Effects of Low-Intensity Pulsed Ultrasound-Induced Blood–Brain Barrier Opening in P301S Mice Modeling Alzheimer’s Disease Tauopathies
Abstract
1. Introduction
2. Results
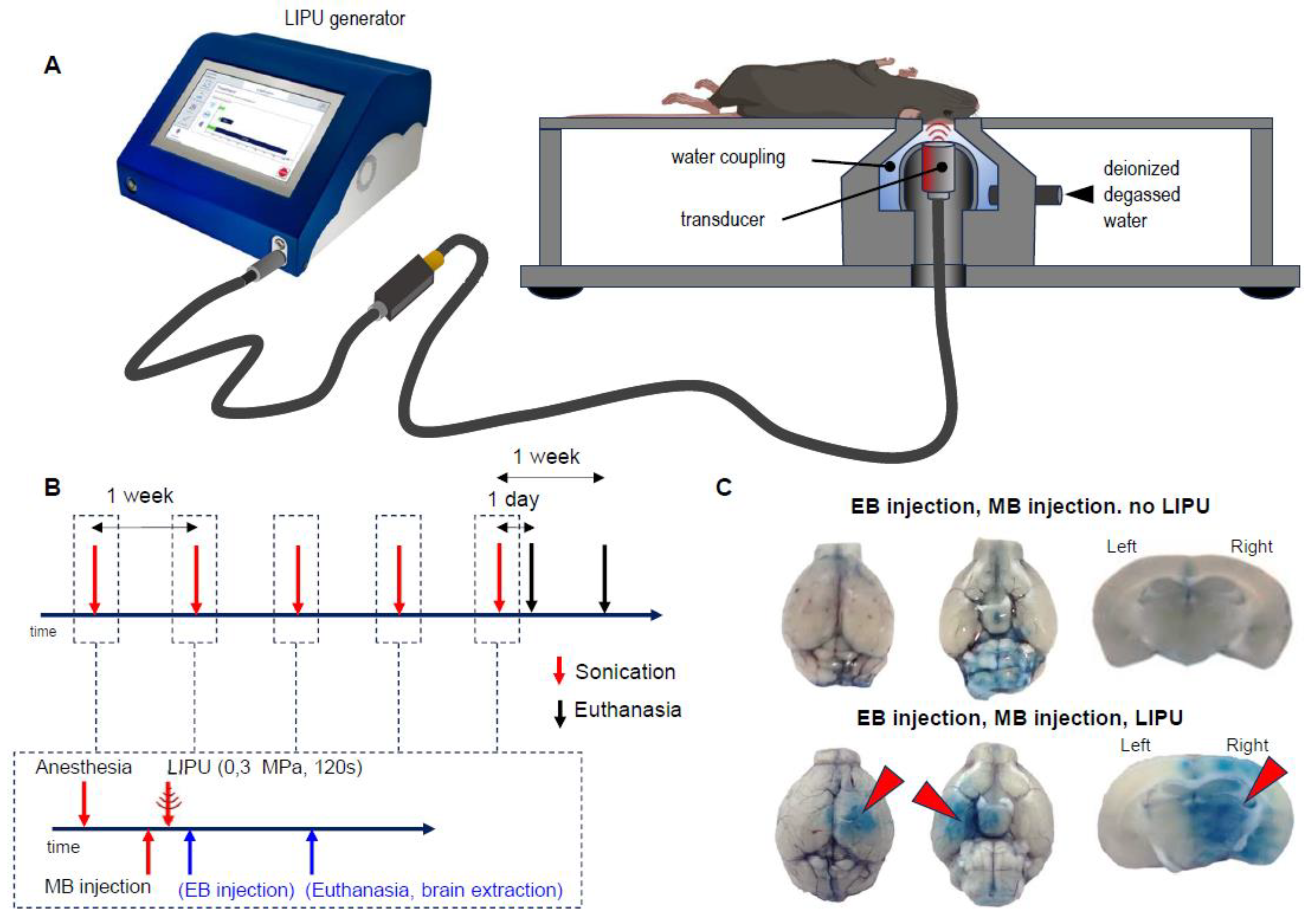
2.1. LIPU Efficiently and Reproducibly Opens the BBB
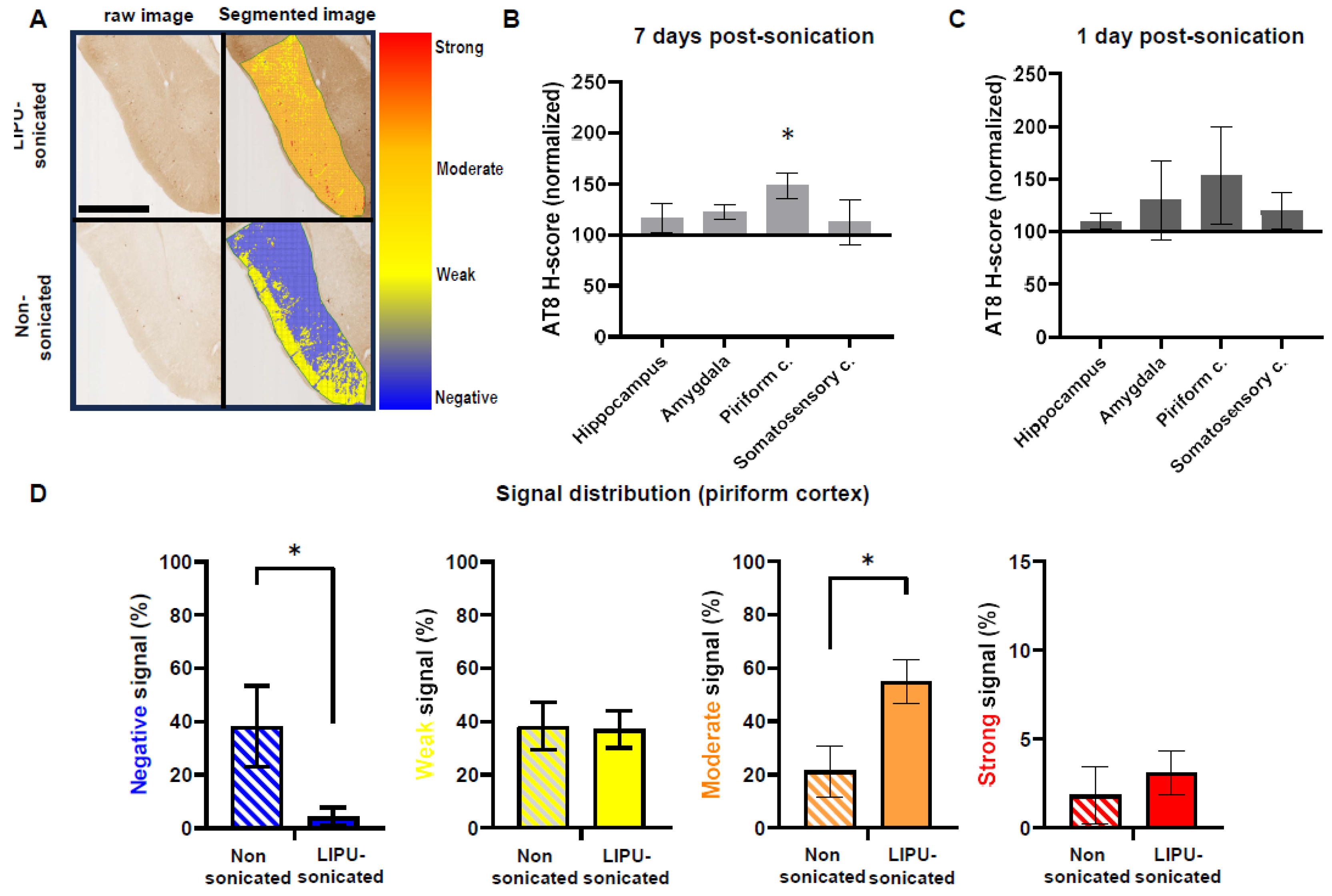
2.2. Lateralization Effect on the Levels of Immune-Staining
2.3. LIPU-Induced BBB Opening Does Not Decrease Tau Pathologies
2.4. LIPU-Mediated BBB Opening Induces Genotype-Dependent Modulation of (Micro)Glial Cells
2.4.1. Decreased Microglial Densities in P301S Tau Transgenic Mice
2.4.2. Increased Microglial Loads in Wild-Type Mice
3. Discussion
3.1. Summary of Results
3.2. Efficiency and Safety of LIPU-Induced BBB Opening
3.3. Comparison with Previous Studies of Ultrasound-Induced BBB Opening in AD Mouse Models
3.4. Hypotheses to Explain the Impact of LIPUs on Microglia and Tau
3.5. Limits
4. Materials and Methods
4.1. Animals
4.2. Low-Intensity Pulsed Ultrasound BBB Opening Procedure
4.3. BBB Opening Assessment
4.4. Mouse Brain Tissue Preparation
4.5. Immunohistochemistry
4.6. Microscopic Morphological Analysis
- (1)
- Segmentation of all ROI in superpixels, which adapt their shape to local staining contrasts instead of using a rigid and regularly spaced grid. We made use of the 10 µm QuPath tool ‘SLIC superpixels’;
- (2)
- Intensity analysis of each superpixel was classified on the basis of thresholds remaining constant across images of the same experience, including sonicated and non-sonicated mice: no signal (negative), weak signal, moderate signal, strong signal;
- (3)
- Calculation of an H-score [47] as follows:
- SPW:
- number of superpixels with weak signals;
- SPM:
- number of superpixels with moderate signals;
- SPH:
- number of superpixels with high signals;
- SPT:
- number of superpixels (total).
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, M. World Alzheimer Report 2015, The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Cummings, J. Anti-Amyloid Monoclonal Antibodies Are Transformative Treatments That Redefine Alzheimer’s Disease Therapeutics. Drugs 2023, 83, 569–576. [Google Scholar] [CrossRef]
