Gold Nanoparticles for Monitoring of Mesenchymal Stem-Cell-Loaded Bioresorbable Polymeric Wraps for Arteriovenous Fistula Maturation
Abstract
1. Introduction
2. Results
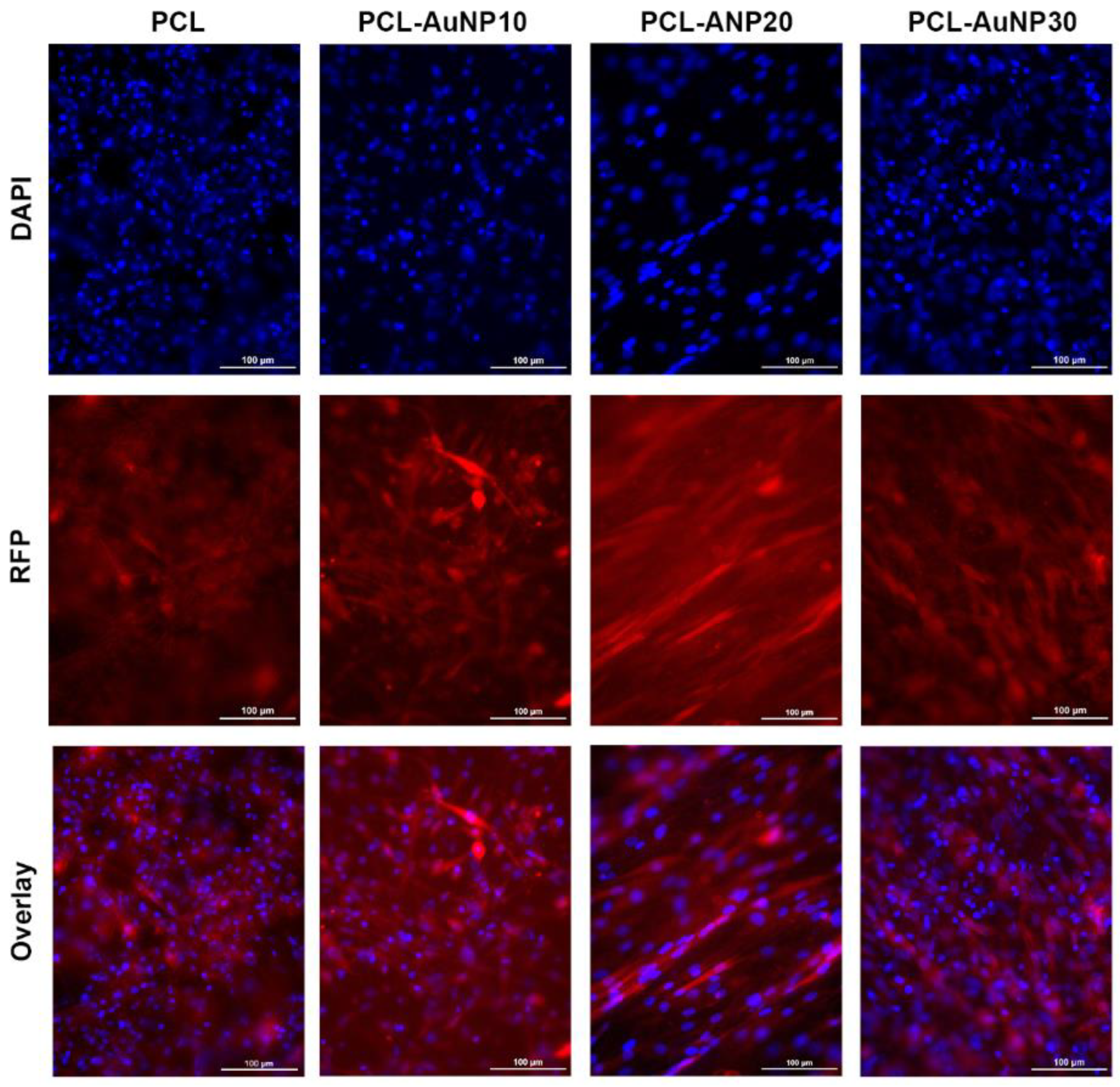
2.1. Physicochemical Characteristics of the Polymeric Wraps
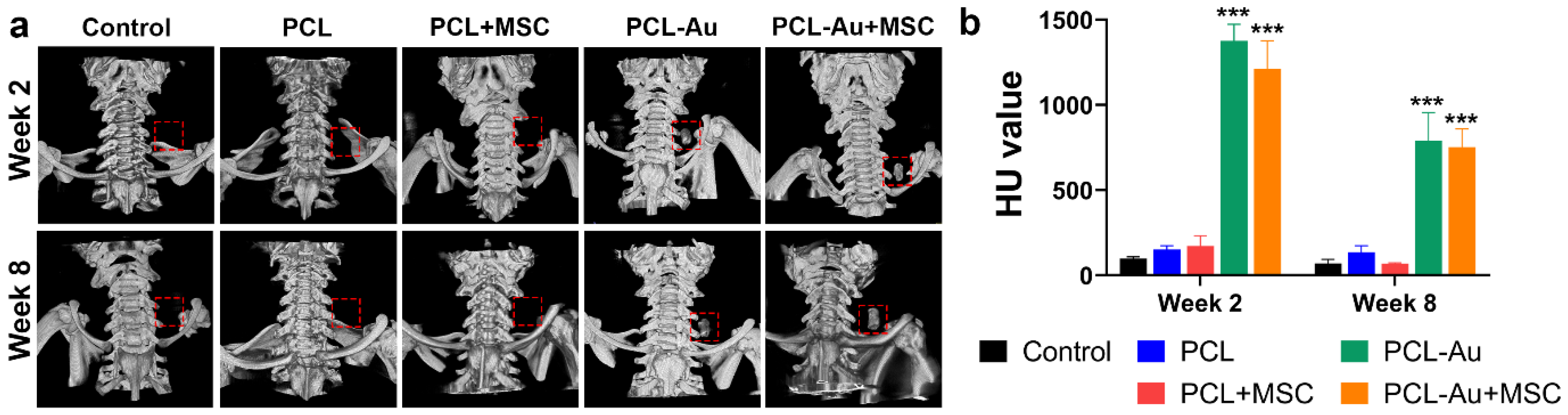
2.2. AuNP-Infused Wraps Demonstrated Higher Radiopacity on Micro-CT Images
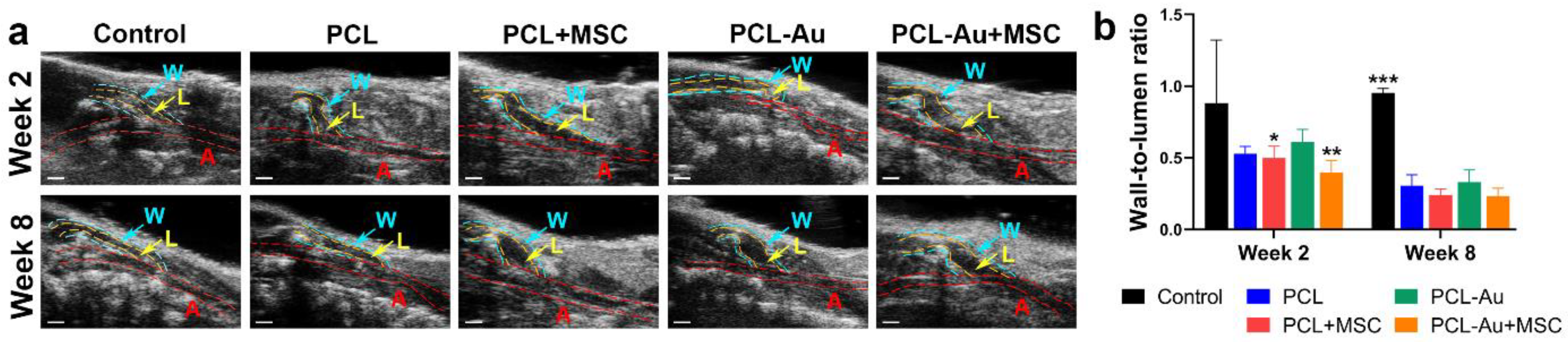
2.3. AuNP-Infused and Non-AuNP Wraps Equally Improved the Wall-to-Lumen Ratio of the Outflow Vein
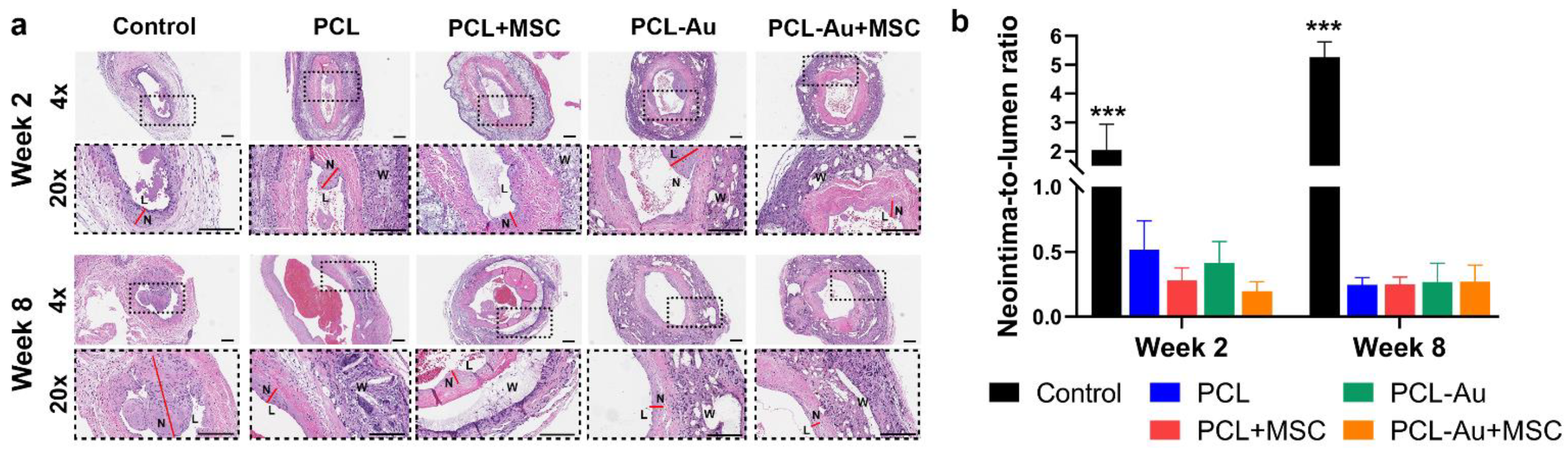
2.4. AuNP-Infused and Non-AuNP Wraps Equally Reduced NIH at the Outflow Vein
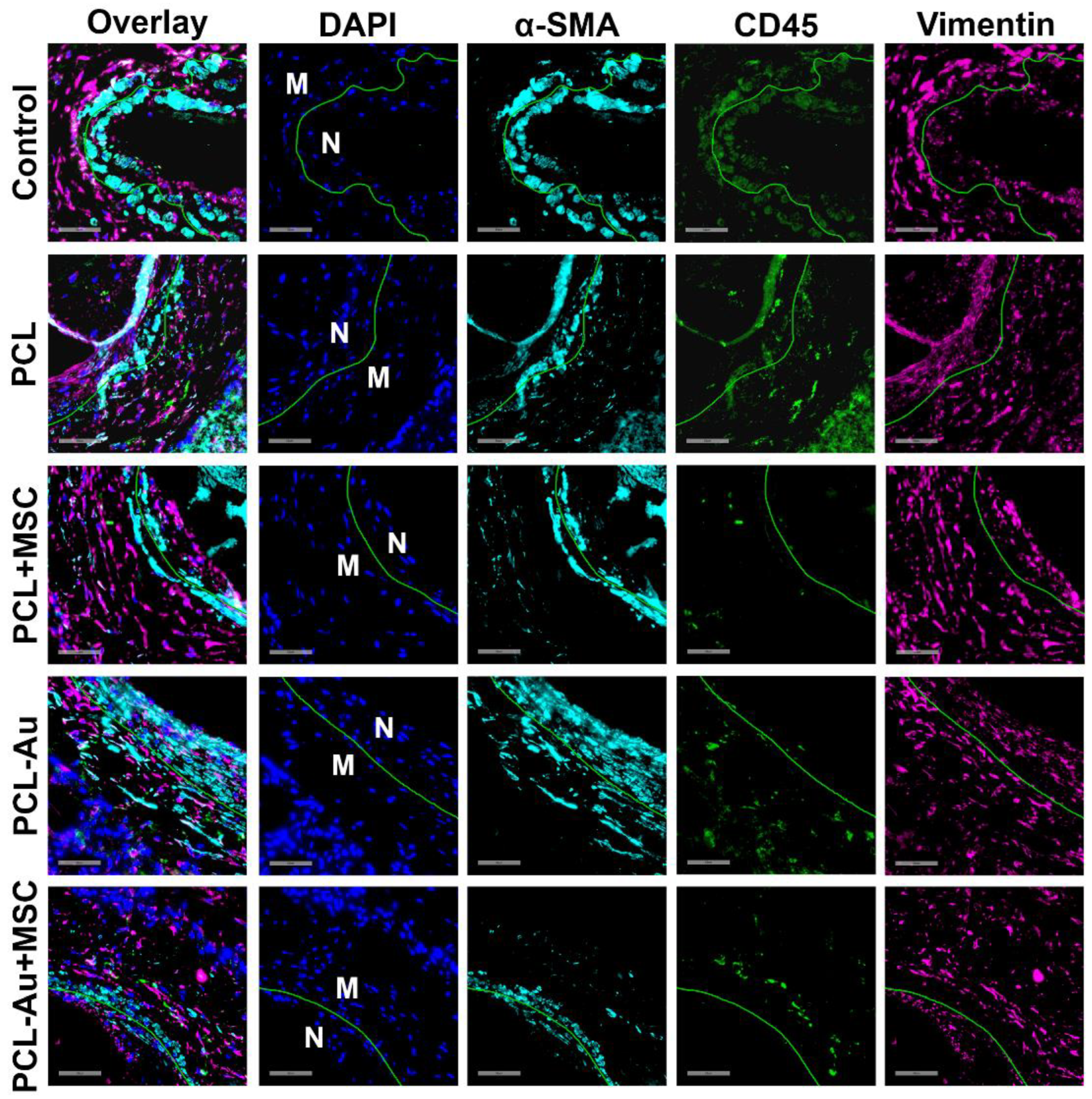
2.5. MSC-Seeded Wraps Reduced Neointimal α-SMA, CD45, and Vimentin Staining
3. Discussion
4. Materials and Methods
4.1. Synthesis of AuNP-Infused Polymeric Wraps
4.2. Characterization of AuNP-Infused Polymeric Wraps
4.3. Cell Culture and MSC Attachment
4.4. AVF Creation and Perivascular Wrap Application
4.5. X-ray and Micro-CT
4.6. Ultrasonography
4.7. Histological Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | alpha smooth muscle actin |
| AuNP | gold nanoparticle |
| AVF | arteriovenous fistula |
| CD45 | cluster of differentiation 45 |
| CKD | chronic kidney disease |
| CT | computed tomography |
| DAPI | 4′,6-diamidino-2-phenylindole |
| 18F-FDG PET | 18F-fluorodeoxyglucose positron emission tomography |
| H&E | hematoxylin and eosin |
| HU | Hounsfield units |
| MSC | mesenchymal stem cell |
| NIH | neointimal hyperplasia |
| PCL | polycaprolactone |
| RFP | red fluorescent protein |
