Four Decades of Carrier Detection and Prenatal Diagnosis in Hemophilia A: Historical Overview, State of the Art and Future Directions
Abstract
1. Introduction
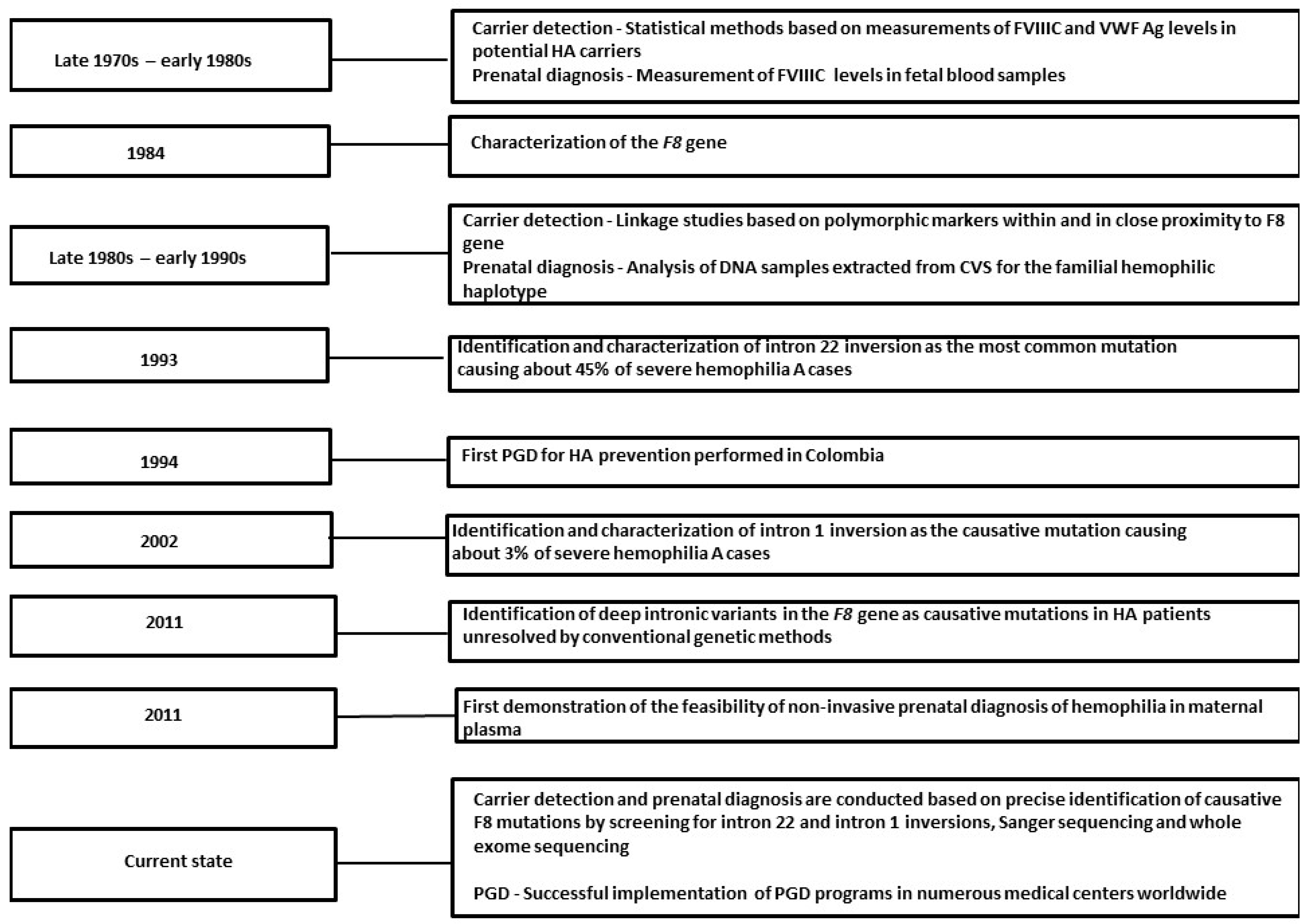
2. Historical Overview of HA Carrier Detection and Prenatal Diagnosis
2.1. Carrier Detection
2.2. Prenatal Diagnosis
2.3. Preimplantation Genetic Diagnosis (PGD)
2.4. Genetic Analysis of Symptomatic HA Carriers
3. Mosaicism in Mothers of Sporadic HA Patients
4. Current Challenges and Future Directions in Genetic Analysis of HA
4.1. Failure to Detect Causative F8 Mutations by Conventional Genetic Techniques
4.2. Risk of Fetal Loss due to Invasive Prenatal Diagnosis
4.3. Symptomatic HA Carriers: Detection of Additional Deleterious Mutations and Their Impact on Reproductive Choices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seligsohn, U.; Zivelin, A.; Perez, C.; Modan, M. Detection of Haemophilia A carriers by replicate factor VIII activity and factor VIII antigenicity determinations. Br. J. Haematol. 1979, 42, 433–439. [Google Scholar] [CrossRef]
- Akhcmetcli, M.A.; Aledort, L.M.; Alexaniats, S.; Bulanov, A.G.; Elston, R.C.; Ginter, E.K.; Goussev, A.; Graham, J.B.; Hermans, J.; Larrieu, M.J.; et al. Methods for the detection of hemophilia carriers: A memorandum. Bull. World Health Organ. 1977, 55, 675–702. [Google Scholar]
- Graham, J.B.; Green, P.P.; McGraw, R.A.; Davis, L.M. Application of molecular genetics to prenatal diagnosis and carrier detection in the hemophilias: Some limitations. Blood 1985, 66, 759–764. [Google Scholar] [CrossRef]
- Peake, I.R.; Lillicrap, D.P.; Boulyjenkov, V.; Briet, E.; Chan, V.; Ginter, E.K.; Kraus, E.M.; Ljung, R.; Mannucci, P.M.; Nicolaides, K.; et al. Haemophilia: Strategies for carrier detection and prenatal diagnosis. Bull. World Health Organ. 1993, 71, 429–458. [Google Scholar]
- Peake, I.R.; Lillicrap, D.P.; Boulyjenkov, V.; Briet, E.; Chan, V.; Ginter, E.K.; Kraus, E.M.; Ljung, R.; Mannucci, P.M.; Nicolaides, K.; et al. Report of a joint WHO/WFH meeting on the control of haemophilia: Carrier detection and prenatal diagnosis. Blood Coagul. Fibrinolysis 1993, 4, 313–344. [Google Scholar] [CrossRef]
- Lavery, S. Preimplantation genetic diagnosis: New reproductive options for carriers of haemophilia. Haemophilia 2004, 10, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.B.; Rizza, C.R.; Chediak, J.; Mannucci, P.M.; Briët, E.; Ljung, R.; Kasper, C.K.; Essien, E.M.; Green, P.P. Carrier detection in hemophilia A: A cooperative international study. I. The carrier phenotype. Blood 1986, 67, 1554–1559. [Google Scholar] [CrossRef]
- Hoyer, L.W.; Carta, C.A.; Mahoney, M.J. Detection of hemophilia carriers during pregnancy. Blood 1982, 60, 1407–1410. [Google Scholar] [CrossRef]
