The Role of ADAMTS Proteoglycanases in Thoracic Aortic Disease
Abstract
1. Introduction
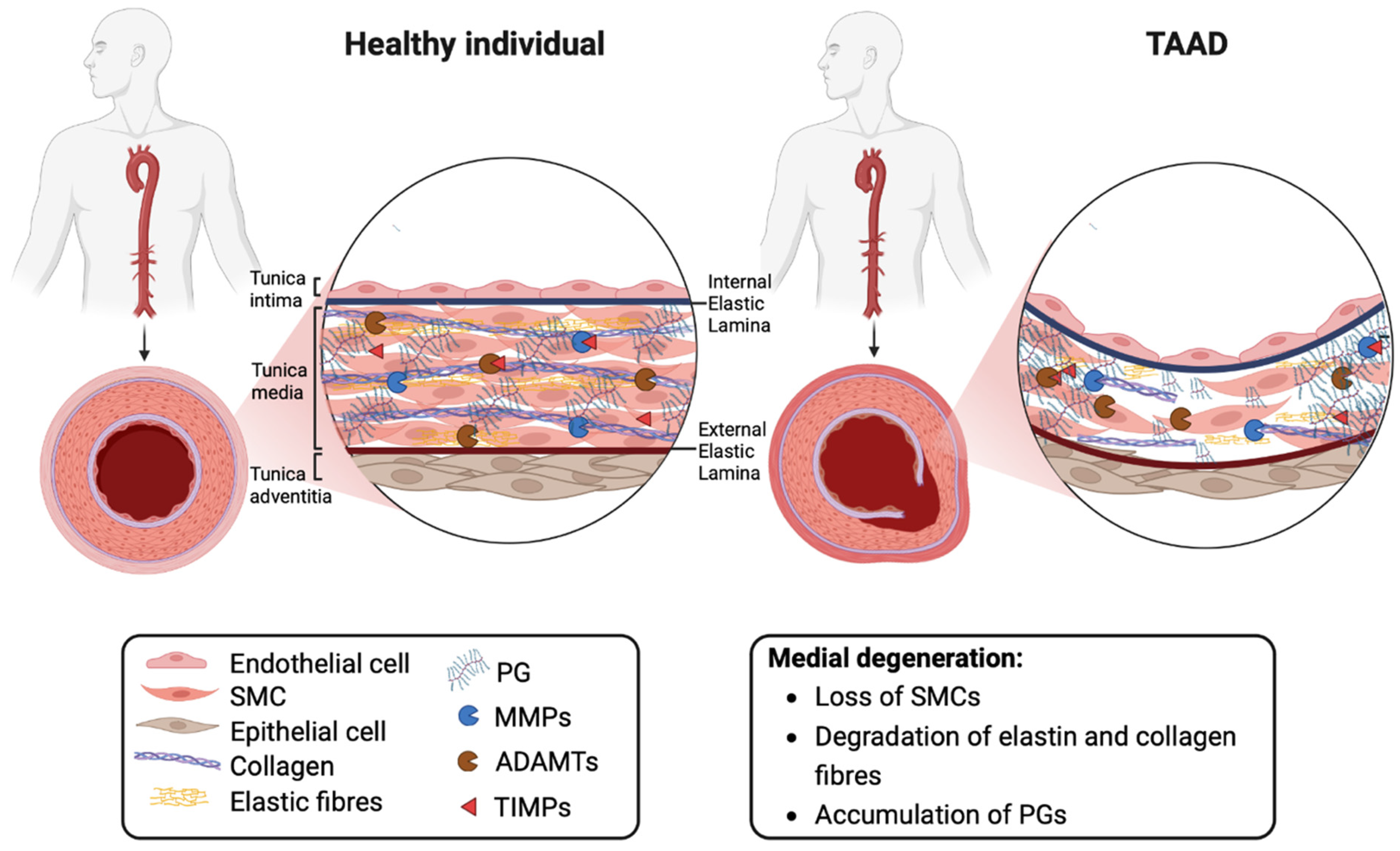
2. Thoracic Aortic Aneurysms and Dissections
3. The Extracellular Matrix
3.1. Proteoglycans and Biomechanical Properties of the Aorta
3.2. Proteoglycans in Aortic Disease
4. The Role of ADAMTS Proteoglycanases in Aortic Disease
5. Levels of ADAMTS Proteases in TAAD
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Alfonso, F.; Boileau, C.; Dirsch, O.; Eber, B.; Haverich, A.; Rakowski, H.; Struyven, J.; Radegran, K.; Sechtem, U.; et al. Diagnosis and management of aortic dissection. Eur. Heart J. 2001, 22, 1642–1681. [Google Scholar] [CrossRef] [PubMed]
- Melvinsdottir, I.H.; Lund, S.H.; Agnarsson, B.A.; Sigvaldason, K.; Gudbjartsson, T.; Geirsson, A. The incidence and mortality of acute thoracic aortic dissection: Results from a whole nation study. Eur. J. Cardiothorac. Surg. 2016, 50, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.; Khanduri, P.; McCord, C.; Ohene-Yeboah, M.; Uranues, S.; Vega Rivera, F.; Mock, C. Global disease burden of conditions requiring emergency surgery. BJS (Br. J. Surg.) 2014, 101, e9–e22. [Google Scholar] [CrossRef]
- Howard, D.P.; Banerjee, A.; Fairhead, J.F.; Perkins, J.; Silver, L.E.; Rothwell, P.M. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013, 127, 2031–2037. [Google Scholar] [CrossRef]
- Cikach, F.S.; Koch, C.D.; Mead, T.J.; Galatioto, J.; Willard, B.B.; Emerton, K.B.; Eagleton, M.J.; Blackstone, E.H.; Ramirez, F.; Roselli, E.E.; et al. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight 2018, 3, e97167. [Google Scholar] [CrossRef]
- Santamaria, S.; de Groot, R. ADAMTS proteases in cardiovascular physiology and disease. Open Biol. 2020, 10, 200333. [Google Scholar] [CrossRef]
- Rienks, M.; Barallobre-Barreiro, J.; Mayr, M. The Emerging Role of the ADAMTS Family in Vascular Diseases. Circ. Res. 2018, 123, 1279–1281. [Google Scholar] [CrossRef]
- Novak, R.; Hrkac, S.; Salai, G.; Bilandzic, J.; Mitar, L.; Grgurevic, L. The Role of ADAMTS-4 in Atherosclerosis and Vessel Wall Abnormalities. J. Vasc. Res. 2022, 59, 69–77. [Google Scholar] [CrossRef]
- Rose, K.W.J.; Taye, N.; Karoulias, S.Z.; Hubmacher, D. Regulation of ADAMTS Proteases. Front. Mol. Biosci. 2021, 8, 701959. [Google Scholar] [CrossRef]
- Vriz, O.; Driussi, C.; Bettio, M.; Ferrara, F.; D Andrea, A.; Bossone, E. Aortic root dimensions and stiffness in healthy subjects. Am. J. Cardiol. 2013, 112, 1224–1229. [Google Scholar] [CrossRef]
- Tsamis, A.; Krawiec, J.T.; Vorp, D.A. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: A review. J. R. Soc. Interface 2013, 10, 20121004. [Google Scholar] [CrossRef]
- Elefteriades, J.A.; Sang, A.; Kuzmik, G.; Hornick, M. Guilt by association: Paradigm for detecting a silent killer (thoracic aortic aneurysm). Open Heart 2015, 2, e000169. [Google Scholar] [CrossRef]
