Foliar Application of Chelated Sugar Alcohol Calcium Improves Photosynthesis and Tuber Quality under Drought Stress in Potatoes (Solanum tuberosum L.)
Abstract
1. Introduction
2. Results
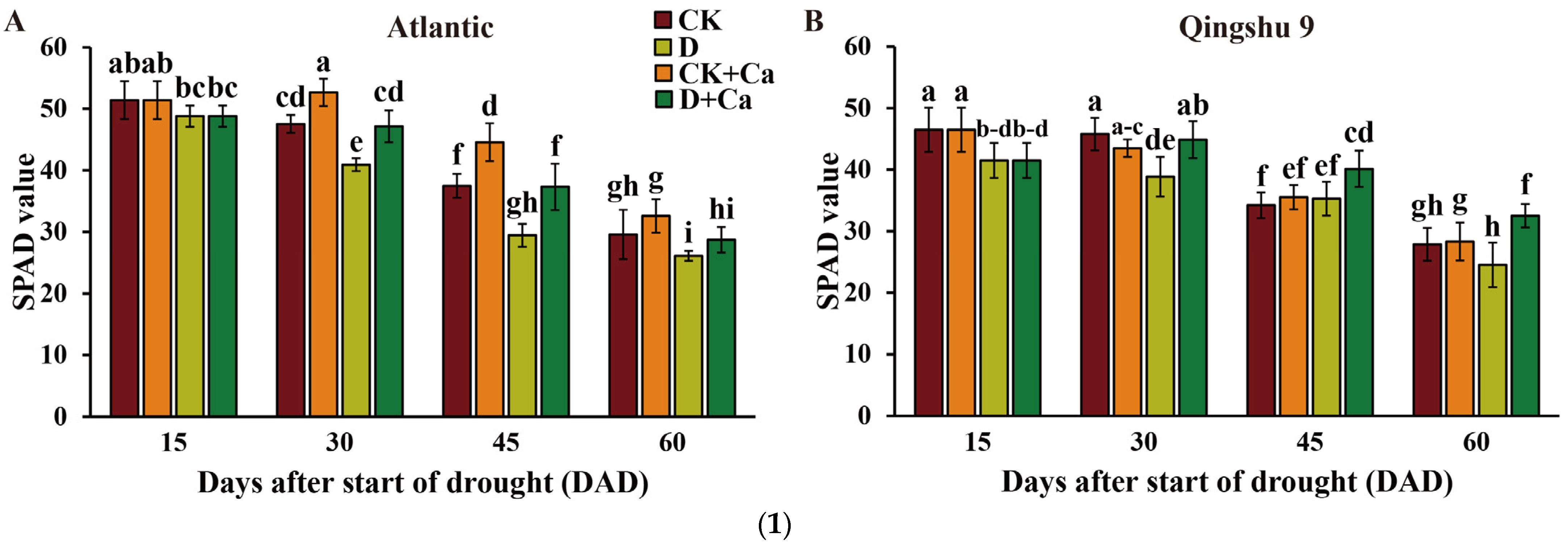
2.1. Foliar Application of Calcium Improved Photosynthetic Parameters of the Potato under Drought Stress
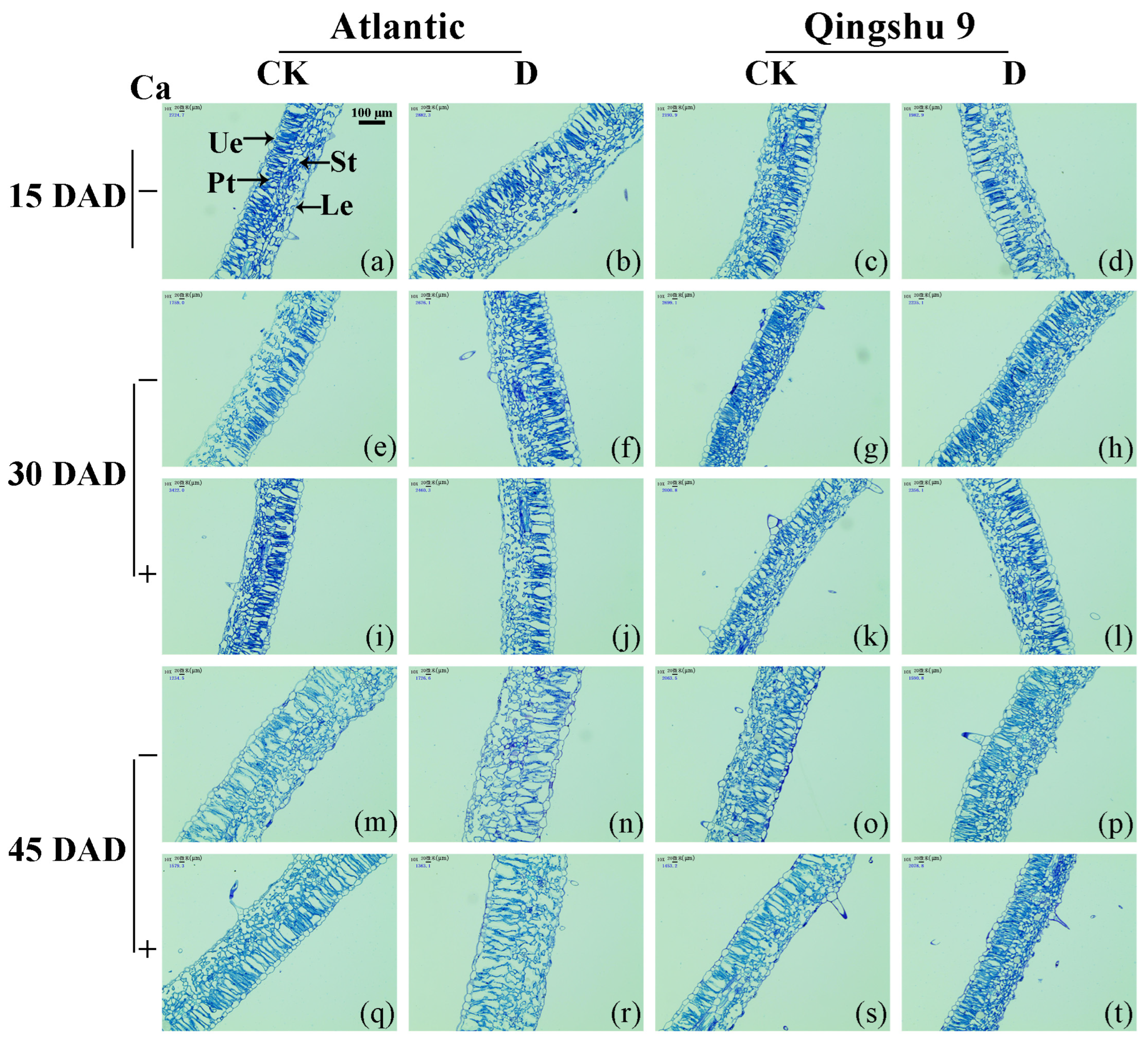
2.2. Foliar Application of Calcium Improved the Leaf Anatomy of the Potato under Drought Stress
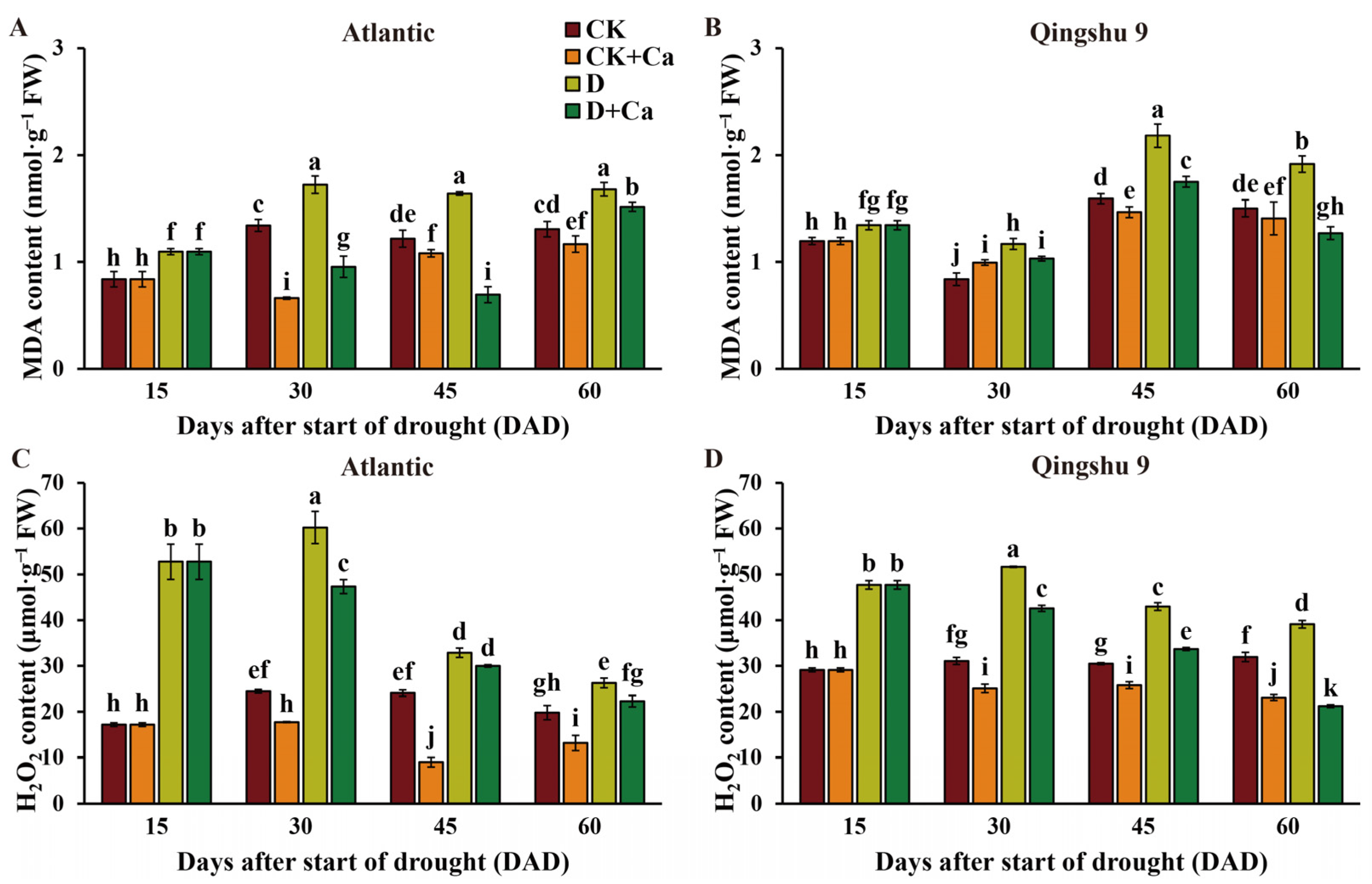
2.3. Foliar Application of Calcium Attenuated Membrane Lipid Peroxidation in the Potato under Drought Stress
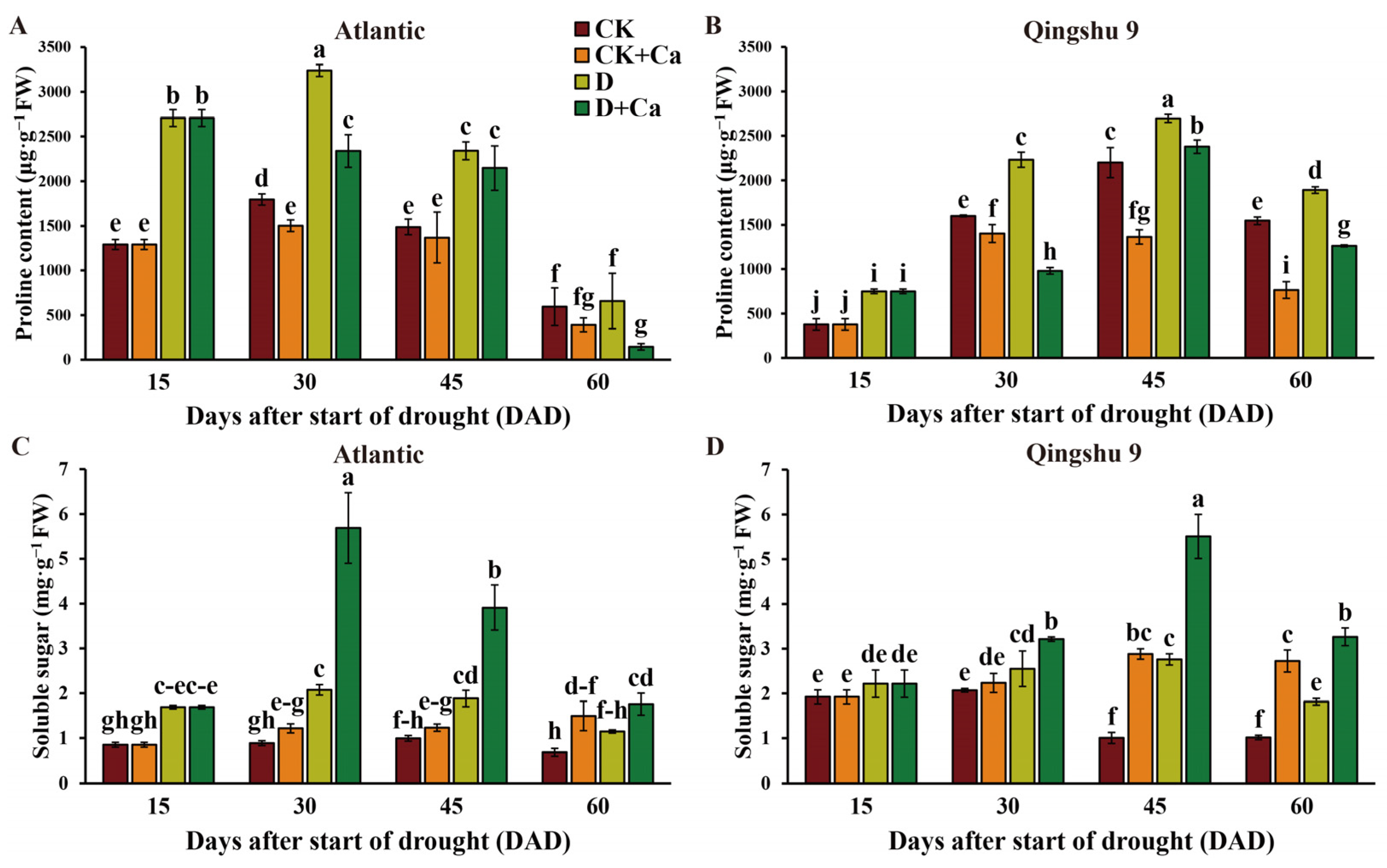
2.4. Foliar Application of Calcium Altered Levels of Osmoregulatory Substances of the Potato under Drought Conditions
2.5. Foliar Application of Calcium Promoted Antioxidant Enzymes in the Potato under Drought Conditions
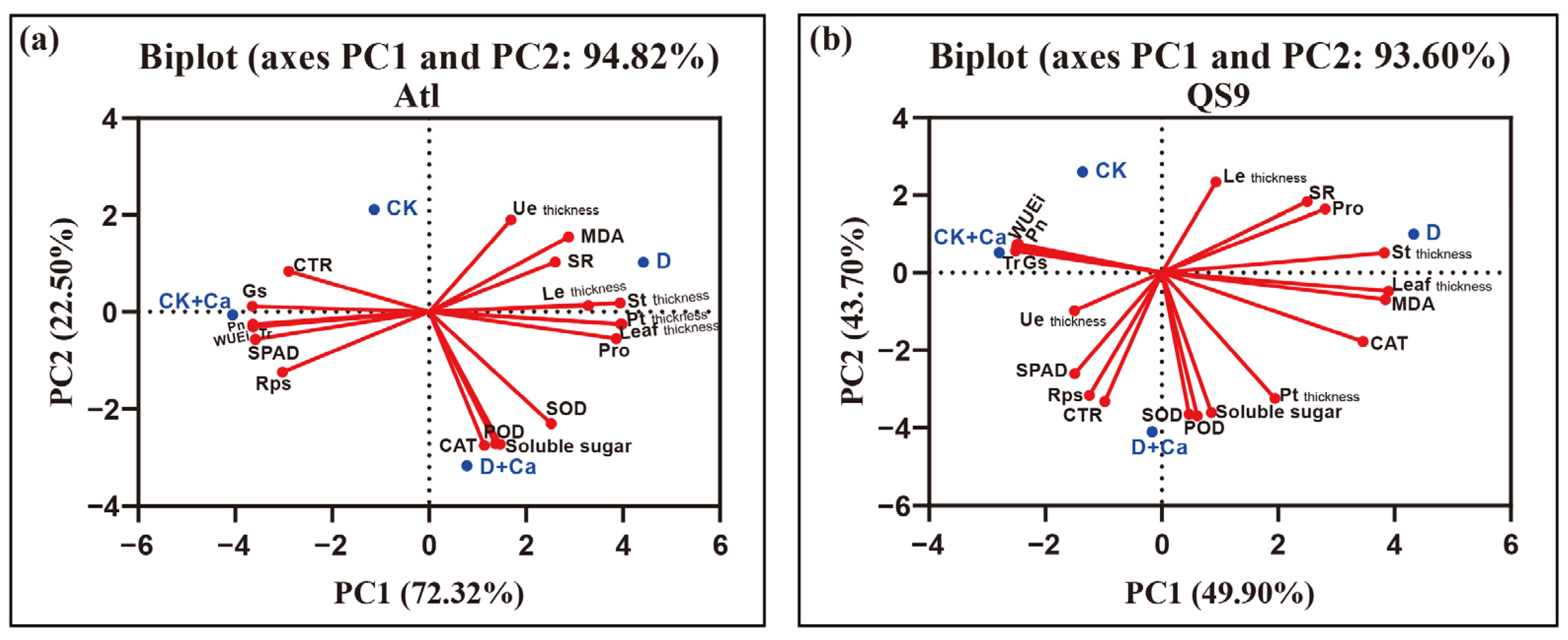
2.6. PCA Analysis
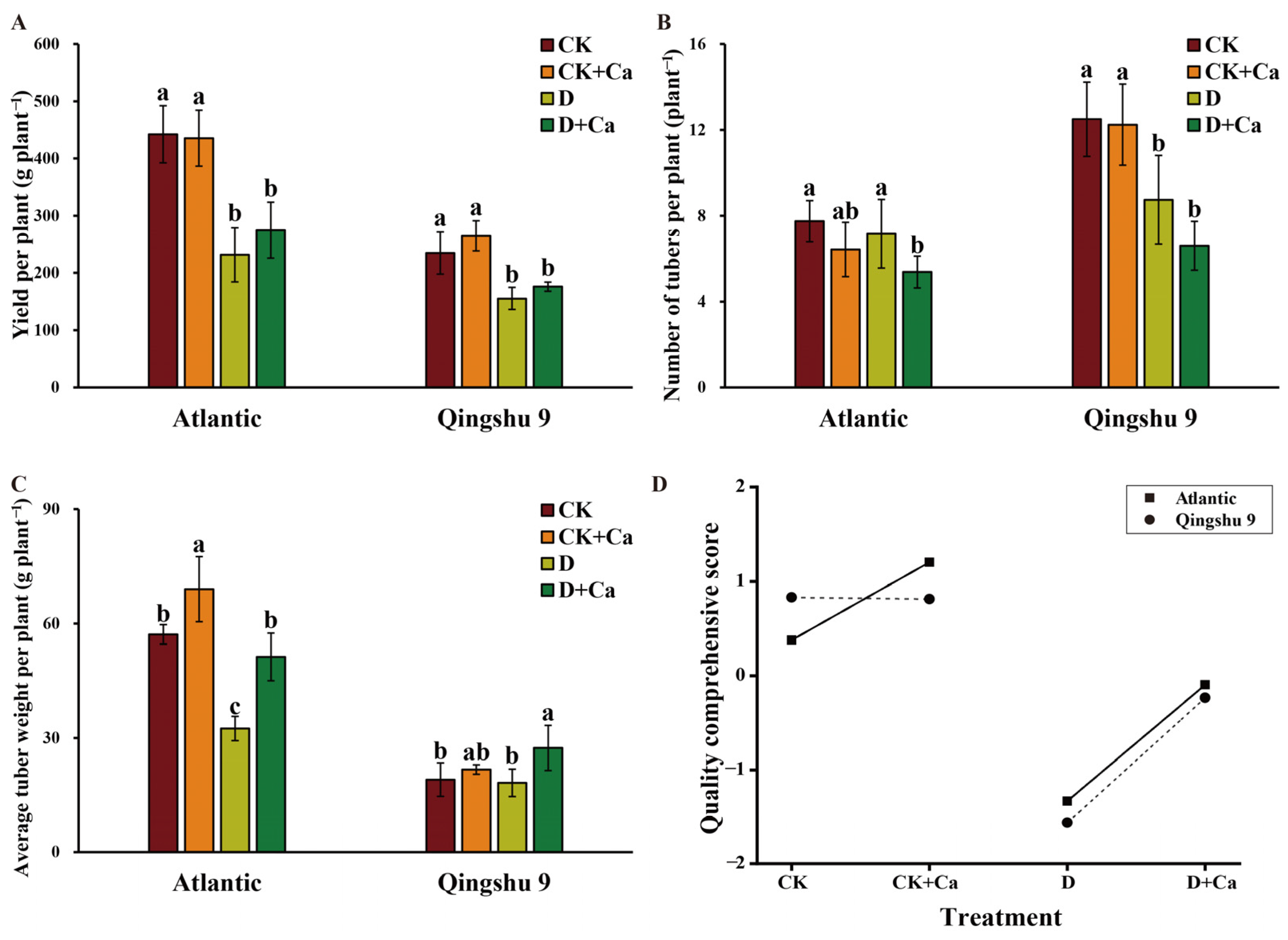
2.7. Foliar Application of Calcium Improved Yield and Tuber Quality of the Potato under Drought Conditions
2.8. Effects of Foliar Application of Calcium on Gene Expression of the Potato under Drought Conditions
3. Discussion
4. Materials and Methods
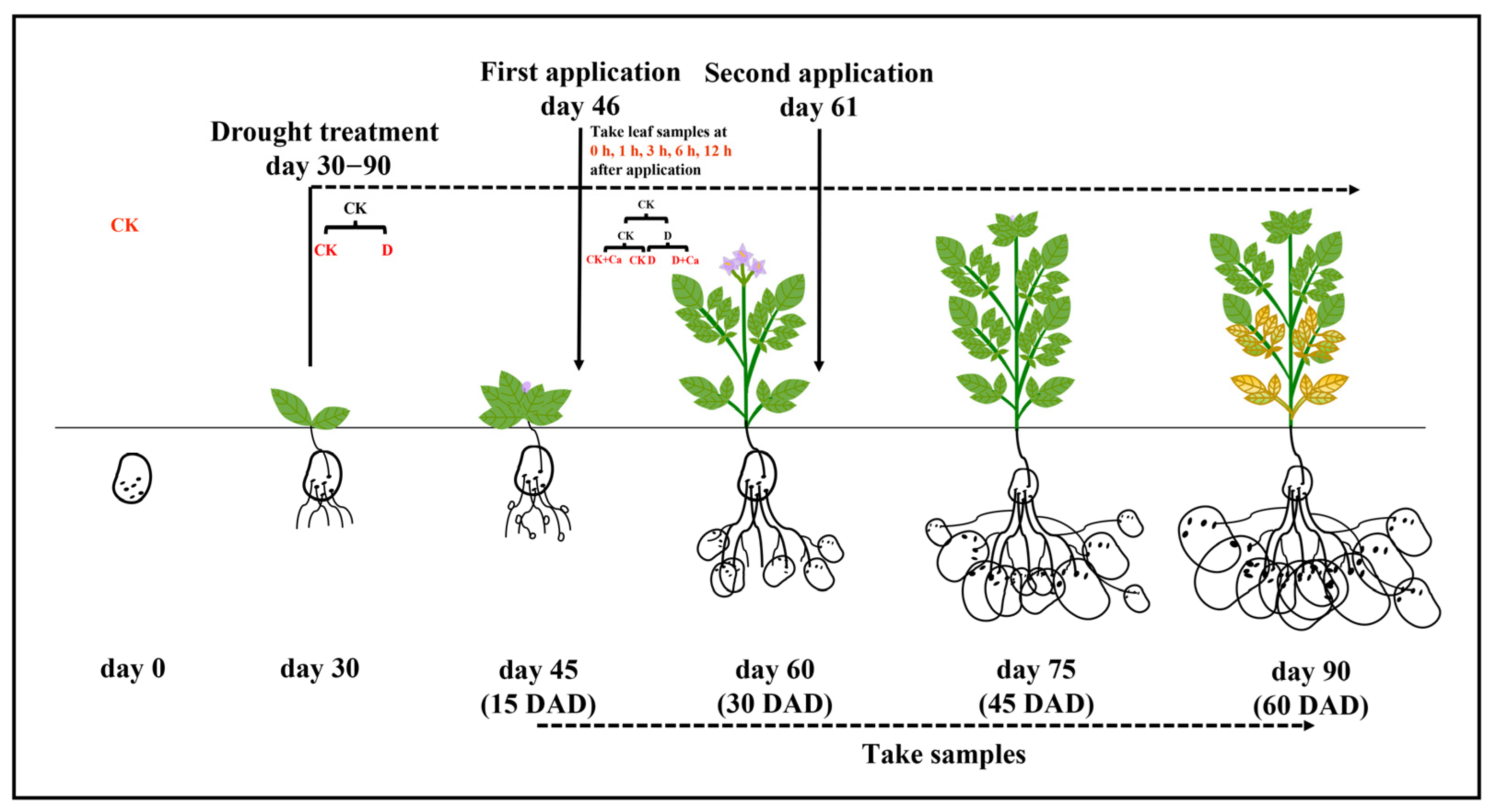
4.1. Plant Material and Experimental Design
4.2. Determination of Photosynthetic Parameters
4.3. Observation of Leaf Microscopic Structure
4.4. Determination of Malondialdehyde (MDA) Content and Hydrogen Peroxide (H2O2)
4.5. Measurement of Osmoregulation Substances
4.6. Measurement of Antioxidant Enzyme Activities
4.7. Measurement of Yield and Tuber Quality Analysis
4.8. Analysis of Gene Expression
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huseynova, I.M.; Rustamova, S.M.; Aliyeva, D.R.; Babayev, H.G.; Aliyev, J.A. Photosynthesis, Antioxidant Protection, and Drought Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1. [Google Scholar]
- Rahdari, P.; Hoseini, S.M.; Tavakoli, S. The studying effect of drought stress on germination, proline, sugar, lipid, protein and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Med. Plants Res. 2012, 6, 1539–1547. [Google Scholar]
