Acanthamoeba castellanii Uncoupling Protein: A Complete Sequence, Activity, and Role in Response to Oxidative Stress
Abstract
1. Introduction
2. Results
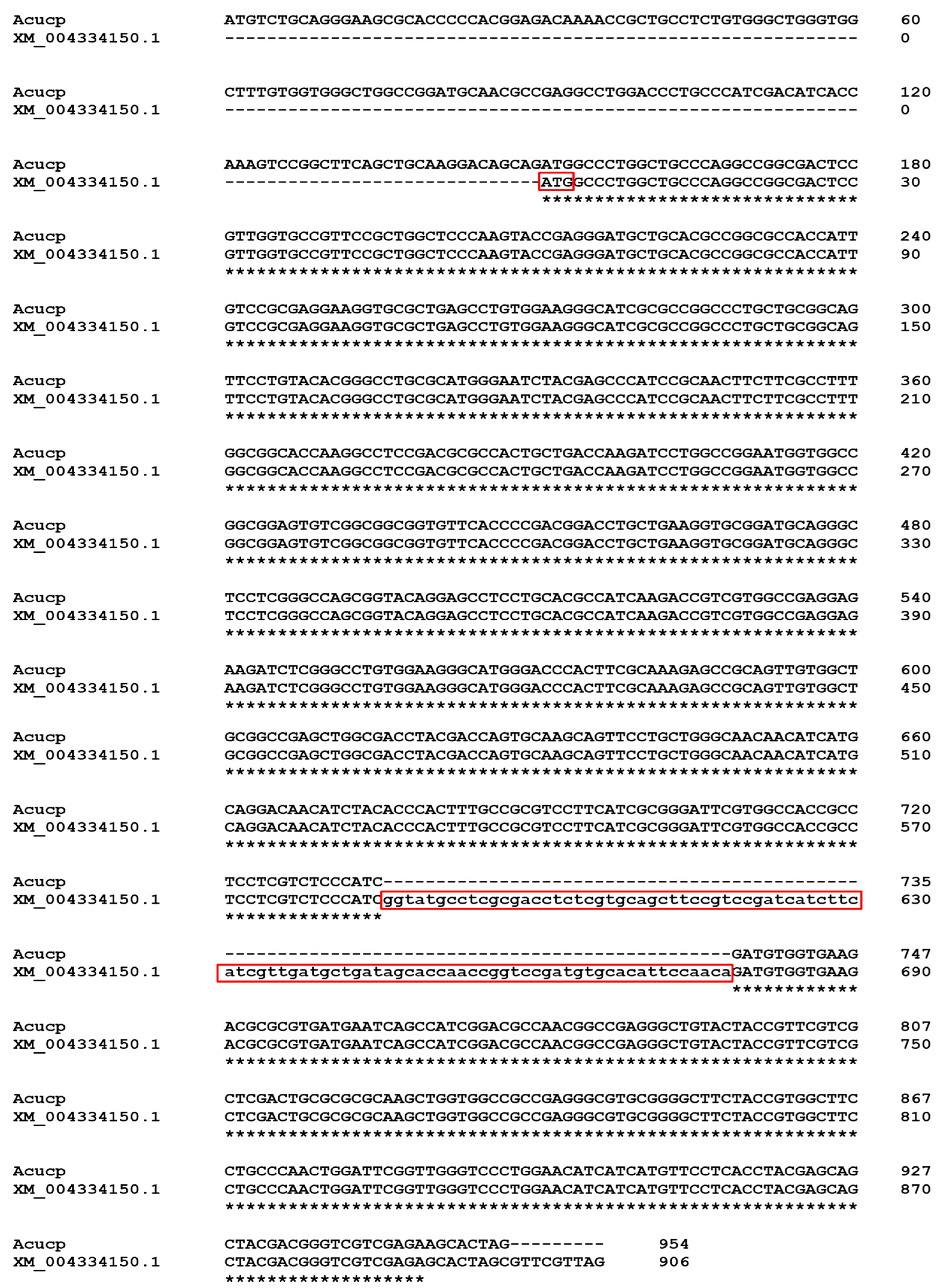
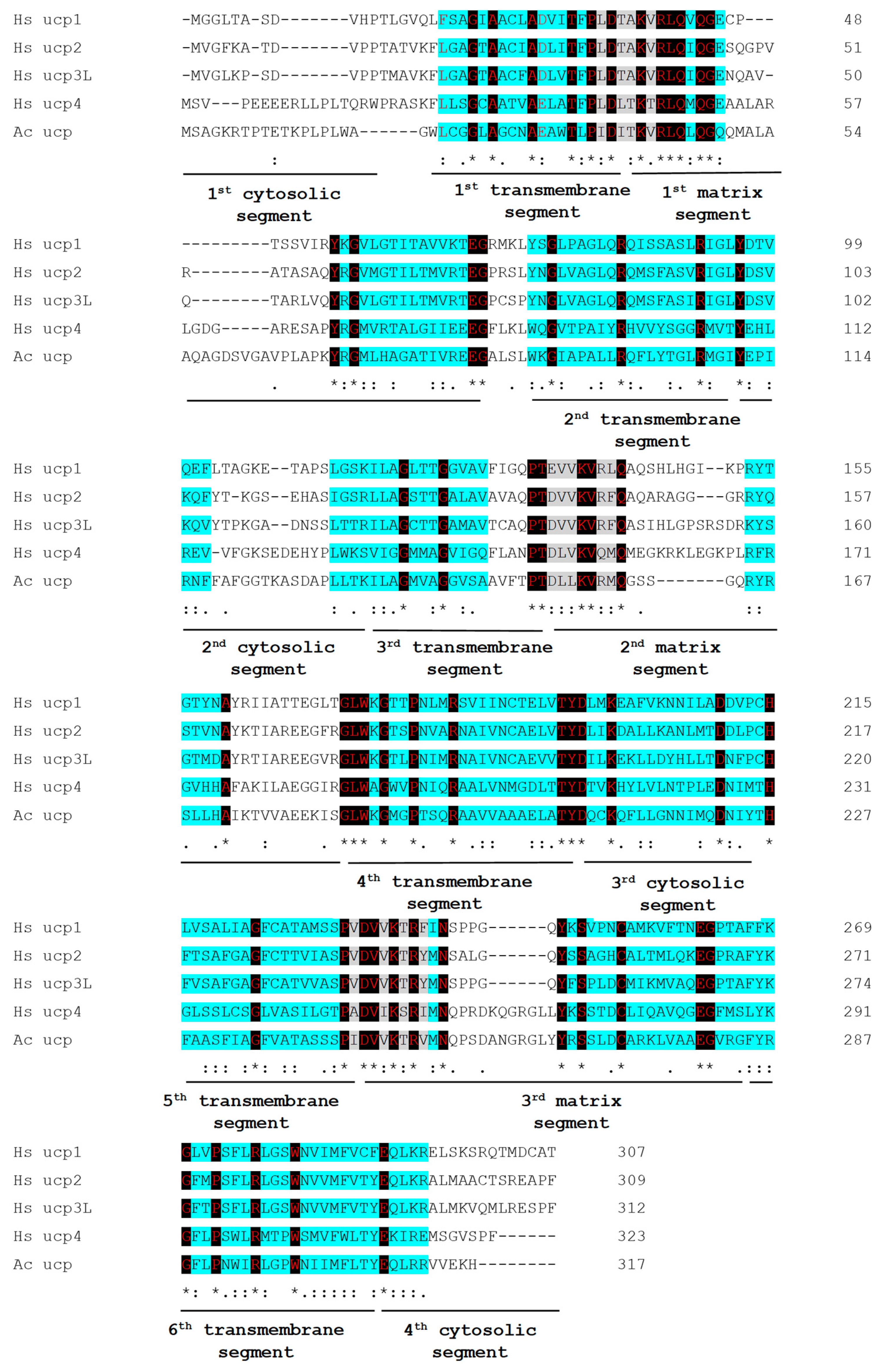
2.1. Acucp Coding Sequence and Translated Protein Sequence Analysis
2.2. Detection of Expression of AcUCP in Yeast S. cerevisiae
2.3. Effect of AcUCP Expression on Yeast Growth
2.4. The Effect of Protonophoric Activity of AcUCP on Oxygen Uptake and Membrane Potential in Yeast Mitochondria
2.5. AcUCP Expression Does Not Change Theoxaloacetate and Dicarboxylate Transport Activity in Yeast Mitochondria
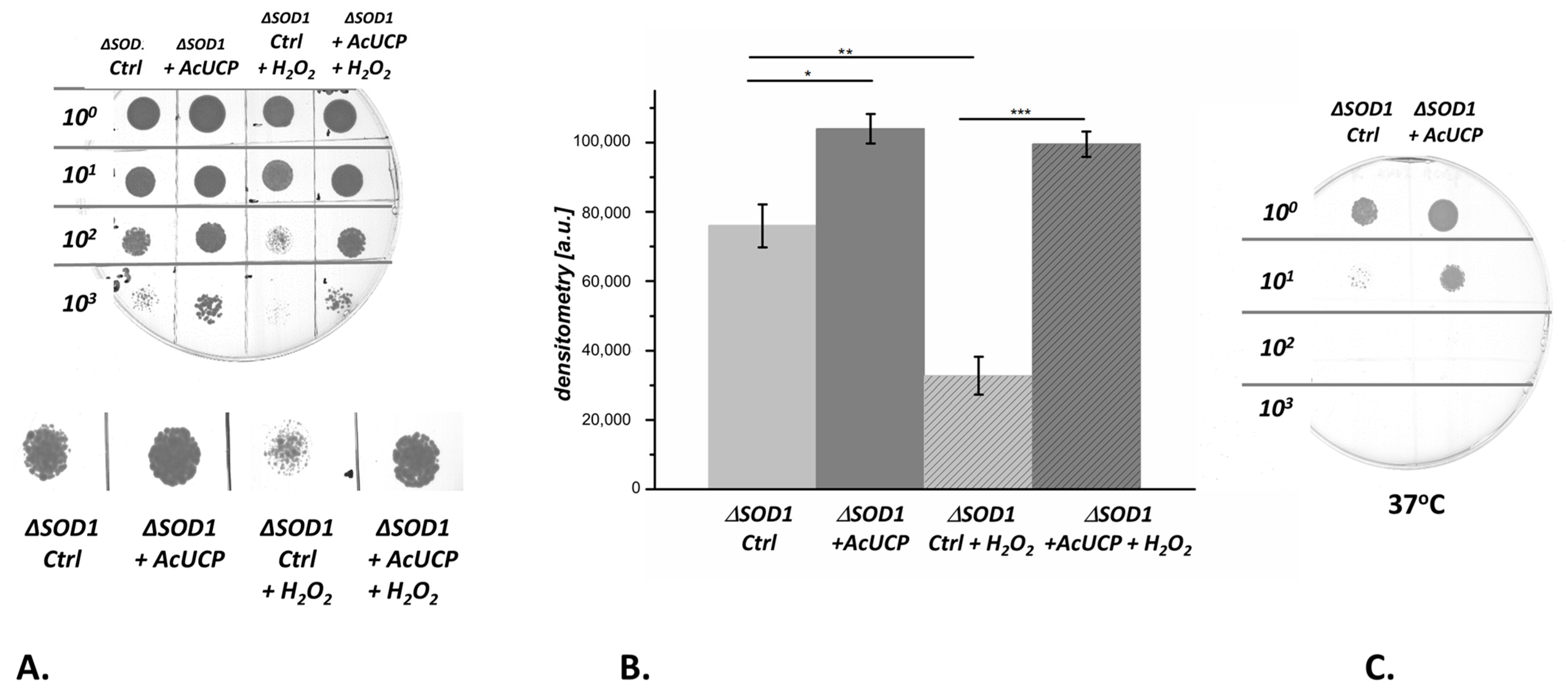
2.6. The Influence of AcUCP Expression on Yeast Oxidative Stress Response
2.7. Respiration of ΔSOD1 Control Cells and ΔSOD1 Cells Expressing AcUCP
2.8. Superoxide Anion Level in ΔSOD1 Cells Is Influenced by AcUCP Expression
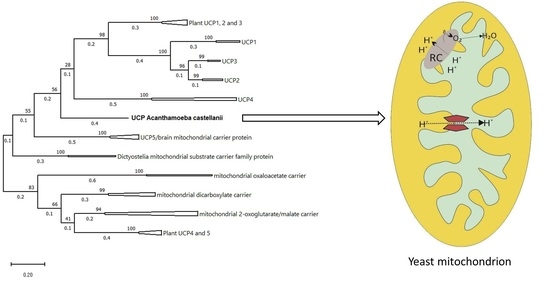
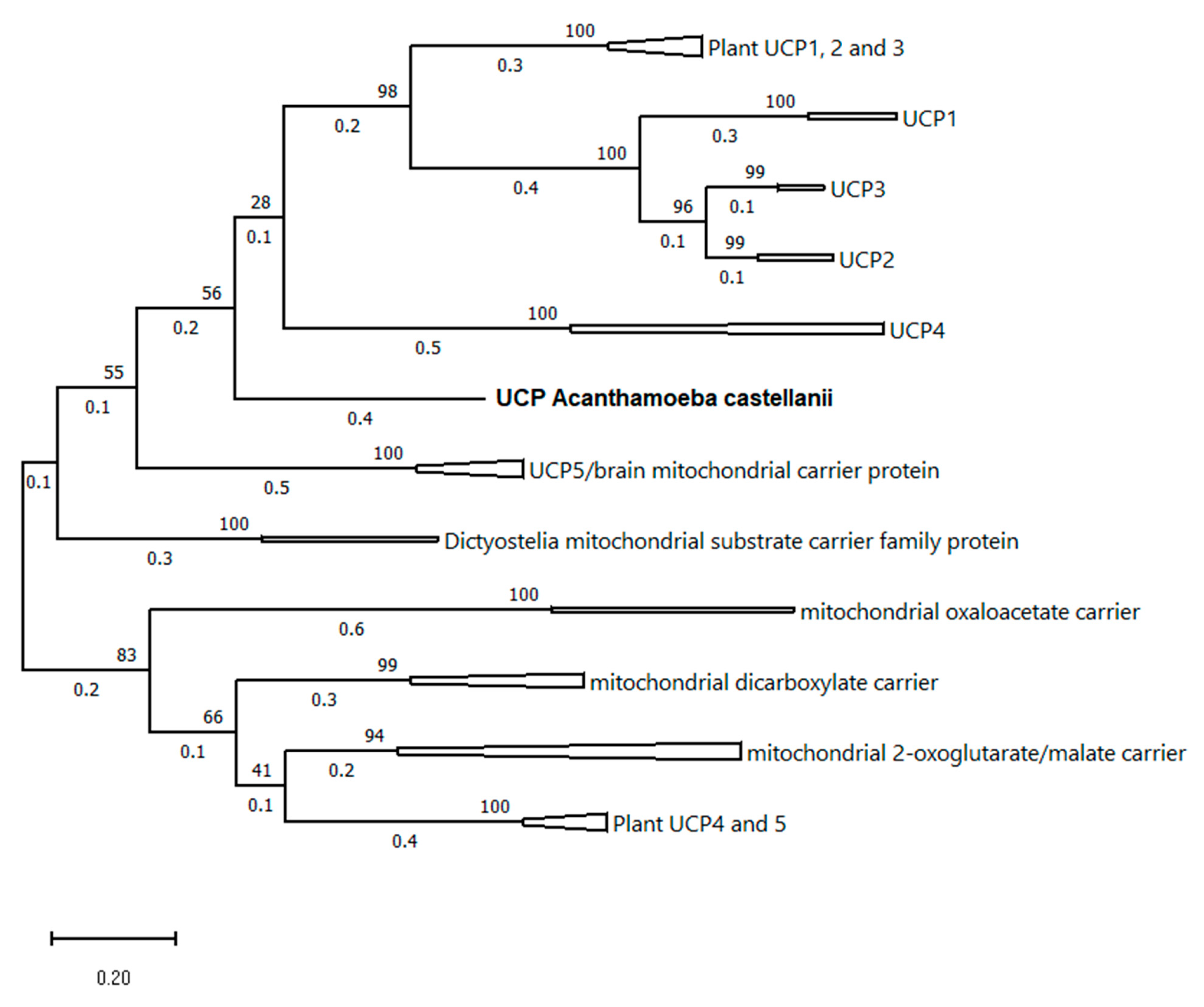
2.9. AcUCP Phylogeny
3. Discussion
4. Materials and Methods
4.1. RACE 5′ and 3′ Ends of Acucp Coding Sequence Obtaining, Cloning in pYES2 Vector, Yeast Transformation
4.2. Yeast Culture and Isolation of Mitochondria
4.3. AcUCP Immunological Detection
4.4. Mitochondrial Oxygen Consumption and Membrane Potential Measurements
4.5. Mitochondrial Transport of Succinate and Sulfate
4.6. Viability of Yeast Cells under Oxidative Stress
4.7. Superoxide Anion Radical Level Measurement Using MitoSoxRed Fluorescent Dye
4.8. Statistical Analysis
4.9. Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woyda-Ploszczyca, A.M.; Jarmuszkiewicz, W. The conserved regulation of mitochondrial uncoupling proteins: From unicellular eukaryotes to mammals. Biochim. Biophys. Acta 2017, 1858, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, A.; Smith, M.D.; Jelokhani-Niaraki, M. Uncoupling proteins and regulated proton leak in mitochondria. Int. J. Mol. Sci. 2022, 23, 1528. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, M. Mechanism and evolution of the uncoupling protein of brown adipose tissue. Trends Biochem. Sci. 1990, 15, 108–112. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Rial, E. A history of the first uncoupling protein, UCP1. J. Bioenerg. Biomembr. 1999, 31, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Fleury, C.; Neverova, M.; Collins, S.; Raimbault, S.; Champigny, O.; Levi-Meyrueis, C.; Bouillaud, F.; Seldin, M.F.; Surwit, R.S.; Ricquier, D.; et al. Uncoupling protein-2: A novel gene linked to obesity and hyperinsulinemia. Nat. Genet. 1997, 15, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Boss, O.; Samec, S.; Paoloni-Giacobino, A.; Rossier, C.; Dulloo, A.; Seydoux, J.; Muzzin, P.; Giacobino, J.P. Uncoupling protein-3: A new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 1997, 408, 39–42. [Google Scholar] [CrossRef]
