Adipose Tissue-Derived Products May Present Inflammatory Properties That Affect Chondrocytes and Synoviocytes from Patients with Knee Osteoarthritis
Abstract
1. Introduction
2. Results
2.1. Analysis of the MF-AT Inflammatory Factors
2.2. MF-AT Inflammatory Factors and Chondrocytes/Synoviocytes Gene Expression Levels
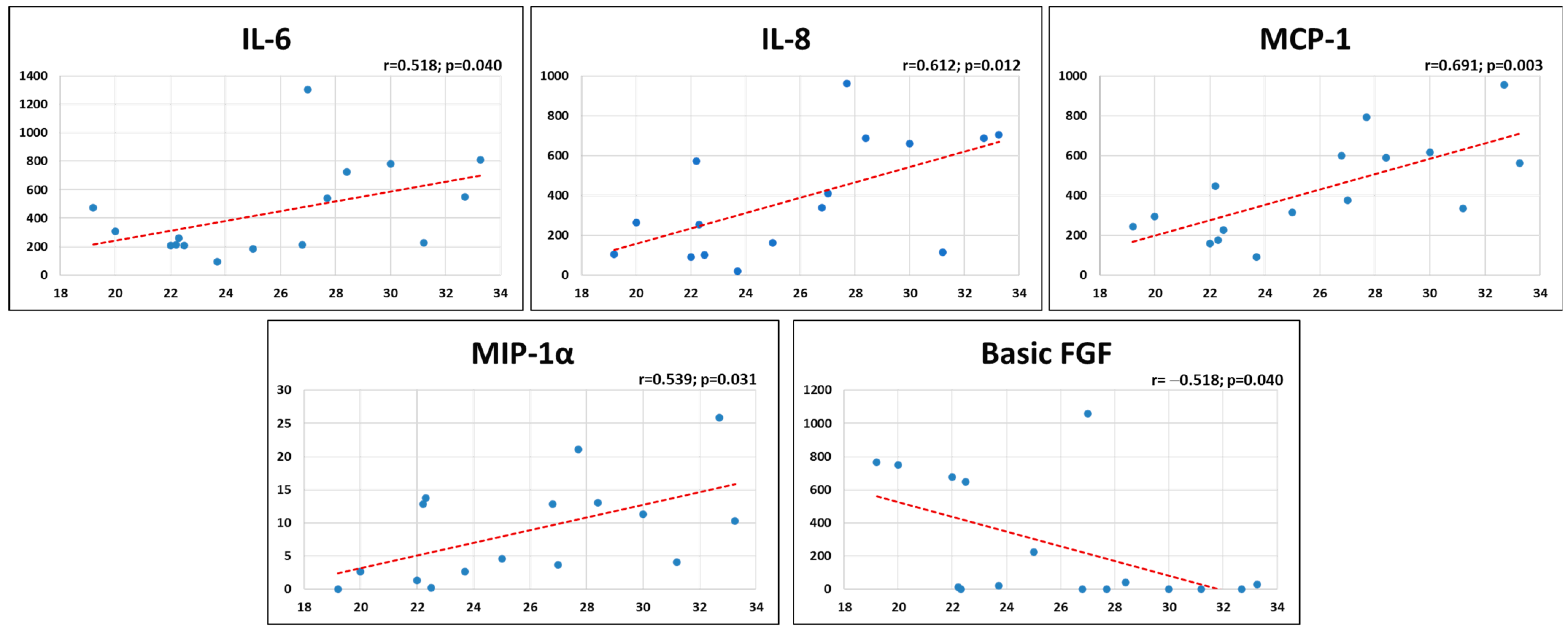
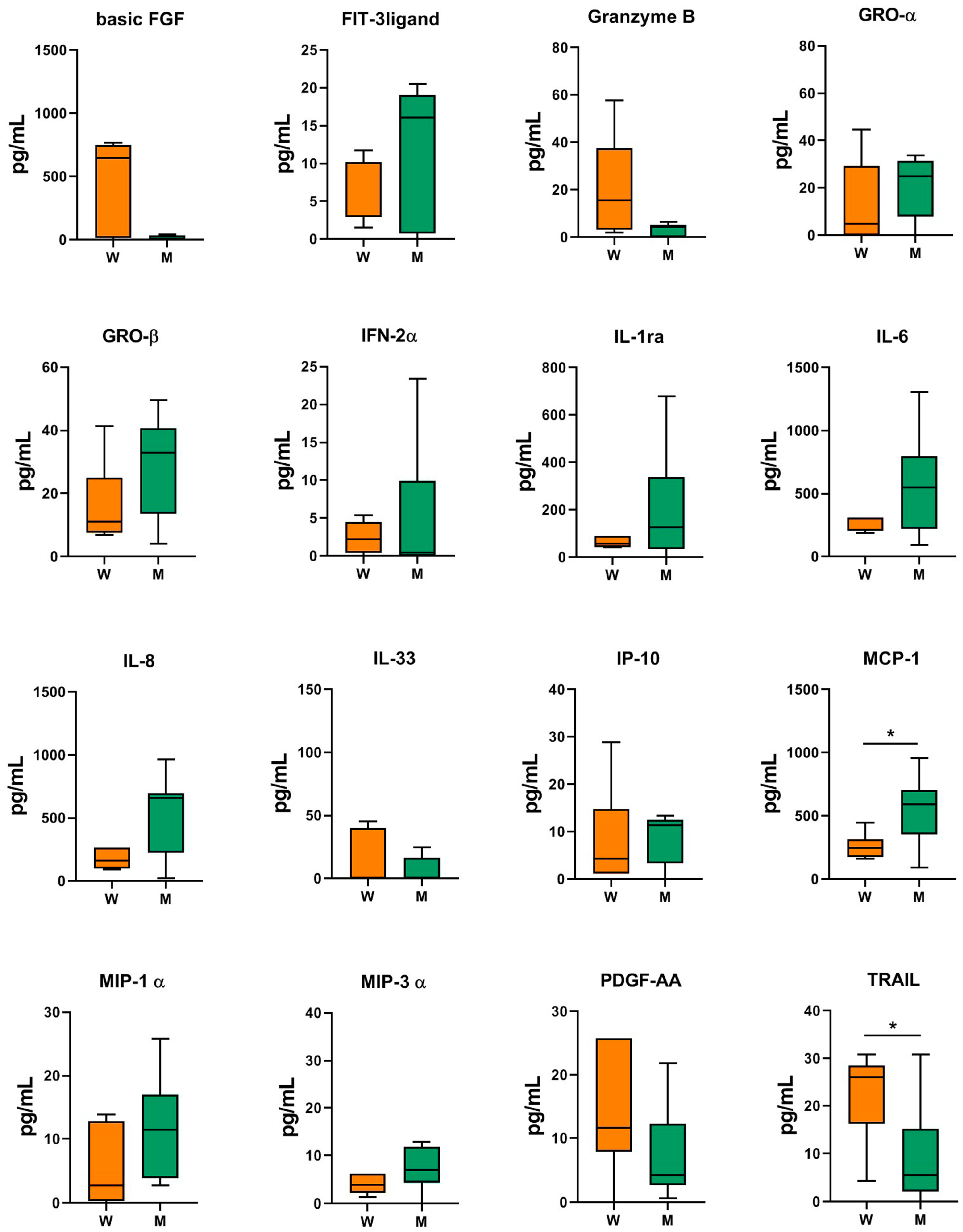
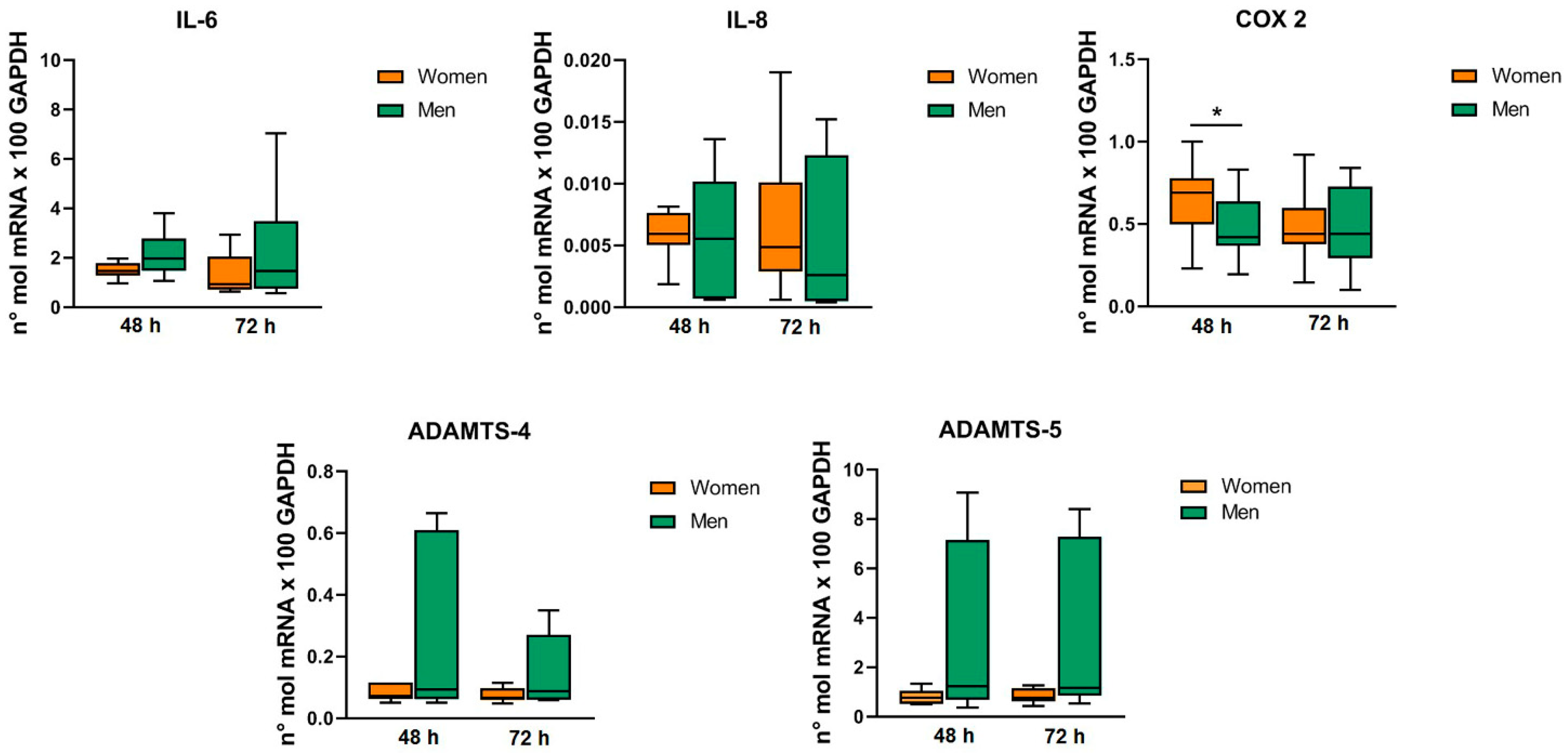
2.3. Patients’ Characteristics and MF-AT Inflammatory Molecules
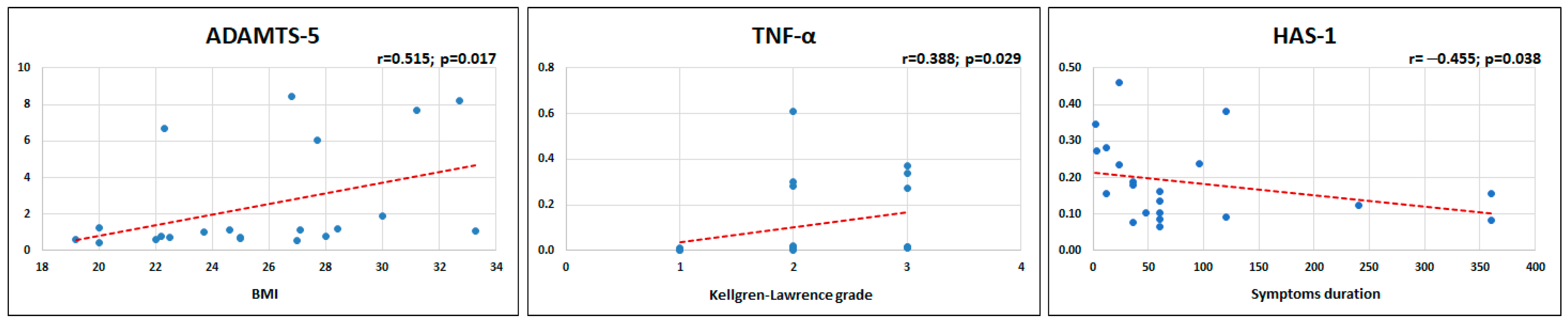
2.4. Patients’ Characteristics and MF-AT Effects on Gene Expression
3. Discussion
4. Materials and Methods
4.1. Adipose Tissue Harvesting and Processing
4.2. Release of Inflammatory Factors from Adipose Tissue
4.3. Chondrocyte and Synoviocyte Isolation and Co-Colture Experiments
4.4. MF-AT Effects on Gene Expression in Chondrocytes and Synoviocytes
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAMTS | a disintegrin and metalloproteinase with thrombospondin motifs |
| BMI | body mass index |
| CD | cluster of differentiation |
| COX | cyclooxygenase |
| DMEM | Dulbecco’s modified eagle medium |
| EGF | epidermal growth factor |
| FGF | fibroblast growth factor |
| Flt-3 ligand | Fms-like tyrosine kinase 3 ligand |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| G-CSF | granulocyte colony-stimulating factor |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GRO | growth-related oncogene |
| HAS | hyaluronan synthase |
| IFN-α2 | interferon alpha-2 |
| IL | interleukin |
| IL-1ra | interleukin-1 receptor antagonist |
| MCP-1 | monocyte chemoattractant protein-1 |
| MF-AT | micro-fragmented adipose tissue |
| MIP | macrophage inflammatory protein |
| MMPs | matrix metalloproteinases |
| mRNA | messenger ribonucleic acid |
| MSCs | mesenchymal stromal cells |
| OA | osteoarthritis |
| PD-L1 | programmed death-ligand 1 |
| PDGF | platelet-derived growth factor |
| RT-PCR | reverse transcriptase-polymerase chain reaction |
| TGF | transforming growth factor |
| TNF | tumor necrosis factor |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand |
| VEGF | vascular endothelial growth factor |