- Olmos, E.; Sanchez-Blanco, M.J.; Ferrandez, T.; Alarcon, J.J. Subcellular effects of drought stress in Rosmarinus officinalis. Plant Biol. 2007, 9, 77–84. [Google Scholar] [CrossRef]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought Stress in Plants: Causes, Consequences, and Tolerance. In Drought Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1, pp. 1–16. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Miao, Y.; Zhu, Z.; Guo, Q.; Ma, H.; Zhu, L. Alternate wetting and drying irrigation-mediated changes in the growth, photosynthesis and yield of the medicinal plant Tulipa edulis. Ind. Crops Prod. 2015, 66, 81–88. [Google Scholar] [CrossRef]
- Maggio, A.; Miyazaki, S.; Veronese, P.; Fujita, T.; Ibeas, J.I.; Damsz, B.; Narasimhan, M.L.; Hasegawa, P.M.; Joly, R.J.; Bressan, R.A. Does proline accumulation play an active role in stress-induced growth reduction? Plant J. Cell Mol. Biol. 2002, 31, 699–712. [Google Scholar] [CrossRef]
- Demiral, T.; Turkan, I. Does exogenous glycinebetaine affect antioxidative system of rice seedlings under NaCl treatment? J. Plant Physiol. 2004, 161, 1089–1100. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Xu, L.; Islam, F.; Ali, B.; Pei, Z.; Li, J.; Ghani, M.A.; Zhou, W. Silicon and water-deficit stress differentially modulate physiology and ultrastructure in wheat (Triticum aestivum L.). 3 Biotech 2017, 7, 273. [Google Scholar] [CrossRef]
- Hu, C.A.; Delauney, A.J.; Verma, D.P. A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc. Natl. Acad. Sci. USA 1992, 89, 9354–9358. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Szoke, A.; Miao, G.H.; Hong, Z.; Verma, D.P. Subcellular location of delta-pyrroline-5-carboxylate reductase in root/nodule and leaf of soybean. Plant Physiol. 1992, 99, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide Dismutase and Stress Tolerance. Annu. Rev. Plant Physiol. Plant Mol. Bio. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Ben Amor, N.; Megdiche, W.; Jimenez, A.; Sevilla, F.; Abdelly, C. The effect of calcium on the antioxidant systems in the halophyte Cakile maritima under salt stress. Acta Physiol. Plant. 2010, 32, 453–461. [Google Scholar] [CrossRef]
- Willekens, H.; Chamnongpol, S.; Davey, M.; Schraudner, M.; Langebartels, C.; Van Montagu, M.; Inze, D.; Van Camp, W. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997, 16, 4806–4816. [Google Scholar] [CrossRef]
- Sofo, A.; Dichio, B.; Xiloyannis, C.; Masia, A. Antioxidant defences in olive trees during drought stress: Changes in activity of some antioxidant enzymes. Funct. Plant Biol. FPB 2005, 32, 45–53. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding Oxidative Stress and Antioxidant Functions to Enhance Photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef]