| SEM | scanning electron microscope |
| TEM | transmission electron microscope |
| US | ultrasound |
References
- International Society of Nephrology. ISN Global Kidney Health Atlas. Available online: https://www.theisn.org/initiatives/global-kidney-health-atlas/ (accessed on 15 September 2022).
- United States Renal Data System. 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2022. [Google Scholar]
- Lok, C.E.; Huber, T.S.; Lee, T.; Shenoy, S.; Yevzlin, A.S.; Abreo, K.; Allon, M.; Asif, A.; Astor, B.C.; Glickman, M.H.; et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am. J. Kidney Dis. 2020, 75 (Suppl. S2), S1–S164. [Google Scholar] [CrossRef] [PubMed]
- Wasse, H.; Huang, R.; Naqvi, N.; Smith, E.; Wang, D.; Husain, A. Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from AVF creation in CKD patients. J. Vasc. Access. 2012, 13, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Simone, S.; Loverre, A.; Cariello, M.; Divella, C.; Castellano, G.; Gesualdo, L.; Pertosa, G.; Grandaliano, G. Arteriovenous fistula stenosis in hemodialysis patients is characterized by an increased adventitial fibrosis. J. Nephrol. 2014, 27, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Hammes, M. Hemodynamic and biologic determinates of arteriovenous fistula outcomes in renal failure patients. Biomed. Res. Int. 2015, 2015, 171674. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Misra, S. New Insights into Dialysis Vascular Access: Molecular Targets in Arteriovenous Fistula and Arteriovenous Graft Failure and Their Potential to Improve Vascular Access Outcomes. Clin. J. Am. Soc. Nephrol. 