- Mao, W.; Yu, X.X.; Zhong, A.; Li, W.; Brush, J.; Sherwood, S.W.; Adams, S.H.; Pan, G. UCP4, a novel brain-specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Lett. 1999, 443, 326–330. [Google Scholar] [CrossRef]
- Yu, X.X.; Mao, W.; Zhong, A.; Schow, P.; Brush, J.; Sherwood, S.W.; Adams, S.H.; Pan, G. Characterization of novel UCP5/BMCP1 isoforms and differential regulation of UCP4 and UCP5 expression through dietary or temperature manipulation. FASEB J. 2000, 14, 1611–1618. [Google Scholar] [CrossRef]
- Vianna, C.R.; Hagen, T.; Zhang, C.Y.; Bachman, E.; Boss, O.; Gereben, B.; Moriscot, A.S.; Lowell, B.B.; Bicudo, J.E.; Bianco, A.C. Cloning and functional characterization of an uncoupling protein homolog in hummingbirds. Physiol. Genom. 2001, 5, 137–145. [Google Scholar] [CrossRef]
- Jastroch, M.; Wuertz, S.; Kloas, W.; Klingenspor, M. Uncoupling protein 1 in fish uncovers an ancient evolutionary history of mammalian nonshivering thermogenesis. Physiol. Genom. 2005, 222, 150–156. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Sokolov, E.P. Evolution of mitochondrial uncoupling proteins: Novel invertebrate UCP homologues suggest early evolutionary divergence of the UCP family. FEBS Lett. 2005, 579, 313–317. [Google Scholar] [CrossRef]
- Vercesi, A.E.; Borecký, J.; Maia Ide, G.; Arruda, P.; Cuccovia, I.M.; Chaimovich, H. Plant uncoupling mitochondrial proteins. Annu. Rev. Plant Biol. 2006, 57, 383–404. [Google Scholar] [CrossRef]
- Hughes, J.; Criscuolo, F. Evolutionary history of the UCP gene family: Gene duplication and selection. BMC Evol. Biol. 2008, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Rey, B.; Sibille, B.; Romestaing, C.; Belouze, M.; Letexier, D.; Servais, S.; Barre, H.; Duchamp, C.; Voituron, Y. Reptilian uncoupling protein: Functionality and expression in sub-zero temperatures. J. Exp. Biol. 2008, 211, 1456–1462. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jarmuszkiewicz, W.; Woyda-Ploszczyca, A.; Antos-Krzeminska, N.; Sluse, F.E. Mitochondrial uncoupling proteins in unicellular eukaryotes. Biochim. Biophys. Acta 2010, 1797, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Slocinska, M.; Barylski, J.; Jarmuszkiewicz, W. Uncoupling proteins of invertebrates: A review. IUBMB Life 2016, 68, 691–699. [Google Scholar] [CrossRef]
