Antioxidants Amelioration Is Insufficient to Prevent Acrylamide and Alpha-Solanine Synergistic Toxicity in BEAS-2B Cells
Abstract
1. Introduction
2. Results
2.1. Cell Morphology and Protein Expression of AKT and Its Downstream Genes
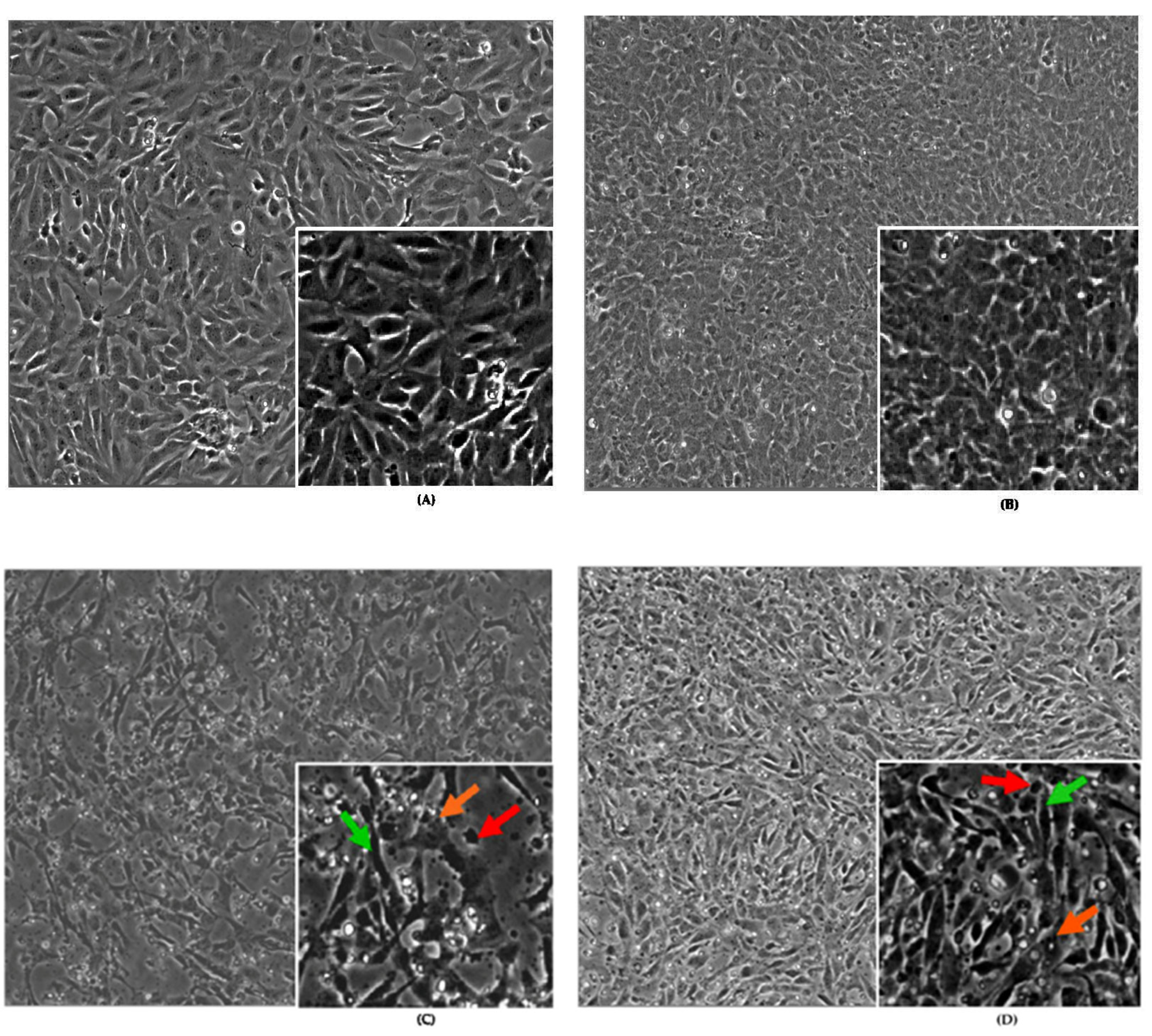
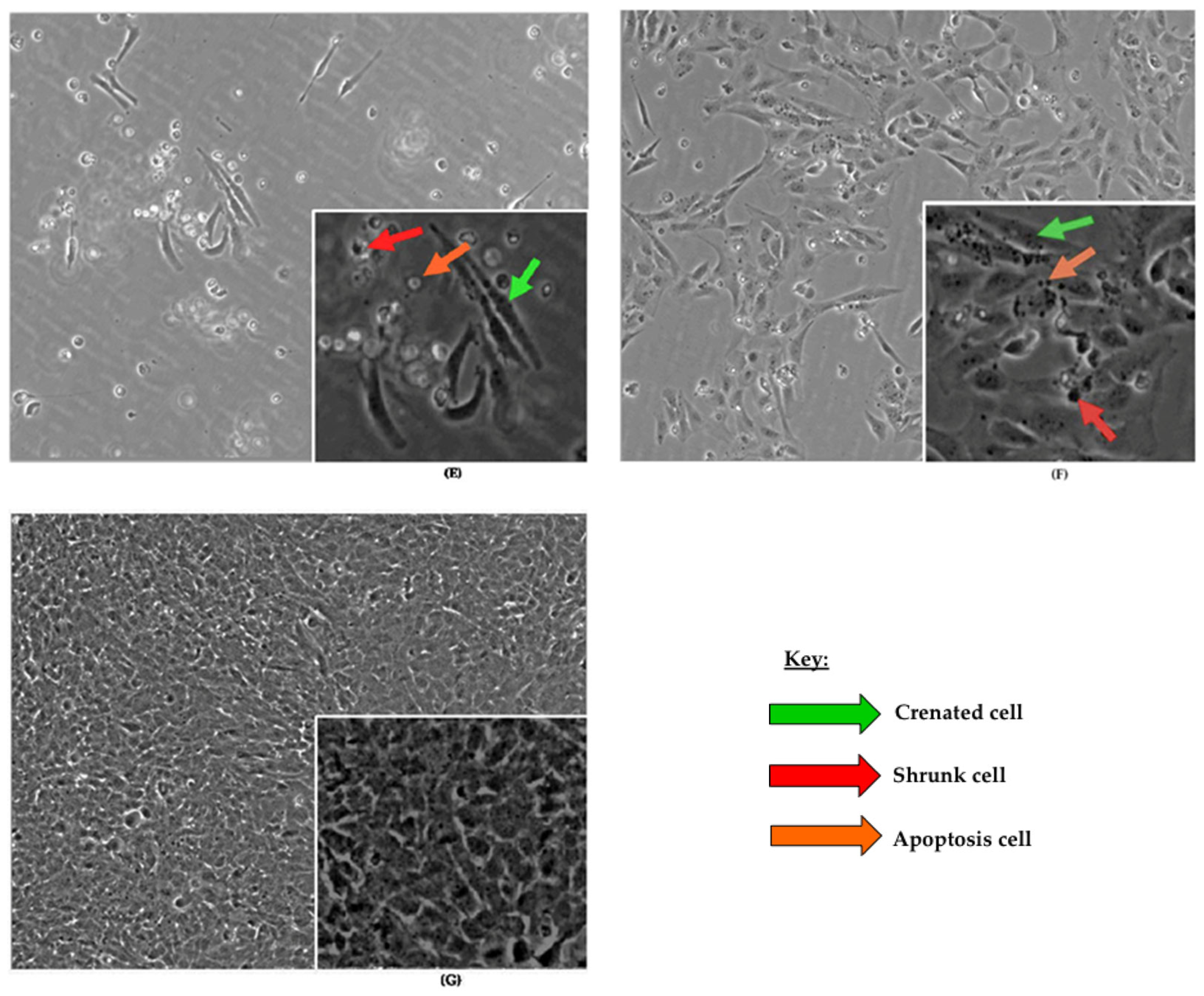
2.1.1. Effects of Antioxidants, Toxicants, and Toxins on Cell Morphology
