.; Unichenko, P.; Bedner, P.; Steinhauser, C.; Henneberger, C. Overview Article Astrocytes as Initiators of Epilepsy. Neurochem. Res. 2023, 48, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154 (Suppl. S1), S10–S28. [Google Scholar] [CrossRef]
- Ghazisaeidi, S.; Muley, M.M.; Salter, M.W. Neuropathic Pain: Mechanisms, Sex Differences, and Potential Therapies for a Global Problem. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Gotra, P.; Bhardwaj, N.; Ludhiadch, A.; Singh, G.; Munshi, A. Epilepsy and Migraine Shared Genetic and Molecular Mechanisms: Focus on Therapeutic Strategies. Mol. Neurobiol. 2021, 58, 3874–3883. [Google Scholar] [CrossRef]
- Zarcone, D.; Corbetta, S. Shared mechanisms of epilepsy, migraine and affective disorders. Neurol. Sci. 2017, 38 (Suppl. S1), 73–76. [Google Scholar] [CrossRef]
- Pelzer, N.; de Boer, I.; van den Maagdenberg, A.; Terwindt, G.M. Neurological and psychiatric comorbidities of migraine: Concepts and future perspectives. Cephalalgia 2023, 43, 3331024231180564. [Google Scholar] [CrossRef]
- McMahon, S.B.; Malcangio, M. Current challenges in glia-pain biology. Neuron 2009, 64, 46–54. [Google Scholar] [CrossRef]
- Afridi, R.; Suk, K. Microglial Responses to Stress-Induced Depression: Causes and Consequences. Cells 2023, 12, 1521. [Google Scholar] [CrossRef]
- Charles, A.C.; Merrill, J.E.; Dirksen, E.R.; Sanderson, M.J. Intercellular signaling in glial cells: Calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron 1991, 6, 983–992. [Google Scholar] [CrossRef]
- Basarsky, T.A.; Duffy, S.N.; Andrew, R.D.; MacVicar, B.A. Imaging spreading depression and associated intracellular calcium waves in brain slices. J. Neurosci. 1998, 18, 7189–7199. [Google Scholar] [CrossRef] [PubMed]
- Chuquet, J.; Hollender, L.; Nimchinsky, E.A. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J. Neurosci. 2007, 27, 4036–4044. [Google Scholar] [CrossRef]
- Leo, L.; Gherardini, L.; Barone, V.; De Fusco, M.; Pietrobon, D.; Pizzorusso, T.; Casari, G. Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2. PLoS Genet. 2011, 7, e1002129. [Google Scholar] [CrossRef]
- Aizawa, H.; Sun, W.; Sugiyama, K.; Itou, Y.; Aida, T.; Cui, W.; Toyoda, S.; Terai, H.; Yanagisawa, M.; Tanaka, K. Glial glutamate transporter GLT-1 determines susceptibility to spreading depression in the mouse cerebral cortex. Glia 2020, 68, 2631–2642. [Google Scholar] [CrossRef] [PubMed]
- Enger, R.; Dukefoss, D.B.; Tang, W.; Pettersen, K.H.; Bjørnstad, D.M.; Helm, P.J.; Jensen, V.; Sprengel, R.; Vervaeke, K.; Ottersen, O.P.; et al. Deletion of Aquaporin-4 Curtails Extracellular Glutamate Elevation in Cortical Spreading Depression in Awake Mice. Cereb. Cortex 2017, 27, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Kraig, R.P.; Dong, L.M.; Thisted, R.; Jaeger, C.B. Spreading depression increases immunohistochemical staining of glial fibrillary acidic protein. J. Neurosci. 1991, 11, 2187–2198. [Google Scholar] [CrossRef]
- Sukhotinsky, I.; Dilekoz, E.; Wang, Y.; Qin, T.; Eikermann-Haerter, K.; Waeber, C.; Ayata, C. Chronic daily cortical spreading depressions suppress spreading depression susceptibility. Cephalalgia 2011, 31, 1601–1608. [Google Scholar] [CrossRef]
- Gehrmann, J.; Mies, G.; Bonnekoh, P.; Banati, R.; Iijima, T.; Kreutzberg, G.W.; Hossmann, K.-A. Microglial reaction in the rat cerebral cortex induced by cortical spreading depression. Brain Pathol. 1993, 3, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Takashima, T.; Takashima-Hirano, M.; Wada, Y.; Shukuri, M.; Tamura, Y.; Doi, H.; Onoe, H.; Kataoka, Y.; Watanabe, Y. 11C-PK11195 PET for the in vivo evaluation of neuroinflammation in the rat brain after cortical spreading depression. J. Nucl. Med. 2009, 50, 1904–1911. [Google Scholar] [CrossRef]