- Harb, A.; Awad, D.; Samarah, N. Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare L.) under controlled severe drought. J. Plant Interact. 2015, 10, 109–116. [Google Scholar] [CrossRef]
- Pavia, I.; Roque, J.; Rocha, L.; Ferreira, H.; Castro, C.; Carvalho, A.; Silva, E.; Brito, C.; Goncalves, A.; Lima-Brito, J.; et al. Zinc priming and foliar application enhances photoprotection mechanisms in drought-stressed wheat plants during anthesis. Plant Physiol. Biochem. 2019, 140, 27–42. [Google Scholar] [CrossRef]
- Weisany, W.; Mohammadi, M.; Tahir, N.A.-r.; Aslanian, N.; Omer, D.A. Changes in Growth and Nutrient Status of Maize (Zea mays L.) in Response to Two Zinc Sources Under Drought Stress. J. Soil Sci. Plant Nutr. 2021, 21, 3367–3377. [Google Scholar] [CrossRef]
- Shabbir, R.N.; Waraich, E.A.; Ali, H.; Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Awan, M.I.; Ahmad, S.; Irfan, M.; Hussain, S.; et al. Supplemental exogenous NPK application alters biochemical processes to improve yield and drought tolerance in wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2016, 23, 2651–2662. [Google Scholar] [CrossRef]
- Lv, X.; Ding, Y.; Long, M.; Liang, W.; Gu, X.; Liu, Y.; Wen, X. Effect of Foliar Application of Various Nitrogen Forms on Starch Accumulation and Grain Filling of Wheat (Triticum aestivum L.) Under Drought Stress. Front. Plant Sci. 2021, 12, 645379. [Google Scholar] [CrossRef] [PubMed]
- Hong-Bo, S.; Li-Ye, C.; Ming-An, S. Calcium as a versatile plant signal transducer under soil water stress. BioEssays News Rev. Mol. Cell. Dev. Biol. 2008, 30, 634–641. [Google Scholar] [CrossRef]
- Shao, H.-B.; Song, W.-Y.; Chu, L.-Y. Advances of calcium signals involved in plant anti-drought. Comptes Rendus Biol. 2008, 331, 587–596. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.K.; Dutta, B.K. CaCl2 improves post-drought recovery potential in Camellia sinensis (L) O. Kuntze. Plant Cell Rep. 2011, 30, 495–503. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Improving tolerance against drought in canola by penconazole and calcium. Pestic. Biochem. Physiol. 