2.1.2. Change in Nucleic AcidsChanges in DNA
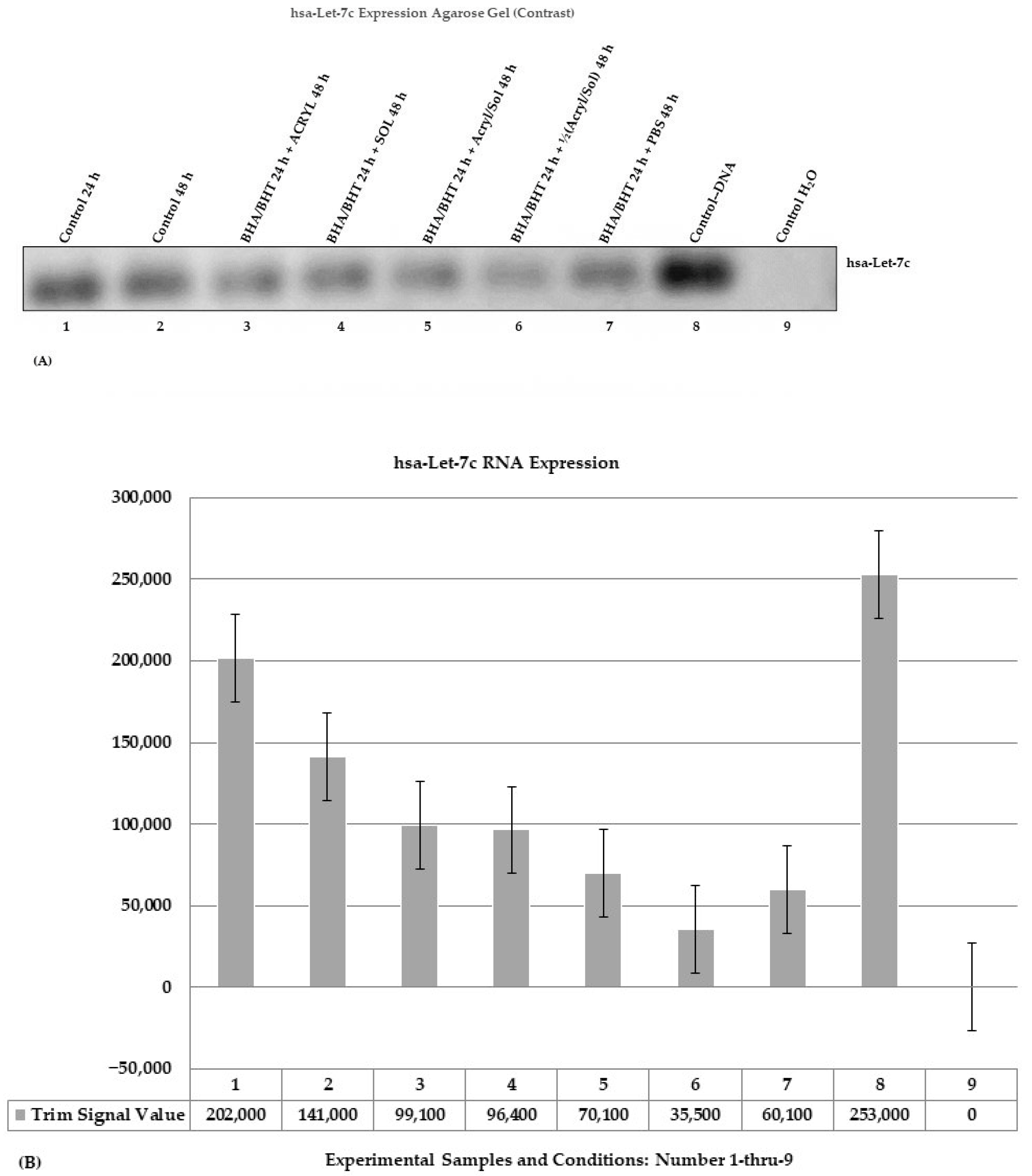
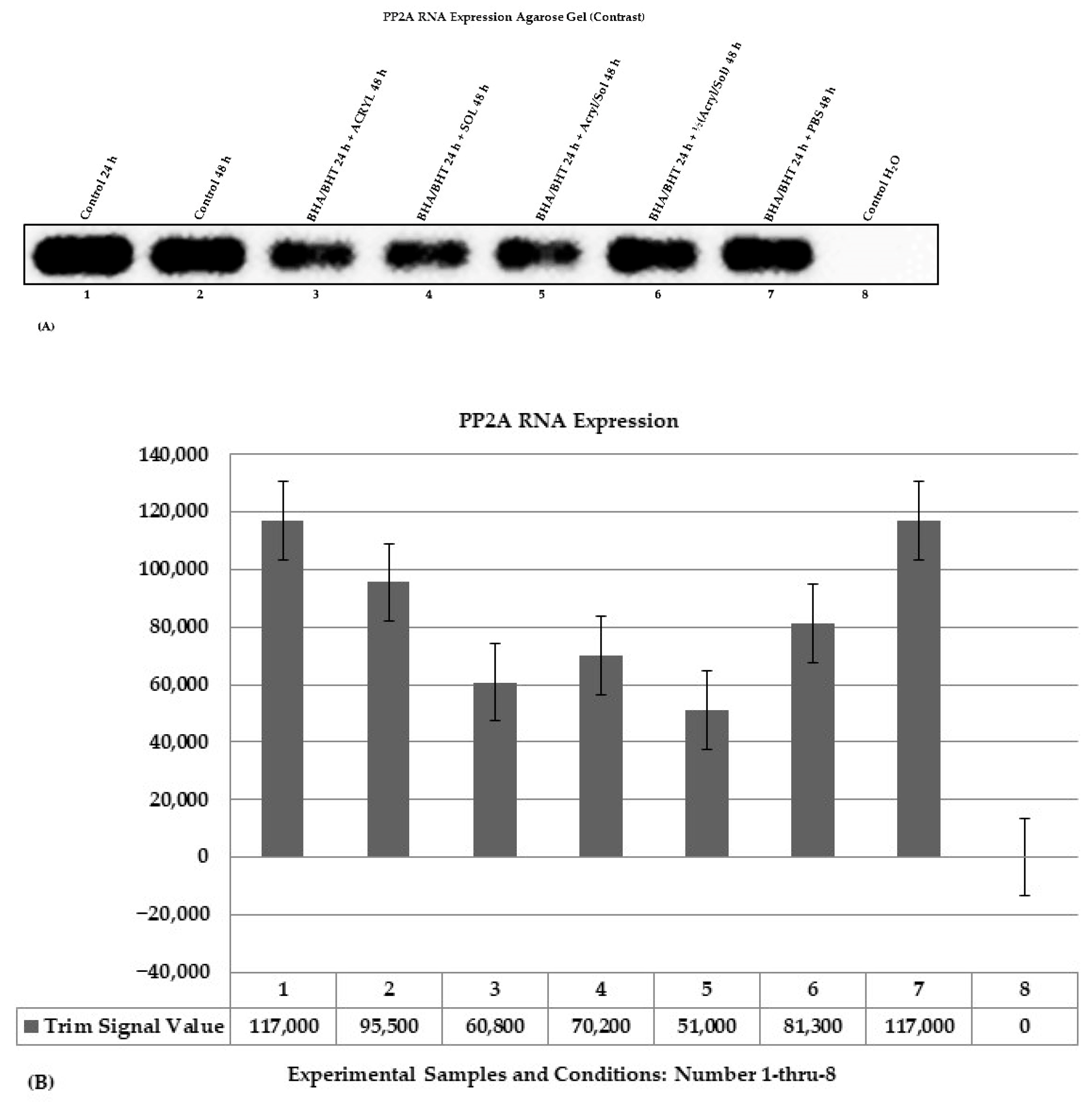
Change in RNA
- D2S123 (Forward Primer: 5′-AAACAGGATGCCTGCCTTTA-3′ and Reverse Primer: 5′-GGACTTTCCACCTATGGGAC-3′ (>chr2: 51,061,299 + 51,061,509, size 211 bp).
- AKT2 (Forward Primer: 5′-CTTTGTCATACGCTGCCTGCAGT-3′ and Reverse Primer: 5′-TCTCCTCACACCAGGCTTGCTC-3′ (>chr19: 40,255,100 + 40,255,229, size 130 bp).
- Mitochondrial CO1 (MT-CO1) (Forward Primer: 5′-GGAGCTTTGGCAACTGACT-3′ and Reverse Primer: 5′-CTGCTAGGTGTAAGGAGAAGATGG-3′ (>chrM: 6130 + 6363, size 234 bp).
Change in RNA
- The hsa-Let-7c primer set (Forward Primer: 5′-GTTGTATGGTTTAGAGTTACAC-3′ and Reverse Primer: 5′-GCTCCAAGGAAAGCTAGAAGGTT-3′ (>chr21: 16,539,849 + 16,539,911, size 63 bp).