- Coady, M.A.; Rizzo, J.A.; Hammond, G.L.; Mandapati, D.; Darr, U.; Kopf, G.S.; Elefteriades, J.A. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J. Thorac. Cardiovasc. Surg. 1997, 113, 476–491; discussion 489–491. [Google Scholar] [CrossRef]
- Benedetto, U.; Sinha, S.; Dimagli, A.; Cooper, G.; Mariscalco, G.; Uppal, R.; Moorjani, N.; Krasopoulos, G.; Kaura, A.; Field, M.; et al. Decade-long trends in surgery for acute Type A aortic dissection in England: A retrospective cohort study. Lancet Reg. Health Eur. 2021, 7, 100131. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black Iii, J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 80, e223–e393. [Google Scholar] [CrossRef] [PubMed]
- Pape, L.A.; Tsai, T.T.; Isselbacher, E.M.; Oh, J.K.; O’gara, P.T.; Evangelista, A.; Fattori, R.; Meinhardt, G.; Trimarchi, S.; Bossone, E.; et al. Aortic diameter > or =5.5 cm is not a good predictor of type A aortic dissection: Observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007, 116, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Bonser, R.S.; Ranasinghe, A.M.; Loubani, M.; Evans, J.D.; Thalji, N.M.A.; Bachet, J.E.; Carrel, T.P.; Czerny, M.; Di Bartolomeo, R.; Grabenwöger, M.; et al. Evidence, Lack of Evidence, Controversy, and Debate in the Provision and Performance of the Surgery of Acute Type A Aortic Dissection. J. Am. Coll. Cardiol. 2011, 58, 2455–2474. [Google Scholar] [CrossRef]
- Suzuki, T.; Distante, A.; Zizza, A.; Trimarchi, S.; Villani, M.; Uriarte, J.A.S.; Schinosa, L.D.L.T.; Renzulli, A.; Sabino, F.; Nowak, R.; et al. Diagnosis of Acute Aortic Dissection by D-Dimer. Circulation 2009, 119, 2702–2707. [Google Scholar] [CrossRef]
- Salmasi, M.Y.; Hartley, P.; Hussein, M.; Jarral, O.; Pepper, J.; Nienaber, C.; Athanasiou, T. Diagnosis and management of acute Type-A aortic dissection in emergency departments: Results of a UK national survey. Int. J. Cardiol. 2020, 300, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, A.M.; Bonser, R.S. Biomarkers in Acute Aortic Dissection and Other Aortic Syndromes. J. Am. Coll. Cardiol. 2010, 56, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Balmforth, D.; Harky, A.; Adams, B.; Yap, J.; Shipolini, A.; Roberts, N.; Uppal, R.; Bashir, M. Is there a role for biomarkers in thoracic aortic aneurysm disease? Gen. Thorac. Cardiovasc. Surg. 2019, 67, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.F.; Touat, Z.; Leclercq, A.; Al Haj Zen, A.; Jondeau’, G.; Franc, B.; Philippe, M.; Meilhac, O.; Gutierrez, P.S.; Michel, J.-B. Tissue diffusion and retention of metalloproteinases in ascending aortic aneurysms and dissections. Hum. Pathol. 2009, 40, 306–313. [Google Scholar] [CrossRef]
- Farkas, E.A. Biomarkers for diagnosis in thoracic aortic disease: CON. Cardiol. Clin. 2010, 28, 213–220. [Google Scholar] [CrossRef]