- Nicholls, D.G. Mitochondrial proton leaks and uncoupling proteins. Biochim. Biophys. Acta Bioenerg. 2021, 18627, 148428. [Google Scholar] [CrossRef]
- Jarmuszkiewicz, W.; Sluse-Goffart, C.M.; Hryniewiecka, L.; Sluse, F.E. Identification and characterization of a protozoan uncoupling protein in Acanthamoeba castellanii. J. Biol. Chem. 1999, 274, 23198–23202. [Google Scholar] [CrossRef]
- Jarmuszkiewicz, W.; Antos, N.; Swida, A.; Czarna, M.; Sluse, F.E. The effect of growth at low temperature on the activity and expression of the uncoupling protein in Acanthamoeba castellanii mitochondria. FEBS Lett. 2004, 569, 178–184. [Google Scholar] [CrossRef][Green Version]
- Jarmuszkiewicz, W.; Swida, A.; Czarna, M.; Antos, N.; Sluse-Goffart, C.M.; Sluse, F.E. In phosphorylating Acanthamoeba castellanii mitochondria the sensitivity of uncoupling protein activity to GTP depends on the redox state of quinone. J. Bioenerg. Biomembr. 2005, 37, 97–107. [Google Scholar] [CrossRef]
- Swida, A.; Czarna, M.; Woyda-Płoszczyca, A.; Kicinska, A.; Sluse, F.E.; Jarmuszkiewicz, W. Fatty acid efficiency profile in uncoupling of Acanthamoeba castellanii mitochondria. J. Bioenerg. Biomembr. 2007, 39, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Woyda-Ploszczyca, A.M.; Jarmuszkiewicz, W. Hydroxynonenal, a lipid peroxidation end product, stimulates uncoupling protein activity in Acanthamoeba castellanii mitochondria; the sensitivity of the inducible activity to purine nucleotides depends on the membranous ubiquinone redox state. J. Bioenerg. Biomembr. 2012, 44, 525–538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Woyda-Ploszczyca, A.; Jarmuszkiewicz, W. Hydroxynonenal-stimulated activity of the uncoupling protein in Acanthamoeba castellanii mitochondria under phosphorylating conditions. Biol. Chem. 2013, 394, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Swida, A.; Woyda-Ploszczyca, A.; Jarmuszkiewicz, W. Redox state of quinone affects sensitivity of Acanthamoeba castellanii mitochondrial uncoupling protein to purine nucleotides. Biochem. J. 2008, 413, 359–367. [Google Scholar] [CrossRef][Green Version]
- Woyda-Ploszczyca, A.; Jarmuszkiewicz, W. Ubiquinol QH2 functions as a negative regulator of purine nucleotide inhibition of Acanthamoeba castellanii mitochondrial uncoupling protein. Biochim. Biophys. Acta 2011, 1807, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Jarmuszkiewicz, W.; Navet, R.; Alberici, L.C.; Douette, P.; Sluse-Goffart, C.M.; Sluse, E.; Vercesi, A.E. Redox state of endogenous coenzyme q modulates the inhibition of linoleic acid-induced uncoupling by guanosine triphosphate in isolated skeletal muscle mitochondria. J. Bioenerg. Biomembr. 2004, 36, 493–502. [Google Scholar] [CrossRef]