- Villain, N.; Planche, V.; Levy, R. High-Clearance Anti-Amyloid Immunotherapies in Alzheimer’s Disease. Part 1: Meta-Analysis and Review of Efficacy and Safety Data, and Medico-Economical Aspects. Rev. Neurol. 2022, 178, 1011–1030. [Google Scholar] [CrossRef]
- Salloway, S.; Chalkias, S.; Barkhof, F.; Burkett, P.; Barakos, J.; Purcell, D.; Suhy, J.; Forrestal, F.; Tian, Y.; Umans, K.; et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients with Early Alzheimer Disease. JAMA Neurol. 2022, 79, 13–21. [Google Scholar] [CrossRef]
- Espay, A.J.; Herrup, K.; Daly, T. Finding the Falsification Threshold of the Toxic Proteinopathy Hypothesis in Neurodegeneration. Handb. Clin. Neurol. 2023, 192, 143–154. [Google Scholar] [CrossRef]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Tredici, K.D.; et al. Correlation of Alzheimer Disease Neuropathologic Changes with Cognitive Status: A Review of the Literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s Disease Drug Development Pipeline: 2023. Alzheimer’s Dement. Transl. Res. Clin. Interv. (TRCI) 2023, 9, e12385. [Google Scholar] [CrossRef]
- Mummery, C.J.; Börjesson-Hanson, A.; Blackburn, D.J.; Vijverberg, E.G.B.; De Deyn, P.P.; Ducharme, S.; Jonsson, M.; Schneider, A.; Rinne, J.O.; Ludolph, A.C.; et al. Tau-Targeting Antisense Oligonucleotide MAPTRx in Mild Alzheimer’s Disease: A Phase 1b, Randomized, Placebo-Controlled Trial. Nat. Med. 2023, 29, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Teng, E.; Manser, P.T.; Pickthorn, K.; Brunstein, F.; Blendstrup, M.; Sanabria Bohorquez, S.; Wildsmith, K.R.; Toth, B.; Dolton, M.; Ramakrishnan, V.; et al. Safety and Efficacy of Semorinemab in Individuals with Prodromal to Mild Alzheimer Disease. JAMA Neurol. 2022, 79, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Bohrmann, B.; Baumann, K.; Benz, J.; Gerber, F.; Huber, W.; Knoflach, F.; Messer, J.; Oroszlan, K.; Rauchenberger, R.; Richter, W.F.; et al. Gantenerumab: A Novel Human Anti-Aβ Antibody Demonstrates Sustained Cerebral Amyloid-β Binding and Elicits Cell-Mediated Removal of Human Amyloid-β. J. Alzheimers Dis. 2012, 28, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2019, 11, 373. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR Imaging–Guided Focal Opening of the Blood-Brain Barrier in Rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Sheikov, N.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.; Hynynen, K. Cellular Mechanisms of the Blood-Brain Barrier Opening Induced by Ultrasound in Presence of Microbubbles. Ultrasound Med. Biol. 2004, 30, 979–989. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of Focused Ultrasound Applied with an Ultrasound Contrast Agent on the Tight Junctional Integrity of the Brain Microvascular Endothelium. Ultrasound Med. Biol. 2008, 34, 1093–1104. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Dmello, C.; Chen, L.; Arrieta, V.A.; Gonzalez-Buendia, E.; Kane, J.R.; Magnusson, L.P.; Baran, A.; James, C.D.; Horbinski, C.; et al. Ultrasound-Mediated Delivery of Paclitaxel for Glioma: A Comparative Study of Distribution, Toxicity, and Efficacy of Albumin-Bound Versus Cremophor Formulations. Clin. Cancer Res. 2020, 26, 477–486. [Google Scholar] [CrossRef]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.-Y.; et al. Clinical Trial of Blood-Brain Barrier Disruption by Pulsed Ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood–Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Baik, K.; Jeon, S.; Chang, W.S.; Ye, B.S.; Chang, J.W. Extensive Frontal Focused Ultrasound Mediated Blood–Brain Barrier Opening for the Treatment of Alzheimer’s Disease: A Proof-of-Concept Study. Transl. Neurodegener. 2021, 10, 44. [Google Scholar] [CrossRef]
- Rezai, A.R.; Ranjan, M.; Haut, M.W.; Carpenter, J.; D’Haese, P.-F.; Mehta, R.I.; Najib, U.; Wang, P.; Claassen, D.O.; Chazen, J.L.; et al. Focused Ultrasound–Mediated Blood-Brain Barrier Opening in Alzheimer’s Disease: Long-Term Safety, Imaging, and Cognitive Outcomes. J. Neurosurg. 2022, 139, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, S.; Burgos, N.; Canney, M.; Matthews, D.; Houot, M.; Santin, M.D.; Desseaux, C.; Bouchoux, G.; Stroer, S.; Martin, C.; et al. Pilot Study of Repeated Blood-Brain Barrier Disruption in Patients with Mild Alzheimer’s Disease with an Implantable Ultrasound Device. Alzheimers Res. Ther. 2022, 14, 40. [Google Scholar] [CrossRef]
