BASIDIN as a New Protein Effector of the Phytopathogen Causing Witche’s Broom Disease in Cocoa
Abstract
1. Introduction
2. Results
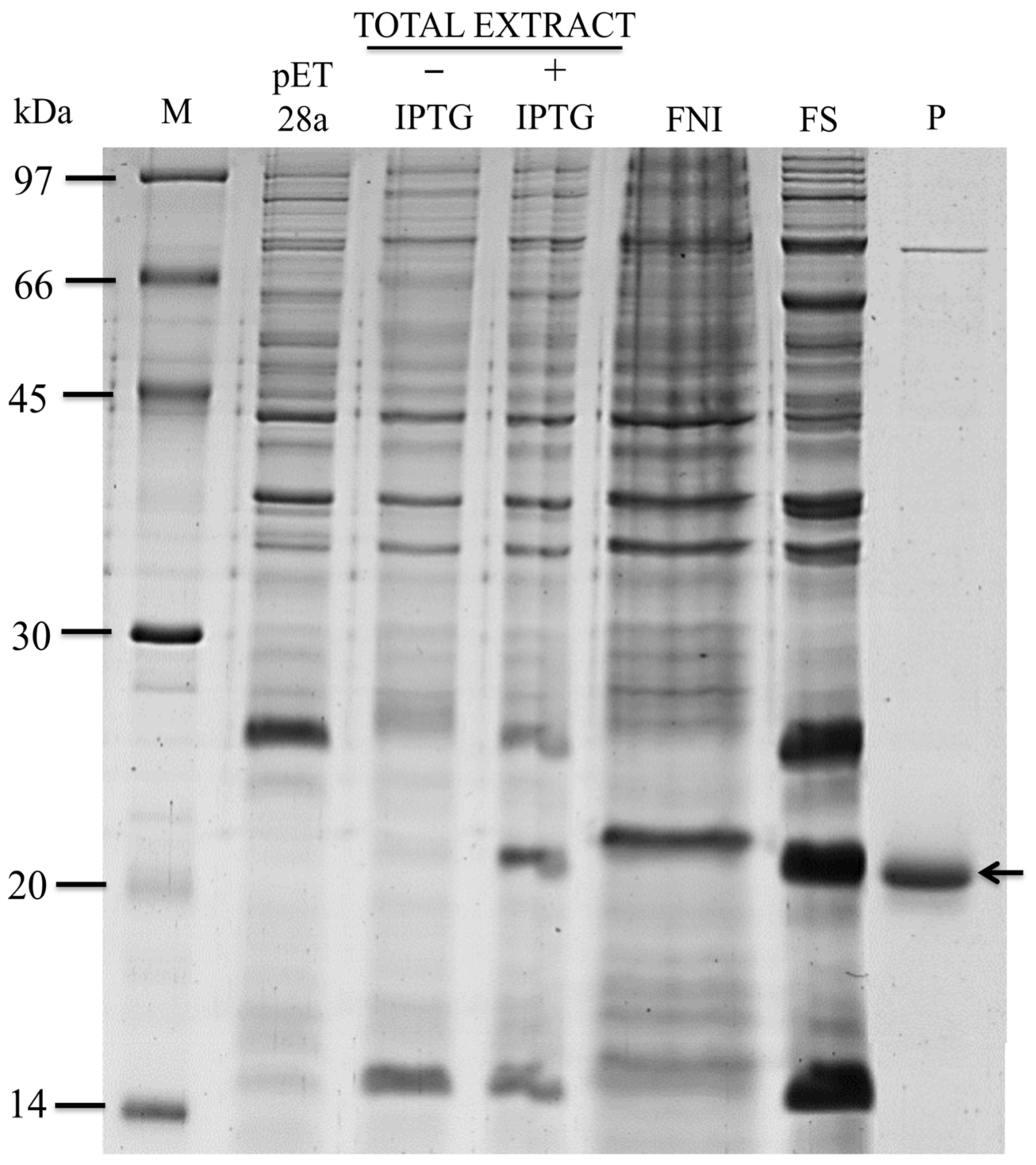
2.1. In Silico Selection, Expression, and Purification of the Recombinant Effector Candidate
2.2. Bioassay with rBASIDIN on Tomato Leaves
2.3. H2O2 Production and Electrolyte Leakage of Tomato Leaves Sprayed with rBASIDIN
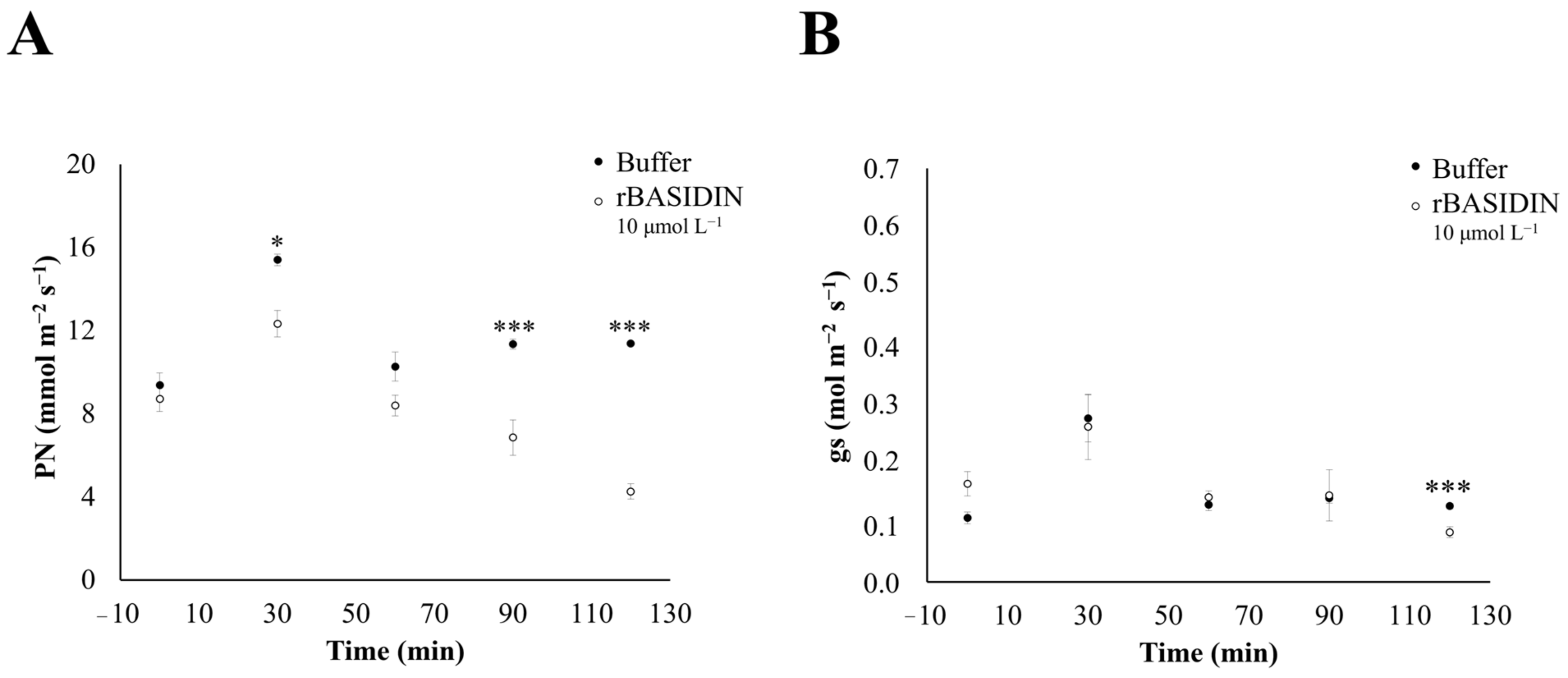
2.4. The Influence of rBASIDIN on Gas Exchange
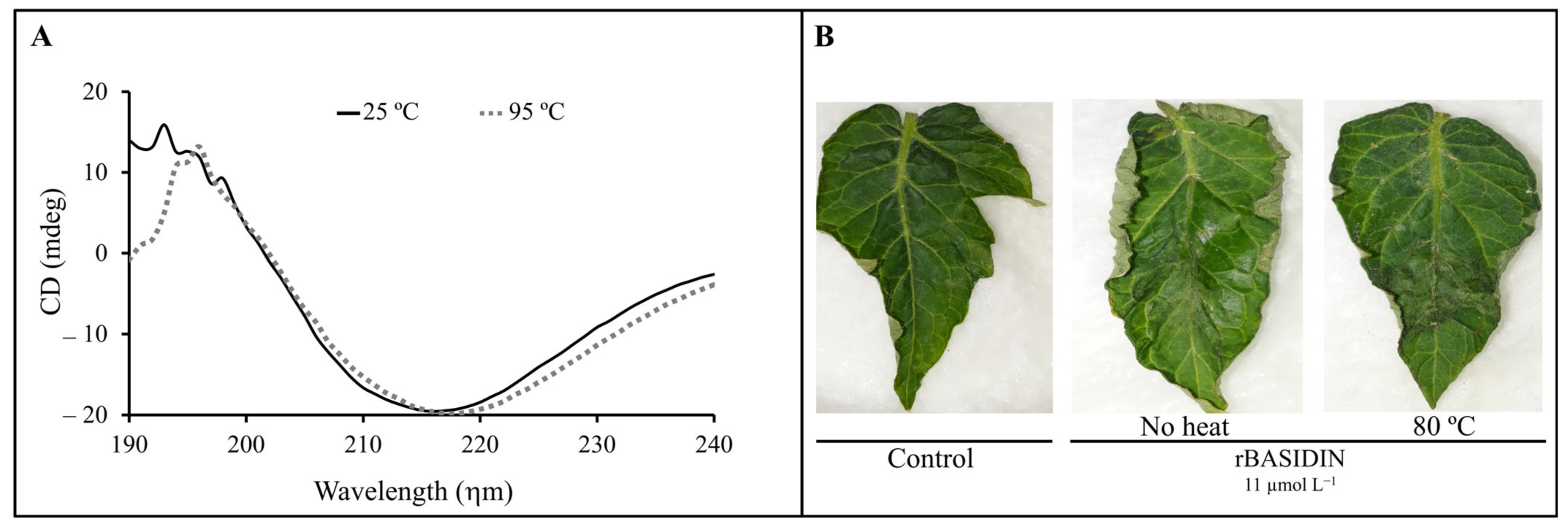
2.5. Circular Dichroism (CD) Analysis
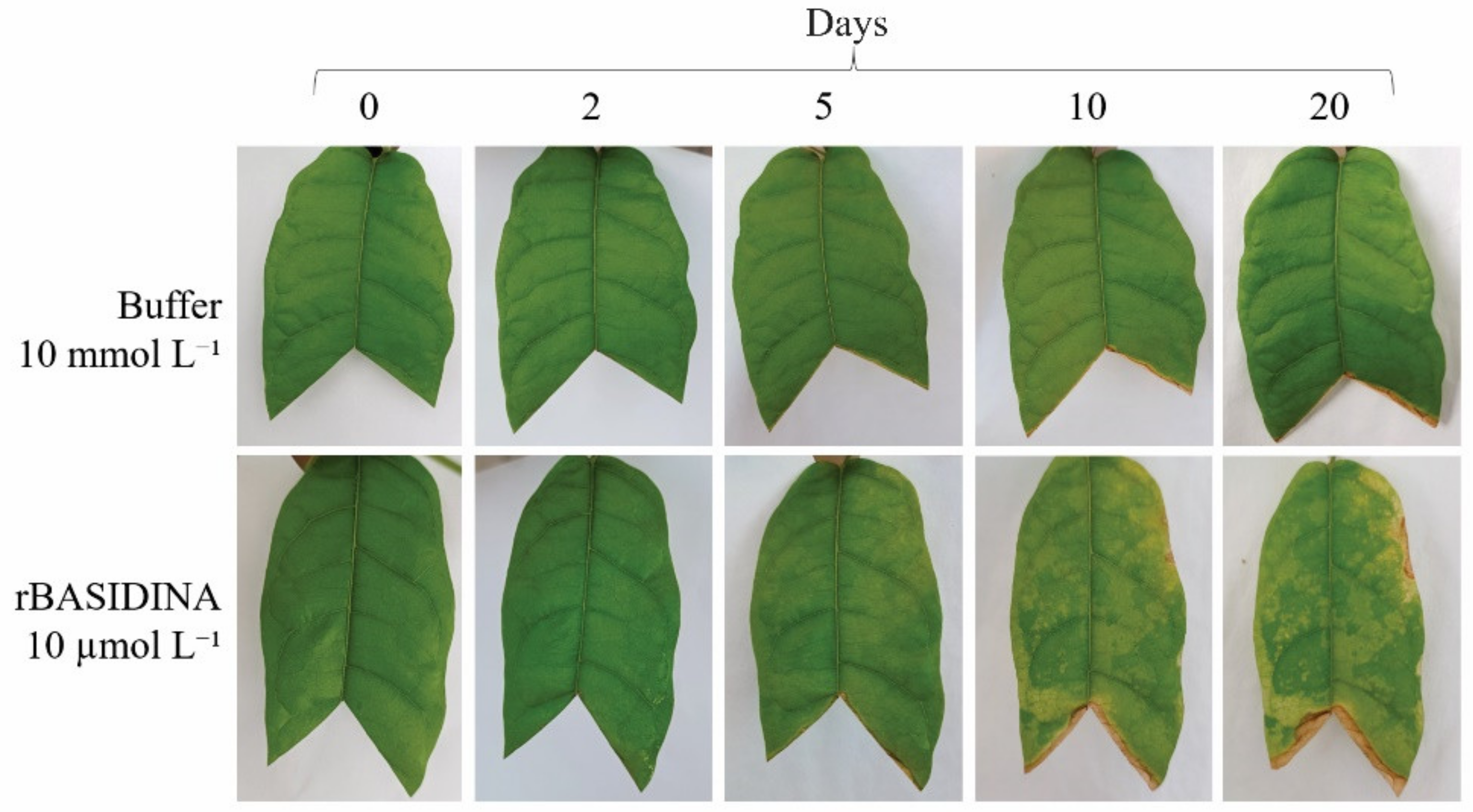
2.6. Sensitivity Test of T. cacao to Recombinant Protein rBASIDIN
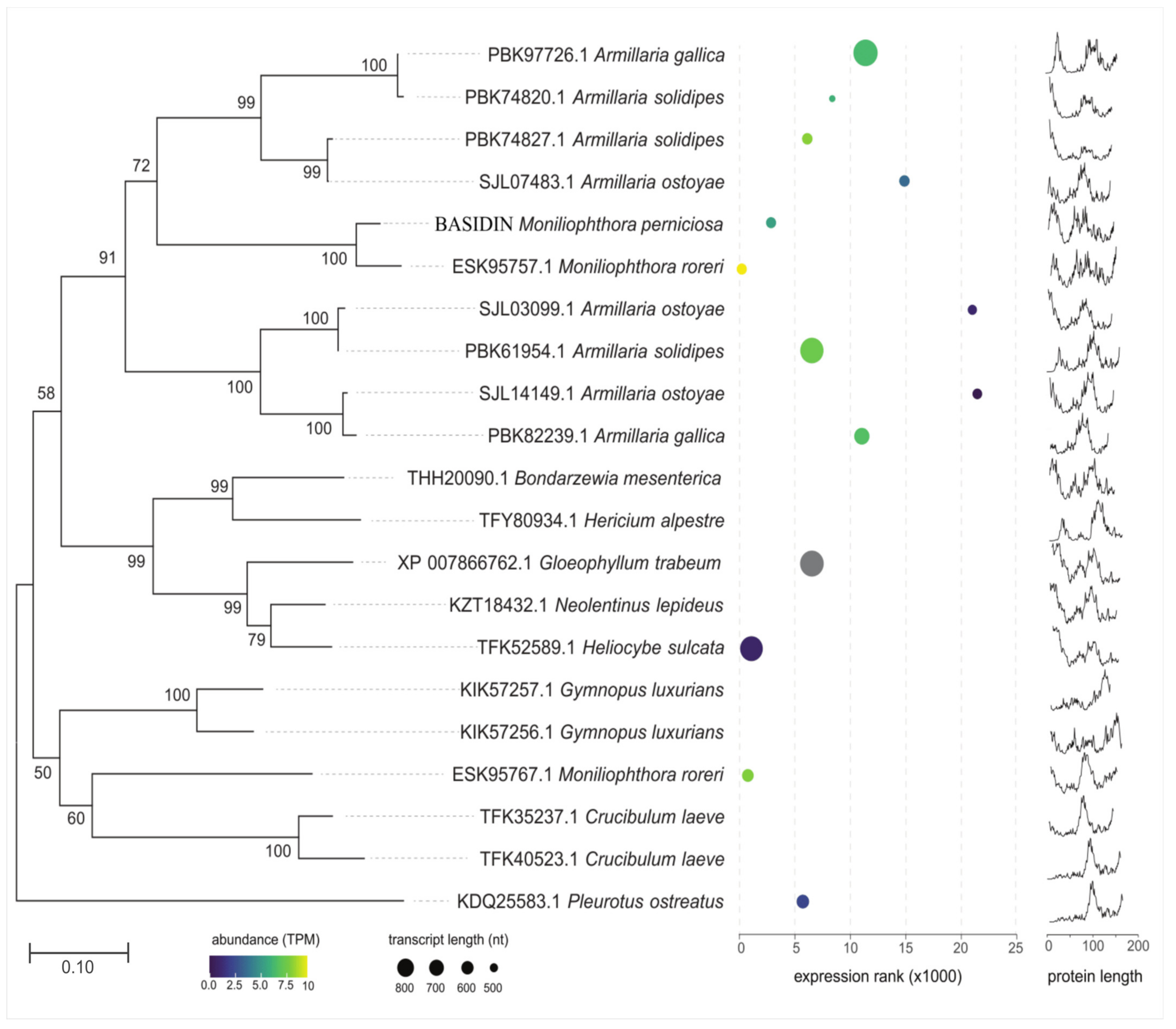
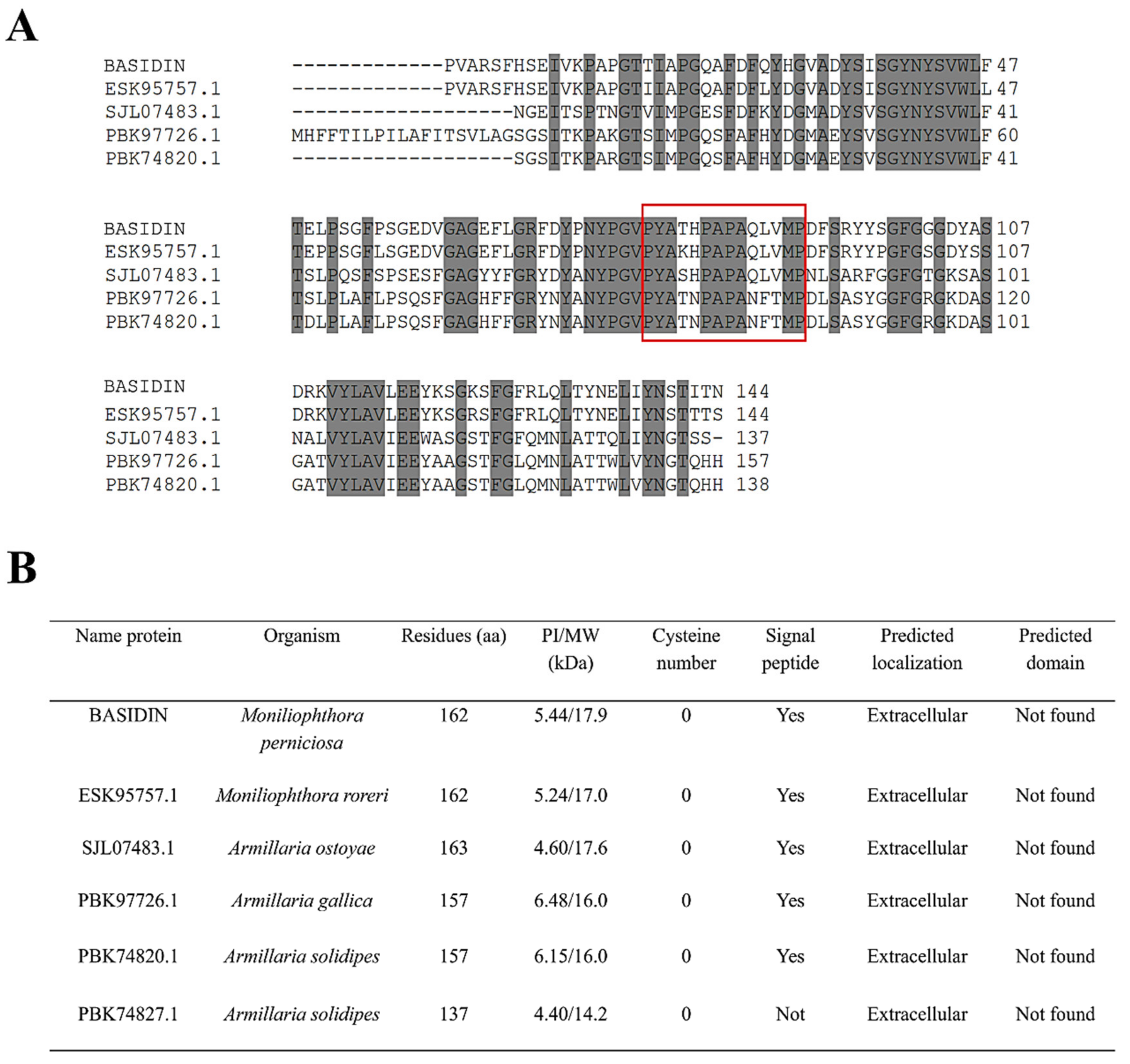
2.7. Evolutionary Origin and Expression of the Effector Candidate BASIDIN
3. Discussion
3.1. Biochemical Characterization and Selection of BASIDIN
3.2. BASIDIN Induces Wilting, Curling, and Necrosis Symptoms in Tomato Leaves
3.3. BASIDIN Causes Damage to Leaf Membranes and Induces Oxidative Stress in Tomato Plants
3.4. BASIDIN Causes Photosynthetic Inhibition of the Plants
3.5. BASIDIN Is Thermostable and Rich in β-Sheets
3.6. rBASIDINA Induced Symptoms in Cacao
3.7. BASIDIN Is a New Basidiomycete-Specific Effector Protein
4. Materials and Methods
4.1. Selection of BASIDIN
4.2. Expression of BASIDIN in Escherichia coli
4.3. Bioassay with Tomato Leaves (Micro-Tom)
4.4. Application of rBASIDIN on Theobroma cacao Leaves
4.5. H2O2Accumulation Test
4.6. Electrolyte Extravasation
4.7. Gas Exchange
4.8. Circular Dichroism (CD) Analysis
4.9. Phylogenetic Analyses
4.10. Analysis of the Transcripts
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rincones, J.; Mazotti, G.D.; Griffith, G.W.; Pomela, A.; Figueira, A.; Leal, G.A.; Queiroz, M.V.; Pereira, J.F.; Azevedo, R.A.; Pereira, G.A.G.; et al. Genetic Variability and Chromosome-Length Polymorphisms of the Witches’ Broom Pathogen Crinipellis perniciosa from Various Plant Hosts in South America. Mycol. Res. 2006, 110, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Darwiche, R.; El Atab, O.; Baroni, R.M.; Teixeira, P.J.P.L.; Mondego, J.M.C.; Pereira, G.A.G.; Schneiter, R. Plant Pathogenesis-Related Proteins of the Cacao Fungal Pathogen Moniliophthora perniciosa Differ in Their Lipid-Binding Specificities. J. Biol. Chem. 2017, 292, 20558–20569. [Google Scholar] [CrossRef] [PubMed]
- Marelli, J.-P.; Maximova, S.; Gramacho, K.; Kang, S.; Guiltinan, M. Infection Biology of Moniliophthora perniciosa on Theobroma cacao and Alternate Solanaceous Hosts. Trop. Plant Biol. 2009, 2, 149–160. [Google Scholar] [CrossRef]
- Meinhardt, L.W.; Rincones, J.; Bailey, B.A.; Aime, M.C.; Griffith, G.W.; Zhang, D.; Pereira, G.A. Moniliophthora perniciosa, the Causal Agent of Witches’ Broom Disease of Cacao: What’s New from This Old Foe? Mol. Plant Pathol. 2008, 9, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, M.A.G.; Nazário, C.C. Importância Econômica e Evolução da Cultura do Cacau no Brasil e na Região dos Tabuleiros Costeiros da Bahia entre 1990 e 2002. 28. Aracajú. 2004; ISSN 1678-1953. [Google Scholar]