- Kobrinsky, N.L.; Watson, C.M.; Cheang, M.S.; Bishop, A.J. Improved hemophilia A carrier detection by DDAVP stimulation of factor VIII. J. Pediatr. 1984, 104, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Gitschier, J.; Wood, W.I.; Goralka, T.M.; Wion, K.L.; Chen, E.Y.; Eaton, D.H.; Vehar, G.A.; Capon, D.J.; Lawn, R.M. Characterization of the human factor VIII gene. Nature 1984, 312, 326–330. [Google Scholar] [CrossRef]
- Southern, E.M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 1975, 98, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Kogan, S.C.; Doherty, M.; Gitschier, J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N. Engl. J. Med. 1987, 317, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Tuddenham, E.G.; Goldman, E.; McGraw, A.; Kernoff, P.B. Haemophilia A: Carrier detection and prenatal diagnosis by linkage analysis using DNA polymorphism. J. Clin. Pathol. 1987, 40, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, P.; Kruse, T.A.; Schwartz, M.; Rasmussen, P.B.; Din, N. A new HindIII restriction fragment length polymorphism in the hemophilia A locus. Hum Genet. 1987, 76, 127–128. [Google Scholar] [CrossRef]
- Janco, R.L.; Phillips, J.A., 3rd; Orlando, P.; Davies, K.E.; Old, J.; Antonarakis, S.E. Carrier testing strategy in haemophilia A. Lancet 1986, 327, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Lalloz, M.R.A.; McVey, J.H.; Pattinson, J.K.; Tuddenham, E.G. Haemophilia A diagnosis by analysis of a hypervariable dinucleotide repeat within the factor VIII gene. Lancet 1991, 338, 207–211. [Google Scholar] [CrossRef]
- Lalloz, M.R.A.; Schwaab, R.; McVey, J.H.; Michaelides, K.; Tuddenham, E.G.D. Haemophilia A diagnosis by simultaneous analysis of two variable dinucleotide tandem repeats within the factor VIII gene. Br. J. Haematol. 1994, 86, 804–809. [Google Scholar]
- Harraway, J.R.; Smith, M.P.; George, P.M. A highly informative, multiplexed assay for the indirect detection of hemophilia A using five-linked microsatellites. J. Thromb. Haemost. 2006, 4, 587–590. [Google Scholar] [CrossRef]
- Higuchi, M.; Kazazian, H.H., Jr.; Kasch, L.; Warren, T.C.; McGinniss, M.J.; Phillips, J.A., 3rd; Kasper, C.; Janco, R.; Antonarakis, S.E. Molecular characterization of severe hemophilia A suggests that about half the mutations are not within the coding regions and splice junctions of the factor VIII gene. Proc. Natl. Acad. Sci. USA 1991, 88, 7405–7409. [Google Scholar] [CrossRef]
- Naylor, J.A.; Green, P.M.; Rizza, C.R.; Giannelli, F. Analysis of factor VIII mRNA reveals defects in everyone of 28 haemophilia A patients. Hum. Mol. Genet. 1993, 2, 11–17. [Google Scholar] [CrossRef]
- Lakich, D.; Kazazian, H.H., Jr.; Antonarakis, S.E.; Gitschier, J. Inversions disrupting the factor VIII gene are a common cause of severe haemophilia A. Nat. Genet. 1993, 5, 236–241. [Google Scholar] [CrossRef]