- Dubey, S.; Heinen, S.; Krantic, S.; McLaurin, J.; Branch, D.R.; Hynynen, K.; Aubert, I. Clinically Approved IVIg Delivered to the Hippocampus with Focused Ultrasound Promotes Neurogenesis in a Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2020, 117, 32691–32700. [Google Scholar] [CrossRef]
- Jordão, J.F.; Ayala-Grosso, C.A.; Markham, K.; Huang, Y.; Chopra, R.; McLaurin, J.; Hynynen, K.; Aubert, I. Antibodies Targeted to the Brain with Image-Guided Focused Ultrasound Reduces Amyloid-β Plaque Load in the TgCRND8 Mouse Model of Alzheimer’s Disease. PLoS ONE 2010, 5, e10549. [Google Scholar] [CrossRef]
- Nisbet, R.M.; Van der Jeugd, A.; Leinenga, G.; Evans, H.T.; Janowicz, P.W.; Götz, J. Combined Effects of Scanning Ultrasound and a Tau-Specific Single Chain Antibody in a Tau Transgenic Mouse Model. Brain 2017, 140, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Leinenga, G.; Koh, W.K.; Götz, J. A Comparative Study of the Effects of Aducanumab and Scanning Ultrasound on Amyloid Plaques and Behavior in the APP23 Mouse Model of Alzheimer Disease. Alz. Res. Ther. 2021, 13, 76. [Google Scholar] [CrossRef]
- Jordão, J.F.; Thévenot, E.; Markham-Coultes, K.; Scarcelli, T.; Weng, Y.-Q.; Xhima, K.; O’Reilly, M.; Huang, Y.; McLaurin, J.; Hynynen, K.; et al. Amyloid-β Plaque Reduction, Endogenous Antibody Delivery and Glial Activation by Brain-Targeted, Transcranial Focused Ultrasound. Exp. Neurol. 2013, 248, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Leinenga, G.; Götz, J. Scanning Ultrasound Removes Amyloid-β and Restores Memory in an Alzheimer’s Disease Mouse Model. Sci. Transl. Med. 2015, 7, 278ra33. [Google Scholar] [CrossRef]
- Burgess, A.; Dubey, S.; Yeung, S.; Hough, O.; Eterman, N.; Aubert, I.; Hynynen, K. Alzheimer Disease in a Mouse Model: MR Imaging-Guided Focused Ultrasound Targeted to the Hippocampus Opens the Blood-Brain Barrier and Improves Pathologic Abnormalities and Behavior. Radiology 2014, 273, 736–745. [Google Scholar] [CrossRef]