- The PP2A primer set (Forward Primer: 5′-CAAATGGAAGCGTTCTCAGGCATAC-3′ and Reverse Primer: 5′-TTCCTCATGAACCTCATTCCACATCTC-3′ (cDNA NM_178001.3 and chr9: 129,111,415 − 129,148,946) (>chr9: 129,111,711 + 129,111,841, size 130 bp).
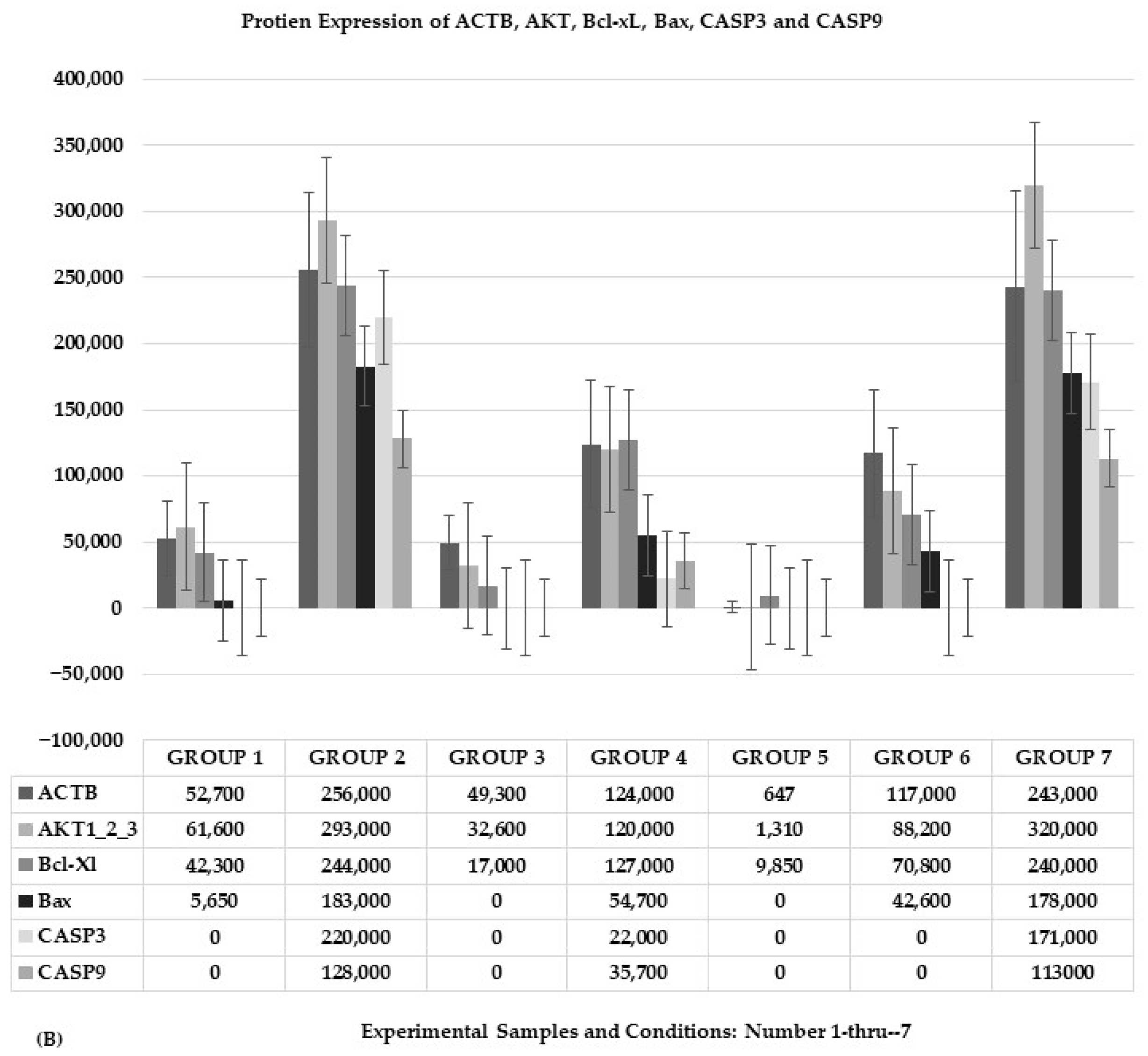
2.1.3. Protein Expression of AKT and Its Downstream Genes
2.1.4. Signal Value of Protein Expression and Statistical Significance Test of One-Way ANOVA: AKT, Bcl-xL, Bax, CASP3, and CASP9
3. Discussion
4. Materials and Methods
4.1. Cell Culture of BEAS-2B Cells and Cell Exposure
4.2. Cell Morphological Analysis
4.3. DNA, RNA, and Proteins Extractions
4.3.1. DNA
4.3.2. Total RNA
4.3.3. Protein
4.3.4. Complementary DNA (cDNA) Synthesis
4.3.5. Polymerase Chain Reaction (PCR) and Gel Electrophoresis
4.3.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gross, L. Diverse toxic chemicals disrupt cell function through a common path. PLoS Biol. 2007, 5, e41. [Google Scholar] [CrossRef]
- Yamashoji, S.; Matsuda, T. Synergistic cytotoxicity induced by alpha-solanine and alpha-chaconine. Food Chem. 2013, 141, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Frisan, T. Bacterial genotoxins: The long journey to the nucleus of mammalian cells. Biochim. Biophys. Acta Biomembr. 2016, 1858, 567–575. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Alsherbiny, M.A.; Abd-Elsalam, W.H.; El Badawy, S.A.; Taher, E.; Fares, M.; Torres, A.; Chang, D.; Li, C.G. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: A comprehensive review. Food Chem. Toxicol. 2019, 123, 72–97. [Google Scholar] [CrossRef]
- Dorrigiv, M.; Zareiyan, A.; Hosseinzadeh, H. Garlic (Allium sativum) as an antidote or a protective agent against natural or chemical toxicities: A comprehensive update review. Phytother. Res. 2020, 34, 1770–1797. [Google Scholar] [CrossRef]
- Pan, X.; Zhu, L.; Lu, H.; Wang, D.; Lu, Q.; Yan, H. Melatonin Attenuates Oxidative Damage Induced by Acrylamide In Vitro and In Vivo. Oxidative Med. Cell Longev. 2015, 2015, 703709. [Google Scholar] [CrossRef]
- Amirshahrokhi, K. Acrylamide exposure aggravates the development of ulcerative colitis in mice through activation of NF-κB, inflammatory cytokines, iNOS, and oxidative stress. Iran. J. Basic Med. Sci. 2021, 24, 312–321. [Google Scholar] [CrossRef]
- Meng, X.-Q.; Zhang, W.; Zhang, F.; Yin, S.-Y.; Xie, H.-Y.; Zhou, L.; Zheng, S.-S. Solanine-induced reactive oxygen species inhibit the growth of human hepatocellular carcinoma HepG2 cells. Oncol. Lett. 2016, 11, 2145–2151. [Google Scholar] [CrossRef]
- Park, J.; Kamendulis, L.M.; Friedman, M.A.; Klaunig, J.E. Acrylamide-induced cellular transformation. Toxicol. Sci. 2002, 65, 177–183. [Google Scholar] [CrossRef]
- Ordóñez-Vásquez, A.; Jaramillo-Gómez, L.; Duran-Correa, C.; Escamilla-García, E.; De La Garza-Ramos, M.A.; Suárez-Obando, F. A Reliable and Reproducible Model for Assessing the Effect of Different Concentrations of α-Solanine on Rat Bone Marrow Mesenchymal Stem Cells. Bone Marrow Res. 2017, 2017, 2170306. [Google Scholar] [CrossRef] [PubMed]
- Charoenprasert, S.; Mitchell, A. Influence of California-style black ripe olive processing on the formation of acrylamide. J. Agric. Food Chem. 2014, 62, 8716–8721. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y.; Yoshida, M.; Isagawa, S.; Yamazaki, K.; Ono, H. Acrylamide in roasted barley grains: Presence, correlation with colour and decrease during storage. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jin, C.; Zhang, Y. Investigation of variations in the acrylamide and Nε-(carboxymethyl) lysine contents in cookies during baking. J. Food Sci. 2014, 79, T1030–T1038. [Google Scholar] [CrossRef]
- Lim, P.K.; Jinap, S.; Sanny, M.; Tan, C.P.; Khatib, A. The influence of deep frying using various vegetable oils on acrylamide formation in sweet potato (Ipomoea batatas L. Lam) chips. J. Food Sci. 2013, 79, T115–T121. [Google Scholar] [CrossRef]