2016, 11, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, A.; Remuzzi, A.; Franzoni, M.; Misra, S. The molecular mechanisms of hemodialysis vascular access failure. Kidney Int. 2016, 89, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Barcena, A.J.R.; Perez, J.V.D.; Liu, O.; Mu, A.; Heralde, F.M.; Huang, S.Y.; Melancon, M.P. Localized perivascular therapeutic approaches to inhibit venous neointimal hyperplasia in arteriovenous fistula access for hemodialysis use. Biomolecules 2022, 12, 1367. [Google Scholar] [CrossRef] [PubMed]
- Barcena, A.J.R.; Perez, J.V.D.; Bernardino, M.; Damasco, J.A.; Cortes, A.; Del Mundo, H.; San Valentin, E.M.D.; Klusman, C.; Canlas, G.; Heralde, F.M.; et al. Bioresorbable mesenchymal stem cell–loaded electrospun polymeric scaffold inhibits neointimal hyperplasia following arteriovenous fistula formation in a rat model of chronic kidney disease. Adv. Healthc. Mater. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.V.D.; Jacobsen, M.C.; Damasco, J.A.; Melancon, A.; Huang, S.Y.; Layman, R.R.; Melancon, M.P. Optimization of the differentiation and quantification of high-Z nanoparticles incorporated in medical devices for CT-guided interventions. Med. Phys. 2021, 48, 300–312. [Google Scholar] [CrossRef] [PubMed]
- San Valentin, E.M.D.; Barcena, A.J.R.; Klusman, C.; Martin, B.; Melancon, M.P. Nano-embedded medical devices and delivery systems in interventional radiology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 15, e1841. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef] [PubMed]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold nanoparticles (GNPs) in biomedical and clinical applications: A review. Nano Select 2022, 3, 792–828. [Google Scholar] [CrossRef]
