Emerging Role of Oral Mesenchymal Stem/Stromal Cells and Their Derivates
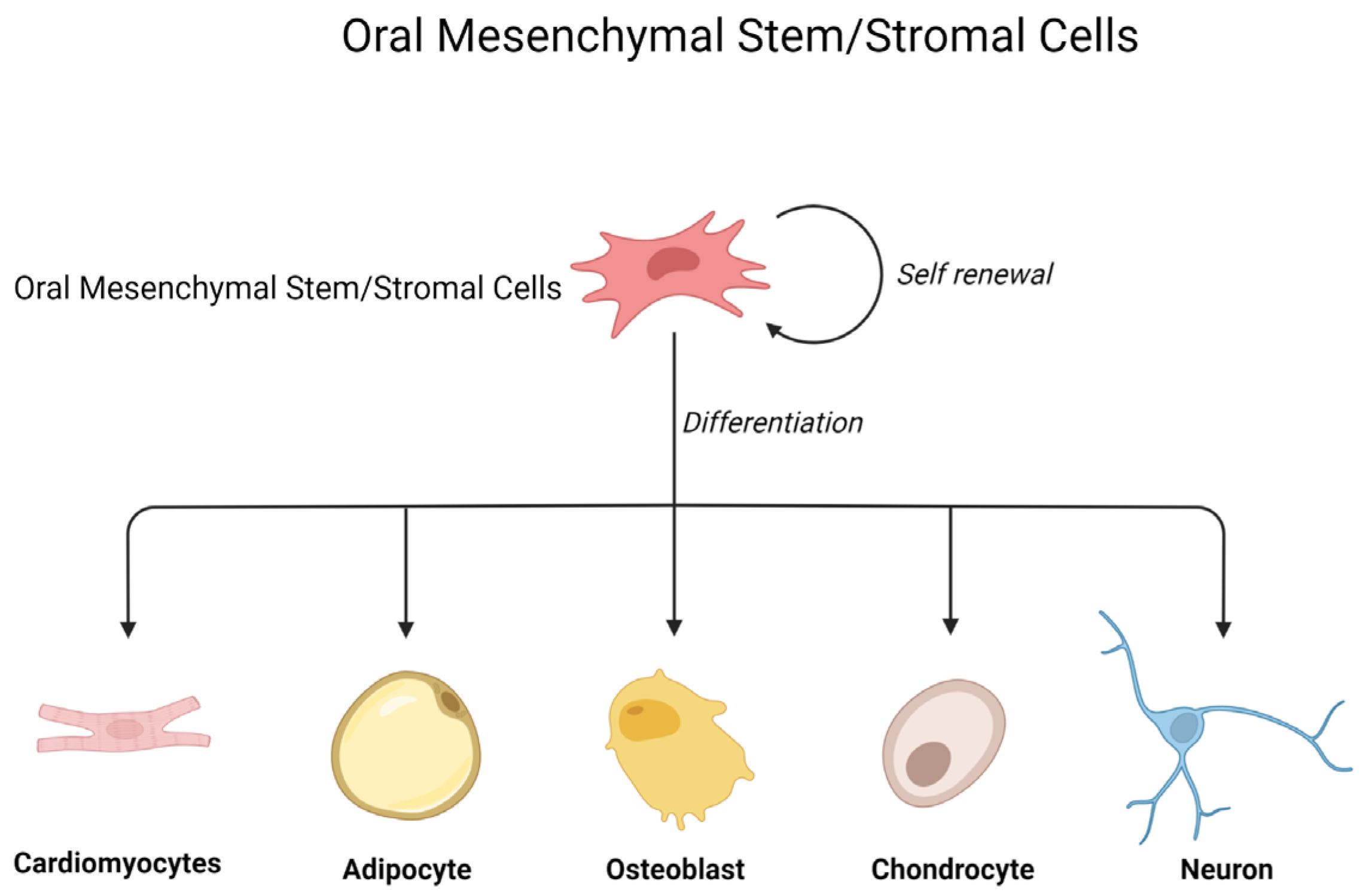
1. Introduction
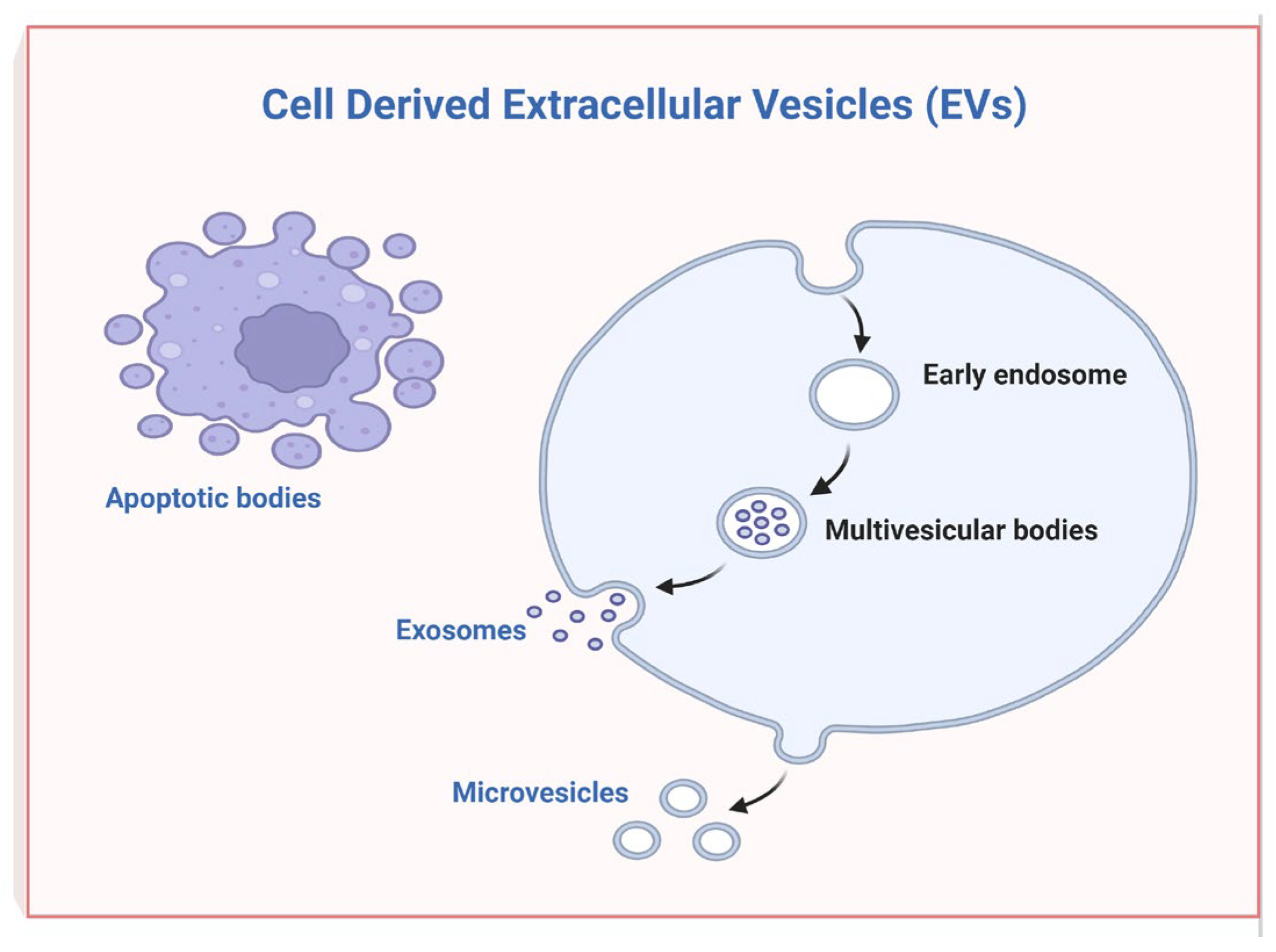
2. Extracellular Vesicles Derived from Oral Mesenchymal Stem Cells and Their Regenerative and Immunomodulation Potential
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, L.L.; Liu, W.; Wu, Y.M.; Sun, W.L.; Dorfer, C.E.; El-Sayed, K.M.F. Oral Mesenchymal Stem/Progenitor Cells: The Immunomodulatory Masters. Stem Cells Int. 2020, 2020, 1327405. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Santonocito, S.; Viglianisi, G.; Tatullo, M.; Isola, G. Impact of Oral Mesenchymal Stem Cells Applications as a Promising Therapeutic Target in the Therapy of Periodontal Disease. Int. J. Mol. Sci. 2022, 23, 13419. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Fonticoli, L.; Della Rocca, Y.; Oliva, S.; Rajan, T.S.; Trubiani, O.; Murmura, G.; Diomede, F.; Pizzicannella, J. Enhanced Extracellular Matrix Deposition on Titanium Implant Surfaces: Cellular and Molecular Evidences. Biomedicines 2021, 9, 1710. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ouchi, T.; Cao, Y.B.; Zhao, Z.H.; Men, Y. Dental-Derived Mesenchymal Stem Cells: State of the Art. Front. Cell Dev. Biol. 2021, 9, 654559. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.S.; Marconi, G.D.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef] [PubMed]
- Muzes, G.; Sipos, F. Mesenchymal Stem Cell-Derived Secretome: A Potential Therapeutic Option for Autoimmune and Immune-Mediated Inflammatory Diseases. Cells 2022, 11, 2300. [Google Scholar] [CrossRef] [PubMed]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front. Cell Dev. Biol. 