- Teixeira, P.J.P.L.; Thomazella, D.P.D.T.; Reis, O.; do Prado, P.F.V.; do Rio, M.C.S.; Fiorin, G.L.; José, J.; Costa, G.G.L.; Negri, V.A.; Mondego, J.M.C.; et al. High-Resolution Transcript Profiling of the Atypical Biotrophic Interaction between Theobroma cacao and the Fungal Pathogen Moniliophthora perniciosa. Plant Cell. 2014, 26, 4245–4269. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.S.; da Fonseca, R.R.; Batista, T.M.; Barreto, M.A.; Argolo, C.S.; de Carvalho, M.R.; do Amaral, D.O.J.; Silva, E.M.; de, A.; Arévalo-Gardini, E.; et al. Genome Sequence and Effectorome of Moniliophthora perniciosa and Moniliophthora roreri Subpopulations. BMC Genom. 2018, 19, 509. [Google Scholar] [CrossRef] [PubMed]
- Selin, C.; de Kievit, T.R.; Belmonte, M.F.; Fernando, W.G.D. Elucidating the Role of Effectors in Plant-Fungal Interactions: Progress and Challenges. Front. Microbiol. 2016, 7, 600. [Google Scholar] [CrossRef]
- Maximo, H.J.; Dalio, R.J.D.; Dias, R.O.; Litholdo, C.G.; Felizatti, H.L.; Machado, M.A. PpCRN7 and PpCRN20 of Phythophthora parasitica Regulate Plant Cell Death Leading to Enhancement of Host Susceptibility. BMC Plant Biol. 2019, 19, 544. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Song, N.; Zhao, M.; Liu, R.; Feng, H.; Wang, X.; Kang, Z. Puccinia striiformis f. Sp. tritici MicroRNA-like RNA 1 (Pst-MilR1), an Important Pathogenicity Factor of Pst, Impairs Wheat Resistance to Pst by Suppressing the Wheat Pathogenesis-Related 2 Gene . New Phytol. 2017, 215, 338–350. [Google Scholar] [CrossRef]
- Fiorin, G.L.; Sanchéz-Vallet, A.; de Toledo Thomazella, D.P.; do Prado, P.F.V.; do Nascimento, L.C.; de Oliveira Figueira, A.V.; Thomma, B.P.; Pereira, G.A.G.; Teixeira, P.J.P.L. Suppression of Plant Immunity by Fungal Chitinase-like Effectors. Curr. Biol. 2018, 28, 3023–3030.e5. [Google Scholar] [CrossRef]
- Dong, S.; Stam, R.; Cano, L.M.; Song, J.; Sklenar, J.; Yoshida, K.; Bozkurt, T.O.; Oliva, R.; Liu, Z.; Tian, M.; et al. Effector Specialization in a Lineage of the Irish Potato Famine Pathogen. Science 2014, 343, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Varden, F.A.; De la Concepcion, J.C.; Maidment, J.H.; Banfield, M.J. Taking the Stage: Effectors in the Spotlight. Curr. Opin. Plant Biol. 2017, 38, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Dalio, R.J.D.; Herlihy, J.; Oliveira, T.S.; McDowell, J.M.; Machado, M. Effector Biology in Focus: A Primer for Computational Prediction and Functional Characterization. MPMI 2017, 31, 22–33. [Google Scholar] [CrossRef]