- Bagnall, R.D.; Waseem, N.; Green, P.M.; Giannelli, F. Recurrent inversion breaking intron 1 of the factor VIII gene is a frequent cause of severe hemophilia A. Blood 2002, 99, 168–174. [Google Scholar] [CrossRef]
- Rossetti, L.C.; Radic, C.P.; Larripa, I.B.; De Brasi, C.D. Genotyping the hemophilia inversion hotspot by use of inverse PCR. Clin. Chem. 2005, 51, 1154–1158. [Google Scholar] [CrossRef]
- Pezeshkpoor, B.; Zimmer, N.; Marquardt, N.; Nanda, I.; Haaf, T.; Budde, U.; Oldenburg, J.; El-Maarri, O. Deep intronic ‘mutations’ cause hemophilia A: Application of next generation sequencing in patients without detectable mutation in F8 cDNA. J. Thromb. Haemost. 2013, 11, 1679–1687. [Google Scholar] [CrossRef]
- Inaba, H.; Shinozawa, K.; Amano, K.; Fukutake, K. Identification of deep intronic individual variants in patients with hemophilia A by next-generation sequencing of the whole factor VIII gene. Res. Pract. Thromb. Haemost. 2017, 1, 264–274. [Google Scholar] [CrossRef]
- Ljung, R.C. Prenatal diagnosis of haemophilia. Haemophilia 1999, 5, 84–87. [Google Scholar] [CrossRef]
- Tedgard, U. Carrier testing and prenatal diagnosis of haemophilia-utilisation and psychological consequences. Haemophilia 1998, 4, 365–369. [Google Scholar]
- Jarrett, K.L.; Michaelis, R.C.; Phelan, M.C.; Vincent, V.A.; Best, R.G. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46,XX products of conception consisting of villi or a combination of villi and membranous material. Am. J. Obstet. Gynecol. 2001, 185, 198–203. [Google Scholar] [CrossRef]
- Honda, H.; Miharu, N.; Ohashi, Y.; Samura, O.; Kinutani, M.; Hara, T.; Ohama, K. Fetal gender determination in early pregnancy through qualitative and quantitative analysis of fetal DNA in maternal serum. Hum. Genet. 2002, 110, 75–79. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Amirizadeh, N.; Rabiee, M.; Rahimi-Sharbaf, F.; Pourfathollah, A.A. Noninvasive Fetal Sex Determination by Real-Time PCR and TaqMan Probes. Rep. Biochem. Mol. Biol. 2020, 9, 315–323. [Google Scholar] [CrossRef]
- Karimi, M.; Peyvandi, F.; Siboni, S.; Ardeshiri, R.; Gringeri, A.; Mannucci, P.M. Comparison of attitudes towards prenatal diagnosis and termination of pregnancy for haemophilia in Iran and Italy. Haemophilia 2004, 10, 367–369. [Google Scholar] [CrossRef]
- Lavery, S. Preimplantation genetic diagnosis of haemophilia. Br. J. Haematol. 2009, 144, 303–307. [Google Scholar] [CrossRef]
- Laurie, A.D.; Hill, A.M.; Harraway, J.R.; Fellowes, A.P.; Phillipson, G.T.; Benny, P.S.; Smith, M.P.; George, P.M. Preimplantation genetic diagnosis for hemophilia A using indirect linkage analysis and direct genotyping approaches. J. Thromb. Haemost. 2010, 8, 783–789. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, M.; Tan, A.S.C.; Cheah, F.S.H.; Mathew, J.; Wong, P.C.; Chong, S.S. Single-tube tetradecaplex panel of highly polymorphic microsatellite markers < 1 Mb from F8 for simplified preimplantation genetic diagnosis of hemophilia A. J. Thromb. Haemost. 2017, 15, 1473–1483. [Google Scholar]
