Evaluation of Immune Infiltrates in Ovarian Endometriosis and Endometriosis-Associated Ovarian Cancer: Relationship with Histological and Clinical Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Samples Preparation
2.3. Immunohistochemistry
2.4. Immunofluorescence
2.5. Statistical Analysis
3. Results
3.1. Immunohistochemical Analysis of TILs Distribution in OE and EAOC
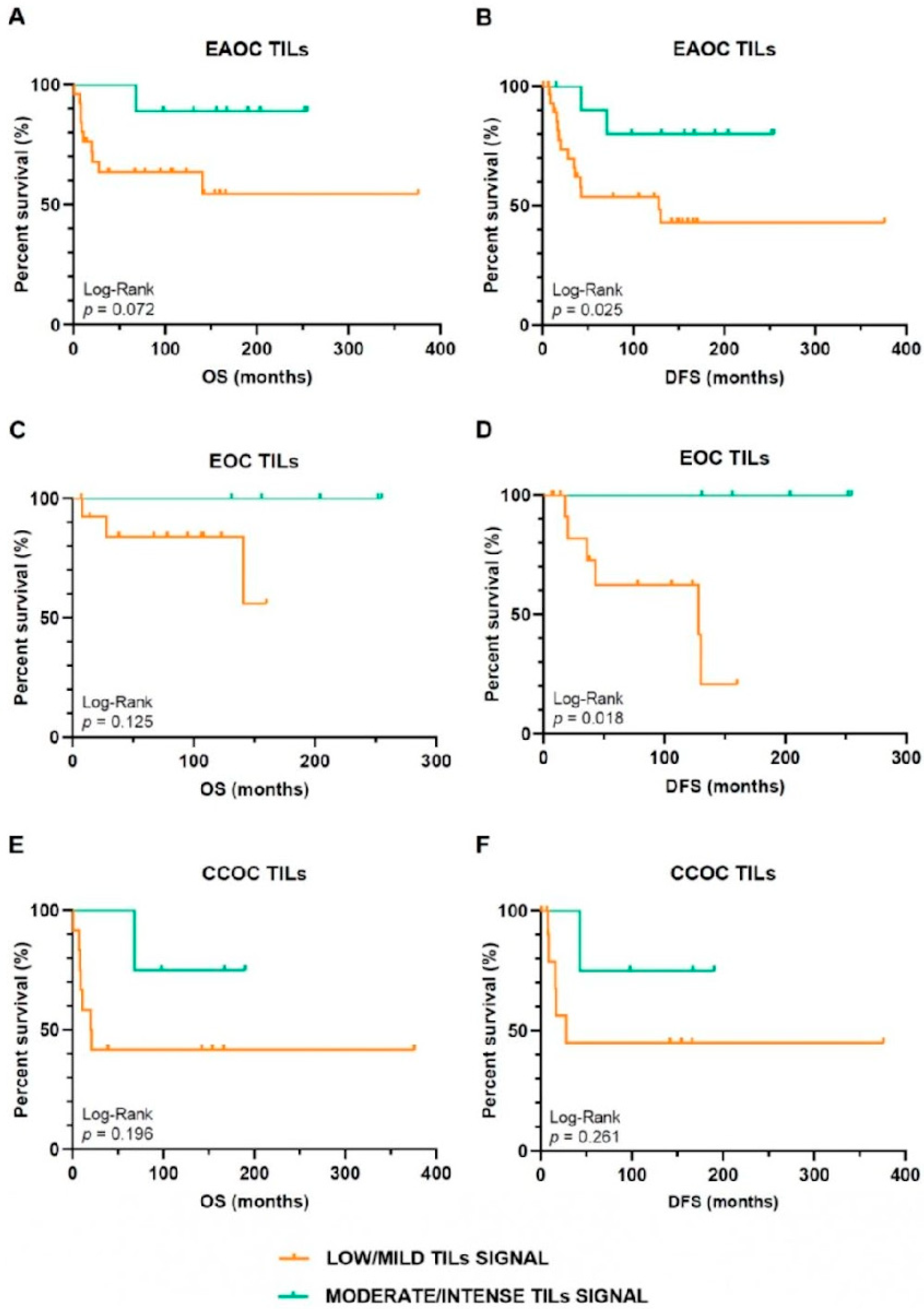
3.2. Oncological Outcomes Depending on TILs Infiltration Signal in EAOC Patients
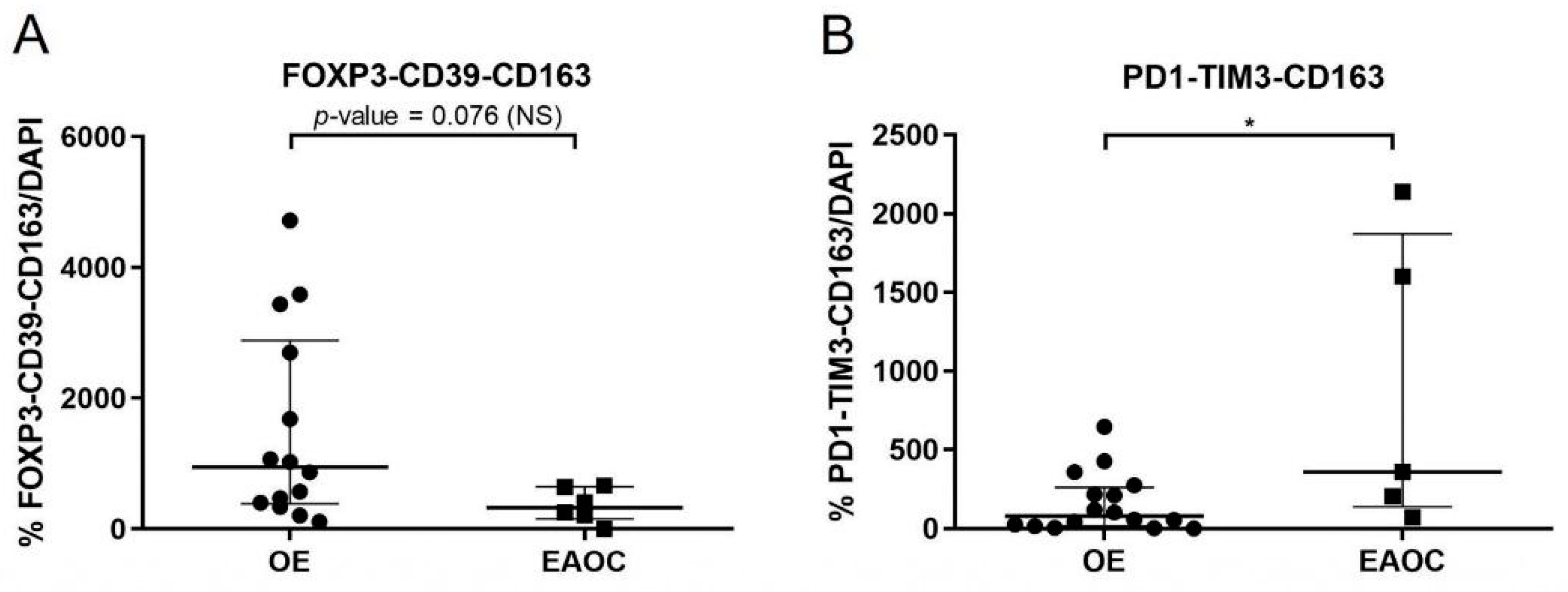
3.3. Analyses of T-Cell Exhaustion Markers in EAOC
3.4. Comparison of T-Cell Exhaustion Markers in EAOC vs. OE
4. Discussion
Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vallvé-Juanico, J.; Houshdaran, S.; Giudice, L.C. The Endometrial Immune Environment of Women with Endometriosis. Hum. Reprod. Update 2019, 25, 564–591. [Google Scholar] [CrossRef]
- Leenen, S.; Hermens, M.; de Vos van Steenwijk, P.J.; Bekkers, R.L.M.; van Esch, E.M.G. Immunologic Factors Involved in the Malignant Transformation of Endometriosis to Endometriosis-Associated Ovarian Carcinoma. Cancer Immunol. Immunother. 2021, 70, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between Endometriosis and Risk of Histological Subtypes of Ovarian Cancer: A Pooled Analysis of Case-Control Studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-J.J.; William, J.; Bulun, S. Endometriosis and Ovarian Cancer: A Review of Clinical, Pathologic, and Molecular Aspects. Int. J. Gynecol. Pathol. 2011, 30, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Hermens, M.; van Altena, A.M.; van der Aa, M.; Bulten, J.; van Vliet, H.A.A.M.; Siebers, A.G.; Bekkers, R.L.M. Ovarian Cancer Prognosis in Women with Endometriosis: A Retrospective Nationwide Cohort Study of 32,419 Women. Am. J. Obstet. Gynecol. 2021, 224, 284.e1–284.e10. [Google Scholar] [CrossRef] [PubMed]
- Santoiemma, P.P.; Powell, D.J. Tumor Infiltrating Lymphocytes in Ovarian Cancer. Cancer Biol. Ther. 2015, 16, 807–820. [Google Scholar] [CrossRef]
- Khalique, S.; Nash, S.; Mansfield, D.; Wampfler, J.; Attygale, A.; Vroobel, K.; Kemp, H.; Buus, R.; Cottom, H.; Roxanis, I.; et al. Quantitative Assessment and Prognostic Associations of the Immune Landscape in Ovarian Clear Cell Carcinoma. Cancers 2021, 13, 3854. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.P.; Huang, X.; Vlad, A.M. Chronic Inflammation in Endometriosis and Endometriosis-Associated Ovarian Cancer: New Roles for the “Old” Complement Pathway. Oncoimmunology 2015, 4, e1002732. [Google Scholar] [CrossRef]