- Mondego, J.M.; Carazzolle, M.F.; Costa, G.G.; Formighieri, E.F.; Parizzi, L.P.; Rincones, J.; Cotomacci, C.; Carraro, D.M.; Cunha, A.F.; Carrer, H.; et al. A Genome Survey of Moniliophthora perniciosa Gives New Insights into Witches’ Broom Disease of Cacao. BMC Genom. 2008, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Sędzielewska Toro, K.; Brachmann, A. The Effector Candidate Repertoire of the Arbuscular mycorrhizal Fungus Rhizophagus clarus. BMC Genom. 2016, 17, 101. [Google Scholar] [CrossRef]
- Tompa, P.; Fersht, A. Structure and Function of Intrinsically Disordered Proteins; Chapman and Hall/CRC: New York, NY, USA, 2009; ISBN 978-0-429-19310-1. [Google Scholar]
- Sambi, I.; Gatti-Lafranconi, P.; Longhi, S.; Lotti, M. How Disorder Influences Order and Vice Versa—Mutual Effects in Fusion Proteins Containing an Intrinsically Disordered and a Globular Protein. FEBS J. 2010, 277, 4438–4451. [Google Scholar] [CrossRef]
- Garcia, O.; Macedo, J.A.N.; Tibúrcio, R.; Zaparoli, G.; Rincones, J.; Bittencourt, L.M.C.; Ceita, G.O.; Micheli, F.; Gesteira, A.; Mariano, A.C.; et al. Characterization of Necrosis and Ethylene-Inducing Proteins (NEP) in the Basidiomycete Moniliophthora perniciosa, the Causal Agent of Witches’ Broom in Theobroma cacao. Mycol. Res. 2007, 111, 443–455. [Google Scholar] [CrossRef]
- Villela-Dias, C.; Camillo, L.R.; de Oliveira, G.A.; Sena, J.A.; Santiago, A.S.; de Sousa, S.T.; Mendes, J.S.; Pirovani, C.P.; Alvim, F.C.; Costa, M.G. Nep1-like Protein from Moniliophthora perniciosa Induces a Rapid Proteome and Metabolome Reprogramming in Cells of Nicotiana benthamiana. Physiol. Plant. 2013, 150, 1–17. [Google Scholar] [CrossRef]
- Bailey, B.A. Purification of a Protein from Culture Filtrates of Fusarium oxysporum That Induces Ethylene and Necrosis in Leaves of Erythroxylum coca. Phytopathology 1995, 85, 1250–1255. [Google Scholar] [CrossRef]
- Ellis, M.L.; Lanubile, A.; Garcia, C.; Munkvold, G.P. Association of Putative Fungal Effectors in Fusarium oxysporum with Wilt Symptoms in Soybean. Phytopathology 2016, 106, 762–773. [Google Scholar] [CrossRef]
- Wang, R.; Ning, Y.; Shi, X.; He, F.; Zhang, C.; Fan, J.; Jiang, N.; Zhang, Y.; Zhang, T.; Hu, Y.; et al. Immunity to Rice Blast Disease by Suppression of Effector-Triggered Necrosis. Curr. Biol. 2016, 26, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.T.; Yao, N. The Role and Regulation of Programmed Cell Death in Plant–Pathogen Interactions. Cell. Microbiol. 2004, 6, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Malinovsky, F.G.; Brodersen, P.; Fiil, B.K.; McKinney, L.V.; Thorgrimsen, S.; Beck, M.; Nielsen, H.B.; Pietra, S.; Zipfel, C.; Robatzek, S.; et al. Lazarus1, a DUF300 Protein, Contributes to Programmed Cell Death Associated with Arabidopsis Acd11 and the Hypersensitive Response. PLoS ONE 2010, 5, e12586. [Google Scholar] [CrossRef]