- Fernandez, R.M.; Pecina, A.; Sanchez, B.; Lozano-Arana, M.D.; Garcia-Lozano, J.C.; Perez-Garrido, R.; Nunez, R.; Borrego, S.; Antinolo, G. Experience of Preimplantation Genetic Diagnosis for Hemophilia at the University Hospital Virgen Del Rocío in Spain: Technical and Clinical Overview. Biomed. Res. Int. 2015, 2015, 406096. [Google Scholar] [CrossRef]
- Perez, J.; Lucena, E.; Hughes, M.; Lizcano, L.; Kasper, C.K. Preimplantation genetic diagnosis--a historical annotation: First PGD baby turns fifteen. Haemophilia 2011, 17 (Suppl. 1), 18–19. [Google Scholar] [CrossRef]
- Chen, M.; Chang, S.P.; Ma, G.C.; Lin, W.H.; Chen, H.F.; Chen, S.U.; Tsai, H.D.; Tsai, F.P.; Shen, M.C. Preimplantation genetic diagnosis of hemophilia A. Thromb. J. 2016, 14 (Suppl. 1), 79–86. [Google Scholar] [CrossRef]
- Pavlova, A.; Brondke, H.; Müsebeck, J.; Pollmann, H.; Srivastava, A.; Oldenburg, J. Molecular mechanisms underlying hemophilia A phenotype in seven females. J. Thromb. Haemost. 2009, 7, 976–982. [Google Scholar] [CrossRef]
- Janczar, S.; Babol-Pokora, K.; Jatczak-Pawlik, I.; Taha, J.; Klukowska, A.; Laguna, P.; Windyga, J.; Odnoczko, E.; Zdziarska, J.; Iwaniec, T.; et al. Six molecular patterns leading to hemophilia A phenotype in 18 females from Poland. Thromb. Res. 2020, 193, 9–14. [Google Scholar] [CrossRef]
- Panarello, C.; Acquila, M.; Caprino, D.; Gimelli, G.; Pecorara, M.; Mori, P.G. Concomitant Turner syndrome and hemophilia A in a female with an idic(X)(p11) heterozygous at locus DXS52. Cytogenet. Cell. Genet. 1992, 59, 241–242. [Google Scholar] [CrossRef]
- Chuansumrit, A.; Sasanakul, W.; Goodeve, A.; Treratvirapong, T.; Parinayok, R.; Pintadit, P.; Hathirat, P. Inversion of intron 22 of the factor VIII gene in a girl with severe hemophilia A and Turner’s syndrome. Thromb. Haemost. 1999, 82, 1379. [Google Scholar]
- Mazurier, C.; Parquet-Gernez, A.; Gaucher, C.; Lavergne, J.M.; Goudemand, J. Factor VIII deficiency not induced by FVIII gene mutation in a female first cousin of two brothers with haemophilia A. Br. J. Haematol. 2002, 119, 390–392. [Google Scholar] [CrossRef]
- Miller, C.H.; Bean, C.J. Genetic causes of haemophilia in women and girls. Haemophilia 2021, 27, e164–e179. [Google Scholar] [CrossRef]
- Di Michele, D.M.; Gibb, C.; Lefkowitz, J.M.; Ni, Q.; Gerber, L.M.; Ganguly, A. Severe and moderate haemophilia A and B in US females. Haemophilia 2014, 20, e136–e143. [Google Scholar] [CrossRef]
- Favier, R.; Lavergne, J.M.; Costa, J.M.; Caron, C.; Mazurier, C.; Viemont, M.; Delpech, M.; Valleix, S. Unbalanced X-chromosome inactivation with a novel FVIII gene mutation resulting in severe hemophilia A in a female. Blood 2000, 96, 4373–4375. [Google Scholar] [CrossRef]