References
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef]
- Gimble, J.; Guilak, F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy 2003, 5, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Review: Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [PubMed]
- Chen, F.H.; Tuan, R.S. Mesenchymal stem cells in arthritic diseases. Arthritis Res. Ther. 2008, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Via, A.G.; Frizziero, A.; Oliva, F. Biological properties of mesenchymal Stem Cells from different sources. Muscles Ligaments Tendons J. 2012, 2, 154–162. [Google Scholar]
- Jang, Y.; Koh, Y.G.; Choi, Y.J.; Kim, S.H.; Yoon, D.S.; Lee, M.; Lee, J.W. Characterization of adipose tissue-derived stromal vascular fraction for clinical application to cartilage regeneration. In Vitro Cell Dev. Biol. Anim. 2015, 51, 142–150. [Google Scholar] [CrossRef]
- Carelli, S.; Messaggio, F.; Canazza, A.; Hebda, D.M.; Caremoli, F.; Latorre, E.; Grimoldi, M.G.; Colli, M.; Bulfamante, G.; Tremolada, C.; et al. Characteristics and Properties of Mesenchymal Stem Cells Derived From Microfragmented Adipose Tissue. Cell Transplant. 2015, 24, 1233–1252. [Google Scholar] [CrossRef]
- Aust, L.; Devlin, B.; Foster, S.J.; Halvorsen, Y.D.; Hicok, K.; du Laney, T.; Sen, A.; Willingmyre, G.D.; Gimble, J.M. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 2004, 6, 7–14. [Google Scholar] [CrossRef]
- Perucca Orfei, C.; Boffa, A.; Sourugeon, Y.; Laver, L.; Magalon, J.; Sanchez, M.; Tischer, T.; Filardo, G.; de Girolamo, L. Cell-based therapies have disease-modifying effects on osteoarthritis in animal models. A systematic review by the ESSKA Orthobiologic Initiative. Part 1: Adipose tissue-derived cell-based injectable therapies. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 641–655. [Google Scholar] [CrossRef]
- Lu, L.; Dai, C.; Zhang, Z.; Du, H.; Li, S.; Ye, P.; Fu, Q.; Zhang, L.; Wu, X.; Dong, Y.; et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: A prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res. Ther. 2019, 10, 143. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, H.J.; Kim, K.I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Gadelkarim, M.; Abd Elmegeed, A.; Allam, A.H.; Awad, A.K.; Shehata, M.A.; AbouEl-Enein, A.; Alsadek, M.E.; Abo Deeb, M.; Afifi, A.M. Safety and efficacy of adipose-derived mesenchymal stem cells for knee osteoarthritis: A systematic review and m-analysis. Jt. Bone Spine 2022, 89, 105404. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, S.; Andriolo, L.; Boffa, A.; Poggi, A.; Cenacchi, A.; Busacca, M.; Kon, E.; Filardo, G.; Di Martino, A. Microfragmented Adipose Tissue Versus Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Prospective Randomized Controlled Trial at 2-Year Follow-up. Am. J. Sports Med. 2022, 50, 2881–2892. [Google Scholar] [CrossRef]
- Van Genechten, W.; Vuylsteke, K.; Martinez, P.R.; Swinnen, L.; Sas, K.; Verdonk, P. Autologous Micro-Fragmented Adipose Tissue (MFAT) to Treat Symptomatic Knee Osteoarthritis: Early Outcomes of a Consecutive Case Series. J. Clin. Med. 2021, 10, 2231. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Vezzani, B.; Shaw, I.; Lesme, H.; Yong, L.; Khan, N.; Tremolada, C.; Peault, B. Higher Pericyte Content and Secretory Activity of Microfragmented Human Adipose Tissue Compared to Enzymatically Derived Stromal Vascular Fraction. Stem Cells Transl. Med. 2018, 7, 876–886. [Google Scholar] [CrossRef]
- Toussirot, E. Mini-Review: The Contribution of Adipokines to Joint Inflammation in Inflammatory Rheumatic Diseases. Front. Endocrinol. 2020, 11, 606560. [Google Scholar] [CrossRef]
- Thijssen, E.; van Caam, A.; van der Kraan, P.M. Obesity and osteoarthritis, more than just wear and tear: Pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology 2015, 54, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919; quiz 920. [Google Scholar] [CrossRef] [PubMed]
- Zagotta, I.; Dimova, E.Y.; Debatin, K.M.; Wabitsch, M.; Kietzmann, T.; Fischer-Posovszky, P. Obesity and inflammation: Reduced cytokine expression due to resveratrol in a human in vitro model of inflamed adipose tissue. Front. Pharmacol. 2015, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef] [PubMed]