- Nero, C.; Romito, I.; Spadola, S.; Romito, A.; Turco, L.C.; Cosentino, F.; De Ninno, M.; Catena, U.; De Cicco Nardone, A.; Moroni, R.; et al. Infiltrating T Lymphocytes and Programmed Cell Death Protein-1/Programmed Death-Ligand 1 Expression in Endometriosis-Associated Ovarian Cancer. Fertil. Steril. 2021, 117, 160–168. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Revised American Society for Reproductive Medicine Classification of Endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [CrossRef] [PubMed]
- Gilly, F.N.; Cotte, E.; Brigand, C.; Monneuse, O.; Beaujard, A.C.; Freyer, G.; Glehen, O. Quantitative Prognostic Indices in Peritoneal Carcinomatosis. Eur. J. Surg. Oncol. 2006, 32, 597–601. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of Cancer Immunity and the Cancer-Immune Set Point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Scheerer, C.; Bauer, P.; Chiantera, V.; Sehouli, J.; Kaufmann, A.; Mechsner, S. Characterization of Endometriosis-Associated Immune Cell Infiltrates (EMaICI). Arch. Gynecol. Obstet. 2016, 294, 657–664. [Google Scholar] [CrossRef]
- Devlin, M.-J.; Miller, R.; Laforets, F.; Kotantaki, P.; Garsed, D.W.; Kristeleit, R.; Bowtell, D.D.; McDermott, J.; Maniati, E.; Balkwill, F.R. The Tumor Microenvironment of Clear-Cell Ovarian Cancer. Cancer Immunol. Res. 2022, 10, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.Z.; Ye, J.; Yee, C.V.; Lim, D.; Ngoi, N.Y.L.; Tan, D.S.P.; Huang, R.Y.-J. Analysis of Gene Expression Signatures Identifies Prognostic and Functionally Distinct Ovarian Clear Cell Carcinoma Subtypes. EBioMedicine 2019, 50, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Howitt, B.E.; Strickland, K.C.; Sholl, L.M.; Rodig, S.; Ritterhouse, L.L.; Chowdhury, D.; D’Andrea, A.D.; Matulonis, U.A.; Konstantinopoulos, P.A. Clear Cell Ovarian Cancers with Microsatellite Instability: A Unique Subset of Ovarian Cancers with Increased Tumor-Infiltrating Lymphocytes and PD-1/PD-L1 Expression. Oncoimmunology 2017, 6, e1277308. [Google Scholar] [CrossRef] [PubMed]