- Swida-Barteczka, A.; Woyda-Ploszczyca, A.; Sluse, F.E.; Jarmuszkiewicz, W. Uncoupling protein 1 inhibition by purine nucleotides is under the control of the endogenous ubiquinone redox state. Biochem. J. 2009, 424, 297–306. [Google Scholar] [CrossRef]
- Navet, R.; Douette, P.; Puttine-Marique, F.; Sluse-Goffart, C.M.; Jarmuszkiewicz, W.; Sluse, F.E. Regulation of uncoupling protein activity in phosphorylating potato tuber mitochondria. FEBS Lett. 2005, 579, 4437–4442. [Google Scholar] [CrossRef] [PubMed]
- Czarna, M.; Jarmuszkiewicz, W. Activation of alternative oxidase and uncoupling protein lowers hydrogen peroxide formation in amoeba Acanthamoeba castellanii mitochondria. FEBS Lett. 2005, 579, 3136–3140. [Google Scholar] [CrossRef]
- Czarna, M.; Sluse, F.E.; Jarmuszkiewicz, W. Mitochondrial function plasticity in Acanthamoeba castellanii during growth in batch culture. J. Bioenerg. Biomembr. 2007, 39, 149–157. [Google Scholar] [CrossRef]
- Cavalheiro, R.A.; Fortes, F.; Borecky, J.; Faustinoni, V.C.; Schreiber, A.Z.; Vercesi, A.E. Respiration, oxidative phosphorylation, and uncoupling protein in Candida albicans. Braz. J. Med. Biol. Res. 2004, 37, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Jarmuszkiewicz, W.; Milani, G.; Fortes, F.; Schreiber, A.Z.; Sluse, F.E.; Vercesi, A.E. First evidence and characterization of an uncoupling protein in fungi kingdom: CpUCP of Candida parapsilosis. FEBS Lett. 2000, 467, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Luevano-Martinez, L.A.; Moyano, E.; de Lacoba, M.G.; Rial, E.; Uribe-Carvajal, S. Identification of the mitochondrial carrier that provides Yarrowia lipolytica with a fatty acid-induced and nucleotide-sensitive uncoupling protein-like activity. Biochim. Biophys. Acta 2010, 1797, 81–88. [Google Scholar] [CrossRef]
- Uyemura, S.A.; Luo, S.; Moreno, S.N.; Docampo, R. Oxidative phosphorylation, Ca2+ transport, and fatty acid-induced uncoupling in malaria parasites mitochondria. J. Biol. Chem. 2000, 275, 9709–9715. [Google Scholar] [CrossRef] [PubMed]
- Uyemura, S.A.; Luo, S.; Vieira, M.; Moreno, S.N.; Docampo, R. Oxidative phosphorylation and rotenone-insensitive malate- and NADH-quinone oxidoreductases in Plasmodium yoelii yoelii mitochondria in situ. J. Biol. Chem. 2004, 279, 385–393. [Google Scholar] [CrossRef]
- Jarmuszkiewicz, W.; Behrendt, M.; Navet, R.; Sluse, F.E. Uncoupling protein and alternative oxidase of Dictyostelium discoideum: Occurrence, properties and protein expression during vegetative life and starvation-induced early development. FEBS Lett. 2002, 532, 459–464. [Google Scholar] [CrossRef]