- Ma, A.; Zhang, D.; Wang, G.; Wang, K.; Li, Z.; Gao, Y.; Li, H.; Bian, C.; Cheng, J.; Han, Y.; et al. Verticillium dahliae Effector VDAL Protects MYB6 from Degradation by Interacting with PUB25 and PUB26 E3 Ligases to Enhance Verticillium Wilt Resistance. Plant Cell. 2021, 33, 3675–3699. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.N.; Ferreira-Silva, S.L.; de Vasconcelos Fontenele, A.; Ribeiro, R.V.; Viégas, R.A.; Silveira, J.A.G. Photosynthetic Changes and Protective Mechanisms against Oxidative Damage Subjected to Isolated and Combined Drought and Heat Stresses in Jatropha Curcas Plants. J. Plant Physiol. 2010, 167, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.-J.; Zhang, B.; Shi, W.-W.; Li, H.-Y. Hydrogen Peroxide in Plants: A Versatile Molecule of the Reactive Oxygen Species Network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Van Breusegem, F.; Vranová, E.; Dat, J.F.; Inzé, D. The Role of Active Oxygen Species in Plant Signal Transduction. Plant Sci. 2001, 161, 405–414. [Google Scholar] [CrossRef]
- Pungartnik, C.; Melo, S.C.O.; Basso, T.S.; Macena, W.G.; Cascardo, J.C.M.; Brendel, M. Reactive Oxygen Species and Autophagy Play a Role in Survival and Differentiation of the Phytopathogen Moniliophthora perniciosa. Fungal Genet. Biol. 2009, 46, 461–472. [Google Scholar] [CrossRef]
- Chaparro-Garcia, A.; Schwizer, S.; Sklenar, J.; Yoshida, K.; Petre, B.; Bos, J.I.B.; Schornack, S.; Jones, A.M.E.; Bozkurt, T.O.; Kamoun, S. Phytophthora infestans RXLR-WY Effector AVR3a Associates with Dynamin-Related Protein 2 Required for Endocytosis of the Plant Pattern Recognition Receptor FLS2. PLoS ONE 2015, 10, e0137071. [Google Scholar] [CrossRef]
- Fang, A.; Gao, H.; Zhang, N.; Zheng, X.; Qiu, S.; Li, Y.; Zhou, S.; Cui, F.; Sun, W. A Novel Effector Gene SCRE2 Contributes to Full Virulence of Ustilaginoidea virens to Rice. Front. Microbiol. 2019, 10, 845. [Google Scholar] [CrossRef]
- Bot, P.; Mun, B.-G.; Imran, Q.M.; Hussain, A.; Lee, S.-U.; Loake, G.; Yun, B.-W. Differential Expression of AtWAKL10 in Response to Nitric Oxide Suggests a Putative Role in Biotic and Abiotic Stress Responses. PeerJ. 2019, 7, e7383. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Sapir, T.; Rouillard, P.; Ferrand, M.; Jiménez-Gómez, J.M. Interaction between Photoperiod and Variation in Circadian rhythms in Tomato. BMC Plant Biol. 2022, 22, 187. [Google Scholar] [CrossRef]
- Yarkhunova, Y.; Guadagno, C.R.; Rubin, M.J.; Davis, S.J.; Ewers, B.E.; Weinig, C. Circadian Rhythms Are Associated with Variation in Photosystem II Function and Photoprotective Mechanisms. PlantCell Environ. 2018, 41, 2518–2529. [Google Scholar] [CrossRef]
- Wen, Z.; Raffaello, T.; Zeng, Z.; Pavicic, M.; Asiegbu, F.O. Chlorophyll Fluorescence Imaging for Monitoring Effects of Heterobasidion parviporum Small Secreted Protein Induced Cell Death and in Planta Defense Gene Expression. Fungal Genet. Biol. 2019, 126, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Tang, C.; Wang, X.; Sun, S.; Zhao, J.; Kang, Z.; Wang, X. An Effector Protein of the Wheat Stripe Rust Fungus Targets Chloroplasts and Suppresses Chloroplast Function. Nat. Commun. 2019, 10, 5571. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yang, L.; Zhu, Q.; Wu, H.; He, Y.; Liu, Y.; Xu, J.; Jiang, D.; Zhang, S. Active photosynthetic inhibition mediated by MPK3/MPK6 is critical to effector-triggered immunity. PLoS Biol. 2018, 16, e2004122. [Google Scholar] [CrossRef]