- Milne, K.; Köbel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.H.; Nelson, B.H. Systematic Analysis of Immune Infiltrates in High-Grade Serous Ovarian Cancer Reveals CD20, FoxP3 and TIA-1 as Positive Prognostic Factors. PLoS ONE 2009, 4, e6412. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ Tumor-Infiltrating Lymphocytes and a High CD8+/Regulatory T Cell Ratio Are Associated with Favorable Prognosis in Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Hwang, W.-T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic Significance of Tumor-Infiltrating T Cells in Ovarian Cancer: A Meta-Analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Matsumura, N.; Brown, J.B.; Wang, Z.; Yamaguchi, K.; Abiko, K.; Yoshioka, Y.; Hamanishi, J.; Baba, T.; Koshiyama, M.; et al. Prediction of Taxane and Platinum Sensitivity in Ovarian Cancer Based on Gene Expression Profiles. Gynecol. Oncol. 2016, 141, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.R.; Milne, K.; Nelson, B.H. PD-1 and CD103 Are Widely Coexpressed on Prognostically Favorable Intraepithelial CD8 T Cells in Human Ovarian Cancer. Cancer Immunol. Res. 2015, 3, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.R.; Milne, K.; Kroeger, D.R.; Nelson, B.H. PD-L1 Expression Is Associated with Tumor-Infiltrating T Cells and Favorabl Prognosis in High-Grade Serous Ovarian Cancer. Gynecol. Oncol. 2016, 141, 293–302. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Iwasaki, M.; Okazaki, T.; Tanaka, Y.; Yamaguchi, K.; Higuchi, T.; Yagi, H.; Takakura, K.; Minato, N.; et al. Programmed Cell Death 1 Ligand 1 and Tumor-Infiltrating CD8+ T Lymphocytes Are Prognostic Factors of Human Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 3360–3365. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.D.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M.; et al. Antitumor Activity and Safety of Pembrolizumab in Patients with Advanced Recurrent Ovarian Cancer: Results from the Phase II KEYNOTE-100 Study. Ann. Oncol. 2019, 30, 1080–1087. [Google Scholar] [CrossRef]
- Sia, T.Y.; Manning-Geist, B.; Gordhandas, S.; Murali, R.; Marra, A.; Liu, Y.L.; Friedman, C.F.; Hollmann, T.J.; Zivanovic, O.; Chi, D.S.; et al. Treatment of Ovarian Clear Cell Carcinoma with Immune Checkpoint Blockade: A Case Series. Int. J. Gynecol. Cancer 2022, 32, 1017–1024. [Google Scholar] [CrossRef]
- Sawada, M.; Goto, K.; Morimoto-Okazawa, A.; Haruna, M.; Yamamoto, K.; Yamamoto, Y.; Nakagawa, S.; Hiramatsu, K.; Matsuzaki, S.; Kobayashi, E.; et al. PD-1+Tim3+tumor-Infiltrating CD8 T Cells Sustain the Potential for IFN-Γproduction, but Lose Cytotoxic Activity in Ovarian Cancer. Int. Immunol. 2020, 32, 397–405. [Google Scholar] [CrossRef]