- Eichinger, L.; Pachebat, J.A.; Glockner, G.; Rajandream, M.A.; Sucgang, R.; Berriman, M.; Song, J.; Olsen, R.; Szafranski, K.; Xu, Q.; et al. The genome of the social amoeba Dictyostelium discoideum. Nature 2005, 435, 43–57. [Google Scholar] [CrossRef]
- Jarmuszkiewicz, W.; Wagner, A.M.; Wagner, M.J.; Hryniewiecka, L. Immunological identification of the alternative oxidase of Acanthamoeba castellanii mitochondria. FEBS Lett. 1997, 411, 110–114. [Google Scholar] [CrossRef]
- Antos-Krzeminska, N.; Jarmuszkiewicz, W. External NADPH dehydrogenases in Acanthamoeba castellanii mitochondria. Protist 2014, 165, 580–593. [Google Scholar] [CrossRef]
- Clarke, M.; Lohan, A.J.; Liu, B.; Lagkouvardos, I.; Roy, S.; Zafar, N.; Bertelli, C.; Schilde, C.; Kianianmomeni, A.; Burglin, T.R.; et al. Genome of Acanthamoeba castellanii highlights extensive lateral gene transfer and early evolution of tyrosine kinase signaling. Genome Biol. 2013, 14, R11. [Google Scholar] [CrossRef]
- Hanak, P.; Jezek, P. Mitochondrial uncoupling proteins and phylogenesis-UCP4 as the ancestral uncoupling protein. FEBS Lett. 2001, 495, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Murdza-Inglis, D.L.; Patel, H.V.; Freeman, K.B.; Jezek, P.; Orosz, D.E.; Garlid, K.D. Functional reconstitution of rat uncoupling protein following its high level expression in yeast. J. Biol. Chem. 1991, 26618, 11871–11875. [Google Scholar] [CrossRef]
- Ito, K.; Matsukawa, K.; Kato, Y. Functional analysis of skunk cabbage SfUCPB, a unique uncoupling protein lacking the fifth transmembrane domain, in yeast cells. Biochem. Biophys. Res. Commun. 2006, 3491, 383–390. [Google Scholar] [CrossRef]
- Wang, C.; Sun, G.; Chen, K.; Lv, Z.; Peng, S.; Jiang, X.; Xiang, Y.; Zhang, C. Molecular cloning of lamprey uncoupling protein and assessment of its uncoupling activity using a yeast heterologous expression system. Mitochondrion 2010, 10, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Sun, G.; Lv, Z.; Wang, C.; Jiang, X.; Li, D.; Zhang, C. Molecular cloning of amphioxus uncoupling protein and assessment of its uncoupling activity using a yeast heterologous expression system. Biochem. Biophys. Res. Commun. 2010, 4004, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Panek, A.; Pietrow, O.; Filipkowski, P.; Synowiecki, J. Effects of the polyhistidine tag on kinetics and other properties of trehalose synthase from Deinococcus geothermalis. Acta Biochim. Pol. 2013, 60, 163–166. [Google Scholar] [CrossRef]