2018, 149, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Gopi, R.; Somasundaram, R.; Panneerselvam, R. Water deficit stress mitigation by calcium chloride in Catharanthus roseus: Effects on oxidative stress, proline metabolism and indole alkaloid accumulation. Colloids Surf. B Biointerfaces 2007, 60, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Hui, Y.; Zhang, L.; Su, B.; Wang, R. Foliar application of chelated sugar alcohol calcium fertilizer for regulating the growth and quality of wine grapes. Int. J. Agric. Biol. Eng. 2022, 15, 153–158. [Google Scholar] [CrossRef]
- Su, F.; Li, Y.; Liu, S.; Liu, Z.; Nie, S.; Xu, Q.; Qin, F.; Li, F.; Lyu, D.; Xu, H.-l. Application of Xerophytophysiology and Signal Transduction in Plant Production: Partial Root-Zone Drying in Potato Crops. Potato Res. 2020, 63, 41–56. [Google Scholar] [CrossRef]
- Boguszewska-Mankowska, D.; Zarzynska, K.; Nosalewicz, A. Drought Differentially Affects Root System Size and Architecture of Potato Cultivars with Differing Drought Tolerance. Am. J. Potato Res. 2020, 97, 54–62. [Google Scholar] [CrossRef]
- Huntenburg, K.; Dodd, I.C.; Stalham, M. Agronomic and physiological responses of potato subjected to soil compaction and/or drying. Ann. Appl. Biol. 2021, 178, 328–340. [Google Scholar] [CrossRef]
- Andrea, A.-V.; Muriel, Q.; Stanley, L.; Juan Pablo, M.; Carolina, L.X. Tuber yield and quality responses of potato to moderate temperature increase during Tuber bulking under two water availability scenarios. Field Crops Res. 2020, 251, 107786. [Google Scholar] [CrossRef]
- Ierna, A.; Mauromicale, G. Tuber yield and irrigation water productivity in early potatoes as affected by irrigation regime. Agric. Water Manag. 2012, 115, 276–284. [Google Scholar] [CrossRef]
- Bi, Z.; Wang, Y.; Li, P.; Sun, C.; Qin, T.; Bai, J. Evolution and expression analysis of CDPK genes under drought stress in two varieties of potato. Biotechnol. Lett. 2021, 43, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Wang, Y.; Li, P.; Li, C.; Liu, Y.; Sun, C.; Yao, P.; Liu, Y.; Liu, Z.; Bai, J. Transcriptomics Analysis Reveals a More Refined Regulation Mechanism of Methylation in a Drought-Tolerant Variety of Potato. Genes 2022, 13, 2260. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Li, J.; Kong, L.; Hu, H.; Tian, J.; Liu, Y.; Wei, A. miRNAs and their target genes regulate the antioxidant system of Zanthoxylum bungeanum under drought stress. Plant Physiol. Biochem. 2020, 150, 196–203. [Google Scholar] [CrossRef]