- Mojska, H.; Gielecińska, I. Studies of acrylamide level in coffee and coffee substitutes: Influence of raw material and manufacturing conditions. Rocz. Panstw. Zakl. Hig. 2013, 64, 173–181. [Google Scholar]
- Jiang, L.; Cao, J.; An, Y.; Geng, C.; Qu, S.; Jiang, L.; Zhong, L. Genotoxicity of acrylamide in human hepatoma G2 (HepG2) cells. Toxicol. In Vitro 2007, 21, 1486–1492. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Besaratinia, A.; Pfeifer, G.P. A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis 2007, 28, 519–528. [Google Scholar] [CrossRef]
- Barber, D.S.; LoPachin, R.M. Proteomic analysis of acrylamide-protein adduct formation in rat brain synaptosomes. Toxicol. Appl. Pharmacol. 2004, 201, 120–136. [Google Scholar] [CrossRef]
- Tian, S.-M.; Ma, Y.-X.; Shi, J.; Lou, T.-Y.; Liu, S.-S.; Li, G.-Y. Acrylamide neurotoxicity on the cerebrum of weaning rats. Neural Regen. Res. 2015, 10, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Katen, A.L.; Roman, S.D. The genetic consequences of paternal acrylamide exposure and potential for amelioration. Mutat. Res. Mol. Mech. Mutagen. 2015, 777, 91–100. [Google Scholar] [CrossRef]
- Nixon, B.J.; Katen, A.L.; Stanger, S.J.; Schjenken, J.E.; Nixon, B.; Roman, S.D. Mouse spermatocytes express CYP2E1 and respond to acrylamide exposure. PLoS ONE 2014, 9, e94904. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, Y.; Jia, L.; Jiang, L.-P.; Geng, C.-Y.; Yao, X.-F.; Kong, Y.; Jiang, B.-N.; Zhong, L.-F. Curcumin attenuates acrylamide-induced cytotoxicity and genotoxicity in HepG2 cells by ROS scavenging. J. Agric. Food Chem. 2008, 56, 12059–12063. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Park, B.; Rhee, D.-K.; Pyo, S. Correction to Acrylamide Induces Senescence in Macrophages through a Process Involving ATF3, ROS, p38/JNK, and a Telomerase-Independent Pathway. Chem. Res. Toxicol. 2018, 31, 1426. [Google Scholar] [CrossRef]
- Chico Galdo, V.; Massart, C.; Jin, L.; Vanvooren, V.; Caillet-Fauquet, P.; Andry, G.; Lothaire, P.; Dequanter, D.; Friedman, M.; Van Sande, J. Acrylamide, an in vivo thyroid carcinogenic agent, induces DNA damage in rat thyroid cell lines and primary cultures. Mol. Cell. Endocrinol. 2006, 257-258, 6–14. [Google Scholar] [CrossRef]
- Gao, S.-Y.; Wang, Q.-J.; Ji, Y.-B. Effect of solanine on the membrane potential of mitochondria in HepG2 cells and [Ca2+]i in the cells. World J. Gastroenterol. 2006, 12, 3359–3367. [Google Scholar] [CrossRef]
- Lorenzen, J.H.; Balbyshev, N.F.; Lafta, A.M.; Casper, H.; Tian, X.; Sagredo, B. Resistant potato selections contain leptine and inhibit development of the colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2001, 94, 1260–1267. [Google Scholar] [CrossRef]
- Mensinga, T.T.; Sips, A.J.A.M.; Rompelberg, C.J.M.; van Twillert, K.; Meulenbelt, J.; van den Top, H.J.; van Egmond, H.P. Potato glycoalkaloids and adverse effects in humans: An ascending dose study. Regul. Toxicol. Pharmacol. 2005, 41, 66–72. [Google Scholar] [CrossRef]
- Langkilde, S.; Schrøder, M.; Stewart, D.; Meyer, O.; Conner, S.; Davies, H.; Poulsen, M. Acute toxicity of high doses of the glycoalkaloids, alpha-solanine and alpha-chaconine, in the syrian golden hamster. J. Agric. Food Chem. 2008, 56, 8753–8760. [Google Scholar] [CrossRef]
- Silva-Beltran, N.P.; Chaidez-Quiroz, C.; Lopez-Cuevas, O.; Ruiz-Cruz, S.; Lopez-Mata, M.A.; Del-Toro-Sanchez, C.L.; Marquez-Rios, E.; Ornelas-Paz, J.D.J. Phenolic Compounds of Potato Peel Extracts: Their Antioxidant Activity and Protection against Human Enteric Viruses. J. Microbiol. Biotechnol. 2017, 27, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-R.; Kozukue, N.; Han, J.-S.; Park, J.-H.; Chang, E.-Y.; Baek, E.-J.; Chang, J.-S.; Friedman, M. Glycoalkaloids and metabolites inhibit the growth of human colon (HT29) and liver (HepG2) cancer cells. J. Agric. Food Chem. 2004, 52, 2832–2839. [Google Scholar] [CrossRef]
