HIV and SIV Envelope Glycoproteins Interact with Glycolipids and Lipids
Abstract
1. Introduction
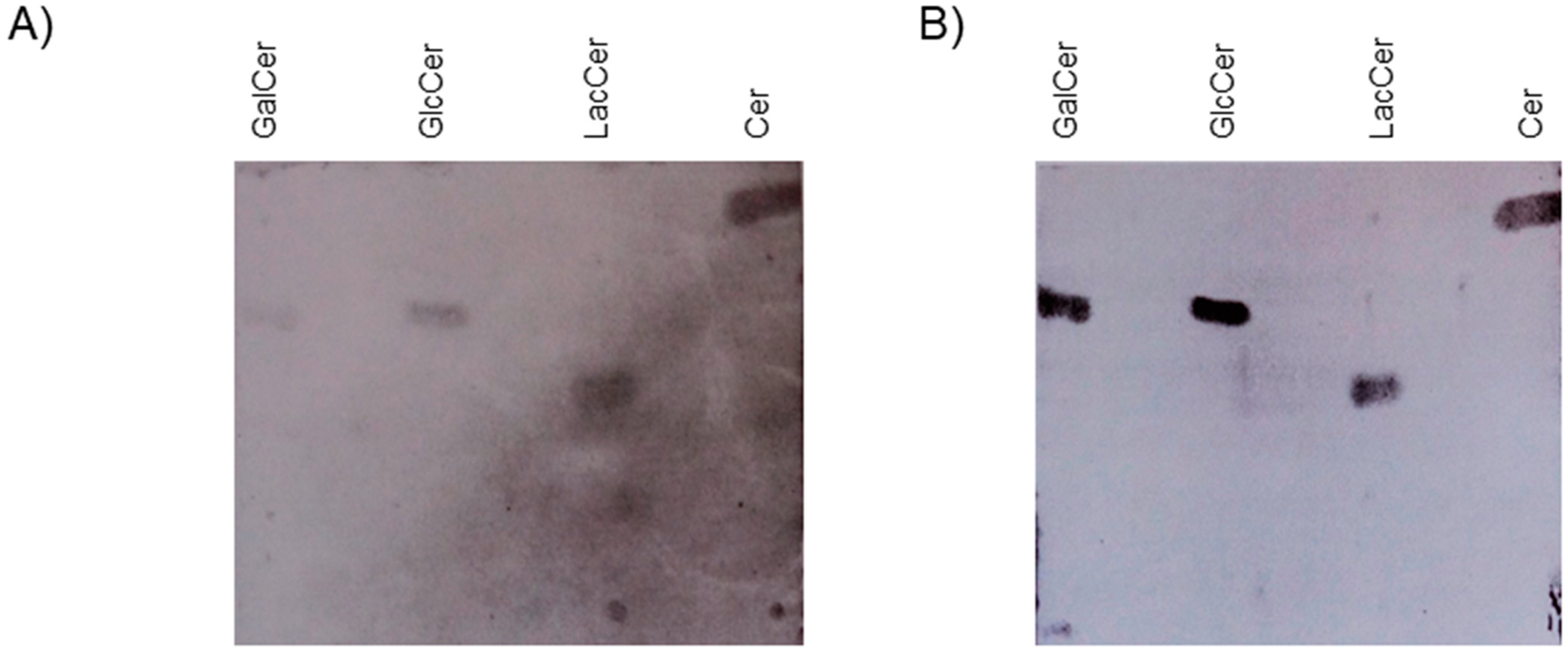
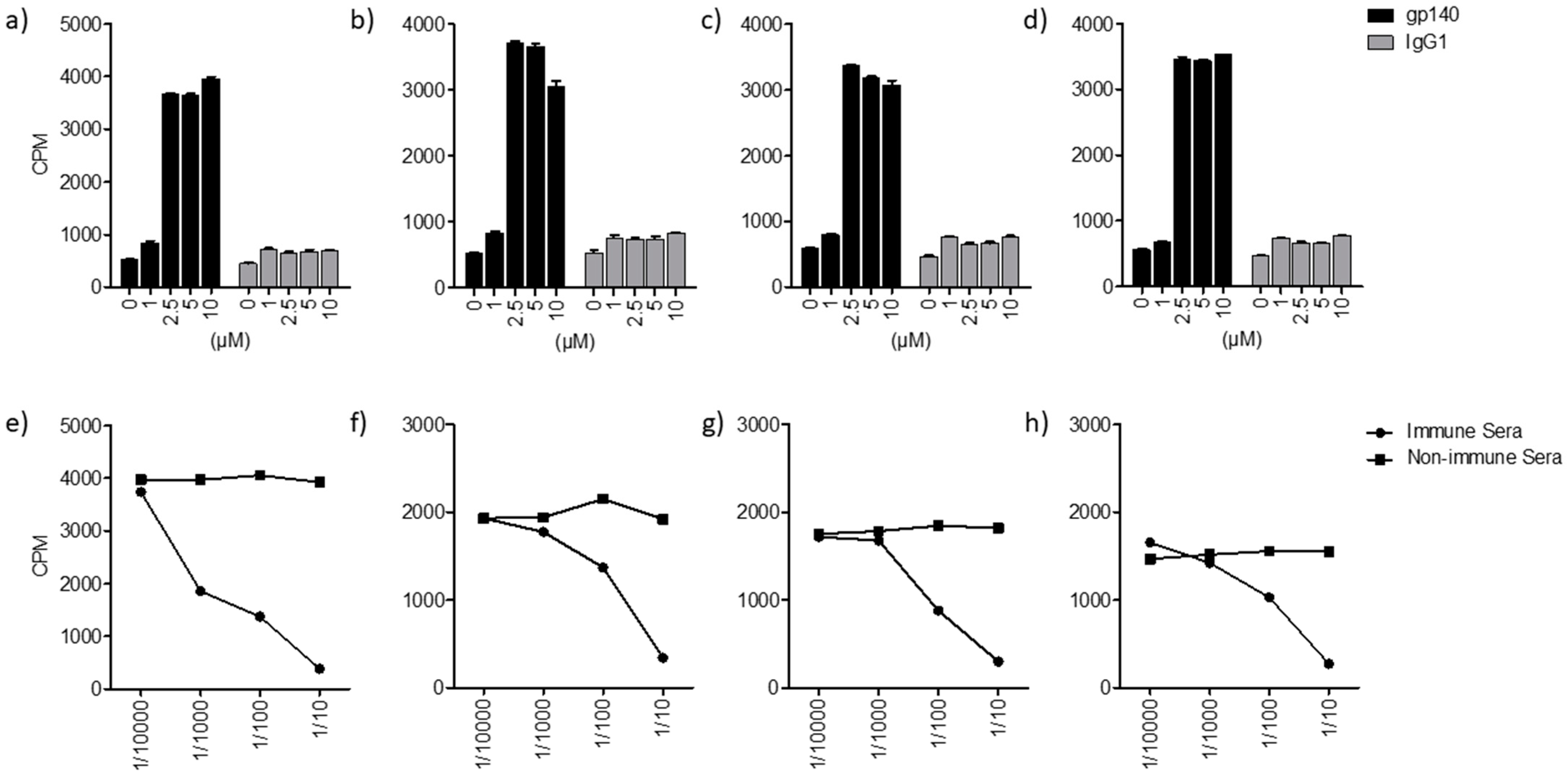
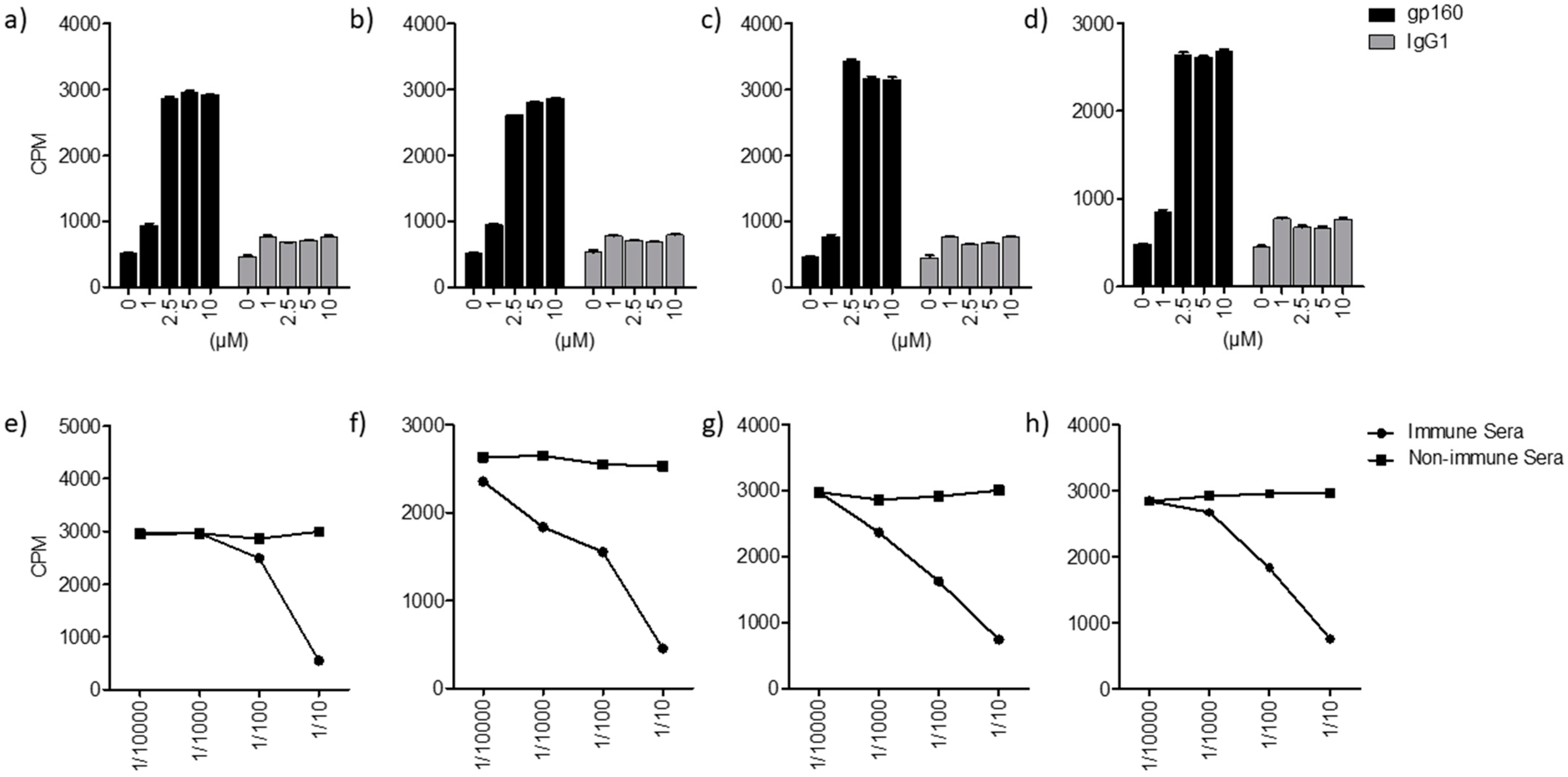
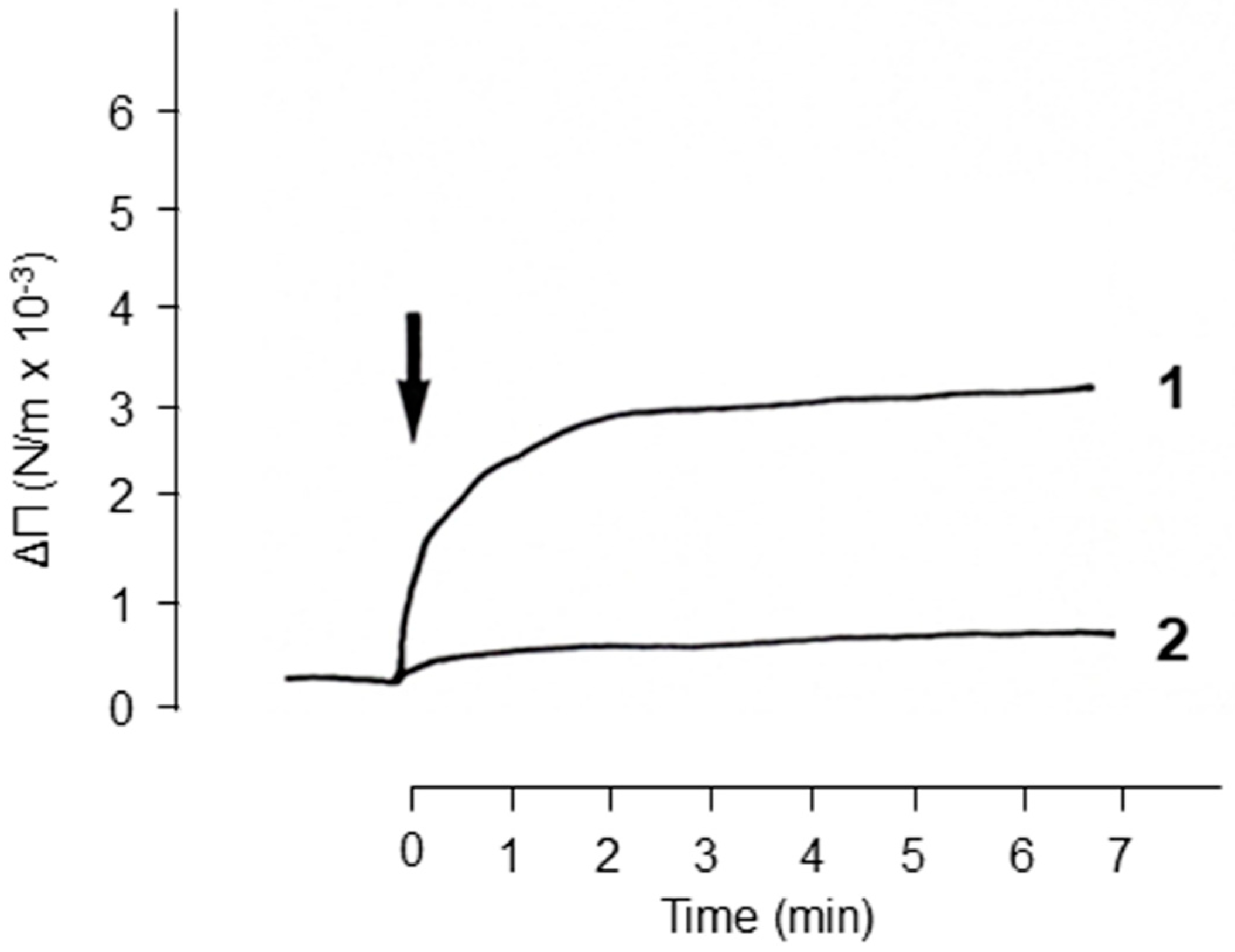
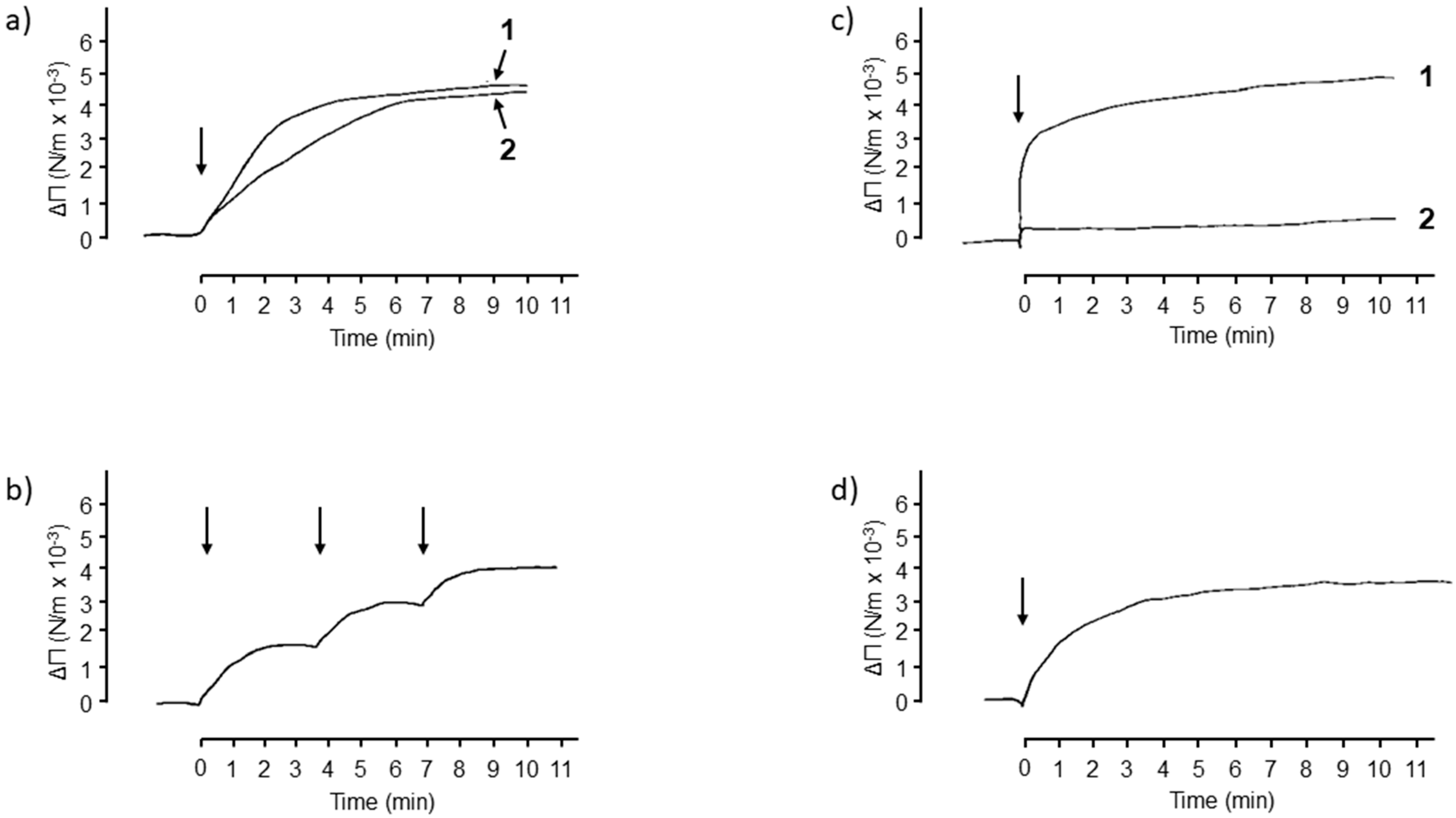
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harouse, J.M.; Bhat, S.; Spitalnik, S.L.; Laughlin, M.; Stefano, K.; Silberberg, D.H.; Gonzalez-Scarano, F. Inhibition of entry of hiv-1 in neural cell lines by antibodies against galactosyl ceramide. Science 1991, 253, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Hammache, D.; Delezay, O.; Yahi, N.; Andre-Barres, C.; Rico-Lattes, I.; Lattes, A. Synthetic soluble analogs of galactosylceramide (galcer) bind to the v3 domain of hiv-1 gp120 and inhibit hiv-1-induced fusion and entry. J. Biol. Chem. 1997, 272, 7245–7252. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.E.; Adhikary, S.; Kolokoltsov, A.A.; Davey, R.A. Ebolavirus requires acid sphingomyelinase activity and plasma membrane sphingomyelin for infection. J. Virol. 2012, 86, 7473–7483. [Google Scholar] [CrossRef]
- Audi, A.; Soudani, N.; Dbaibo, G.; Zaraket, H. Depletion of host and viral sphingomyelin impairs influenza virus infection. Front. Microbiol. 2020, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Pastenkos, G.; Miller, J.L.; Pritchard, S.M.; Nicola, A.V. Role of sphingomyelin in alphaherpesvirus entry. J. Virol. 2019, 93, 1110–1128. [Google Scholar] [CrossRef]
- Mori, Y.; Sakata, M.; Sakai, S.; Okamoto, T.; Nakatsu, Y.; Taguwa, S.; Otsuki, N.; Maeda, Y.; Hanada, K.; Matsuura, Y.; et al. Membrane sphingomyelin in host cells is essential for nucleocapsid penetration into the cytoplasm after hemifusion during rubella virus entry. mBio 2022, 13, e0169822. [Google Scholar] [CrossRef]