- Ve, T.; Williams, S.J.; Catanzariti, A.-M.; Rafiqi, M.; Rahman, M.; Ellis, J.G.; Hardham, A.R.; Jones, D.A.; Anderson, P.A.; Dodds, P.N.; et al. Structures of the Flax-Rust Effector AvrM Reveal Insights into the Molecular Basis of Plant-Cell Entry and Effector-Triggered Immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 17594–17599. [Google Scholar] [CrossRef]
- Verli, H. Bioinformática: Da biologia à Flexibilidade Molecular; Sociedade Brasileira de Bioquímica e Biologia Molecular: São Paulo, Brazil, 2014; ISBN 978-85-69288-00-8. [Google Scholar]
- Franceschetti, M.; Maqbool, A.; Jiménez-Dalmaroni, M.J.; Pennington, H.G.; Kamoun, S.; Banfield, M.J. Effectors of Filamentous Plant Pathogens: Commonalities amid Diversity. Microbiol. Mol. Biol. Rev. 2017, 81, e00066-16. [Google Scholar] [CrossRef]
- He, J.; Ye, W.; Choi, D.S.; Wu, B.; Zhai, Y.; Guo, B.; Duan, S.; Wang, Y.; Gan, J.; Ma, W.; et al. Structural Analysis of Phytophthora Suppressor of RNA Silencing 2 (PSR2) Reveals a Conserved Modular Fold Contributing to Virulence. Proc. Natl. Acad. Sci. USA. 2019, 116, 8054–8059. [Google Scholar] [CrossRef]
- Boutemy, L.S.; King, S.R.F.; Win, J.; Hughes, R.K.; Clarke, T.A.; Blumenschein, T.M.A.; Kamoun, S.; Banfield, M.J. Structures of Phytophthora RXLR Effector Proteins: A Conserved but Adaptable Fold Underpins Functional Diversity. J. Biol. Chem. 2011, 286, 35834–35842. [Google Scholar] [CrossRef]
- Szilágyi, A.; Závodszky, P. Structural Differences between Mesophilic, Moderately Thermophilic and Extremely Thermophilic Protein Subunits: Results of a Comprehensive Survey. Structure 2000, 8, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Charoenkwan, P.; Chotpatiwetchkul, W.; Lee, V.S.; Nantasenamat, C.; Shoombuatong, W. A Novel Sequence-Based Predictor for Identifying and Characterizing Thermophilic Proteins Using Estimated Propensity Scores of Dipeptides. Sci. Rep. 2021, 11, 23782. [Google Scholar] [CrossRef] [PubMed]
- Basso, L.G.M.; Vicente, E.F.; Crusca, E., Jr.; Cilli, E.M.; Costa-Filho, A.J. SARS-CoV Fusion Peptides Induce Membrane Surface Ordering and Curvature. Sci. Rep. 2016, 6, 37131. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.J.P.L.; Thomazella, D.P.T.; Vidal, R.O.; do Prado, P.F.V.; Reis, O.; Baroni, R.M.; Franco, S.F.; Mieczkowski, P.; Pereira, G.A.G.; Mondego, J.M.C. The Fungal Pathogen Moniliophthora perniciosa Has Genes Similar to Plant PR-1 That Are Highly Expressed during Its Interaction with Cacao. PLoS ONE 2012, 7, e45929. [Google Scholar] [CrossRef]