- Li, R.; Tee, C.-S.; Jiang, Y.-L.; Jiang, X.-Y.; Venkatesh, P.N.; Sarojam, R.; Ye, J. A terpenoid phytoalexin plays a role in basal defense of Nicotiana benthamiana against Potato virus X. Sci. Rep. 2015, 5, 9682. [Google Scholar] [CrossRef] [PubMed]
- Karaboga Arslan, A.K.; Yerer, M.B. alpha-Chaconine and alpha-Solanine Inhibit RL95-2 Endometrium Cancer Cell Proliferation by Reducing Expression of Akt (Ser473) and ERα (Ser167). Nutrients 2018, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.H.; Jeon, Y.J.; Han, H.J.; Park, S.H.; Baek, K.M.; Chang, W.; Kim, J.S.; Kim, L.K.; Lee, Y.-M.; Lee, S.; et al. Protective effect of butylated hydroxylanisole against hydrogen peroxide-induced apoptosis in primary cultured mouse hepatocytes. J. Vet. Sci. 2015, 16, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.G.; Andreazza, A.C.; Salvador, M. Sequestering ability of butylated hydroxytoluene, propyl gallate, resveratrol, and vitamins C and E against ABTS, DPPH, and hydroxyl free radicals in chemical and biological systems. J. Agric. Food Chem. 2003, 51, 1077–1080. [Google Scholar] [CrossRef]
- Botterweck, A.A.; Verhagen, H.; Goldbohm, R.; Kleinjans, J.; van den Brandt, P. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: Results from analyses in the Netherlands Cohort Study. Food Chem. Toxicol. 2000, 38, 599–605. [Google Scholar] [CrossRef]
- Hocman, G. Chemoprevention of cancer: Phenolic antioxidants (BHT, BHA). Int. J. Biochem. 1988, 20, 639–651. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt Signaling and Redox Metabolism in Cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef]
- Salmon, T.B.; Evert, B.A.; Song, B.; Doetsch, P.W. Biological consequences of oxidative stress-induced DNA damage in Saccharomyces cerevisiae. Nucleic Acids Res. 2004, 32, 3712–3723. [Google Scholar] [CrossRef]
- Maynard, S.; Schurman, S.H.; Harboe, C.; de Souza-Pinto, N.C.; Bohr, V.A. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 2009, 30, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivera, J.C.; Sherman, M.W.; Wang, D.S.; Chuvalo-Abraham, J.C.L.; Ruiz, L.H.; Contreras, L.M. RNA oxidation in chromatin modification and DNA-damage response following exposure to formaldehyde. Sci. Rep. 2020, 10, 16545. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Turner, K.M.; Yung, W.K.A.; Chen, K.; Zhang, W. Role of AKT signaling in DNA repair and clinical response to cancer therapy. Neuro Oncol. 2014, 16, 1313–1323. [Google Scholar] [CrossRef]
- Xu, N.; Lao, Y.; Zhang, Y.; Gillespie, D.A. Akt: A Double-edged sword in cell proliferation and genome stability. J. Oncol. 2012, 2012, 951724. [Google Scholar] [CrossRef]
- Kacar, S.; Sahinturk, V.; Kutlu, H.M. Effect of acrylamide on BEAS-2B normal human lung cells: Cytotoxic, oxidative, apoptotic and morphometric analysis. Acta Histochem. 2019, 121, 595–603. [Google Scholar] [CrossRef]
- Kacar, S.; Vejselova, D.; Kutlu, M.; Sahinturk, V. Acrylamide-derived cytotoxic, anti-proliferative, and apoptotic effects on A549 cells. Hum. Exp. Toxicol. 2017, 37, 468–474. [Google Scholar] [CrossRef]
- Sahinturk, V.; Kacar, S.; Vejselova, D.; Kutlu, M. Acrylamide exerts its cytotoxicity in NIH/3T3 fibroblast cells by apoptosis. Toxicol. Ind. Health 2018, 34, 481–489. [Google Scholar] [CrossRef]
- Pan, X.; Wu, X.; Yan, D.; Peng, C.; Rao, C.; Yan, H. Acrylamide-induced oxidative stress and inflammatory response are alleviated by N-acetylcysteine in PC12 cells: Involvement of the crosstalk between Nrf2 and NF-κB pathways regulated by MAPKs. Toxicol. Lett. 2018, 288, 55–64. [Google Scholar] [CrossRef]
- Kacar, S.; Sahinturk, V. The Protective Agents Used against Acrylamide Toxicity: An In Vitro Cell Culture Study-Based Review. Cell J. 2021, 23, 367–381. [Google Scholar] [CrossRef]
- Hasanain, M.; Bhattacharjee, A.; Pandey, P.; Ashraf, R.; Singh, N.; Sharma, S.; Vishwakarma, A.L.; Datta, D.; Mitra, K.; Sarkar, J. α-Solanine induces ROS-mediated autophagy through activation of endoplasmic reticulum stress and inhibition of Akt/mTOR pathway. Cell Death Dis. 2015, 6, e1860. [Google Scholar] [CrossRef]