- Grinberg, Y.Y.; Milton, J.G.; Kraig, R.P. Spreading depression sends microglia on Levy flights. PLoS ONE 2011, 6, e19294. [Google Scholar] [CrossRef]
- Pusic, K.M.; Pusic, A.D.; Kemme, J.; Kraig, R.P. Spreading depression requires microglia and is decreased by their M2a polarization from environmental enrichment. Glia 2014, 62, 1176–1194. [Google Scholar] [CrossRef]
- Yang, L.; Xu, M.; Bhuiyan, S.A.; Li, J.; Zhao, J.; Cohrs, R.J.; Susterich, J.T.; Signorelli, S.; Green, U.; Stone, J.R.; et al. Human and mouse trigeminal ganglia cell atlas implicates multiple cell types in migraine. Neuron 2022, 110, 1806–1821.e8. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Grell, A.S.; Warfvinge, K. Expression of the CGRP Family of Neuropeptides and their Receptors in the Trigeminal Ganglion. J. Mol. Neurosci. 2020, 70, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, S.D.; Warfvinge, K.; Ohlsson, L.; Edvinsson, L. Expression of Pituitary Adenylate Cyclase-activating Peptide, Calcitonin Gene-related Peptide and Headache Targets in the Trigeminal Ganglia of Rats and Humans. Neuroscience 2018, 393, 319–332. [Google Scholar] [CrossRef]
- Edvinsson, J.C.; Reducha, P.V.; Sheykhzade, M.; Warfvinge, K.; Haanes, K.A.; Edvinsson, L. Neurokinins and their receptors in the rat trigeminal system: Differential localization and release with implications for migraine pain. Mol. Pain 2021, 17, 17448069211059400. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Ossipov, M.H.; Johnson, K.W. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 2017, 158, 543–559. [Google Scholar] [CrossRef]
- Long, T.; He, W.; Pan, Q.; Zhang, S.; Zhang, Y.; Liu, C.; Liu, Q.; Qin, G.; Chen, L.; Zhou, J. Microglia P2X4 receptor contributes to central sensitization following recurrent nitroglycerin stimulation. J. Neuroinflamm. 2018, 15, 245. [Google Scholar] [CrossRef]
- Long, T.; He, W.; Pan, Q.; Zhang, S.; Zhang, D.; Qin, G.; Chen, L.; Zhou, J. Microglia P2X4R-BDNF signalling contributes to central sensitization in a recurrent nitroglycerin-induced chronic migraine model. J. Headache Pain 2020, 21, 4. [Google Scholar] [CrossRef]
- Jing, F.; Zhang, Y.; Long, T.; He, W.; Qin, G.; Zhang, D.; Chen, L.; Zhou, J. P2Y12 receptor mediates microglial activation via RhoA/ROCK pathway in the trigeminal nucleus caudalis in a mouse model of chronic migraine. J. Neuroinflamm. 2019, 16, 217. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Y.; Jing, F.; Long, T.; Qin, G.; Zhang, D.; Chen, L.; Zhou, J. P2X7R-mediated autophagic impairment contributes to central sensitization in a chronic migraine model with recurrent nitroglycerin stimulation in mice. J. Neuroinflamm. 2021, 18, 5. [Google Scholar] [CrossRef]
- He, W.; Long, T.; Pan, Q.; Zhang, S.; Zhang, Y.; Zhang, D.; Qin, G.; Chen, L.; Zhou, J. Microglial NLRP3 inflammasome activation mediates IL-1beta release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model. J. Neuroinflamm. 2019, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Wang, Y.; Pan, Q.; Tian, R.; Zhang, D.; Qin, G.; Zhou, J.; Chen, L. MicroRNA-155-5p promotes neuroinflammation and central sensitization via inhibiting SIRT1 in a nitroglycerin-induced chronic migraine mouse model. J. Neuroinflamm. 2021, 18, 287. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.G.; Gao, Y.Y.; Yin, Z.Q.; Wang, X.R.; Meng, X.S.; Zou, T.F.; Duan, Y.J.; Chen, Y.L.; Liao, C.Z.; Xie, Z.L.; et al. Roxadustat alleviates nitroglycerin-induced migraine in mice by regulating HIF-1alpha/NF-kappaB/inflammation pathway. Acta Pharmacol. Sin. 2022, 44, 308–320. [Google Scholar] [CrossRef]
- Rasmussen, B.K.; Olesen, J. Migraine with aura and migraine without aura: An epidemiological study. Cephalalgia 1992, 12, 221–228; discussion 186. [Google Scholar] [CrossRef]