- Tian, L.; Lee, P.; Singhana, B.; Chen, A.; Qiao, Y.; Lu, L.; Martinez, J.O.; Tasciotti, E.; Melancon, A.; Huang, S.; et al. Radiopaque resorbable inferior vena cava filter infused with gold nanoparticles. Sci. Rep. 2017, 7, 2147. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cui, W.; Li, X.; Jin, Y. Electrospun Fibrous Mats with High Porosity as Potential Scaffolds for Skin Tissue Engineering. Biomacromolecules 2008, 9, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.; Pagliari, S.; Rinaldi, A.; Forte, G.; Fiaccavento, R.; Pagliari, F.; Franzese, O.; Minieri, M.; Di Nardo, P.; Licoccia, S.; et al. Multiscale three-dimensional scaffolds for soft tissue engineering via multimodal electrospinning. Acta Biomater. 2010, 6, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chaudhry, M.A.; Nie, Y.; Xie, Z.; Shapiro, J.I.; Liu, J. A Mouse 5/6th Nephrectomy Model That Induces Experimental Uremic Cardiomyopathy. J. Vis. Exp. 2017, 129, e55825. [Google Scholar] [CrossRef]
- Wong, C.Y.; de Vries, M.R.; Wang, Y.; van der Vorst, J.R.; Vahrmeijer, A.L.; van Zonneveld, A.-J.; Hamming, J.F.; Roy-Chaudhury, P.; Rabelink, T.J.; Quax, P.H.A.; et al. A Novel Murine Model of Arteriovenous Fistula Failure: The Surgical Procedure in Detail. J. Vis. Exp. 2016, 108, e53294. [Google Scholar] [CrossRef]

| Wrap Type | Diameter (μm) | Pore Size (μm) | Porosity (Calculated, %) | Porosity (Intrusion, %) | Tg (°C) | Tm (°C) | Maximum Stress (MPa) |