- Xu, C.; Li, X.; Zhang, L. The Effect of Calcium Chloride on Growth, Photosynthesis, and Antioxidant Responses of Zoysia japonica under Drought Conditions. PLoS ONE 2013, 8, e68214. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Shaw, A.K.; Bhardwaj, P.K.; Ghosh, S.; Roy, S.; Saha, S.; Sherpa, A.R.; Saha, S.K.; Hossain, Z. beta-aminobutyric acid mediated drought stress alleviation in maize (Zea mays L.). Environ. Sci. Pollut. Res. 2016, 23, 2437–2453. [Google Scholar] [CrossRef]
- Leng, X.; Xue, L.; Wang, J.; Li, S.; Yang, Z.; Ren, H.; Yao, X.; Wu, Z.; Li, J. Physiological Responses of Handeliodendron bodinieri (Levl.) Rehd. to Exogenous Calcium Supply under Drought Stress. Forests 2020, 11, 69. [Google Scholar] [CrossRef]
- Yang, M.; Tan, L.; Xu, Y.; Zhao, Y.; Cheng, F.; Ye, S.; Jiang, W. Effect of Low pH and Aluminum Toxicity on the Photosynthetic Characteristics of Different Fast-Growing Eucalyptus Vegetatively Propagated Clones. PLoS ONE 2015, 10, e0130963. [Google Scholar] [CrossRef]
- Ashkani, J.; Pakniyat, H.; Ghotbi, V. Genetic evaluation of several physiological traits for screening of suitable spring safflower (Carthamus tinctorius L.) genotypes under stress and non-stress irrigation regimes. Pak. J. Biol. Sci. PJBS 2007, 10, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Schollert, M.; Kivimaenpaa, M.; Michelsen, A.; Blok, D.; Rinnan, R. Leaf anatomy, BVOC emission and CO2 exchange of arctic plants following snow addition and summer warming. Ann. Bot. 2017, 119, 433–445. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qi, M.; Huang, Z.; Xu, X.; Begum, N.; Qin, C.; Zhang, C.; Ahmad, N.; Mustafa, N.S.; Ashraf, M.; et al. Improving growth and photosynthetic performance of drought stressed tomato by application of nano-organic fertilizer involves up-regulation of nitrogen, antioxidant and osmolyte metabolism. Ecotoxicol. Environ. Saf. 2021, 216, 112195. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. NMCD 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Yonny, M.E.; Rodriguez Torressi, A.; Nazareno, M.A.; Cerutti, S. Development of a Novel, Sensitive, Selective, and Fast Methodology to Determine Malondialdehyde in Leaves of Melon Plants by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Anal. Methods Chem. 2017, 2017, 4327954. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Luo, Q.; Tian, Y.; Meng, F. Physiological and proteomic analyses of the drought stress response in Amygdalus Mira (Koehne) Yu et Lu roots. Bmc Plant Biol. 2017, 17, 53. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Alegre, L.J.P.P. The xanthophyll cycle is induced by light irrespective of water status in field-grown lavender (Lavandula stoechas) plants. Physiol. Plant. 2010, 108, 147–151. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Penconazole and calcium ameliorate drought stress in canola by upregulating the antioxidative enzymes. Funct. Plant Biol. 2020, 47, 825–839. [Google Scholar] [CrossRef]
- Serraj, R.; Sinclair, T.R. Osmolyte accumulation: Can it really help increase crop yield under drought conditions? Plant Cell Environ. 2002, 25, 333–341. [Google Scholar] [CrossRef]
- Hong, Z.; Lakkineni, K.; Zhang, Z.; Verma, D.P. Removal of feedback inhibition of delta(1)-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000, 122, 1129–1136. [Google Scholar] [CrossRef]
- Siripornadulsil, S.; Traina, S.; Verma, D.P.S.; Sayre, R.T. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 2002, 14, 2837–2847. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Naeem, M.S.; Ahmad, R.; Ihsan, M.Z.; Ashraf, M.Y.; Hussain, Y.; Fahad, S. Foliar calcium spray confers drought stress tolerance in maize via modulation of plant growth, water relations, proline content and hydrogen peroxide activity. Arch. Agron. Soil Sci. 2018, 64, 116–131. [Google Scholar] [CrossRef]
- Fazeli, F.; Ghorbanli, M.; Niknam, V.J.B.P. Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol. Plant. 2007, 51, 98–103. [Google Scholar] [CrossRef]
- Vaseva, I.; Akiscan, Y.; Simova-Stoilova, L.; Kostadinova, A.; Nenkova, R.; Anders, I.; Feller, U.; Demirevska, K. Antioxidant response to drought in red and white clover. Acta Physiol. Plant. 2012, 34, 1689–1699. [Google Scholar] [CrossRef]
- Hasanagić, D.; Koleska, I.; Kojic, D.; Vlaisavljevic, S.; Janjic, N.; Kukavica, B. Long term drought effects on tomato leaves: Anatomical, gas exchange and antioxidant modifications. Acta Physiol. Plant. 2020, 42, 121. [Google Scholar] [CrossRef]
- Ariharasutharsan, G.; Karthikeyan, A.; Renganathan, V.G.; Marthandan, V.; Dhasarathan, M.; Ambigapathi, A.; Akilan, M.; Palaniyappan, S.; Mariyammal, I.; Pandiyan, M.; et al. Distinctive Physio-Biochemical Properties and Transcriptional Changes Unfold the Mungbean Cultivars Differing by Their Response to Drought Stress at Flowering Stage. Horticulturae 2022, 8, 424. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.M.; Zhang, Q.F.; Li, J. Enhanced chilling tolerance in Zoysia matrella by pre-treatment with salicylic acid, calcium chloride, hydrogen peroxide or 6-benzylaminopurine. Biol. Plant. 2009, 53, 179–182. [Google Scholar] [CrossRef]