- Janczar, S.; Kosinska, J.; Ploski, R.; Pastorczak, A.; Wegner, O.; Zalewska-Szewczyk, B.; Paige, A.J.; Borowiec, M.; Mlynarski, W. Haemophilia A and cardiovascular morbidity in a female SHAM syndrome carrier due to skewed X chromosome inactivation. Eur. J. Med. Genet. 2016, 59, 43–47. [Google Scholar] [CrossRef]
- Dardik, R.; Avishai, E.; Lalezari, S.; Barg, A.A.; Levy-Mendelovich, S.; Budnik, I.; Barel, O.; Khavkin, Y.; Kenet, G.; Livnat, T. Molecular Mechanisms of Skewed X-Chromosome Inactivation in Female Hemophilia Patients-Lessons from Wide Genome Analyses. Int. J. Mol. Sci. 2021, 22, 9074. [Google Scholar] [CrossRef]
- Matsumaru, S.; Oguni, H.; Ogura, H.; Shimojima, K.; Nagata, S.; Kanno, H.; Yamamoto, T. A novel PGK1 mutation associated with neurological dysfunction and the absence of episodes of hemolytic anemia or myoglobinuria. Intractable Rare Dis. Res. 2017, 6, 132–136. [Google Scholar] [CrossRef]
- Pajerowski, A.G.; Shapiro, M.J.; Gwin, K.; Sundsbak, R.; Nelson-Holte, M.; Medina, K.; Shapiro, V.S. Adult hematopoietic stem cells require NKAP for maintenance and survival. Blood 2010, 116, 2684–2693. [Google Scholar] [CrossRef]
- Fiordaliso, S.K.; Iwata-Otsubo, A.; Ritter, A.L.; Quesnel-Vallières, M.; Fujiki, K.; Nishi, E.; Hancarova, M.; Miyake, N.; Morton, J.E.V.; Lee, S.; et al. Missense mutations in NKAP cause a disorder of transcriptional regulation characterized by Marfanoid habitus and cognitive impairment. Am. J. Hum. Genet. 2019, 105, 987–995. [Google Scholar] [CrossRef]
- Rafi, S.K.; Fernández-Jaén, A.; Alvarez, S.; Nadeau, O.W.; Butler, M.G. High functioning autism with missense mutations in Synaptotagmin-Like Protein 4 (SYTL4) and Transmembrane Protein 187 (TMEM187) genes: SYTL4- protein modeling, protein protein interaction, expression profiling and microRNA studies. Int. J. Mol. Sci. 2019, 20, 3358. [Google Scholar] [CrossRef]
- Wang, H.; Ishizaki, R.; Xu, J.; Kasai, K.; Kobayashi, E.; Gomi, H.; Izumi, T. The Rab27a effector exophilin7 promotes fusion of secretory granules that have not been docked to the plasma membrane. Mol. Biol. Cell 2013, 24, 319–330. [Google Scholar] [CrossRef]
- Habuchi, H.; Tanaka, M.; Habuchi, O.; Yoshida, K.; Suzuki, H.; Ban, K.; Kimata, K. The occurrence of three isoforms of heparan sulfate 6-O-sulfotransferase having different specificities for hexuronic acid adjacent to the targeted N-sulfoglucosamine. J. Biol. Chem. 2000, 275, 2859–2868. [Google Scholar] [CrossRef]
- Paganini, L.; Hadi, L.A.; Chetta, M.; Rovina, D.; Fontana, L.; Colapietro, P.; Bonaparte, E.; Pezzani, L.; Marchisio, P.; Tabano, S.M.; et al. A HS6ST2 gene variant associated with X-linked intellectual disability and severe myopia in two male twins. Clin. Genet. 2019, 95, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Dejosez, M.; Levine, S.S.; Frampton, G.M.; Whyte, W.A.; Stratton, S.A.; Barton, M.C.; Gunaratne, P.H.; Young, R.A.; Zwaka, T.P. Ronin/Hcf-1 binds to a hyperconserved enhancer element and regulates genes involved in the growth of embryonic stem cells. Genes Dev. 2010, 24, 1479–1484. [Google Scholar] [CrossRef]