- Dong, J.; Bao, J.; Feng, R.; Zhao, Z.; Lu, Q.; Wang, G.; Li, H.; Su, D.; Zhou, J.; Jing, Q.; et al. Circulating microRNAs: A novel potential biomarker for diagnosing acute aortic dissection. Sci. Rep. 2017, 7, 12784. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Huang, B.; Yang, Y.M.; Zhang, L.; Su, W.J.; Tian, L.; Lu, T.Y.; Zhang, S.; Fan, X.H.; Hui, R.T. Differential expression of microRNAs in aortic tissue and plasma in patients with acute aortic dissection. J. Geriatr. Cardiol. 2015, 12, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.A.; Munoz, J.-V.; Patel, T.R.; Loukas, M.; Tubbs, R.S. The anatomy of the aging aorta. Clin. Anat. 2014, 27, 463–466. [Google Scholar] [CrossRef]
- Wight, T.N. A role for proteoglycans in vascular disease. Matrix Biol. 2018, 71, 396–420. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Yamamoto, K.; Okano, H.; Miyagawa, W.; Visse, R.; Shitomi, Y.; Santamaria, S.; Dudhia, J.; Troeberg, L.; Strickland, D.K.; Hirohata, S.; et al. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016, 56, 57–73. [Google Scholar] [CrossRef]
- Yamamoto, K.; Scavenius, C.; Meschis, M.M.; Gremida, A.M.E.; Mogensen, E.H.; Thøgersen, I.B.; Bonelli, S.; Scilabra, S.D.; Jensen, A.; Santamaria, S.; et al. A top-down approach to uncover the hidden ligandome of low-density lipoprotein receptor-related protein 1 in cartilage. Matrix Biol. 2022, 112, 190–218. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ling, Z.; Ren, X. Extracellular matrix dynamics: Tracking in biological systems and their implications. J. Biol. Eng. 2022, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Spinale, F.G.; Ikonomidis, J.S. Transforming growth factor-beta signaling in thoracic aortic aneurysm development: A paradox in pathogenesis. J. Vasc. Res. 2009, 46, 119–137. [Google Scholar] [CrossRef]
- Martin-Blazquez, A.; Heredero, A.; Aldamiz-Echevarria, G.; Martin-Lorenzo, M.; Alvarez-Llamas, G. Non-syndromic thoracic aortic aneurysm: Cellular and molecular insights. J. Pathol. 2021, 254, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Ostberg, N.P.; Zafar, M.A.; Ziganshin, B.A.; Elefteriades, J.A. The Genetics of Thoracic Aortic Aneurysms and Dissection: A Clinical Perspective. Biomolecules 2020, 10, 182. [Google Scholar] [CrossRef]
- Rai, P.; Robinson, L.; Davies, H.A.; Akhtar, R.; Field, M.; Madine, J. Is There Enough Evidence to Support the Role of Glycosaminoglycans and Proteoglycans in Thoracic Aortic Aneurysm and Dissection?-A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9200. [Google Scholar] [CrossRef]
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef]
- Azeloglu, E.U.; Albro, M.B.; Thimmappa, V.A.; Ateshian, G.A.; Costa, K.D. Heterogeneous transmural proteoglycan distribution provides a mechanism for regulating residual stresses in the aorta. Am. J. Physiol.-Heart Circ. Physiol. 2008, 294, H1197–H1205. [Google Scholar] [CrossRef]