- Issam, N.; Kawther, M.; Haythem, M.; Moez, J. Effects of CaCl2 pretreatment on antioxidant enzyme and leaf lipid content of faba bean (Vicia faba L.) seedlings under cadmium stress. Plant Growth Regul. 2012, 68, 37–47. [Google Scholar] [CrossRef]
- Ozgen, S.; Ozgen, M.; Palta, J.P. Influence of supplemental calcium fertilization on potato tuber size and tuber number. HortScience 2003, 619, 542. [Google Scholar]
- Ciccarese, A.; Stellacci, A.M.; Gentilesco, G.; Rubino, P. Effectiveness of pre- and post-veraison calcium applications to control decay and maintain table grape fruit quality during storage. Postharvest Biol. Technol. 2013, 75, 135–141. [Google Scholar] [CrossRef]
- Lötze, E.; Joubert, J.; Theron, K.I.J.S.H. Evaluating pre-harvest foliar calcium applications to increase fruit calcium and reduce bitter pit in ‘Golden Delicious’ apples. Sci. Hortic. 2008, 116, 299–304. [Google Scholar] [CrossRef]
- Tuteja, N.; Mahajan, S. Calcium signaling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Luan, S.; Kudla, J.; Rodriguez-Concepcion, M.; Yalovsky, S.; Gruissem, W. Calmodulins and calcineurin B-like proteins: Calcium sensors for specific signal response coupling in plants. Plant Cell 2002, 14 (Suppl. S1), S389–S400. [Google Scholar] [CrossRef]
- Dekomah, S.D.; Bi, Z.; Dormatey, R.; Wang, Y.; Haider, F.U.; Sun, C.; Yao, P.; Bai, J. The role of CDPKs in plant development, nutrient and stress signaling. Front. Genet. 2022, 13, 996203. [Google Scholar] [CrossRef]
- Quandahor, P.; Lin, C.; Gou, Y.; Coulter, J.A.; Liu, C. Leaf Morphological and Biochemical Responses of Three Potato (Solanum tuberosum L.) Cultivars to Drought Stress and Aphid (Myzus persicae Sulzer) Infestation. Insects 2019, 10, 435. [Google Scholar] [CrossRef]
- Dormatey, R.; Sun, C.; Ali, K.; Qin, T.; Xu, D.; Bi, Z.; Bai, J. Influence of Phosphite Supply in the MS Medium on Root Morphological Characteristics, Fresh Biomass and Enzymatic Behavior in Five Genotypes of Potato (Solanum tuberosum L.). Horticulturae 2021, 7, 265. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. II. Role of electron transfer. Arch. Biochem. Biophys. 1968, 125, 850–857. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Xue, L.; Ren, H.; Long, W.; Leng, X.; Wang, J.; Yao, X.; Li, S. Ecophysiological Responses of Calcicole Cyclobalanopsis glauca (Thunb.) Oerst. to Drought Stress and Calcium Supply. Forests 2018, 9, 667. [Google Scholar] [CrossRef]