- Wiegertjes, R.; van Caam, A.; van Beuningen, H.; Koenders, M.; van Lent, P.; van der Kraan, P.; van de Loo, F.; Blaney Davidson, E. TGF-beta dampens IL-6 signaling in articular chondrocytes by decreasing IL-6 receptor expression. Osteoarthr. Cartil. 2019, 27, 1197–1207. [Google Scholar] [CrossRef]
- Miller, D.; Grant, A.; Durgam, S.; El-Hayek, K.; Flanigan, D.C.; Malanga, G.; Vasileff, W.K.; Baria, M.R. Adipose-Derived Stem Cells, Obesity, and Inflammation: A Systematic Review and Implications for Osteoarthritis Treatment. Am. J. Phys. Med. Rehabil. 2022, 101, 879–887. [Google Scholar] [CrossRef]
- Sawaji, Y.; Hynes, J.; Vincent, T.; Saklatvala, J. Fibroblast growth factor 2 inhibits induction of aggrecanase activity in human articular cartilage. Arthritis Rheum. 2008, 58, 3498–3509. [Google Scholar] [CrossRef]
- Chia, S.L.; Sawaji, Y.; Burleigh, A.; McLean, C.; Inglis, J.; Saklatvala, J.; Vincent, T. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009, 60, 2019–2027. [Google Scholar] [CrossRef]
- Nummenmaa, E.; Hamalainen, M.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Effects of FGF-2 and FGF receptor antagonists on MMP enzymes, aggrecan, and type II collagen in primary human OA chondrocytes. Scand. J. Rheumatol. 2015, 44, 321–330. [Google Scholar] [CrossRef]
- Ellman, M.B.; Yan, D.; Ahmadinia, K.; Chen, D.; An, H.S.; Im, H.J. Fibroblast growth factor control of cartilage homeostasis. J. Cell. Biochem. 2013, 114, 735–742. [Google Scholar] [CrossRef]
- Kim, S.; Ahn, C.; Bong, N.; Choe, S.; Lee, D.K. Biphasic effects of FGF2 on adipogenesis. PLoS ONE 2015, 10, e0120073. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Huang, C.; Liu, H.; Zhang, Q.; Sun, Q.; Jia, Y.; Liu, S.; Dong, M.; Hou, M.; et al. FGF2 disruption enhances thermogenesis in brown and beige fat to protect against adiposity and hepatic steatosis. Mol. Metab. 2021, 54, 101358. [Google Scholar] [CrossRef] [PubMed]
- Zaragosi, L.E.; Ailhaud, G.; Dani, C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells 2006, 24, 2412–2419. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Uchida, K.; Inoue, G.; Matsumoto, T.; Aikawa, J.; Iwase, D.; Mukai, M.; Miyagi, M.; Takaso, M. Vascular endothelial growth factor expression and their action in the synovial membranes of patients with painful knee osteoarthritis. BMC Musculoskelet. Disord. 