- Parker, J.B.; Palchaudhuri, S.; Yin, H.; Wei, J.; Chakravarti, D. A transcriptional regulatory role of the THAP11-HCF-1 complex in colon cancer cell function. Mol. Cell. Biol. 2012, 32, 1654–1670. [Google Scholar] [CrossRef]
- Yu, H.C.; Sloan, J.L.; Scharer, G.; Brebner, A.; Quintana, A.M.; Achilly, N.P.; Manoli, I.; Coughlin, C.R., 2nd; Geiger, E.A.; Schneck, U.; et al. An X-linked cobalamin disorder caused by mutations in transcriptional coregulator HCFC1. Am. J. Hum. Genet. 2013, 93, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Ostendorff, H.P.; Peirano, R.I.; Peters, M.A.; Schlüter, A.; Bossenz, M.; Scheffner, M.; Bach, I. Ubiquitination-dependent cofactor exchange on LIM homeodomain transcription factors. Nature 2002, 416, 99–103. [Google Scholar] [CrossRef]
- Gontan, C.; Achame, E.M.; Demmers, J.; Barakat, T.S.; Rentmeester, E.; van IJcken, W.; Grootegoed, J.A.; Gribnau, J. RNF12 initiates X-chromosome inactivation by targeting REX1 for degradation. Nature 2012, 485, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, H.; Zhou, F.; Schimmel, J.; Pardo, C.G.; Zhang, T.; Barakat, T.S.; Sheppard, K.A.; Mickanin, C.; Porter, J.A.; et al. RNF12 controls embryonic stem cell fate and morphogenesis in zebrafish embryos by targeting Smad7 for degradation. Mol. Cell 2012, 46, 650–661. [Google Scholar] [CrossRef]
- Her, Y.R.; Chung, I.K. Ubiquitin ligase RLIM modulates telomere length homeostasis through a proteolysis of TRF1. J. Biol. Chem. 2009, 284, 8557–8566. [Google Scholar] [CrossRef] [PubMed]
- Ostendorff, H.P.; Tursun, B.; Cornils, K.; Schlüter, A.; Drung, A.; Güngör, C.; Bach, I. Dynamic expression of LIM cofactors in the developing mouse neural tube. Dev. Dyn. 2006, 235, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Tønne, E.; Holdhus, R.; Stansberg, C.; Stray-Pedersen, A.; Petersen, K.; Brunner, H.G.; Gilissen, C.; Hoischen, A.; Prescott, T.; Steen, V.M.; et al. Syndromic X-linked intellectual disability segregating with a missense variant in RLIM. Eur. J. Hum. Genet. 2015, 23, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, S.E.; Rossiter, J.P.; Young, M.; Horst, J.; de Moerloose, P.; Sommer, S.S.; Ketterling, R.P.; Kazazian, H.H., Jr.; Négrier, C.; Vinciguerra, C. Factor VIII gene inversions in severe hemophilia A: Results of an international consortium study. Blood 1995, 86, 2206–2212. [Google Scholar] [CrossRef]
- Oldenburg, J.; Ananyeva, N.M.; Saenko, E.L. Molecular basis of haemophilia A. Haemophilia 2004, 10 (Suppl. 4), 133–139. [Google Scholar] [CrossRef]