- Young, M.M.; Kester, M.; Wang, H.G. Sphingolipids: Regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 2013, 54, 5–19. [Google Scholar] [CrossRef]
- Perry, D.K. Serine palmitoyltransferase: Role in apoptotic de novo ceramide synthesis and other stress responses. Biochim. Biophys. Acta 2002, 1585, 146–152. [Google Scholar] [CrossRef]
- Feng, Y.; Broder, C.C.; Kennedy, P.E.; Berger, E.A. Hiv-1 entry cofactor: Functional cdna cloning of a seven-transmembrane, g protein-coupled receptor. Science 1996, 272, 872–877. [Google Scholar] [CrossRef]
- Deng, H.; Liu, R.; Ellmeier, W.; Choe, S.; Unutmaz, D.; Burkhart, M.; Di Marzio, P.; Marmon, S.; Sutton, R.E.; Hill, C.M.; et al. Identification of a major co-receptor for primary isolates of hiv-1. Nature 1996, 381, 661–666. [Google Scholar] [CrossRef]
- Baba, M.; Snoeck, R.; Pauwels, R.; de Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef]
- Sorice, M.; Parolini, I.; Sansolini, T.; Garofalo, T.; Dolo, V.; Sargiacomo, M.; Tai, T.; Peschle, C.; Torrisi, M.R.; Pavan, A. Evidence for the existence of ganglioside-enriched plasma membrane domains in human peripheral lymphocytes. J. Lipid Res. 1997, 38, 969–980. [Google Scholar] [CrossRef]
- Harouse, J.M.; Kunsch, C.; Hartle, H.T.; Laughlin, M.A.; Hoxie, J.A.; Wigdahl, B.; Gonzalez-Scarano, F. Cd4-independent infection of human neural cells by human immunodeficiency virus type 1. J. Virol. 1989, 63, 2527–2533. [Google Scholar] [CrossRef]
- Adachi, A.; Koenig, S.; Gendelman, H.E.; Daugherty, D.; Gattoni-Celli, S.; Fauci, A.S.; Martin, M.A. Productive, persistent infection of human colorectal cell lines with human immunodeficiency virus. J. Virol. 1987, 61, 209–213. [Google Scholar] [CrossRef]
- Cao, Y.Z.; Friedman-Kien, A.E.; Huang, Y.X.; Li, X.L.; Mirabile, M.; Moudgil, T.; Zucker-Franklin, D.; Ho, D.D. Cd4-independent, productive human immunodeficiency virus type 1 infection of hepatoma cell lines in vitro. J. Virol. 1990, 64, 2553–2559. [Google Scholar] [CrossRef]