- Karakatsani, M.E.; Kugelman, T.; Ji, R.; Murillo, M.; Wang, S.; Niimi, Y.; Small, S.A.; Duff, K.E.; Konofagou, E.E. Unilateral Focused Ultrasound-Induced Blood-Brain Barrier Opening Reduces Phosphorylated Tau from The RTg4510 Mouse Model. Theranostics 2019, 9, 5396–5411. [Google Scholar] [CrossRef]
- Bajracharya, R.; Cruz, E.; Götz, J.; Nisbet, R.M. Ultrasound-Mediated Delivery of Novel Tau-Specific Monoclonal Antibody Enhances Brain Uptake but Not Therapeutic Efficacy. J. Control. Release 2022, 349, 634–648. [Google Scholar] [CrossRef]
- Pandit, R.; Leinenga, G.; Götz, J. Repeated Ultrasound Treatment of Tau Transgenic Mice Clears Neuronal Tau by Autophagy and Improves Behavioral Functions. Theranostics 2019, 9, 3754–3767. [Google Scholar] [CrossRef] [PubMed]
- Mathon, B.; Navarro, V.; Lecas, S.; Roussel, D.; Charpier, S.; Carpentier, A. Safety Profile of Low-Intensity Pulsed Ultrasound–Induced Blood–Brain Barrier Opening in Non-Epileptic Mice and in a Mouse Model of Mesial Temporal Lobe Epilepsy. Ultrasound Med. Biol. 2023, 49, 1327–1336. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Hernández-Verdin, I.; Quissac, E.; Lemaire, N.; Guerin, C.; Guyonnet, L.; Zahr, N.; Mouton, L.; Santin, M.; Petiet, A.; et al. Low-Intensity Pulsed Ultrasound-Mediated Blood-Brain Barrier Opening Increases Anti-Programmed Death-Ligand 1 Delivery and Efficacy in Gl261 Mouse Model. Pharmaceutics 2023, 15, 455. [Google Scholar] [CrossRef]
- Baseri, B.; Choi, J.J.; Tung, Y.-S.; Konofagou, E.E. Multi-Modality Safety Assessment of Blood-Brain Barrier Opening Using Focused Ultrasound and Definity Microbubbles: A Short-Term Study. Ultrasound Med. Biol. 2010, 36, 1445–1459. [Google Scholar] [CrossRef]
- Liu, X.; Naomi, S.S.M.; Sharon, W.L.; Russell, E.J. The Applications of Focused Ultrasound (FUS) in Alzheimer’s Disease Treatment: A Systematic Review on Both Animal and Human Studies. Aging Dis. 2021, 12, 1977–2002. [Google Scholar] [CrossRef] [PubMed]