- Bolay, H.; Moskowitz, M.A. The emerging importance of cortical spreading depression in migraine headache. Rev. Neurol. 2005, 161, 655–657. [Google Scholar] [CrossRef]
- Vila-Pueyo, M.; Cuenca-León, E.; Queirós, A.C.; Kulis, M.; Sintas, C.; Cormand, B.; Martín-Subero, J.I.; Pozo-Rosich, P.; Fernàndez-Castillo, N.; Macaya, A. Genome-wide DNA methylation analysis in an antimigraine-treated preclinical model of cortical spreading depolarization. Cephalalgia 2023, 43, 3331024221146317. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, E.; Takato, M.; Noda, Y. Neuronal and glial activity during spreading depression in cerebral cortex of cat. J. Neurophysiol. 1975, 38, 822–841. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, N.; Attwell, D. Astrocyte calcium signaling: The third wave. Nat. Neurosci. 2016, 19, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.B.; Ducros, A. Sporadic and familial hemiplegic migraine: Pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol. 2011, 10, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Bair, M.; Descalzi, G. Reactive Astrocytes: Critical Players in the Development of Chronic Pain. Front. Psychiatry 2021, 12, 682056. [Google Scholar] [CrossRef]
- Wendt, S.; Wogram, E.; Korvers, L.; Kettenmann, H. Experimental Cortical Spreading Depression Induces NMDA Receptor Dependent Potassium Currents in Microglia. J. Neurosci. 2016, 36, 6165–6174. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.D.; Goadsby, P.J.; Rami, B.; Kurth, T.; Cenk, A.; Charles, A.; Messoud, A.; van den Maagdenberg Arn, M.J.M.; Dodick, D.W. Migraine. Nat. Rev. Dis. Prim. 2022, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, M.; Kubo, A.; Hayashi, Y.; Iwata, K. Peripheral and Central Mechanisms of Persistent Orofacial Pain. Front. Neurosci. 2019, 13, 1227. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.Y.; Dostrovsky, J.O.; Iwata, K.; Sessle, B.J. Role of glia in orofacial pain. Neuroscientist 2011, 17, 303–320. [Google Scholar] [CrossRef]
- Ye, Y.; Salvo, E.; Romero-Reyes, M.; Akerman, S.; Shimizu, E.; Kobayashi, Y.; Michot, B.; Gibbs, J. Glia and Orofacial Pain: Progress and Future Directions. Int. J. Mol. Sci. 2021, 22, 5345. [Google Scholar] [CrossRef]
- Thalakoti, S.; Patil, V.V.; Damodaram, S.; Vause, C.V.; Langford, L.E.; Freeman, S.E.; Durham, P.L. Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache 2007, 47, 1008–1023; discussion 24–25. [Google Scholar] [CrossRef]
- Cherkas, P.S.; Huang, T.Y.; Pannicke, T.; Tal, M.; Reichenbach, A.; Hanani, M. The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain 2004, 110, 290–298. [Google Scholar] [CrossRef]
- Vit, J.P.; Jasmin, L.; Bhargava, A.; Ohara, P.T. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biol. 2006, 2, 247–257. [Google Scholar] [CrossRef]
- Vit, J.P.; Ohara, P.T.; Bhargava, A.; Kelley, K.; Jasmin, L. Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury. J. Neurosci. 2008, 28, 4161–4171. [Google Scholar] [CrossRef]
- Weick, M.; Cherkas, P.; Härtig, W.; Pannicke, T.; Uckermann, O.; Bringmann, A.; Tal, M.; Reichenbach, A.; Hanani, M. P2 receptors in satellite glial cells in trigeminal ganglia of mice. Neuroscience 2003, 120, 969–977. [Google Scholar] [CrossRef]
- Hendrikse, E.R.; Bower, R.L.; Hay, D.L.; Walker, C.S. Molecular studies of CGRP and the CGRP family of peptides in the central nervous system. Cephalalgia 2019, 39, 403–419. [Google Scholar] [CrossRef]
- Ashina, M.; Hansen, J.M.; á Dunga, B.O.; Olesen, J. Human models of migraine-short-term pain for long-term gain. Nat. Rev. Neurol. 2017, 13, 713–724. [Google Scholar] [CrossRef]
- Liang, H.; Hu, H.; Shan, D.; Lyu, J.; Yan, X.; Wang, Y.; Jian, F.; Li, X.; Lai, W.; Long, H. CGRP Modulates Orofacial Pain through Mediating Neuron-Glia Crosstalk. J. Dent. Res. 2021, 100, 98–105. [Google Scholar] [CrossRef]