- Balança, C.-C.C.; Salvioni, A.; Scarlata, C.-M.M.; Michelas, M.; Martinez-Gomez, C.; Gomez-Roca, C.; Sarradin, V.; Tosolini, M.; Valle, C.; Pont, F.; et al. PD-1 Blockade Restores Helper Activity of Tumor-Infiltrating, Exhausted PD-1hiCD39+ CD4 T Cells. JCI Insight 2021, 6, e142513. [Google Scholar] [CrossRef]
- Redjimi, N.; Raffin, C.; Raimbaud, I.; Pignon, P.; Matsuzaki, J.; Odunsi, K.; Valmori, D.; Ayyoub, M. CXCR3+ T Regulatory Cells Selectively Accumulate in Human Ovarian Carcinomas to Limit Type I Immunity. Cancer Res. 2012, 72, 4351–4360. [Google Scholar] [CrossRef]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The Immunopathophysiology of Endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef]
- da Gama Coelho Riccio, L.; Santulli, P.; Marcellin, L.; Abrão, M.S.; Batteux, F.; Chapron, C. Immunology of Endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 39–49. [Google Scholar] [CrossRef]
- Olkowska-Truchanowicz, J.; Sztokfisz-Ignasiak, A.; Zwierzchowska, A.; Janiuk, I.; Dąbrowski, F.; Korczak-Kowalska, G.; Barcz, E.; Bocian, K.; Malejczyk, J. Endometriotic Peritoneal Fluid Stimulates Recruitment of CD4+CD25highFOXP3+ Treg Cells. J. Clin. Med. 2021, 10, 3789. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, D.S.; Diniz, G.; Sayhan, S.; Sayar, C.; Ayaz, D.; Gokcu, M.; Karadeniz, T. The Prognostic Significance of Pdl1 and Foxp3 Expressions in Tumor Cells and the Tumor Microenvironment of Ovarian Epithelial Tumors. Int. J. Clin. Exp. Pathol. 2018, 11, 3884–3890. [Google Scholar] [PubMed]
- Hornburg, M.; Desbois, M.; Lu, S.; Guan, Y.; Lo, A.A.; Kaufman, S.; Elrod, A.; Lotstein, A.; DesRochers, T.M.; Munoz-Rodriguez, J.L.; et al. Single-Cell Dissection of Cellular Components and Interactions Shaping the Tumor Immune Phenotypes in Ovarian Cancer. Cancer Cell 2021, 39, 928–944. [Google Scholar] [CrossRef] [PubMed]
- Crispim, P.C.A.; Jammal, M.P.; Murta, E.F.C.; Nomelini, R.S. Endometriosis: What Is the Influence of Immune Cells? Immunol. Investig. 2021, 50, 372–388. [Google Scholar] [CrossRef] [PubMed]
- Gallego, A.; Mendiola, M.; Hernando, B.; Berjon, A.; Cádiz, A.; Chaves-Urbano, B.; Heredia-Soto, V.; Gutiérrez, A.H.; Hardisson, D.; Macintyre, G.; et al. Prognostic Markers of Inflammation in Endometrioid and Clear Cell Ovarian Cancer. Int. J. Gynecol. Cancer 2022, 32, 1009–1016. [Google Scholar] [CrossRef]