- Dickson, J.M.; Lee, W.J.; Shepherd, P.R.; Buchanan, C.M. Enzyme activity effects of N-terminal His-tag attached to catalytic sub-unit of phosphoinositide-3-kinase. Biosci. Rep. 2013, 336, e00079. [Google Scholar] [CrossRef]
- Freydank, A.C.; Brandt, W.; Drager, B. Protein structure modeling indicates hexahistidine-tag interference with enzyme activity. Proteins 2008, 72, 173–183. [Google Scholar] [CrossRef]
- Majorek, K.A.; Kuhn, M.L.; Chruszcz, M.; Anderson, W.F.; Minor, W. Double trouble-Buffer selection and His-tag presence may be responsible for nonreproducibility of biomedical experiments. Protein Sci. 2014, 23, 1359–1368. [Google Scholar] [CrossRef]
- Woyda-Ploszczyca, A.M.; Jarmuszkiewicz, W. Different effects of guanine nucleotides GDP and GTP on protein-mediated mitochondrial proton leak. PLoS ONE 2014, 9, e98969. [Google Scholar] [CrossRef]
- Vozza, A.; Parisi, G.; De Leonardis, F.; Lasorsa, F.M.; Castegna, A.; Amorese, D.; Marmo, R.; Calcagnile, V.M.; Palmieri, L.; Ricquier, D.; et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Luevano-Martinez, L.A. Uncoupling proteins UCP in unicellular eukaryotes: True UCPs or UCP1-like acting proteins? FEBS Lett. 2012, 586, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Gorgoglione, R.; Porcelli, V.; Santoro, A.; Daddabbo, L.; Vozza, A.; Monné, M.; Di Noia, M.A.; Palmieri, L.; Fiermonte, G.; Palmieri, F. The human uncoupling proteins 5 and 6 UCP5/SLC25A14 and UCP6/SLC25A30 transport sulfur oxyanions phosphate and dicarboxylates. Biochim. Biophys. Acta Bioenerg. 2019, 18609, 724–733. [Google Scholar] [CrossRef]
- Monné, M.; Daddabbo, L.; Gagneul, D.; Obata, T.; Hielscher, B.; Palmieri, L.; Miniero, D.V.; Fernie, A.R.; Weber, A.P.M.; Palmieri, F. Uncoupling proteins 1 and 2 UCP1 and UCP2 from Arabidopsis thaliana are mitochondrial transporters of aspartate, glutamate, and dicarboxylates. J. Biol. Chem. 2018, 29311, 4213–4227. [Google Scholar] [CrossRef]
- Palmieri, L.; Vozza, A.; Honlinger, A.; Dietmeier, K.; Palmisano, A.; Zara, V.; Palmieri, F. The mitochondrial dicarboxylate carrier is essential for the growth of Saccharomyces cerevisiae on ethanol or acetate as the sole carbon source. Mol. Microbiol. 1999, 312, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.; Vozza, A.; Agrimi, G.; De Marco, V.; Runswick, M.J.; Palmieri, F.; Walker, J.E. Identification of the yeast mitochondrial transporter for oxaloacetate and sulfate. J. Biol. Chem. 1999, 274, 22184–22190. [Google Scholar] [CrossRef]
- Stuart, J.A.; Harper, J.A.; Brindle, K.M.; Jekabsons, M.B.; Brand, M.D. A mitochondrial uncoupling artifact can be caused by expression of uncoupling protein 1 in yeast. Biochem. J. 2001, 356, 779–789. [Google Scholar] [CrossRef]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Marechal-Drouard, L.; et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef]