2018, 19, 204. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.D.; Xiao, W.F.; Li, J.; de la Motte, C.A.; Sandy, J.D.; Plaas, A. Deficiency of hyaluronan synthase 1 (Has1) results in chronic joint inflammation and widespread intra-articular fibrosis in a murine model of knee joint cartilage damage. Osteoarthr. Cartil. 2015, 23, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Stuhlmeier, K.M. Prostaglandin E2: A potent activator of hyaluronan synthase 1 in type-B-synoviocytes. Biochim. Biophys. Acta 2007, 1770, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Siiskonen, H.; Oikari, S.; Pasonen-Seppanen, S.; Rilla, K. Hyaluronan synthase 1: A mysterious enzyme with unexpected functions. Front. Immunol. 2015, 6, 43. [Google Scholar] [CrossRef]
- Yoshida, M.; Sai, S.; Marumo, K.; Tanaka, T.; Itano, N.; Kimata, K.; Fujii, K. Expression analysis of three isoforms of hyaluronan synthase and hyaluronidase in the synovium of knees in osteoarthritis and rheumatoid arthritis by quantitative real-time reverse transcriptase polymerase chain reaction. Arthritis Res. Ther. 2004, 6, R514–R520. [Google Scholar] [CrossRef]
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999, 274, 25085–25092. [Google Scholar] [CrossRef]
- Assirelli, E.; Filardo, G.; Mariani, E.; Kon, E.; Roffi, A.; Vaccaro, F.; Marcacci, M.; Facchini, A.; Pulsatelli, L. Effect of two different preparations of platelet-rich plasma on synoviocytes. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2690–2703. [Google Scholar] [CrossRef]
- Momberger, T.S.; Levick, J.R.; Mason, R.M. Hyaluronan secretion by synoviocytes is mechanosensitive. Matrix Biol. 2005, 24, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Thomas, R.; Shihab, P.; Sriraman, D.; Behbehani, K.; Ahmad, R. Obesity Is a Positive Modulator of IL-6R and IL-6 Expression in the Subcutaneous Adipose Tissue: Significance for Metabolic Inflammation. PLoS ONE 2015, 10, e0133494. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; White, A.; Perry, R.J.; Camporez, J.P.; Hidalgo, J.; Shulman, G.I.; Davis, R.J. Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl. Acad. Sci. USA 2020, 117, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Maria Aguilera, C.; Gil-Campos, M.; Canete, R. Altered signalling and gene expression associated with the immune system and the inflammatory response in obesity. Br. J. Nutr. 2007, 98 (Suppl. S1), S121–S126. [Google Scholar] [CrossRef]
- Kobashi, C.; Asamizu, S.; Ishiki, M.; Iwata, M.; Usui, I.; Yamazaki, K.; Tobe, K.; Kobayashi, M.; Urakaze, M. Inhibitory effect of IL-8 on insulin action in human adipocytes via MAP kinase pathway. J. Inflamm. 2009, 6, 25. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Ding, Y.; Xu, P.; Wang, T.; Xu, W.; Lu, H.; Li, J.; Wang, Y.; Li, S.; et al. Adipose Tissues Characteristics of Normal, Obesity, and Type 2 Diabetes in Uygurs Population. J. Diabetes Res. 2015, 2015, 905042. [Google Scholar] [CrossRef]
- Noh, H.J.; Kim, C.S.; Kang, J.H.; Park, J.Y.; Choe, S.Y.; Hong, S.M.; Yoo, H.; Park, T.; Yu, R. Quercetin suppresses MIP-1alpha-induced adipose inflammation by downregulating its receptors CCR1/CCR5 and inhibiting inflammatory signaling. J. Med. Food 2014, 17, 550–557. [Google Scholar] [CrossRef]
- Surmi, B.K.; Webb, C.D.; Ristau, A.C.; Hasty, A.H. Absence of macrophage inflammatory protein-1alpha does not impact macrophage accumulation in adipose tissue of diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E437–E445. [Google Scholar] [CrossRef]
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diabetes Rep. 2018, 18, 69. [Google Scholar] [CrossRef]
- Pettersson, U.S.; Walden, T.B.; Carlsson, P.O.; Jansson, L.; Phillipson, M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef]
- Gavin, K.M.; Bessesen, D.H. Sex Differences in Adipose Tissue Function. Endocrinol. Metab. Clin. N. Am. 2020, 49, 215–228. [Google Scholar] [CrossRef]
- Gobbi, A.; Dallo, I.; Rogers, C.; Striano, R.D.; Mautner, K.; Bowers, R.; Rozak, M.; Bilbool, N.; Murrell, W.D. Two-year clinical outcomes of autologous microfragmented adipose tissue in elderly patients with knee osteoarthritis: A multi-centric, international study. Int. Orthop. 2021, 45, 1179–1188. [Google Scholar] [CrossRef]