- Heinze, K.; Nazeran, T.M.; Lee, S.; Krämer, P.; Cairns, E.S.; Chiu, D.S.; Leung, S.C.; Kang, E.Y.; Meagher, N.S.; Kennedy, C.J.; et al. Validated Biomarker Assays Confirm That ARID1A Loss Is Confounded with MMR Deficiency, CD8+ TIL Infiltration, and Provides No Independent Prognostic Value in Endometriosis-Associated Ovarian Carcinomas. J. Pathol. 2022, 256, 388–401. [Google Scholar] [CrossRef]
- Hudry, D.; Le Guellec, S.; Meignan, S.; Bécourt, S.; Pasquesoone, C.; El Hajj, H.; Martínez-Gómez, C.; Leblanc, É.; Narducci, F.; Ladoire, S. Tumor-Infiltrating Lymphocytes (TILs) in Epithelial Ovarian Cancer: Heterogeneity, Prognostic Impact, and Relationship with Immune Checkpoints. Cancers 2022, 14, 5332. [Google Scholar] [CrossRef]
- Nakamura, M.; Bax, H.J.; Scotto, D.; Souri, E.A.; Sollie, S.; Harris, R.J.; Hammar, N.; Walldius, G.; Winship, A.; Ghosh, S.; et al. Immune Mediator Expression Signatures Are Associated with Improved Outcome in Ovarian Carcinoma. Oncoimmunology 2019, 8, e1593811. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific Recruitment of Regulatory T Cells in Ovarian Carcinoma Fosters Immune Privilege and Predicts Reduced Survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Leffers, N.; Gooden, M.J.M.; de Jong, R.A.; Hoogeboom, B.-N.; ten Hoor, K.A.; Hollema, H.; Boezen, H.M.; van der Zee, A.G.J.; Daemen, T.; Nijman, H.W. Prognostic Significance of Tumor-Infiltrating T-Lymphocytes in Primary and Metastatic Lesions of Advanced Stage Ovarian Cancer. Cancer Immunol. Immunother. 2009, 58, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, N.; Akman, L.; Acar, K.; Demir, S.; Ozkan, S.; Alan, N.; Zekioglu, O.; Terek, M.C.; Ozdemir, N.; Ozsaran, A. Do Tumor-Infiltrating Lymphocytes Really Indicate Favorable Prognosis in Epithelial Ovarian Cancer? Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 215, 55–61. [Google Scholar] [CrossRef] [PubMed]

| EOC (n = 30) | CCOC (n = 24) | p-Value | |
|---|---|---|---|
| Age (years) (mean ± SD) | 52.0 ± 10.3 | 55.4 ± 13.2 | NS (0.301) a |
| BMI (kg/m2) (mean ± SD) | 25.4 ± 4.5 | 26.3 ± 3.5 | NS (0.435) a |
| FIGO stage (N (%)) | NS (0.267) b | ||
| I–II | 24 (80.0) | 16 (66.7) | |
| III–IV | 6 (20.0) | 8 (33.3) | |
| Grade (N (%)) | NS (0.588) b | ||
| G1 | 8 (26.7) | 4 (16.6) | |
| G2 | 18 (60.0) | 15 (62.5) | |
| G3 | 4 (13.3) | 5 (20.8) | |
| Surgical performance (N (%)) | NS (0.695) b | ||
| Complete | 25 (83.3) | 19 (79.2) | |
| Not complete (optimal + suboptimal) | 5 (16.7) | 5 (20.8) | |
| Neoadjuvant treatment (N (%)) | NS (0.816) b | ||
| Yes | 2 (6.7) | 2 (8.3) | |
| No | 28 (93.3) | 22 (91.7) | |