- Bhat, S.; Spitalnik, S.L.; Gonzalez-Scarano, F.; Silberberg, D.H. Galactosyl ceramide or a derivative is an essential component of the neural receptor for human immunodeficiency virus type 1 envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 1991, 88, 7131–7134. [Google Scholar] [CrossRef]
- Meng, G.; Wei, X.; Wu, X.; Sellers, M.T.; Decker, J.M.; Moldoveanu, Z.; Orenstein, J.M.; Graham, M.F.; Kappes, J.C.; Mestecky, J.; et al. Primary intestinal epithelial cells selectively transfer r5 hiv-1 to ccr5+ cells. Nat. Med. 2002, 8, 150–156. [Google Scholar] [CrossRef]
- Delezay, O.; Koch, N.; Yahi, N.; Hammache, D.; Tourres, C.; Tamalet, C.; Fantini, J. Co-expression of cxcr4/fusin and galactosylceramide in the human intestinal epithelial cell line ht-29. AIDS 1997, 11, 1311–1318. [Google Scholar] [CrossRef]
- Tsai, B.; Gilbert, J.M.; Stehle, T.; Lencer, W.; Benjamin, T.L.; Rapoport, T.A. Gangliosides are receptors for murine polyoma virus and sv40. EMBO J. 2003, 22, 4346–4355. [Google Scholar] [CrossRef]
- Smith, A.E.; Lilie, H.; Helenius, A. Ganglioside-dependent cell attachment and endocytosis of murine polyomavirus-like particles. FEBS Lett. 2003, 555, 199–203. [Google Scholar] [CrossRef]
- Yu, L.; Lee, K.K.; Hodges, R.S.; Paranchych, W.; Irvin, R.T. Adherence of pseudomonas aeruginosa and candida albicans to glycosphingolipid (asialo-gm1) receptors is achieved by a conserved receptor-binding domain present on their adhesins. Infect. Immun. 1994, 62, 5213–5219. [Google Scholar] [CrossRef]
- Krivan, H.C.; Roberts, D.D.; Ginsburg, V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence galnac beta 1-4gal found in some glycolipids. Proc. Natl. Acad. Sci. USA 1988, 85, 6157–6161. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.J.; Hannah, J.H.; Leininger, E. Adhesion of bordetella pertussis to sulfatides and to the galnac beta 4gal sequence found in glycosphingolipids. J. Biol. Chem. 1991, 266, 18827–18831. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, C.A. Glycolipid receptors for verotoxin and helicobacter pylori: Role in pathology. Biochim. Biophys. Acta 1999, 1455, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Waddell, T.; Head, S.; Petric, M.; Cohen, A.; Lingwood, C. Globotriosyl ceramide is specifically recognized by the escherichia coli verocytotoxin 2. Biochem. Biophys. Res. Commun. 1988, 152, 674–679. [Google Scholar] [CrossRef]
- Pinyon, R.A.; Paton, J.C.; Paton, A.W.; Botten, J.A.; Morona, R. Refinement of a therapeutic shiga toxin-binding probiotic for human trials. J. Infect. Dis. 2004, 189, 1547–1555. [Google Scholar] [CrossRef]
- Heyningen, S.V. Cholera toxin: Interaction of subunits with ganglioside gm1. Science 1974, 183, 656–657. [Google Scholar] [CrossRef]
- Jacewicz, M.; Clausen, H.; Nudelman, E.; Donohue-Rolfe, A.; Keusch, G.T. Pathogenesis of shigella diarrhea. Xi. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and hela cells and its identification as globotriaosylceramide. J. Exp. Med. 1986, 163, 1391–1404. [Google Scholar] [CrossRef]
- Kiarash, A.; Boyd, B.; Lingwood, C.A. Glycosphingolipid receptor function is modified by fatty acid content. Verotoxin 1 and verotoxin 2c preferentially recognize different globotriaosyl ceramide fatty acid homologues. J. Biol. Chem. 1994, 269, 11138–11146. [Google Scholar] [CrossRef]
- Ali, S.; Brockman, H.L.; Brown, R.E. Structural determinants of miscibility in surface films of galactosylceramide and phosphatidylcholine: Effect of unsaturation in the galactosylceramide acyl chain. Biochemistry 1991, 30, 11198–11205. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Kwon, D.S.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Middel, J.; Cornelissen, I.L.; Nottet, H.S.; KewalRamani, V.N.; Littman, D.R.; et al. Dc-sign, a dendritic cell-specific hiv-1-binding protein that enhances trans-infection of t cells. Cell 2000, 100, 587–597. [Google Scholar] [CrossRef]