- Chen, Z.; Li, C.; Yuan, A.; Gu, T.; Zhang, F.; Fan, X.; Wu, X.; Xiong, X.; Yang, Q. α-Solanine Causes Cellular Dysfunction of Human Trophoblast Cells via Apoptosis and Autophagy. Toxins 2021, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Kahkeshani, N.; Saeidnia, S.; Abdollahi, M. Role of antioxidants and phytochemicals on acrylamide mitigation from food and reducing its toxicity. J. Food Sci. Technol. 2015, 52, 3169–3186. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Shi, J.; Huang, C.; Zhang, G.; Teng, J.; Jiang, Y.; Yang, B. Effect of antioxidants on elimination and formation of acrylamide in model reaction systems. J. Hazard. Mater. 2010, 182, 863–868. [Google Scholar] [CrossRef]
- Hellmann, H.; Goyer, A.; Navarre, D.A. Antioxidants in Potatoes: A Functional View on One of the Major Food Crops Worldwide. Molecules 2021, 26, 2446. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Schutte, R.; Sporns, P.; Doyle, J.; Jewel, L.; Fedorak, R. Potato glycoalkaloids adversely affect intestinal permeability and aggravate inflammatory bowel disease. Inflamm. Bowel Dis. 2002, 8, 340–346. [Google Scholar] [CrossRef]
- Iablokov, V.; Sydora, B.C.; Foshaug, R.; Meddings, J.; Driedger, D.; Churchill, T.; Fedorak, R.N. Naturally occurring glycoalkaloids in potatoes aggravate intestinal inflammation in two mouse models of inflammatory bowel disease. Dig. Dis. Sci. 2010, 55, 3078–3085. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry and anticarcinogenic mechanisms of glycoalkaloids produced by eggplants, potatoes, and tomatoes. J. Agric. Food Chem. 2015, 63, 3323–3337. [Google Scholar] [CrossRef]
- Tang, X.; Guo, Y.; Zhang, S.; Wang, X.; Teng, Y.; Jin, Q.; Jin, Q.; Shen, W.; Wang, R. Solanine Represses Gastric Cancer Growth by Mediating Autophagy Through AAMDC/MYC/ATF4/Sesn2 Signaling Pathway. Drug Des. Dev. Ther. 2023, 17, 389–402. [Google Scholar] [CrossRef]
- Wang, S.; Panter, K.; Gaffield, W.; Evans, R.; Bunch, T. Effects of steroidal glycoalkaloids from potatoes (Solanum tuberosum) on in vitro bovine embryo development. Anim. Reprod. Sci. 2005, 85, 243–250. [Google Scholar] [CrossRef]
- Langkilde, S.; Mandimika, T.; Schrøder, M.; Meyer, O.; Slob, W.; Peijnenburg, A.; Poulsen, M. A 28-day repeat dose toxicity study of steroidal glycoalkaloids, α-solanine and α-chaconine in the Syrian Golden hamster. Food Chem. Toxicol. 2009, 47, 1099–1108. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lee, D.-C.; Chen, M.-S.; Ko, Y.-C.; Chiu, I.-M. Inhibition of neurosphere formation in neural stem/progenitor cells by acrylamide. Cell Transplant. 2015, 24, 779–796. [Google Scholar] [CrossRef]
- Aras, D.; Cakar, Z.; Ozkavukcu, S.; Can, A.; Cinar, O. In Vivo acrylamide exposure may cause severe toxicity to mouse oocytes through its metabolite glycidamide. PLoS ONE 2017, 12, e0172026. [Google Scholar] [CrossRef] [PubMed]
- Concalo-Gamboa da Costa, G.; Churchwell, M.I.; Hamilton, L.P.; Von Tungeln, L.S.; Beland, F.A.; Marques, M.M.; Doerge, D.R. DNA adduct formation from acrylamide via conversion to glycidamide in adult and neonatal mice. Chem. Res. Toxicol. 2003, 16, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Watzek, N.; Böhm, N.; Feld, J.; Scherbl, D.; Berger, F.; Merz, K.H.; Lampen, A.; Reemtsma, T.; Tannenbaum, S.R.; Skipper, P.L.; et al. N7-glycidamide-guanine DNA adduct formation by orally ingested acrylamide in rats: A dose–response study encompassing human diet-related exposure levels. Chem. Res. Toxicol. 2012, 25, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Matsushita, K.; Kuroda, K.; Yokoo, Y.; Kijima, A.; Takasu, S.; Kodama, Y.; Nishikawa, A.; Umemura, T. Acrylamide induces specific DNA adduct formation and gene mutations in a carcinogenic target site, the mouse lung. Mutagenesis 2015, 30, 227–235. [Google Scholar] [CrossRef]