| Adjuvant treatment (N (%)) | NS (0.462) b | ||
| Yes | 28 (93.3) | 21 (87.5) | |
| No | 2 (6.7) | 3 (12.5) | |
| Recurrence (N (%)) | NS (0.793) b | ||
| Yes | 9 (30.0) | 8 (33.3) | |
| No | 21 (70.0) | 16 (66.7) | |
| Exitus (N (%)) | NS (0.081) b | ||
| Yes | 7 (23.3) | 11 (45.8) | |
| No | 23 (76.7) | 13 (54.2) | |
| OS (months) (median; Q1–Q3) | 123.0; 59.5–159.0 | 57.5; 12.0–163.0 | 0.030 c |
| DFS (months) (median; Q1–Q3) | 106.0; 28.0–158.0 | 38.5; 10.5–163.0 | NS (0.269) c |
| OE (n = 43) | EAOC (n = 54) | EOC (n = 30) | CCOC (n = 24) | p-Value (EAOC vs. OE) | p-Value (EOC vs. COCC) | |
|---|---|---|---|---|---|---|
| CD3 signal (N (%)) | ||||||
| Yes No | 40 (100.0) 0 (0.0) | 48 (98.0) 1 (2.0) | 26 (96.3) 1 (3.7) | 22(100.0) 0 (0.0) | NS (0.551) a | NS (0.551) a |
| Low/mild Moderate/intense | 13 (32.5) 27 (67.5) | 36 (78.3) 10 (21.7) | 19 (79.2) 5 (20.8) | 17 (77.3) 5 (22.7) | <0.001 a | NS (0.876) b |
| Intraepithelial Stromal Intraepithelial and stromal | 32 (80.0) 0 (0.0) 8 (2.0) | 16 (33.3) 2 (4.2) 30 (62.5) | 7 (26.9) 2 (7.7) 17 (65.4) | 9 (40.9) 0 (0.0) 13 (59.1) | <0.001 a | NS (0.291) a |
| CD4 signal (N (%)) | ||||||
| Yes No | 36 (94.7) 2 (5.3) | 39 (86.7) 6 (13.3) | 21 (87.5) 3 (12.5) | 18 (85.7) 3 (14.3) | NS (0.279) a | NS (0.600) a |
| Low/mild Moderate/intense | 17 (51.5) 16 (48.5) | 24 (77.4) 7 (22.6) | 14 77.8) 4 (22.2) | 10 (77.0) 3 (23.0) | 0.031 a | NS (0.955) b |
| Intraepithelial Stromal Intraepithelial and stromal | 23 (63.9) 4 (11.1) 9 (20.9) | 14 (35.9) 8 (20.5) 17 (43.6) | 7 (33.3) 3 (14.3) 11 (52.4) | 7 (38.9) 5 (27.8) 6 (33.3) | NS (0.053) a | NS (0.417) a |
| CD8 signal (N (%)) | ||||||
| Yes No | 36 (90.0) 4 (10.0) | 34 (69.4) 15 (30.6) | 20 (76.9) 6 (23.1) | 14 (60.8) 9 (39.2) | 0.018 b | NS (0.224) b |
| Low/mild Moderate/intense | 16 (47.1) 18 (52.9) | 26 (86.7) 4 (13.3) | 14 (87.5) 2 (12.5) | 12 (85.7) 2 (14.3) | 0.001 b | NS (0.886) b |
| Intraepithelial Stromal Intraepithelial and stromal | 25 (69.4) 2 (5.6) 9 (20.9) | 18 (52.9) 4 (11.8) 12 (35.3) | 8 (40.0) 4 (20.0) 8 (40.0) | 10 (71.4) 0 (0.0) 4 (28.6) | NS (0.336) a | NS (0.098) a |
| TILs (N (%)) | ||||||
| Low/mild Moderate/intense | 4 (11.1) 32 (88.9) | 30 (73.2) 11 (26.8) | 0.001 a | |||
| TILs Signal | |||
|---|---|---|---|
| Low/Mild | Moderate/Intense | p-Value | |
| Exitus | |||
| Yes | 11 (33.7) | 1 (9.1) | NS (0.128) a |
| No | 19 (63.3) | 10 (90.9) | |
| Recurrence | |||
| Yes | 11 (63.7) | 1 (9.1) | NS (0.128) a |