- Vause, C.V.; Durham, P.L. CGRP stimulation of iNOS and NO release from trigeminal ganglion glial cells involves mitogen-activated protein kinase pathways. J. Neurochem. 2009, 110, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Noseda, R.; Schain, A.J.; Melo-Carrillo, A.; Tien, J.; Stratton, J.; Mai, F.; Strassman, A.M.; Burstein, R. Fluorescently-labeled fremanezumab is distributed to sensory and autonomic ganglia and the dura but not to the brain of rats with uncompromised blood brain barrier. Cephalalgia 2020, 40, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Afroz, S.; Arakaki, R.; Iwasa, T.; Oshima, M.; Hosoki, M.; Inoue, M.; Baba, O.; Okayama, Y.; Matsuka, Y. CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception. Int. J. Mol. Sci. 2019, 20, 711. [Google Scholar] [CrossRef] [PubMed]
- Messlinger, K.; Balcziak, L.K.; Russo, A.F. Cross-talk signaling in the trigeminal ganglion: Role of neuropeptides and other mediators. J. Neural Transm. 2020, 127, 431–444. [Google Scholar] [CrossRef]
- Martin, F.C.; Anton, P.A.; Gornbein, J.A.; Shanahan, F.; Merrill, J.E. Production of interleukin-1 by microglia in response to substance P: Role for a non-classical NK-1 receptor. J. Neuroimmunol. 1993, 42, 53–60. [Google Scholar] [CrossRef]
- Martin, F.C.; Charles, A.C.; Sanderson, M.J.; Merrill, J.E. Substance P stimulates IL-1 production by astrocytes via intracellular calcium. Brain Res. 1992, 599, 13–18. [Google Scholar] [CrossRef]
- Wienrich, M.; Kettenmann, H. Activation of substance P receptors leads to membrane potential responses in cultured astrocytes. Glia 1989, 2, 155–160. [Google Scholar] [CrossRef]
- May, A.; Goadsby, P.J. Substance P receptor antagonists in the therapy of migraine. Expert. Opin. Investig. Drugs 2001, 10, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B. Tracing transformation: Chronic migraine classification, progression, and epidemiology. Neurology 2009, 72 (Suppl. S5), S3–S7. [Google Scholar] [CrossRef]
- Torres-Ferrus, M.; Gallardo, V.J.; Alpuente, A.; Caronna, E.; Gine-Cipres, E.; Pozo-Rosich, P. Patterns of response to anti-calcitonin gene-related peptide monoclonal antibodies during first 6 months of treatment in resistant migraine patients: Impact on outcome. Eur. J. Neurol. 2023, 30, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Milligan, E.D.; Watkins, L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.D.; Suter, M.R.; Ji, R.R.; Decosterd, I. Glial cells and chronic pain. Neuroscientist 2010, 16, 519–531. [Google Scholar] [CrossRef]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef]
- Rezaei, M.; Karimian, L.; Shafaghi, B.; Noubarani, M.; Salecheh, M.; Dehghani, M.S.; Eskandari, M.R.; Pourahmad, J. Evaluation of Molecular and Cellular Alterations Induced by Neuropathic Pain in Rat Brain Glial cells. Iran. J. Pharm. Res. 2021, 20, 359–370. [Google Scholar]
- Ou, M.; Chen, Y.; Liu, J.; Zhang, D.; Yang, Y.; Shen, J.; Miao, C.; Tang, S.-J.; Liu, X.; Mulkey, D.K.; et al. Spinal astrocytic MeCP2 regulates Kir4.1 for the maintenance of chronic hyperalgesia in neuropathic pain. Prog. Neurobiol. 2023, 224, 102436. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.X.; Zhai, M.N.; Zhu, M.; He, C.; Wang, H.; Wang, J.; Zhang, Z.J. Inflammation in pathogenesis of chronic pain: Foe and friend. Mol. Pain 2023, 19, 17448069231178176. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Masuda, T.; Kohno, K. Microglial diversity in neuropathic pain. Trends Neurosci. 2023, 46, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.A.; Smith, M.L.; McGuire, B.; Tarash, I.; Evans, C.J.; Charles, A. Characterization of a novel model of chronic migraine. Pain 2014, 155, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Harriott, A.M.; Strother, L.C.; Vila-Pueyo, M.; Holland, P.R. Animal models of migraine and experimental techniques used to examine trigeminal sensory processing. J. Headache Pain 2019, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Zou, Q.; Wang, Y.; Cai, Z.; Tang, Y. Activation of microglial GLP-1R in the trigeminal nucleus caudalis suppresses central sensitization of chronic migraine after recurrent nitroglycerin stimulation. J. Headache Pain 2021, 22, 86. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, Y.; Tian, R.; Wen, Q.; Qin, G.; Zhang, D.; Chen, L.; Zhang, Y.; Zhou, J. Sphingosine-1 phosphate receptor 1 contributes to central sensitization in recurrent nitroglycerin-induced chronic migraine model. J. Headache Pain 2022, 23, 25. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef]
- Ledeboer, A.; Sloane, E.M.; Milligan, E.D.; Frank, M.G.; Mahony, J.H.; Maier, S.F.; Watkins, L.R. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 2005, 115, 71–83. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Zarzuelo, A.; Galvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef]
- Lai, A.Y.; Todd, K.G. Hypoxia-activated microglial mediators of neuronal survival are differentially regulated by tetracyclines. Glia 2006, 53, 809–816. [Google Scholar] [CrossRef]
- Tawfik, V.; Regan, M.; Haenggeli, C.; La Croix-Fralish, M.; Nutile-McMenemy, N.; Perez, N.; Rothstein, J.; De Leo, J. Propentofylline-induced astrocyte modulation leads to alterations in glial glutamate promoter activation following spinal nerve transection. Neuroscience 2008, 152, 1086–1092. [Google Scholar] [CrossRef]
- Ji, R.R.; Kawasaki, Y.; Zhuang, Z.Y.; Wen, Y.R.; Decosterd, I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: Review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2006, 2, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, A.; Ito, A.; Yoshikawa, M.; Sawada, M. Ibudilast suppresses TNFalpha production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Res. 1999, 837, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Kwok, Y.H.; Swift, J.E.; Gazerani, P.; Rolan, P. A double-blind, randomized, placebo-controlled pilot trial to determine the efficacy and safety of ibudilast, a potential glial attenuator, in chronic migraine. J. Pain Res. 2016, 9, 899–907. [Google Scholar] [CrossRef]
- O’Carroll, S.J.; Cook, W.H.; Young, D. AAV Targeting of Glial Cell Types in the Central and Peripheral Nervous System and Relevance to Human Gene Therapy. Front. Mol. Neurosci. 2020, 13, 618020. [Google Scholar] [CrossRef]
- Bossuyt, J.; Van Den Herrewegen, Y.; Nestor, L.; Buckinx, A.; De Bundel, D.; Smolders, I. Chemogenetic modulation of astrocytes and microglia: State-of-the-art and implications in neuroscience. Glia 2023, 71, 2071–2095. [Google Scholar] [CrossRef]
- Valori, C.F.; Guidotti, G.; Brambilla, L.; Rossi, D. Astrocytes: Emerging Therapeutic Targets in Neurological Disorders. Trends Mol. Med. 2019, 25, 750–759. [Google Scholar] [CrossRef]
- Almad, A.A.; Maragakis, N.J. Glia: An emerging target for neurological disease therapy. Stem Cell Res. Ther. 2012, 3, 37. [Google Scholar] [CrossRef][Green Version]
- Baloh, R.H.; Johnson, J.P.; Avalos, P.; Allred, P.; Svendsen, S.; Gowing, G.; Roxas, K.; Wu, A.; Donahue, B.; Osborne, S.; et al. Transplantation of human neural progenitor cells secreting GDNF into the spinal cord of patients with ALS: A phase 1/2a trial. Nat. Med. 2022, 28, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef] [PubMed]

| Migraine Phase | Glial Cell | Evidence of Implication | Proposed Function of Glia in Migraine | Ref. |
|---|---|---|---|---|
| Cortical spreading depolarisation (CSD)—aura | Astrocyte | Calcium waves inhibition blocks vascular changes without altering CSD propagation | Regulation of vascular response, but not the propagation, of CSD | [30,31,32] |