|---|---|---|---|---|---|---|---|
| PCL | 2.07 ± 1.03 | 168.34 ± 177.94 | 93.21 ± 1.23 | 76.75 ± 8.06 | 52.53 | −69.61 | 9.17 ± 0.31 |
| PCL-AuNP10 | 0.53 ± 0.26 | 98.34 ± 76.13 | 53.63 ± 20.62 | 74.31 ± 6.15 | 54.32 | −68.19 | 13.41 ± 0.52 |
| PCL-AuNP20 | 0.25 ± 0.07 | 81.57 ± 86.11 | 65.52 ± 1.13% | 71.13 ± 1.98 | 52.44 | −69.71 | 9.01 ± 0.42 |
| PCL-AuNP30 | 0.27 ± 0.09 | 62.91 ± 53.40 | 64.02 ± 8.34 | 76.03 ± 3.69 | 52.37 | −69.64 | 12.42 ± 1.40 |
| Wrap Type | Radiopacity (HU) | Wall-to-Lumen Ratio | Neointima-to-Lumen Ratio | |||
|---|---|---|---|---|---|---|
| Week 2 | Week 8 | Week 2 | Week 8 | Week 2 | Week 8 | |
| Control | 98.07 ± 9.88 | 69.27 ± 23.81 | 0.88 ± 0.44 | 0.95 ± 0.03 | 2.05 ± 0.89 | 5.26 ± 0.53 |
| PCL | 152.0 ± 21.21 | 135.8 ± 37.19 | 0.53 ± 0.05 | 0.31 ± 0.08 | 0.52 ± 0.22 | 0.25 ± 0.06 |
| PCL + MSC | 171.9 ± 60.22 | 67.68 ± 5.61 | 0.5 ± 0.08 | 0.24 ± 0.04 | 0.28 ± 0.10 | 0.25 ± 0.06 |
| PCL-Au | 1375 ± 97.79 | 790.1 ± 164.0 | 0.61 ± 0.09 | 0.33 ± 0.08 | 0.34 ± 0.24 | 0.27 ± 0.15 |
| PCL-Au + MSC | 1212 ± 164.1 | 751.9 ± 107.1 | 0.4 ± 0.09 | 0.23 ± 0.05 | 0.20 ± 0.08 | 0.27 ± 0.13 |
| Wrap Type | α-SMA | CD45 | Vimentin | DAPI |
|---|---|---|---|---|
| Control | 1.0 | 0.76 | 0.74 | 0.79 |
| PCL | 1.21 | 0.30 | 0.71 | 0.69 |
| PCL + MSC | 0.98 | 0.17 | 0.09 | 0.64 |
| PCL-Au | 1.74 | 0.08 | 0.36 | 0.66 |
| PCL-Au + MSC | 0.92 | 0.07 | 0.22 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcena, A.J.R.; Perez, J.V.D.; Damasco, J.A.; Bernardino, M.R.; San Valentin, E.M.D.; Klusman, C.; Martin, B.; Cortes, A.; Canlas, G.M.; Del Mundo, H.C.; et al. Gold Nanoparticles for Monitoring of Mesenchymal Stem-Cell-Loaded Bioresorbable Polymeric Wraps for Arteriovenous Fistula Maturation. Int. J. Mol. Sci. 2023, 24, 11754. https://doi.org/10.3390/ijms241411754