- Kuan, P.X.; Ho, H.L.; Shuhaili, M.S.; Siti, A.A.; Gudum, H.R. Gender differences in body mass index, body weight perception and weight loss strategies among undergraduates in Universiti Malaysia Sarawak. Malays. J. Nutr. 2011, 17, 67–75. [Google Scholar] [PubMed]
- Park, J.S.; Park, G.; Hong, H.S. Age affects the paracrine activity and differentiation potential of human adipose-derived stem cells. Mol. Med. Rep. 2021, 23, 160. [Google Scholar] [CrossRef]
- Collins, K.H.; Lenz, K.L.; Pollitt, E.N.; Ferguson, D.; Hutson, I.; Springer, L.E.; Oestreich, A.K.; Tang, R.; Choi, Y.R.; Meyer, G.A.; et al. Adipose tissue is a critical regulator of osteoarthritis. Proc. Natl. Acad. Sci. USA 2021, 118, e2021096118. [Google Scholar] [CrossRef] [PubMed]

| Inflammatory Factors | Mean (pg/mL) | SD |
|---|---|---|
| Basic FGF | 264.1 | 372.6 |
| Flt-3 Ligand | 9.1 | 7.8 |
| Granzyme B | 12.6 | 17.1 |
| GROα | 16.7 | 19.7 |
| GROβ | 23.0 | 15.1 |
| IFN-α2 | 4.1 | 6.4 |
| IL-1ra | 149.9 | 175.4 |
| IL-6 | 444.1 | 326.2 |
| IL-8 | 383.2 | 290.5 |
| IL-33 | 15.5 | 27.9 |
| IP-10 | 9.9 | 9.4 |
| MCP-1 | 424.2 | 243.2 |
| MIP-1α | 8.8 | 7.6 |
| MIP-3α | 7.4 | 7.6 |
| PDGF-AA | 10.9 | 8.8 |
| TRAIL | 14.7 | 11.3 |
| Inflammatory Factors | Gene Expression (48 h) | Gene Expression (72 h) |
|---|---|---|
| IL-8 | IL-6: rho = 0.591; p = 0.016 | IL-6: rho = 0.574; p = 0.020 |
| MCP-1 | None | ADAMTS-5: rho = 0.547; p = 0.028 |
| MIP-1α | ADAMTS-5: rho = 0.736; p = 0.001 | ADAMTS-5: rho = 0.745; p = 0.001 |
| Basic FGF | IL-8: rho = 0.630; p = 0.009 COX-2: rho = 0.876; p = 0.0008 ADAMTS-4: rho = −0.603; p = 0.013 ADAMTS-5: rho = −0.959; p < 0.0005 | IL-8: rho = 0.524; p = 0.030 COX-2: rho = 0.536; p = 0.030 ADAMTS-4: rho = −0.638; p = 0.008 ADAMTS-5: rho = −0.929; p < 0.0005 |
| Inflammatory Factors | Gene Expression (48 h) | Gene Expression (72 h) |
|---|---|---|
| Basic FGF | HAS-1: rho = 0.844; p < 0.0005 TNF-α: rho = −0.624; p = 0.010 IL-6: rho = −0.494; p = 0.052 IL-8: rho = −0.515; p = 0.041 VEGF: rho = −0.521; p = 0.039 | HAS-1: rho = 0.807; p < 0.0005 TNF-α: rho = −0.805; p < 0.0005 IL-6: rho = −0.509; p = 0.044 IL-8: rho = −0.569; p = 0.021 VEGF: rho = −0.639; p = 0.008 |