| Astrocyte | Their regulation of extracellular glutamate concentration increases the susceptibility to CSD | Modulation of CSD initiation | [33,34,35] | |
| Astrocyte | Acute and chronic CSD induce astrocytosis, which is reverted after CSD termination | Implication in CSD | [36,37] | |
| Microglia | CSD activates, and induces the migration and motility of cortical microglia | Implication in CSD | [38,39,40] | |
| Microglia | In vitro depletion inhibits the induction of spreading depolarization | Essential for CSD initiation | [41] | |
| Satellite glial cell | Transcriptional activation in TG 1.5 h after induction of CSD | Implication in CSD | [42] | |
| Orofacial pain—headache | Satellite glial cell | Expression of CGRP receptor in TG | Essential for headache induction | [43,44] |
| Satellite glial cell | Expression of substance P receptor and PACAP and its receptors in TG | Regulation of headache induction via different neuropeptides | [44,45] | |
| Chronification | Satellite glial cell | Existence of a CGRP-mediated positive feedback between them and neurons in TG | Potential implication in migraine chronification and target of anti-CGRP treatments | [46] |
| Microglia | Activation in TCC in the NTG-induced chronic migraine mice | Modulation of central sensitization | [47,48,49,50] | |
| Microglia | Expression of inflammatory molecules in TCC in the NTG-induced chronic migraine mice | Modulation of central sensitization | [51] | |
| Microglia | Expression of microRNA miR-155-5p in the TCC of chronic migraine mice that is correlated with hyperalgesia levels | Modulation of central sensitization | [52] | |
| Microglia | Systemic minocycline reduces hind-paw allodynia and microglia activation in NTG-induced chronic migraine mice | Modulation of central sensitization | [47] | |
| Microglia | Systemic roxadustat reduces NTG-induced hyperalgesia, inflammatory cytokine levels and microglia activation in NTG-induced chronic migraine mice. | Modulation of central sensitization | [53] |
| Approach | Type of Approach | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| AAV vectors | Delivery system | Allows the delivery of gene therapy specifically into the glial cells of interest | Currently, they have only been used to deliver gene therapy in the nervous system to target neurons, not glia | [104,105] |
| Nanoparticles | Delivery system | Cross the blood–brain barrier after systemic administration and release the content into the glial cells of interest | They have not been used to deliver specific therapies in glial cells yet | [106] |
| Cell replacement | Therapeutic approach | Replacement of dysfunctional glia restores an appropriate homeostatic environment | It has been tested in neurons, not glia, for ALS treatment | [107,108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vila-Pueyo, M.; Gliga, O.; Gallardo, V.J.; Pozo-Rosich, P. The Role of Glial Cells in Different Phases of Migraine: Lessons from Preclinical Studies. Int. J. Mol. Sci. 2023, 24, 12553. https://doi.org/10.3390/ijms241612553
Vila-Pueyo M, Gliga O, Gallardo VJ, Pozo-Rosich P. The Role of Glial Cells in Different Phases of Migraine: Lessons from Preclinical Studies. International Journal of Molecular Sciences. 2023; 24(16):12553. https://doi.org/10.3390/ijms241612553
Chicago/Turabian StyleVila-Pueyo, Marta, Otilia Gliga, Víctor José Gallardo, and Patricia Pozo-Rosich. 2023. "The Role of Glial Cells in Different Phases of Migraine: Lessons from Preclinical Studies" International Journal of Molecular Sciences 24, no. 16: 12553. https://doi.org/10.3390/ijms241612553
APA StyleVila-Pueyo, M., Gliga, O., Gallardo, V. J., & Pozo-Rosich, P. (2023). The Role of Glial Cells in Different Phases of Migraine: Lessons from Preclinical Studies. International Journal of Molecular Sciences, 24(16), 12553. https://doi.org/10.3390/ijms241612553