- McReynolds, K.D.; Hadd, M.J.; Gervay-Hague, J. Synthesis of biotinylated glycoconjugates and their use in a novel elisa for direct comparison of hiv-1 gp120 recognition of galcer and related carbohydrate analogues. Bioconjug Chem. 1999, 10, 1021–1031. [Google Scholar] [CrossRef]
- Long, D.; Berson, J.F.; Cook, D.G.; Doms, R.W. Characterization of human immunodeficiency virus type 1 gp120 binding to liposomes containing galactosylceramide. J. Virol. 1994, 68, 5890–5898. [Google Scholar] [CrossRef]
- Bhat, S.; Mettus, R.V.; Reddy, E.P.; Ugen, K.E.; Srikanthan, V.; Williams, W.V.; Weiner, D.B. The galactosyl ceramide/sulfatide receptor binding region of hiv-1 gp120 maps to amino acids 206–275. AIDS Res. Hum. Retroviruses 1993, 9, 175–181. [Google Scholar] [CrossRef]
- Cook, D.G.; Fantini, J.; Spitalnik, S.L.; Gonzalez-Scarano, F. Binding of human immunodeficiency virus type i (hiv-1) gp120 to galactosylceramide (galcer): Relationship to the v3 loop. Virology 1994, 201, 206–214. [Google Scholar] [CrossRef]
- Delezay, O.; Hammache, D.; Fantini, J.; Yahi, N. Spc3, a v3 loop-derived synthetic peptide inhibitor of hiv-1 infection, binds to cell surface glycosphingolipids. Biochemistry 1996, 35, 15663–15671. [Google Scholar] [CrossRef]
- Santosuosso, M.; Righi, E.; Lindstrom, V.; Leblanc, P.R.; Poznansky, M.C. Hiv-1 envelope protein gp120 is present at high concentrations in secondary lymphoid organs of individuals with chronic hiv-1 infection. J. Infect. Dis. 2009, 200, 1050–1053. [Google Scholar] [CrossRef]
- Hammache, D.; Pieroni, G.; Yahi, N.; Delezay, O.; Koch, N.; Lafont, H.; Tamalet, C.; Fantini, J. Specific interaction of hiv-1 and hiv-2 surface envelope glycoproteins with monolayers of galactosylceramide and ganglioside gm3. J. Biol. Chem. 1998, 273, 7967–7971. [Google Scholar] [CrossRef]