| No | 19 (63.3) | 8 (90.9) | |
| OS ≥ 68 months | |||
| Yes | 12 (54.5) | 9 (100.0) | 0.030 a |
| No | 10 (45.5) | 0 (0.0) | |
| OS ≥ 68 months in patients with adjuvant therapy | |||
| Yes | 10 (52.6) | 9 (100.0) | 0.026 a |
| No | 9 (47.4) | 0 (0.0) | |
| DFS ≥ 45 months | |||
| Yes | 10 (41.7) | 10 (90.9) | 0.010 a |
| No | 14 (58.3) | 1 (9.1) | |
| DFS ≥ 45 months in patients with adjuvant therapy | |||
| Yes | 8 (38.1) | 10 (90.9) | 0.008 a |
| No | 13 (61.9) | 1 (9.1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagnolo, E.; Martinez, A.; Mascarós-Martínez, A.; Marí-Alexandre, J.; Carbonell, M.; González-Cantó, E.; Pena-Burgos, E.M.; Mc Cormack, B.A.; Tomás-Pérez, S.; Gilabert-Estellés, J.; et al. Evaluation of Immune Infiltrates in Ovarian Endometriosis and Endometriosis-Associated Ovarian Cancer: Relationship with Histological and Clinical Features. Int. J. Mol. Sci. 2023, 24, 12083. https://doi.org/10.3390/ijms241512083
Spagnolo E, Martinez A, Mascarós-Martínez A, Marí-Alexandre J, Carbonell M, González-Cantó E, Pena-Burgos EM, Mc Cormack BA, Tomás-Pérez S, Gilabert-Estellés J, et al. Evaluation of Immune Infiltrates in Ovarian Endometriosis and Endometriosis-Associated Ovarian Cancer: Relationship with Histological and Clinical Features. International Journal of Molecular Sciences. 2023; 24(15):12083. https://doi.org/10.3390/ijms241512083
Chicago/Turabian StyleSpagnolo, Emanuela, Alejandra Martinez, Andrea Mascarós-Martínez, Josep Marí-Alexandre, María Carbonell, Eva González-Cantó, Eva Manuela Pena-Burgos, Bárbara Andrea Mc Cormack, Sarai Tomás-Pérez, Juan Gilabert-Estellés, and et al. 2023. "Evaluation of Immune Infiltrates in Ovarian Endometriosis and Endometriosis-Associated Ovarian Cancer: Relationship with Histological and Clinical Features" International Journal of Molecular Sciences 24, no. 15: 12083. https://doi.org/10.3390/ijms241512083
APA StyleSpagnolo, E., Martinez, A., Mascarós-Martínez, A., Marí-Alexandre, J., Carbonell, M., González-Cantó, E., Pena-Burgos, E. M., Mc Cormack, B. A., Tomás-Pérez, S., Gilabert-Estellés, J., López-Carrasco, A., Hidalgo, P., Ángeles, M. A., Redondo, A., Gallego, A., & Hernández, A. (2023). Evaluation of Immune Infiltrates in Ovarian Endometriosis and Endometriosis-Associated Ovarian Cancer: Relationship with Histological and Clinical Features. International Journal of Molecular Sciences, 24(15), 12083. https://doi.org/10.3390/ijms241512083