- Criscuolo, F.; Gonzalez-Barroso, M.d.M.; Le Maho, Y.; Ricquier, D.; Bouillaud, F. Avian uncoupling protein expressed in yeast mitochondria prevents endogenous free radical damage. Proc. Biol. Sci. 2005, 2721565, 803–810. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Brand, M.D.; Esteves, T.C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2002, 2, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Heidkaemper, D.; Winkler, E.; Müller, V.; Frischmuth, K.; Liu, Q.; Caskey, T.; Klingenberg, M. The bulk of UCP3 expressed in yeast cells is incompetent for a nucleotide regulated H+ transport. FEBS Lett. 2000, 480, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Stuart, J.A.; Harper, J.A.; Brindle, K.M.; Jekabsons, M.B.; Brand, M.D. Physiological levels of mammalian uncoupling protein 2 do not uncouple yeast mitochondria. J. Biol. Chem. 2001, 276, 18633–18639. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.A.; Stuart, J.A.; Jekabsons, M.B.; Roussel, D.; Brindle, K.M.; Dickinson, K.; Jones, R.B.; Brand, M.D. Artifactual uncoupling by uncoupling protein 3 in yeast mitochondria at the concentrations found in mouse and rat skeletal-muscle mitochondria. Biochem. J. 2002, 361, 49–56. [Google Scholar] [CrossRef]
- Samartsev, V.N.; Smirnov, A.V.; Zeldi, I.P.; Markova, O.V.; Mokhova, E.N.; Skulachev, V.P. Involvement of the aspartate/glutamate antiporter in fatty acid-induced uncoupling of liver mitochondria. Biochim. Biophys. Acta 1997, 1319, 251–257. [Google Scholar] [CrossRef]
- Wieckowski, M.R.; Wojtczak, L. Involvement of a dicarboxylate carrier in the protonophore effect of long-chain fatty acids in mitochondria. Biochem. Biophys. Res. Commun. 1997, 232, 414–417. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Hirtz, C.; Carrera, G.; Cazenave, R.; Troly, M.; Salvayre, R.; Penicaud, L.; Casteilla, L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997, 11, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; Costa, A.D.; Vercesi, A.E. Activation of the potato plant uncoupling mitochondrial protein inhibits reactive oxygen species generation by the respiratory chain. FEBS Lett. 1998, 425, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Casteilla, L.; Rigoulet, M.; Penicaud, L. Mitochondrial ROS metabolism: Modulation by uncoupling proteins. IUBMB Life 2001, 52, 181–188. [Google Scholar] [CrossRef]