- Alfsen, A.; Iniguez, P.; Bouguyon, E.; Bomsel, M. Secretory iga specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of hiv-1. J. Immunol. 2001, 166, 6257–6265. [Google Scholar] [CrossRef]
- Alfsen, A.; Bomsel, M. Hiv-1 gp41 envelope residues 650-685 exposed on native virus act as a lectin to bind epithelial cell galactosyl ceramide. J. Biol. Chem. 2002, 277, 25649–25659. [Google Scholar] [CrossRef]
- Symington, F.W.; Hedges, D.L.; Hakomori, S. Glycolipid antigens of human polymorphonuclear neutrophils and the inducible hl-60 myeloid leukemia line. J. Immunol. 1985, 134, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Hug, P.; Lin, H.M.; Korte, T.; Xiao, X.; Dimitrov, D.S.; Wang, J.M.; Puri, A.; Blumenthal, R. Glycosphingolipids promote entry of a broad range of human immunodeficiency virus type 1 isolates into cell lines expressing cd4, cxcr4, and/or ccr5. J. Virol. 2000, 74, 6377–6385. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Maurer, B.J.; Reynolds, C.P.; Cabot, M.C. N-(4-hydroxyphenyl)retinamide elevates ceramide in neuroblastoma cell lines by coordinate activation of serine palmitoyltransferase and ceramide synthase. Cancer Res. 2001, 61, 5102–5105. [Google Scholar] [PubMed]
- Finnegan, C.M.; Rawat, S.S.; Puri, A.; Wang, J.M.; Ruscetti, F.W.; Blumenthal, R. Ceramide, a target for antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2004, 101, 15452–15457. [Google Scholar] [CrossRef] [PubMed]
- Voisset, C.; Lavie, M.; Helle, F.; Op De Beeck, A.; Bilheu, A.; Bertrand-Michel, J.; Terce, F.; Cocquerel, L.; Wychowski, C.; Vu-Dac, N.; et al. Ceramide enrichment of the plasma membrane induces cd81 internalization and inhibits hepatitis c virus entry. Cell Microbiol. 2008, 10, 606–617. [Google Scholar] [CrossRef]
- Tani, H.; Shiokawa, M.; Kaname, Y.; Kambara, H.; Mori, Y.; Abe, T.; Moriishi, K.; Matsuura, Y. Involvement of ceramide in the propagation of japanese encephalitis virus. J. Virol. 2010, 84, 2798–2807. [Google Scholar] [CrossRef]