- Lu, Y.; Xin, Y.; Dai, J.; Wu, X.; You, G.; Ding, Q.; Wu, W.; Wang, X. Spectrum and origin of mutations in sporadic cases of haemophilia A in China. Haemophilia 2018, 24, 291–298. [Google Scholar] [CrossRef]
- Martensson, A.; Ivarsson, S.; Letelier, A.; Manderstedt, E.; Halldén, C.; Ljung, R. Origin of mutation in sporadic cases of severe haemophilia A in Sweden. Clin. Genet. 2016, 90, 63–68. [Google Scholar] [CrossRef]
- Lannoy, N.; Hermans, C. Genetic mosaicism in haemophilia: A practical review to help evaluate the risk of transmitting the disease. Haemophilia 2020, 26, 375–383. [Google Scholar] [CrossRef]
- Dericquebourg, A.; Fretigny, M.; Leuci, A.; Zawadzki, C.; Huguenin, Y.; Castet, S.M.; Dargaud, Y.; Vinciguerra, C.; Jourdy, Y. Whole F8 gene sequencing combined with splicing functional analyses led to a substantial increase of the molecular diagnosis yield for non-severe haemophilia A. Haemophilia 2023. [Google Scholar] [CrossRef]
- Bach, J.E.; Müller, C.R.; Rost, S. Mini-gene assays confirm the splicing effect of deep intronic variants in the factor VIII gene. Thromb. Haemost. 2016, 115, 222–224. [Google Scholar]
- Lassalle, F.; Jourdy, Y.; Jouan, L.; Swystun, L.; Gauthier, J.; Zawadzki, C.; Goudemand, J.; Susen, S.; Rivard, G.E.; Lillicrap, D. The challenge of genetically unresolved haemophilia A patients: Interest of the combination of whole F8 gene sequencing and functional assays. Haemophilia 2020, 26, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of fetal DNA in maternal plasma and serum. Lancet 1997, 350, 485–487. [Google Scholar] [CrossRef]
- Shaw, J.; Scotchman, E.; Chandler, N.; Chitty, L.S. Preimplantation Genetic Testing: Non-invasive prenatal testing for aneuploidy, copy-number variants and single-gene disorders. Reproduction 2020, 160, A1–A11. [Google Scholar] [CrossRef] [PubMed]
- Hudecova, I.; Jiang, P.; Davies, J.; Lo, Y.M.D.; Kadir, R.A.; Chiu, R.W.K. Noninvasive detection of F8 int22h-related inversions and sequence variants in maternal plasma of hemophilia carriers. Blood 2017, 130, 340–347. [Google Scholar] [CrossRef]
- Tsui, N.B.; Kadir, R.A.; Chan, K.C.; Chi, C.; Mellars, G.; Tuddenham, E.G.; Leung, T.Y.; Lau, T.K.; Chiu, R.W.; Lo, Y.M. Noninvasive prenatal diagnosis of hemophilia by microfluidics digital PCR analysis of maternal plasma DNA. Blood 2011, 117, 3684–3691. [Google Scholar] [CrossRef]
- Lalezari, S.; Barg, A.A.; Dardik, R.; Luboshitz, J.; Bashari, D.; Avishai, E.; Kenet, G. Women with hemophilia: Case series of reproductive choices and review of literature. TH Open 2021, 5, e183–e187. [Google Scholar] [CrossRef]
- Johnsen, J.M.; Fletcher, S.N.; Huston, H.; Roberge, S.; Martin, B.K.; Kircher, M.; Josephson, N.C.; Shendure, J.; Ruuska, S.; Koerper, M.A.; et al. Novel approach to genetic analysis and results in 3000 hemophilia patients enrolled in the My Life, Our Future initiative. Blood Adv. 2017, 18, 824–834. [Google Scholar] [CrossRef] [PubMed]