- Zaparoli, G.; Barsottini, M.R.D.O.; de Oliveira, J.F.; Dyszy, F.; Teixeira, P.J.P.L.; Barau, J.G.; Garcia, O.; Costa-Filho, A.J.; Ambrosio, A.L.B.; Pereira, G.A.G.; et al. The Crystal Structure of Necrosis- and Ethylene-Inducing Protein 2 from the Causal Agent of Cacao’s Witches’ Broom Disease Reveals Key Elements for Its Activity. Biochemistry 2011, 50, 9901–9910. [Google Scholar] [CrossRef]
- Bailey, B.A.; Bae, H.; Strem, M.D.; Antúnez de Mayolo, G.; Guiltinan, M.J.; Verica, J.A.; Maximova, S.N.; Bowers, J.H. Developmental Expression of Stress Response Genes in Theobroma cacao Leaves and Their Response to Nep1 Treatment and a Compatible Infection by Phytophthora megakarya. Plant Physiol. Biochem. 2005, 43, 611–622. [Google Scholar] [CrossRef]
- Nakayama, L.H.I.; Soares, M.K.M.; Appezzato-Da-Glória, B. Contribuição ao estudo anatômico da folha e do caule do cacaueiro (Theobroma cacao L.). Sci. Agric. 1996, 53, 73. [Google Scholar] [CrossRef]
- Elias, S.R.M.; Assis, R.M.; Stacciarini-Seraphin, E.; Rezende, M.H. Anatomia foliar emplantasjovens de Solanum lycocarpum A.St.-Hil. (Solanaceae). Braz. J. Bot. 2003, 26, 169–174. [Google Scholar] [CrossRef]
- Drakulic, J.; Gorton, C.; Perez-Sierra, A.; Clover, G.; Beal, L. Associations between Armillaria Species and Host Plants in U.K. Gardens. Plant Dis. 2017, 101, 1903–1909. [Google Scholar] [CrossRef]
- Bailey, B.A.; Evans, H.C.; Phillips-Mora, W.; Ali, S.S.; Meinhardt, L.W. Moniliophthora roreri, Causal Agent of Cacao Frosty Pod Rot. Mol. Plant Pathol. 2018, 19, 1580–1594. [Google Scholar] [CrossRef]
- Marelli, J.-P.; Guest, D.I.; Bailey, B.A.; Evans, H.C.; Brown, J.K.; Junaid, M.; Barreto, R.W.; Lisboa, D.O.; Puig, A.S. Chocolate Under Threat from Old and New Cacao Diseases. Phytopathology 2019, 109, 1331–1343. [Google Scholar] [CrossRef]
- Nakazawa, T.; Izuno, A.; Horii, M.; Kodera, R.; Nishimura, H.; Hirayama, Y.; Tsunematsu, Y.; Miyazaki, Y.; Awano, T.; Muraguchi, H.; et al. Effects of Pex1 Disruption on Wood Lignin Biodegradation, Fruiting Development and the Utilization of Carbon Sources in the White-Rot Agaricomycete Pleurotus ostreatus and Non-Wood Decaying Coprinopsis cinerea. Fungal Genet. Biol. 2017, 109, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, J.-J.; Wang, M.; Chen, Y.-Y.; Cui, B.-K. Phylogeny and Biogeography of the Remarkable Genus Bondarzewia (Basidiomycota, Russulales). Sci. Rep. 2016, 6, 34568. [Google Scholar] [CrossRef]
- Rupcic, Z.; Rascher, M.; Kanaki, S.; Köster, R.W.; Stadler, M.; Wittstein, K. Two New Cyathane Diterpenoids from Mycelial Cultures of the Medicinal Mushroom Hericium erinaceus and the Rare Species, Hericium flagellum. Int. J. Mol. Sci. 2018, 19, 740. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; Bianco, C.; Cennamo, G.; Giardina, P.; Marino, G.; Monti, M.; Sannia, G. Purification, Characterization, and Functional Role of a Novel Extracellular Protease from Pleurotus ostreatus. Appl. Environ. Microbiol. 2001, 67, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Atashi, A.; Soleimani, M.; Mashhadikhan, M.; Barahimi, A.; Maghari, A. Anti-Invasive and Antiproliferative Effects of Pleurotus ostreatus Extract on Acute Leukemia Cell Lines. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 95–102. [Google Scholar] [CrossRef]