- Leinenga, G.; Götz, J. Safety and Efficacy of Scanning Ultrasound Treatment of Aged APP23 Mice. Front. Neurosci. 2018, 12, 55. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.-M.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M.-Y. Synapse Loss and Microglial Activation Precede Tangles in a P301S Tauopathy Mouse Model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Terwel, D.; Lasrado, R.; Snauwaert, J.; Vandeweert, E.; Van Haesendonck, C.; Borghgraef, P.; Van Leuven, F. Changed Conformation of Mutant Tau-P301L Underlies the Moribund Tauopathy, Absent in Progressive, Nonlethal Axonopathy of Tau-4R/2N Transgenic Mice. J. Biol. Chem. 2005, 280, 3963–3973. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, Y.; Park, E.-J.; Kwon, S.; Kim, H.; Lee, J.Y.; Lee, D.S. Improvement of Glymphatic-Lymphatic Drainage of Beta-Amyloid by Focused Ultrasound in Alzheimer’s Disease Model. Sci. Rep. 2020, 10, 16144. [Google Scholar] [CrossRef]
- Bolós, M.; Llorens-Martín, M.; Perea, J.R.; Jurado-Arjona, J.; Rábano, A.; Hernández, F.; Avila, J. Absence of CX3CR1 Impairs the Internalization of Tau by Microglia. Mol. Neurodegener. 2017, 12, 59. [Google Scholar] [CrossRef]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of Microglia and Inhibition of Exosome Synthesis Halt Tau Propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Maphis, N.; Xu, G.; Kokiko-Cochran, O.N.; Jiang, S.; Cardona, A.; Ransohoff, R.M.; Lamb, B.T.; Bhaskar, K. Reactive Microglia Drive Tau Pathology and Contribute to the Spreading of Pathological Tau in the Brain. Brain 2015, 138, 1738–1755. [Google Scholar] [CrossRef]
- Clavaguera, F.; Bolmont, T.; Crowther, R.A.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M.; et al. Transmission and Spreading of Tauopathy in Transgenic Mouse Brain. Nat. Cell Biol. 2009, 11, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.M.O.; Whitesell, J.D.; Bhagwat, N.; Thomas, T.L.; Ajay, A.D.; Nawaby, A.; Delatour, B.; LaFaye, P.; Knox, J.E.; Harris, J.A.; et al. Endogenous Pathology in Tauopathy Mice Progresses via Brain Networks|BioRxiv. Available online: https://www.biorxiv.org/content/10.1101/2023.05.23.541792v1 (accessed on 22 June 2023).