- Roccabianca, S.; Ateshian, G.A.; Humphrey, J.D. Biomechanical roles of medial pooling of glycosaminoglycans in thoracic aortic dissection. Biomech. Model. Mechanobiol. 2014, 13, 13–25. [Google Scholar] [CrossRef]
- Salmasi, M.Y.; Pirola, S.; Sasidharan, S.; Fisichella, S.M.; Redaelli, A.; Jarral, O.A.; O’Regan, D.P.; Oo, A.Y.; Moore, J.E.; Xu, X.Y.; et al. High Wall Shear Stress can Predict Wall Degradation in Ascending Aortic Aneurysms: An Integrated Biomechanics Study. Front. Bioeng. Biotechnol. 2021, 9, 750656. [Google Scholar] [CrossRef]
- Lemire, J.M.; Braun, K.R.; Maurel, P.; Kaplan, E.D.; Schwartz, S.M.; Wight, T.N. Versican/PG-M isoforms in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1630–1639. [Google Scholar] [CrossRef]
- Cattaruzza, S.; Schiappacassi, M.; Ljungberg-Rose, A.; Spessotto, P.; Perissinotto, D.; Mörgelin, M.; Mucignat, M.T.; Colombatti, A.; Perris, R. Distribution of PG-M/versican variants in human tissues and de novo expression of isoform V3 upon endothelial cell activation, migration, and neoangiogenesis in vitro. J. Biol. Chem. 2002, 277, 47626–47635. [Google Scholar] [CrossRef]
- Ameye, L.; Aria, D.; Jepsen, K.; Oldberg, A.; Xu, T.; Young, M.F. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002, 16, 673–680. [Google Scholar] [CrossRef]
- Meester, J.A.; Vandeweyer, G.; Pintelon, I.; Lammens, M.; Van Hoorick, L.; De Belder, S.; Waitzman, K.; Young, L.; Markham, L.W.; Vogt, J.; et al. Loss-of-function mutations in the X-linked biglycan gene cause a severe syndromic form of thoracic aortic aneurysms and dissections. Genet. Med. 2017, 19, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Heegaard, A.M.; Corsi, A.; Danielsen, C.C.; Nielsen, K.L.; Jorgensen, H.L.; Riminucci, M.; Young, M.F.; Bianco, P. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation 2007, 115, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, S.; Yamamoto, K.; Teraz-Orosz, A.; Koch, C.; Apte, S.S.; de Groot, R.; Lane, D.A.; Ahnström, J. Exosites in Hypervariable Loops of ADAMTS Spacer Domains control Substrate Recognition and Proteolysis. Sci. Rep. 2019, 9, 10914. [Google Scholar] [CrossRef] [PubMed]
- Minns, A.; Qi, Y.; Yamamoto, K.; Lee, K.; Ahnström, J.; Santamaria, S. The C-terminal domains of ADAMTS1 contain exosites involved in its proteoglycanase activity. J. Biol. Chem. 2023, 299, 103048. [Google Scholar] [CrossRef] [PubMed]
- Massadeh, S.; Alhashem, A.; van de Laar, I.; Alhabshan, F.; Ordonez, N.; Alawbathani, S.; Khan, S.; Kabbani, M.S.; Chaikhouni, F.; Sheereen, A.; et al. ADAMTS19-associated heart valve defects: Novel genetic variants consolidating a recognizable cardiac phenotype. Clin. Genet. 2020, 98, 56–63. [Google Scholar] [CrossRef]
- Wünnemann, F.; Ta-Shma, A.; Preuss, C.; Leclerc, S.; van Vliet, P.P.; Oneglia, A.; Thibeault, M.; Nordquist, E.; Lincoln, J.; Scharfenberg, F.; et al. Loss of ADAMTS19 causes progressive non-syndromic heart valve disease. Nat. Genet. 2020, 52, 40–47. [Google Scholar] [CrossRef]