- Manjanatha, M.G.; Guo, L.-W.; Shelton, S.D.; Doerge, D.R. Acrylamide-induced carcinogenicity in mouse lung involves mutagenicity: cII gene mutations in the lung of big blue mice exposed to acrylamide and glycidamide for up to 4 weeks. Environ. Mol. Mutagen. 2015, 56, 446–456. [Google Scholar] [CrossRef]
- Martins, C.; Oliveira, N.G.; Pingarilho, M.; da Costa, G.G.; Martins, V.; Marques, M.M.; Beland, F.A.; Churchwell, M.I.; Doerge, D.R.; Rueff, J.; et al. Cytogenetic damage induced by acrylamide and glycidamide in mammalian cells: Correlation with specific glycidamide-DNA adducts. Toxicol. Sci. 2007, 95, 383–390. [Google Scholar] [CrossRef]
- Luo, S.; Tian, G.-J.; Yu, F.-X.; Wen, Z.-D. A narrative review of the antitumor studies of solanine. Transl. Cancer Res. 2021, 10, 1578–1582. [Google Scholar] [CrossRef]
- Martyniuk, C.J.; Feswick, A.; Fang, B.; Koomen, J.M.; Barber, D.S.; Gavin, T.; LoPachin, R.M. Protein targets of acrylamide adduct formation in cultured rat dopaminergic cells. Toxicol. Lett. 2013, 219, 279–287. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, R.; Zhang, G.; Cheng, R.; Bai, Y.; Zhao, H.; Lu, X.; Li, H.; Chen, S.; Li, J.; et al. Anticancer function of α-solanine in lung adenocarcinoma cells by inducing microRNA-138 expression. Tumor Biol. 2016, 37, 6437–6446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Guo, W.; Sun, Q.; Chen, X.; Zang, W.; Dong, Z.; Zhao, G. α-Solanine Modulates the Radiosensitivity of Esophageal Cancer Cells by Inducing MicroRNA 138 Expression. Cell. Physiol. Biochem. 2016, 39, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, L.; Du, X.; Sun, Q.; Wang, Y.; Li, M.; Zang, W.; Liu, K.; Zhao, G. α-solanine enhances the chemosensitivity of esophageal cancer cells by inducing microRNA-138 expression. Oncol. Rep. 2018, 39, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hao, T.; Sun, J.; Wei, P.; Zhang, H. Long noncoding RNA GAS5 modulates α-Solanine-induced radiosensitivity by negatively regulating miR-18a in human prostate cancer cells. Biomed. Pharmacother. 2019, 112, 108656. [Google Scholar] [CrossRef] [PubMed]


| Groups | N | Mean | Std. Dev. | Std. Error |
|---|---|---|---|---|
| AKT | 7 | 1.1483 | 0.4514 | 0.1706 |
| Bcl-xL | 7 | 2.8488 | 5.4624 | 2.0646 |
| Bax | 7 | 0.3373 | 0.314 | 0.1187 |
| CASP3 | 7 | 0.2486 | 0.3725 | 0.1408 |
| CASP9 | 7 | 0.179 | 0.2327 | 0.0879 |
| Source | Degrees of Freedom | Sum of Squares | Mean Square | F-Stat | p-Value |
|---|---|---|---|---|---|
| DF | SS | MS | |||
| Between Groups | 4 | 35.7458 | 8.9364 | 1.4731 | 0.2351 |
| Within Groups | 30 | 181.9985 | 6.0666 | ||
| Total: | 34 | 217.7442 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eltayeb, H.A.; Stewart, L.; Morgem, M.; Johnson, T.; Nguyen, M.; Earl, K.; Sodipe, A.; Jackson, D.; Olufemi, S.-E. Antioxidants Amelioration Is Insufficient to Prevent Acrylamide and Alpha-Solanine Synergistic Toxicity in BEAS-2B Cells. Int. J. Mol. Sci. 2023, 24, 11956. https://doi.org/10.3390/ijms241511956
Eltayeb HA, Stewart L, Morgem M, Johnson T, Nguyen M, Earl K, Sodipe A, Jackson D, Olufemi S-E. Antioxidants Amelioration Is Insufficient to Prevent Acrylamide and Alpha-Solanine Synergistic Toxicity in BEAS-2B Cells. International Journal of Molecular Sciences. 2023; 24(15):11956. https://doi.org/10.3390/ijms241511956
Chicago/Turabian StyleEltayeb, Hoda Awad, Leandra Stewart, Mounira Morgem, Tommie Johnson, Michael Nguyen, Kadeshia Earl, Ayodotun Sodipe, Desirée Jackson, and Shodimu-Emmanuel Olufemi. 2023. "Antioxidants Amelioration Is Insufficient to Prevent Acrylamide and Alpha-Solanine Synergistic Toxicity in BEAS-2B Cells" International Journal of Molecular Sciences 24, no. 15: 11956. https://doi.org/10.3390/ijms241511956
APA StyleEltayeb, H. A., Stewart, L., Morgem, M., Johnson, T., Nguyen, M., Earl, K., Sodipe, A., Jackson, D., & Olufemi, S.-E. (2023). Antioxidants Amelioration Is Insufficient to Prevent Acrylamide and Alpha-Solanine Synergistic Toxicity in BEAS-2B Cells. International Journal of Molecular Sciences, 24(15), 11956. https://doi.org/10.3390/ijms241511956