- Ježek, P.; Holendová, B.; Garlid, K.D.; Jabůrek, M. Mitochondrial Uncoupling Proteins: Subtle Regulators of Cellular Redox Signaling. Antioxid. Redox Signal. 2018, 297, 667–714. [Google Scholar] [CrossRef]
- Hass, D.T.; Barnstable, C.J. Uncoupling proteins in the mitochondrial defense against oxidative stress. Prog. Retin. Eye Res. 2021, 83, 100941. [Google Scholar] [CrossRef] [PubMed]
- Hirschenson, J.; Melgar-Bermudez, E.; Mailloux, R.J. The uncoupling proteins: A systematic review on the mechanism used in the prevention of oxidative stress. Antioxidants 2022, 112, 322. [Google Scholar] [CrossRef] [PubMed]
- Woyda-Ploszczyca, A.; Koziel, A.; Antos-Krzeminska, N.; Jarmuszkiewicz, W. Impact of oxidative stress on Acanthamoeba castellanii mitochondrial bioenergetics depends on cell growth stage. J. Bioenerg. Biomembr. 2011, 43, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Keeling, P.J.; Burger, G.; Durnford, D.G.; Lang, B.F.; Lee, R.W.; Pearlman, R.E.; Roger, A.J.; Gray, M.W. The tree of eukaryotes. Trends Ecol. Evol. 2005, 20, 670–676. [Google Scholar] [CrossRef]
- Galganska, H.; Karachitos, A.; Baranek, M.; Budzinska, M.; Jordan, J.; Kmita, H. Viability of Saccharomyces cerevisiae cells following exposure to H2O2 and protective effect of minocycline depend on the presence of VDAC. Eur. J. Pharmacol. 2010, 643, 42–47. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 2153, 403–410. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 2321, 2947–2948. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antos-Krzeminska, N.; Kicinska, A.; Nowak, W.; Jarmuszkiewicz, W. Acanthamoeba castellanii Uncoupling Protein: A Complete Sequence, Activity, and Role in Response to Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 12501. https://doi.org/10.3390/ijms241512501
Antos-Krzeminska N, Kicinska A, Nowak W, Jarmuszkiewicz W. Acanthamoeba castellanii Uncoupling Protein: A Complete Sequence, Activity, and Role in Response to Oxidative Stress. International Journal of Molecular Sciences. 2023; 24(15):12501. https://doi.org/10.3390/ijms241512501
Chicago/Turabian StyleAntos-Krzeminska, Nina, Anna Kicinska, Witold Nowak, and Wieslawa Jarmuszkiewicz. 2023. "Acanthamoeba castellanii Uncoupling Protein: A Complete Sequence, Activity, and Role in Response to Oxidative Stress" International Journal of Molecular Sciences 24, no. 15: 12501. https://doi.org/10.3390/ijms241512501
APA StyleAntos-Krzeminska, N., Kicinska, A., Nowak, W., & Jarmuszkiewicz, W. (2023). Acanthamoeba castellanii Uncoupling Protein: A Complete Sequence, Activity, and Role in Response to Oxidative Stress. International Journal of Molecular Sciences, 24(15), 12501. https://doi.org/10.3390/ijms241512501