- Rawat, S.S.; Zimmerman, C.; Johnson, B.T.; Cho, E.; Lockett, S.J.; Blumenthal, R.; Puri, A. Restricted lateral mobility of plasma membrane cd4 impairs hiv-1 envelope glycoprotein mediated fusion. Mol. Membr. Biol. 2008, 25, 83–94. [Google Scholar] [CrossRef]
- Barklis, E.; Alfadhli, A.; Kyle, J.E.; Bramer, L.M.; Bloodsworth, K.J.; Barklis, R.L.; Leier, H.C.; Petty, R.M.; Zelnik, I.; Metz, O.; et al. Ceramide synthase 2 deletion decreases the infectivity of HIV-1. J. Biol. Chem. 2021, 296, 100340. [Google Scholar] [CrossRef]
- Tornquist, K.; Asghar, M.Y.; Srinivasan, V.; Korhonen, L.; Lindholm, D. Sphingolipids as modulators of sars-cov-2 infection. Front. Cell Dev. Biol. 2021, 9, 689854. [Google Scholar] [CrossRef]
- Carpinteiro, A.; Edwards, M.J.; Hoffmann, M.; Kochs, G.; Gripp, B.; Weigang, S.; Adams, C.; Carpinteiro, E.; Gulbins, A.; Keitsch, S.; et al. Pharmacological inhibition of acid sphingomyelinase prevents uptake of sars-cov-2 by epithelial cells. Cell Rep. Med. 2020, 1, 100142. [Google Scholar] [CrossRef]
- Fenouillet, E.; Clerget-Raslain, B.; Gluckman, J.C.; Guetard, D.; Montagnier, L.; Bahraoui, E. Role of n-linked glycans in the interaction between the envelope glycoprotein of human immunodeficiency virus and its cd4 cellular receptor. Structural enzymatic analysis. J. Exp. Med. 1989, 169, 807–822. [Google Scholar] [CrossRef]
- Benjouad, A.; Babas, T.; Montagnier, L.; Bahraoui, E. N-linked oligosaccharides of simian immunodeficiency virus envelope glycoproteins are dispensable for the interaction with the cd4 receptor. Biochem. Biophys. Res. Commun. 1993, 190, 311–319. [Google Scholar] [CrossRef]
- Guyader, M.; Emerman, M.; Sonigo, P.; Clavel, F.; Montagnier, L.; Alizon, M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature 1987, 326, 662–669. [Google Scholar] [CrossRef]