- Oller, J.; Méndez-Barbero, N.; Ruiz, E.J.; Villahoz, S.; Renard, M.; Canelas, L.I.; Briones, A.M.; Alberca, R.; Lozano-Vidal, N.; Hurlé, M.A.; et al. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat. Med. 2017, 23, 200–212. [Google Scholar] [CrossRef]
- Mittaz, L.; Russell, D.L.; Wilson, T.; Brasted, M.; Tkalcevic, J.; Salamonsen, L.A.; Hertzog, P.J.; Pritchard, M.A. Adamts-1 is essential for the development and function of the urogenital system. Biol. Reprod. 2004, 70, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Zhao, G.; He, L.; Fu, Y.; Yu, C.; Wang, Z.; Zhao, T.; Cao, F.; Gao, Y.; et al. Postnatal deficiency of ADAMTS1 ameliorates thoracic aortic aneurysm and dissection in mice. Exp. Physiol. 2018, 103, 1717–1731. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Hughes, M.; Krishnamoorthy, S.; Zou, S.; Zhang, L.; Wu, D.; Zhang, C.; Curci, J.A.; Coselli, J.S.; Milewicz, D.M.; et al. Critical Role of ADAMTS-4 in the Development of Sporadic Aortic Aneurysm and Dissection in Mice. Sci. Rep. 2017, 7, 12351. [Google Scholar] [CrossRef]
- Santamaria, S. ADAMTS-5: A difficult teenager turning 20. Int. J. Exp. Pathol. 2020, 101, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Barallobre-Barreiro, J.; Mayr, U.; Lu, R.; Didangelos, A.; Baig, F.; Lynch, M.; Catibog, N.; Joshi, A.; Barwari, T.; et al. Role of ADAMTS-5 in Aortic Dilatation and Extracellular Matrix Remodeling. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.E.; Nelson, E.L.; Hozik, B.; Porto, S.C.; Rogers-DeCotes, A.; Fosang, A.; Kern, C.B. Adamts5−/− Mice Exhibit Altered Aggrecan Proteolytic Profiles That Correlate with Ascending Aortic Anomalies. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2067–2081. [Google Scholar] [CrossRef] [PubMed]
- Kern, C.B.; Wessels, A.; McGarity, J.; Dixon, L.J.; Alston, E.; Argraves, W.S.; Geeting, D.; Nelson, C.M.; Menick, D.R.; Apte, S.S. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biol. 2010, 29, 304–316. [Google Scholar] [CrossRef]
- Wang, L.; Ming Wang, L.; Chen, W.; Chen, X. Bicuspid Aortic Valve: A Review of its Genetics and Clinical Significance. J. Heart Valve Dis. 2016, 25, 568–573. [Google Scholar]
- Sybert, V.P.; McCauley, E. Turner’s syndrome. N. Engl. J. Med. 2004, 351, 1227–1238. [Google Scholar] [CrossRef]
- Corbitt, H.; Morris, S.A.; Gravholt, C.H.; Mortensen, K.H.; Tippner-Hedges, R.; Silberbach, M.; Maslen, C.L. TIMP3 and TIMP1 are risk genes for bicuspid aortic valve and aortopathy in Turner syndrome. PLoS Genet. 2018, 14, e1007692. [Google Scholar] [CrossRef]
- Dupuis, L.E.; Osinska, H.; Weinstein, M.B.; Hinton, R.B.; Kern, C.B. Insufficient versican cleavage and Smad2 phosphorylation results in bicuspid aortic and pulmonary valves. J. Mol. Cell Cardiol. 2013, 60, 50–59. [Google Scholar] [CrossRef]