Barcena AJR, Perez JVD, Damasco JA, Bernardino MR, San Valentin EMD, Klusman C, Martin B, Cortes A, Canlas GM, Del Mundo HC, et al. Gold Nanoparticles for Monitoring of Mesenchymal Stem-Cell-Loaded Bioresorbable Polymeric Wraps for Arteriovenous Fistula Maturation. International Journal of Molecular Sciences. 2023; 24(14):11754. https://doi.org/10.3390/ijms241411754
Chicago/Turabian StyleBarcena, Allan John R., Joy Vanessa D. Perez, Jossana A. Damasco, Marvin R. Bernardino, Erin Marie D. San Valentin, Carleigh Klusman, Benjamin Martin, Andrea Cortes, Gino Martin Canlas, Huckie C. Del Mundo, and et al. 2023. "Gold Nanoparticles for Monitoring of Mesenchymal Stem-Cell-Loaded Bioresorbable Polymeric Wraps for Arteriovenous Fistula Maturation" International Journal of Molecular Sciences 24, no. 14: 11754. https://doi.org/10.3390/ijms241411754
APA StyleBarcena, A. J. R., Perez, J. V. D., Damasco, J. A., Bernardino, M. R., San Valentin, E. M. D., Klusman, C., Martin, B., Cortes, A., Canlas, G. M., Del Mundo, H. C., Heralde, F. M., III, Avritscher, R., Fowlkes, N., Bouchard, R. R., Cheng, J., Huang, S. Y., & Melancon, M. P. (2023). Gold Nanoparticles for Monitoring of Mesenchymal Stem-Cell-Loaded Bioresorbable Polymeric Wraps for Arteriovenous Fistula Maturation. International Journal of Molecular Sciences, 24(14), 11754. https://doi.org/10.3390/ijms241411754