- Olatinwo, R.; So, C.-L.; Eberhardt, T.L. Effect of Acaromyces ingoldii Secondary Metabolites on the Growth of Brown-Rot (Gloeophyllumtrabeum) and White-Rot (Trametes versicolor) Fungi. Mycobiology 2019, 47, 506–511. [Google Scholar] [CrossRef]
- Arantes, V.; Goodell, B. Current Understanding of Brown-Rot Fungal Biodegradation Mechanisms: A Review. In Deterioration and Protection of Sustainable Biomaterials; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; Volume 1158, pp. 3–21. ISBN 978-0-8412-3004-0. [Google Scholar]
- Zhao, Z.; Liu, H.; Wang, C.; Xu, J.-R. Erratum to: Comparative Analysis of Fungal Genomes Reveals Different Plant Cell Wall Degrading Capacity in Fungi. BMC Genom. 2014, 15, 6. [Google Scholar] [CrossRef]
- Sipos, G.; Prasanna, A.N.; Walter, M.C.; O’Connor, E.; Bálint, B.; Krizsán, K.; Kiss, B.; Hess, J.; Varga, T.; Slot, J.; et al. Genome Expansion and Lineage-Specific Genetic Innovations in the Forest Pathogenic Fungi Armillaria. Nat. Ecol. Evol. 2017, 1, 1931–1941. [Google Scholar] [CrossRef]
- dos Santos, E.C.; Pirovani, C.P.; Correa, S.C.; Micheli, F.; Gramacho, K.P. The Pathogen Moniliophthora perniciosa Promotes Differential Proteomic Modulation of Cacao Genotypes with Contrasting Resistance to Witches’ Broom Disease. BMC Plant Biol. 2020, 20, 1. [Google Scholar] [CrossRef]
- Aguín-Casal, O.; Sáinz-Osés, M.J.; Pedro Mansilla-Vázquez, J. Armillaria Species Infesting Vineyards in Northwestern Spain. Eur. J. Plant Pathol. 2004, 110, 683–687. [Google Scholar] [CrossRef]
- Leonelli, L.; Pelton, J.; Schoeffler, A.; Dahlbeck, D.; Berger, J.; Wemmer, D.E.; Staskawicz, B. Structural Elucidation and Functional Characterization of the Hyaloperonospora arabidopsidis Effector Protein ATR13. PLoS Pathog. 2011, 7, e1002428. [Google Scholar] [CrossRef] [PubMed]
- Kuppireddy, V.S.; Uversky, V.N.; Toh, S.S.; Tsai, M.-C.; Beckerson, W.C.; Cahill, C.; Carman, B.; Perlin, M.H. Identification and Initial Characterization of the Effectors of an Anther Smut Fungus and Potential Host Target Proteins. Int. J. Mol. Sci. 2017, 18, 2489. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-N.; Liu, H.; Duan, G.-H.; Huang, Y.-M.; Liu, S.; Fang, Z.-G.; Wu, E.-J.; Shang, L.; Zhan, J. The Phytophthora infestans AVR2 Effector Escapes R2 Recognition Through Effector Disordering. Mol. Plant Microbe Interact. 2020, 33, 921–931. [Google Scholar] [CrossRef]
- Catanzariti, A.-M.; Dodds, P.N.; Lawrence, G.J.; Ayliffe, M.A.; Ellis, J.G. Haustorially Expressed Secreted Proteins from Flax Rust Are Highly Enriched for Avirulence Elicitors. Plant Cell. 2006, 18, 243–256. [Google Scholar] [CrossRef]