- Holmes, B.B.; Furman, J.L.; Mahan, T.E.; Yamasaki, T.R.; Mirbaha, H.; Eades, W.C.; Belaygorod, L.; Cairns, N.J.; Holtzman, D.M.; Diamond, M.I. Proteopathic Tau Seeding Predicts Tauopathy In Vivo. Proc. Natl. Acad. Sci. USA 2014, 111, E4376–E4385. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, Y.; Feng, X.; Jia, M.; Ai, N.; Dong, Y.; Zheng, Y.; Fu, L.; Yu, B.; Zhang, H.; et al. The Behavioural and Neuropathologic Sexual Dimorphism and Absence of MIP-3α in Tau P301S Mouse Model of Alzheimer’s Disease. J. Neuroinflamm. 2020, 17, 72. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Meyerholz, D.K.; Beck, A.P. Principles and Approaches for Reproducible Scoring of Tissue Stains in Research. Lab. Investig. 2018, 98, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Firulyova, M.; Manis, M.; Herz, J.; Smirnov, I.; Aladyeva, E.; Wang, C.; Bao, X.; Finn, M.B.; Hu, H.; et al. Microglia-Mediated T Cell Infiltration Drives Neurodegeneration in Tauopathy. Nature 2023, 615, 668–677. [Google Scholar] [CrossRef]

| Genotype | Sex | Experimental Group | Delay between Last Sonication and Euthanasia (Days) | Mean Age at Euthanasia (Months) | n |
|---|---|---|---|---|---|
| P301S | Male | LIPU-sonicated | 7 | 5.7 | 10 |
| Non sonicated | 7 | 5.9 | 7 | ||
| Male | LIPU-sonicated | 1 | 6.5 | 5 | |
| Non sonicated | 1 | 6.5 | 4 | ||
| WT | Male | LIPU-sonicated | 7 | 6.7 | 5 |
| Non sonicated | 7 | 6.7 | 4 | ||
| Female | LIPU-sonicated | 7 | 6.8 | 5 | |
| Non sonicated | 7 | 6.7 | 5 |
| Region of Interest | AT8-Day 7 | AT8-Day 1 | Iba1 | GFAP |
|---|---|---|---|---|
| Hippocampus | ns | ns | ns | ns |
| Amygdala | p < 0.05 | ns | ns | ns |
| Piriform cx | ns | ns | ns | ns |
| Somatosensory cx | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Géraudie, A.; Riche, M.; Lestra, T.; Trotier, A.; Dupuis, L.; Mathon, B.; Carpentier, A.; Delatour, B. Effects of Low-Intensity Pulsed Ultrasound-Induced Blood–Brain Barrier Opening in P301S Mice Modeling Alzheimer’s Disease Tauopathies. Int. J. Mol. Sci. 2023, 24, 12411. https://doi.org/10.3390/ijms241512411
Géraudie A, Riche M, Lestra T, Trotier A, Dupuis L, Mathon B, Carpentier A, Delatour B. Effects of Low-Intensity Pulsed Ultrasound-Induced Blood–Brain Barrier Opening in P301S Mice Modeling Alzheimer’s Disease Tauopathies. International Journal of Molecular Sciences. 2023; 24(15):12411. https://doi.org/10.3390/ijms241512411
Chicago/Turabian StyleGéraudie, Amandine, Maximilien Riche, Thaïs Lestra, Alexandre Trotier, Léo Dupuis, Bertrand Mathon, Alexandre Carpentier, and Benoît Delatour. 2023. "Effects of Low-Intensity Pulsed Ultrasound-Induced Blood–Brain Barrier Opening in P301S Mice Modeling Alzheimer’s Disease Tauopathies" International Journal of Molecular Sciences 24, no. 15: 12411. https://doi.org/10.3390/ijms241512411
APA StyleGéraudie, A., Riche, M., Lestra, T., Trotier, A., Dupuis, L., Mathon, B., Carpentier, A., & Delatour, B. (2023). Effects of Low-Intensity Pulsed Ultrasound-Induced Blood–Brain Barrier Opening in P301S Mice Modeling Alzheimer’s Disease Tauopathies. International Journal of Molecular Sciences, 24(15), 12411. https://doi.org/10.3390/ijms241512411