- Li, L.; Ma, W.; Pan, S.; Li, Y.; Wang, H.; Wang, B.; Khalil, R.A. Mir 126a 5p limits the formation of abdominal aortic aneurysm in mice and decreases ADAMTs 4 expression. J. Cell. Mol. Med. 2020, 24, 7896–7906. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, Y.; Kanematsu, M.; Kurihara, C.; Tsou, T.L.; Nuki, Y.; Liang, E.I.; Makino, H.; Hashimoto, T. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension 2010, 55, 1267–1274. [Google Scholar] [CrossRef]
- Lu, L.; Tong, Y.; Wang, W.; Hou, Y.; Dou, H.; Liu, Z. Characterization and Significance of Monocytes in Acute Stanford Type B Aortic Dissection. J. Immunol. Res. 2020, 2020, 9670360. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, W.; Yu, C.; Zhong, F.; Li, G.; Kong, W.; Zheng, J. A disintegrin and metalloproteinase with thrombospondin motif 1 (ADAMTS1) expression increases in acute aortic dissection. Sci. China Life Sci. 2016, 59, 59–67. [Google Scholar] [CrossRef]
- Güneş, M.F.; Akpinar, M.B.; Cömertoğlu, I.; Akyol, S.; Demirçelik, B.; Gürel, Ö.M.; Aynekin, B.; Erdemli, H.K.; Ateş, M.; Eryonucu, B.; et al. The Investigation of a Disintegrin and Metalloproteinase with ThromboSpondin Motifs (ADAMTS) 1, 5 and 16 in Thoracic Aortic Aneurysms and Dissections. Clin. Lab. 2016, 62, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Taketani, T.; Imai, Y.; Morota, T.; Maemura, K.; Morita, H.; Hayashi, D.; Yamazaki, T.; Nagai, R.; Takamoto, S. Altered patterns of gene expression specific to thoracic aortic aneurysms: Microarray analysis of surgically resected specimens. Int. Heart J. 2005, 46, 265–277. [Google Scholar] [CrossRef]
- Ren, P.; Zhang, L.; Xu, G.; Palmero, L.C.; Albini, P.T.; Coselli, J.S.; Shen, Y.H.; LeMaire, S.A. ADAMTS-1 and ADAMTS-4 levels are elevated in thoracic aortic aneurysms and dissections. Ann. Thorac. Surg. 2013, 95, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, Z.W.; Hu, Z.; Ren, Z.; Hu, X.; Li, L.; Wu, Z.; Wu, H.; Li, B.; Huang, J.; et al. Assessing Serum Levels of ADAMTS1 and ADAMTS4 as New Biomarkers for Patients with Type A Acute Aortic Dissection. Med. Sci. Monit. 2017, 23, 3913–3922. [Google Scholar] [CrossRef]
- Kimura, N.; Futamura, K.; Arakawa, M.; Okada, N.; Emrich, F.; Okamura, H.; Sato, T.; Shudo, Y.; Koyano, T.K.; Yamaguchi, A.; et al. Gene expression profiling of acute type A aortic dissection combined with in vitro assessment. Eur. J. Cardiothorac. Surg. 2017, 52, 810–817. [Google Scholar] [CrossRef]
- Zeng, T.; Gan, J.; Liu, Y.; Shi, L.; Lu, Z.; Xue, Y.; Xiong, R.; Liu, L.; Yang, Z.; Lin, Y.; et al. ADAMTS-5 Decreases in Aortas and Plasma from Aortic Dissection Patients and Alleviates Angiotensin II-Induced Smooth Muscle-Cell Apoptosis. Front. Cardiovasc. Med. 2020, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, J.O.; Brangsch, J.; Kader, A.; Saatz, J.; Mangarova, D.B.; Zacharias, M.; Kempf, W.E.; Schwaar, T.; Ponader, M.; Adams, L.C.; et al. ADAMTS4-specific MR probe to assess aortic aneurysms in vivo using synthetic peptide libraries. Nat. Commun. 2022, 13, 2867. [Google Scholar] [CrossRef] [PubMed]