- Boguszewska, D.; Grudkowska, M.; Zagdanska, B. Drought-Responsive Antioxidant Enzymes in Potato (Solanum tuberosum L.). Potato Res. 2010, 53, 373–382. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [PubMed]
- Upadhyaya, A.; Sankhla, D.; Davis, T.D.; Sankhla, N.; Smith, B.N.J. Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J. Plant Physiol. 1985, 121, 453–461. [Google Scholar] [CrossRef]
- Dekomah, S.D.; Wang, Y.; Qin, T.; Xu, D.; Sun, C.; Yao, P.; Liu, Y.; Bi, Z.; Bai, J. Identification and Expression Analysis of Calcium-Dependent Protein Kinases Gene Family in Potato under Drought Stress. Front. Genet. 2022, 13, 874397. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qin, T.; Pu, Z.; Dekomah, S.D.; Yao, P.; Sun, C.; Liu, Y.; Bi, Z.; Bai, J. Foliar Application of Chelated Sugar Alcohol Calcium Improves Photosynthesis and Tuber Quality under Drought Stress in Potatoes (Solanum tuberosum L.). Int. J. Mol. Sci. 2023, 24, 12216. https://doi.org/10.3390/ijms241512216
Wang Y, Qin T, Pu Z, Dekomah SD, Yao P, Sun C, Liu Y, Bi Z, Bai J. Foliar Application of Chelated Sugar Alcohol Calcium Improves Photosynthesis and Tuber Quality under Drought Stress in Potatoes (Solanum tuberosum L.). International Journal of Molecular Sciences. 2023; 24(15):12216. https://doi.org/10.3390/ijms241512216
Chicago/Turabian StyleWang, Yihao, Tianyuan Qin, Zhuanfang Pu, Simon Dontoro Dekomah, Panfeng Yao, Chao Sun, Yuhui Liu, Zhenzhen Bi, and Jiangping Bai. 2023. "Foliar Application of Chelated Sugar Alcohol Calcium Improves Photosynthesis and Tuber Quality under Drought Stress in Potatoes (Solanum tuberosum L.)" International Journal of Molecular Sciences 24, no. 15: 12216. https://doi.org/10.3390/ijms241512216
APA StyleWang, Y., Qin, T., Pu, Z., Dekomah, S. D., Yao, P., Sun, C., Liu, Y., Bi, Z., & Bai, J. (2023). Foliar Application of Chelated Sugar Alcohol Calcium Improves Photosynthesis and Tuber Quality under Drought Stress in Potatoes (Solanum tuberosum L.). International Journal of Molecular Sciences, 24(15), 12216. https://doi.org/10.3390/ijms241512216