| Symptomatic HA Carrier (Reference) | F8 Mutation | Additional Mutant Gene Potentially Involved in XCI Skewing | Degree of XCI Skewing | HA Severity |
|---|---|---|---|---|
| 1 [38] | c.6872 delCT Thr2272fs | RSK2 (Arg110*) Protein: Ribosomal S6 Kinase 2 | Severe | Severe |
| 2 [47] | Intron 22 inversion | NKAP (NM_024528; c.175C > T; p. Q59*) Protein: NF kappa B Activating Protein | Severe | Moderate |
| 3 [47] | Intron 22 inversion | SYTL4 (NM_001129896; c.1655A > C; p.K552T) Protein: Synaptotagmin Like 4 | Severe | Moderate |
| 4 [47] | IVS5 + 2 T > G | PGK1 (NM_000291.4; c.1061_1062delCT; p.A354fs*4) Protein: Phosphoglycerate Kinase 1 | Severe | Severe |
| 5 [39] | c.6929C > T; p.Thr2310Ile | HS6ST2 (NM_147175.4; c.349C > T; p.Arg117Trp) Protein: Heparan Sulfate 6-O-Sulfotransferase 2 | Severe | Mild |
| 6 [39] | c.1271 + 1G > T | HCFC1 (NM_005334.3; c.5752G > A; p.Gly1918Ser) Protein: Host Cell Factor C1 | Severe | Moderate |
| 7 [39] | c.1812G > C; p.Trp604Cys | RLIM (NM_016120.4; c.25_28del; p.Lys9GlufsTer27) Protein: Ring Finger, LIM Domain Interacting | Severe | Severe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dardik, R.; Janczar, S.; Lalezari, S.; Avishai, E.; Levy-Mendelovich, S.; Barg, A.A.; Martinowitz, U.; Babol-Pokora, K.; Mlynarski, W.; Kenet, G. Four Decades of Carrier Detection and Prenatal Diagnosis in Hemophilia A: Historical Overview, State of the Art and Future Directions. Int. J. Mol. Sci. 2023, 24, 11846. https://doi.org/10.3390/ijms241411846
Dardik R, Janczar S, Lalezari S, Avishai E, Levy-Mendelovich S, Barg AA, Martinowitz U, Babol-Pokora K, Mlynarski W, Kenet G. Four Decades of Carrier Detection and Prenatal Diagnosis in Hemophilia A: Historical Overview, State of the Art and Future Directions. International Journal of Molecular Sciences. 2023; 24(14):11846. https://doi.org/10.3390/ijms241411846
Chicago/Turabian StyleDardik, Rima, Szymon Janczar, Shadan Lalezari, Einat Avishai, Sarina Levy-Mendelovich, Assaf Arie Barg, Uri Martinowitz, Katarzyna Babol-Pokora, Wojciech Mlynarski, and Gili Kenet. 2023. "Four Decades of Carrier Detection and Prenatal Diagnosis in Hemophilia A: Historical Overview, State of the Art and Future Directions" International Journal of Molecular Sciences 24, no. 14: 11846. https://doi.org/10.3390/ijms241411846
APA StyleDardik, R., Janczar, S., Lalezari, S., Avishai, E., Levy-Mendelovich, S., Barg, A. A., Martinowitz, U., Babol-Pokora, K., Mlynarski, W., & Kenet, G. (2023). Four Decades of Carrier Detection and Prenatal Diagnosis in Hemophilia A: Historical Overview, State of the Art and Future Directions. International Journal of Molecular Sciences, 24(14), 11846. https://doi.org/10.3390/ijms241411846