- Mougin, Z.; Huguet Herrero, J.; Boileau, C.; Le Goff, C. ADAMTS Proteins and Vascular Remodeling in Aortic Aneurysms. Biomolecules 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Xu, Y.; Yu, Q. Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro- and antimetastatic activity, respectively. Oncogene 2006, 25, 2452–2467. [Google Scholar] [CrossRef]
- Rao, N.; Ke, Z.; Liu, H.; Ho, C.-J.; Kumar, S.; Xiang, W.; Zhu, Y.; Ge, R. ADAMTS4 and its proteolytic fragments differentially affect melanoma growth and angiogenesis in mice. Int. J. Cancer 2013, 133, 294–306. [Google Scholar] [CrossRef]
- Kumar, S.; Sharghi-Namini, S.; Rao, N.; Ge, R. ADAMTS5 functions as an anti-angiogenic and anti-tumorigenic protein independent of its proteoglycanase activity. Am. J. Pathol. 2012, 181, 1056–1068. [Google Scholar] [CrossRef]

| Enzyme | Disease | Expression | Level | Localization | Reference |
|---|---|---|---|---|---|
| ADAMTS1 | TAAD (type B) | Decreased | mRNA | Tissue | [64] |
| MFS | Decreased | protein | Tissue | [50] | |
| TAAD | Increased | protein | Plasma/Tissue | [65] | |
| TAAD | Increased | protein | Tissue | [66] | |
| TAAD | Unchanged | mRNA | Tissue | [6] | |
| TAAD | Increased | mRNA | Tissue | [67] | |
| TAAD | Increased | mRNA/protein | Tissue | [68] | |
| TAAD (type A) | Increased | mRNA/protein | Serum/Tissue | [69] | |
| ADAMTS4 | TAAD | Increased | mRNA/protein | Tissue | [68] |
| TAAD | Unchanged | mRNA | Tissue | [6] | |
| TAAD | Increased | protein | Tissue | [53] | |
| TAAD (type A) | Increased | mRNA/protein | Serum/Tissue | [69] | |
| ADAMTS5 | TAAD | Increased | protein | Tissue | [66] |
| TAAD | Decreased | mRNA | Tissue | [6] | |
| TAAD | Decreased | mRNA | Tissue | [70] | |
| TAAD | Decreased | protein | Plasma | [71] | |
| ADAMTS9 | TAAD | Unchanged | mRNA | Tissue | [6] |
| ADAMTS20 | TAAD | Unchanged | mRNA | Tissue | [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemberi, M.; Salmasi, Y.; Santamaria, S. The Role of ADAMTS Proteoglycanases in Thoracic Aortic Disease. Int. J. Mol. Sci. 2023, 24, 12135. https://doi.org/10.3390/ijms241512135
Kemberi M, Salmasi Y, Santamaria S. The Role of ADAMTS Proteoglycanases in Thoracic Aortic Disease. International Journal of Molecular Sciences. 2023; 24(15):12135. https://doi.org/10.3390/ijms241512135
Chicago/Turabian StyleKemberi, Marsioleda, Yousuf Salmasi, and Salvatore Santamaria. 2023. "The Role of ADAMTS Proteoglycanases in Thoracic Aortic Disease" International Journal of Molecular Sciences 24, no. 15: 12135. https://doi.org/10.3390/ijms241512135
APA StyleKemberi, M., Salmasi, Y., & Santamaria, S. (2023). The Role of ADAMTS Proteoglycanases in Thoracic Aortic Disease. International Journal of Molecular Sciences, 24(15), 12135. https://doi.org/10.3390/ijms241512135