- Benjouad, A.; Gluckman, J.C.; Rochat, H.; Montagnier, L.; Bahraoui, E. Influence of carbohydrate moieties on the immunogenicity of human immunodeficiency virus type 1 recombinant gp160. J. Virol. 1992, 66, 2473–2483. [Google Scholar] [CrossRef]
- Bahraoui, E.; Clerget-Raslain, B.; Granier, C.; Van Rietschoten, J.; Sabatier, J.M.; Labbe-Julie, C.; Ceard, B.; Rochat, H.; Gluckman, J.C.; Montagnier, L. Accessibility of the highly conserved amino- and carboxy-terminal regions from hiv-1 external envelope glycoproteins. AIDS Res. Hum. Retroviruses 1989, 5, 451–463. [Google Scholar] [CrossRef]
- Moreau, H.; Pieroni, G.; Jolivet-Reynaud, C.; Alouf, J.E.; Verger, R. A new kinetic approach for studying phospholipase c (Clostridium perfringens alpha toxin) activity on phospholipid monolayers. Biochemistry 1988, 27, 2319–2323. [Google Scholar] [CrossRef]
- Verger, R.; De Haas, G.H. Enzyme reactions in a membrane model. 1. A new technique to study enzyme reactions in monolayers. Chem. Phys. Lipids 1973, 10, 127–136. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Planes, R.; Bahraoui, E. HIV and SIV Envelope Glycoproteins Interact with Glycolipids and Lipids. Int. J. Mol. Sci. 2023, 24, 11730. https://doi.org/10.3390/ijms241411730
Planes R, Bahraoui E. HIV and SIV Envelope Glycoproteins Interact with Glycolipids and Lipids. International Journal of Molecular Sciences. 2023; 24(14):11730. https://doi.org/10.3390/ijms241411730
Chicago/Turabian StylePlanes, Rémi, and Elmostafa Bahraoui. 2023. "HIV and SIV Envelope Glycoproteins Interact with Glycolipids and Lipids" International Journal of Molecular Sciences 24, no. 14: 11730. https://doi.org/10.3390/ijms241411730
APA StylePlanes, R., & Bahraoui, E. (2023). HIV and SIV Envelope Glycoproteins Interact with Glycolipids and Lipids. International Journal of Molecular Sciences, 24(14), 11730. https://doi.org/10.3390/ijms241411730