2020, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Chiricosta, L.; Silvestro, S.; Gugliandolo, A.; Marconi, G.D.; Pizzicannella, J.; Bramanti, P.; Trubiani, O.; Mazzon, E. Extracellular Vesicles of Human Periodontal Ligament Stem Cells Contain MicroRNAs Associated to Proto-Oncogenes: Implications in Cytokinesis. Front. Genet. 2020, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Front. Cell Dev. Biol. 2021, 09, 734720. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Liu, Y.; Cui, D.X.; Pan, Y.; Zheng, L.W.; Wan, M. Dental Tissue-Derived Human Mesenchymal Stem Cells and Their Potential in Therapeutic Application. Stem Cells Int. 2020, 2020, 8864572. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Mills, S.J.; Cowin, A.J. Mesenchymal Stem Cell Secretome as an Emerging Cell-Free Alternative for Improving Wound Repair. Int. J. Mol. Sci. 2020, 21, 7038. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Pizzicannella, J.; Gugliandolo, A.; Orsini, T.; Fontana, A.; Ventrella, A.; Mazzon, E.; Bramanti, P.; Diomede, F.; Trubiani, O. Engineered Extracellular Vesicles From Human Periodontal-Ligament Stem Cells Increase VEGF/VEGFR2 Expression During Bone Regeneration. Front. Physiol. 2019, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, S.; Chiricosta, L.; Gugliandolo, A.; Pizzicannella, J.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Extracellular Vesicles Derived from Human Gingival Mesenchymal Stem Cells: A Transcriptomic Analysis. Genes 2020, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Baglio, S.R.; Pegtel, D.M.; Baldini, N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 2012, 3, 359. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Thangavelu, S.R.; Diomede, F.; Bramanti, P.; Conti, P.; Trubiani, O.; Mazzon, E. Anti-inflammatory effects of hypoxia-preconditioned human periodontal ligament cell secretome in an experimental model of multiple sclerosis: A key role of IL-37. FASEB J. 2017, 31, 5592–5608. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.B.; Bunn, K.E.; Pua, H.H.; Rafat, M. Extracellular vesicles: Mediators of intercellular communication in tissue injury and disease. Cell Commun. Signal 2021, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.B.; Chen, H.S.; Zhao, X.T.; Chen, Z.; Zhang, P.P.; Tian, Y.; Wang, Y.W.; Ding, T.; Wang, L.J.; Shen, Y.Q. Advances in mesenchymal stem cell conditioned medium-mediated periodontal tissue regeneration. J. Transl. Med. 2021, 19, 456. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marconi, G.D.; Diomede, F.; Pizzicannella, J.; Trubiani, O. Emerging Role of Oral Mesenchymal Stem/Stromal Cells and Their Derivates. Int. J. Mol. Sci. 2023, 24, 12003. https://doi.org/10.3390/ijms241512003
Marconi GD, Diomede F, Pizzicannella J, Trubiani O. Emerging Role of Oral Mesenchymal Stem/Stromal Cells and Their Derivates. International Journal of Molecular Sciences. 2023; 24(15):12003. https://doi.org/10.3390/ijms241512003
Chicago/Turabian StyleMarconi, Guya Diletta, Francesca Diomede, Jacopo Pizzicannella, and Oriana Trubiani. 2023. "Emerging Role of Oral Mesenchymal Stem/Stromal Cells and Their Derivates" International Journal of Molecular Sciences 24, no. 15: 12003. https://doi.org/10.3390/ijms241512003
APA StyleMarconi, G. D., Diomede, F., Pizzicannella, J., & Trubiani, O. (2023). Emerging Role of Oral Mesenchymal Stem/Stromal Cells and Their Derivates. International Journal of Molecular Sciences, 24(15), 12003. https://doi.org/10.3390/ijms241512003