- Bajji, M.; Lutts, S.; Kinet, J.-M. Water Deficit Effects on Solute Contribution to Osmotic Adjustment as a Function of Leaf Ageing in Three Durum Wheat (Triticum durum Desf.) Cultivars Performing Differently in Arid Conditions. Plant Sci. 2001, 160, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, Scalable Generation of High-Quality Protein Multiple Sequence Alignments Using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER Web Server: Interactive Sequence Similarity Searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Capra, J.A.; Singh, M. Predicting Functionally Important Residues from Sequence Conservation. Bioinformatics 2007, 23, 1875–1882. [Google Scholar] [CrossRef]
- Dunker, A.K.; Lawson, J.D.; Brown, C.J.; Williams, R.M.; Romero, P.; Oh, J.S.; Oldfield, C.J.; Campen, A.M.; Ratliff, C.M.; Hipps, K.W.; et al. Intrinsically Disordered Protein. J. Mol. Graph. Model. 2001, 19, 26–59. [Google Scholar] [CrossRef] [PubMed]
- Dosztányi, Z.; Csizmok, V.; Tompa, P.; Simon, I. IUPred: Web Server for the Prediction of Intrinsically Unstructured Regions of Proteins Based on Estimated Energy Content. Bioinformatics 2005, 21, 3433–3434. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The Rapid Generation of Mutation Data Matrices from Protein Sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon Provides Fast and Bias-Aware Quantification of Transcript Expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farias, K.S.; Ferreira, M.M.; Amaral, G.V.; Zugaib, M.; Santos, A.S.; Gomes, F.P.; Rezende, R.P.; Gramacho, K.P.; Aguiar, E.R.G.R.; Pirovani, C.P. BASIDIN as a New Protein Effector of the Phytopathogen Causing Witche’s Broom Disease in Cocoa. Int. J. Mol. Sci. 2023, 24, 11714. https://doi.org/10.3390/ijms241411714
Farias KS, Ferreira MM, Amaral GV, Zugaib M, Santos AS, Gomes FP, Rezende RP, Gramacho KP, Aguiar ERGR, Pirovani CP. BASIDIN as a New Protein Effector of the Phytopathogen Causing Witche’s Broom Disease in Cocoa. International Journal of Molecular Sciences. 2023; 24(14):11714. https://doi.org/10.3390/ijms241411714
Chicago/Turabian StyleFarias, Keilane Silva, Monaliza Macêdo Ferreira, Geiseane Veloso Amaral, Maria Zugaib, Ariana Silva Santos, Fábio Pinto Gomes, Rachel Passos Rezende, Karina Peres Gramacho, Eric Roberto Guimarães Rocha Aguiar, and Carlos Priminho Pirovani. 2023. "BASIDIN as a New Protein Effector of the Phytopathogen Causing Witche’s Broom Disease in Cocoa" International Journal of Molecular Sciences 24, no. 14: 11714. https://doi.org/10.3390/ijms241411714
APA StyleFarias, K. S., Ferreira, M. M., Amaral, G. V., Zugaib, M., Santos, A. S., Gomes, F. P., Rezende, R. P., Gramacho, K. P., Aguiar, E. R. G. R., & Pirovani, C. P. (2023). BASIDIN as a New Protein Effector of the Phytopathogen Causing Witche’s Broom Disease in Cocoa. International Journal of Molecular Sciences, 24(14), 11714. https://doi.org/10.3390/ijms241411714