| RNA Template | Primer Sequences (5′-3′) | Annealing Temperature (°C) |
|---|---|---|
| GAPDH | 5′-TGGTATCGTGGAAGGACTCATGAC 3′-ATGCCAGTGAGCTTCCCGTTCAGC | 60 |
| IL-6 | 5′-TAGTGAGGAACAAGCCAGAG 3′-GCGCAGAATGAGATGAGTTG | 60 |
| IL-8 | 5′-CCAAACCTTTCCACCC 3′-ACTTCTCCACAACCCT | 60 |
| TNF-α | 5′-AGCCCATGTTGTAGCAAACC 3′-ACCTGGGAGTAGATGAGGTA | 60 |
| COX-2 | 5′-CAGCACTTCACGCATCAGTTT 3′-GCGCAGTTTACGCTGTCTA | 60 |
| HAS-1 | 5′-TGGTGCTTCTCTCGCTCTACG 3′-GAACTTGGCAGGCAGGAGG | 60 |
| HAS-2 | 5′-AAATGGGATGAATTCTTTGTTTATG 3′-GGCGGATGCACAGTAAGGAA | 60 |
| HAS-3 | 5′-CAGCTGATCCAGGCAATCGT 3′-TGGCTGACCGGATTTCCTC | 60 |
| VEGF | 5′-TGATGATTCTGCCCTCCTC 3′-GCCTTGCCTTGCTGCTC | 60 |
| ADAMTS-4 | 5′-CTGCCTACAACCACCG 3′-GCAACCAGAACCGTCC | 60 |
| ADAMTS-5 | 5′-GCACTTCAGCCACCATCAC 3′-AGGCGAGCACAGACATCC | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallo, C.; Boffa, A.; Salerno, M.; Merli, G.; Grigolo, B.; Filardo, G. Adipose Tissue-Derived Products May Present Inflammatory Properties That Affect Chondrocytes and Synoviocytes from Patients with Knee Osteoarthritis. Int. J. Mol. Sci. 2023, 24, 12401. https://doi.org/10.3390/ijms241512401
Cavallo C, Boffa A, Salerno M, Merli G, Grigolo B, Filardo G. Adipose Tissue-Derived Products May Present Inflammatory Properties That Affect Chondrocytes and Synoviocytes from Patients with Knee Osteoarthritis. International Journal of Molecular Sciences. 2023; 24(15):12401. https://doi.org/10.3390/ijms241512401
Chicago/Turabian StyleCavallo, Carola, Angelo Boffa, Manuela Salerno, Giulia Merli, Brunella Grigolo, and Giuseppe Filardo. 2023. "Adipose Tissue-Derived Products May Present Inflammatory Properties That Affect Chondrocytes and Synoviocytes from Patients with Knee Osteoarthritis" International Journal of Molecular Sciences 24, no. 15: 12401. https://doi.org/10.3390/ijms241512401
APA StyleCavallo, C., Boffa, A., Salerno, M., Merli, G., Grigolo, B., & Filardo, G. (2023). Adipose Tissue-Derived Products May Present Inflammatory Properties That Affect Chondrocytes and Synoviocytes from Patients with Knee Osteoarthritis. International Journal of Molecular Sciences, 24(15), 12401. https://doi.org/10.3390/ijms241512401








