Serological and Molecular Characterization of Hepatitis C Virus-Related Cryoglobulinemic Vasculitis in Patients without Cryoprecipitate
Abstract
1. Introduction
2. Results
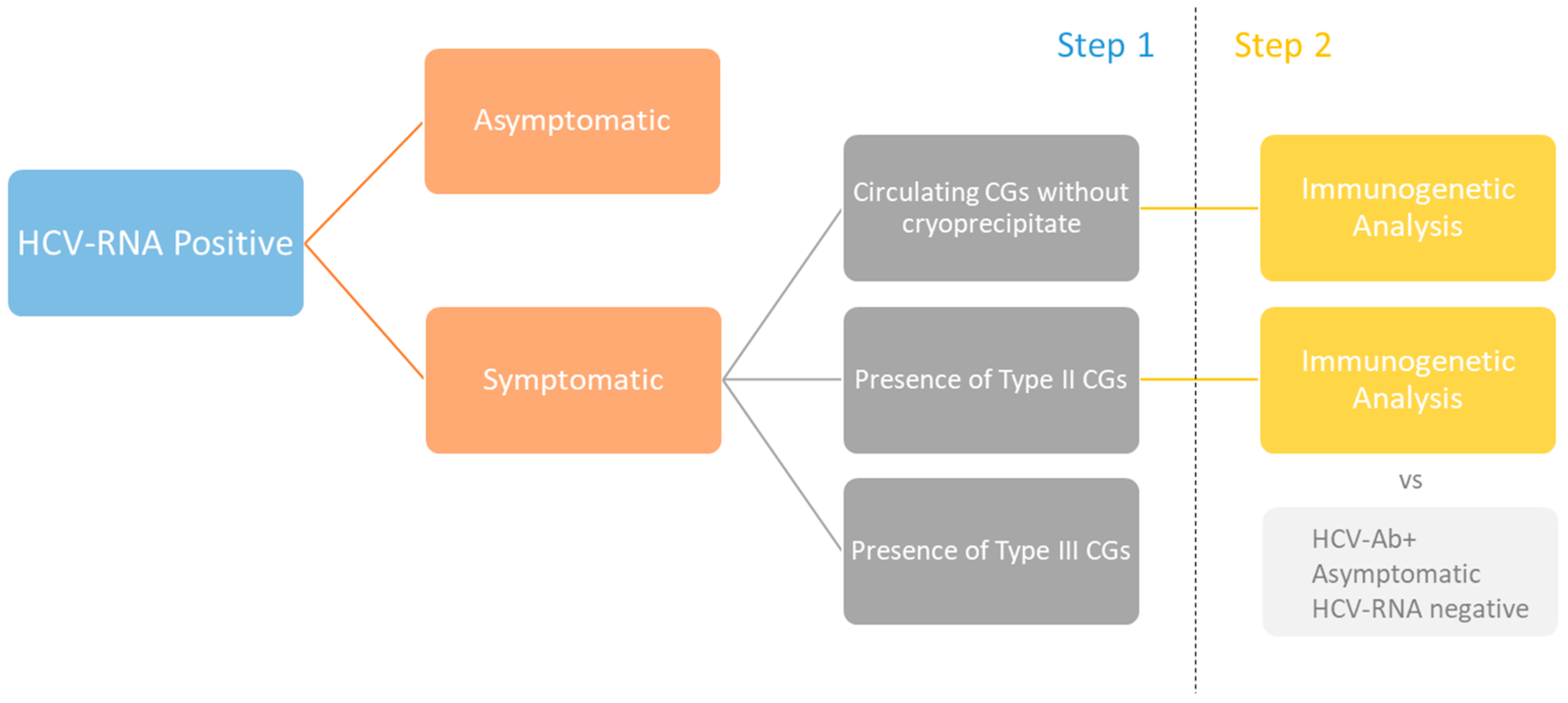
2.1. General Features of HCV-RNA-Positive Patients
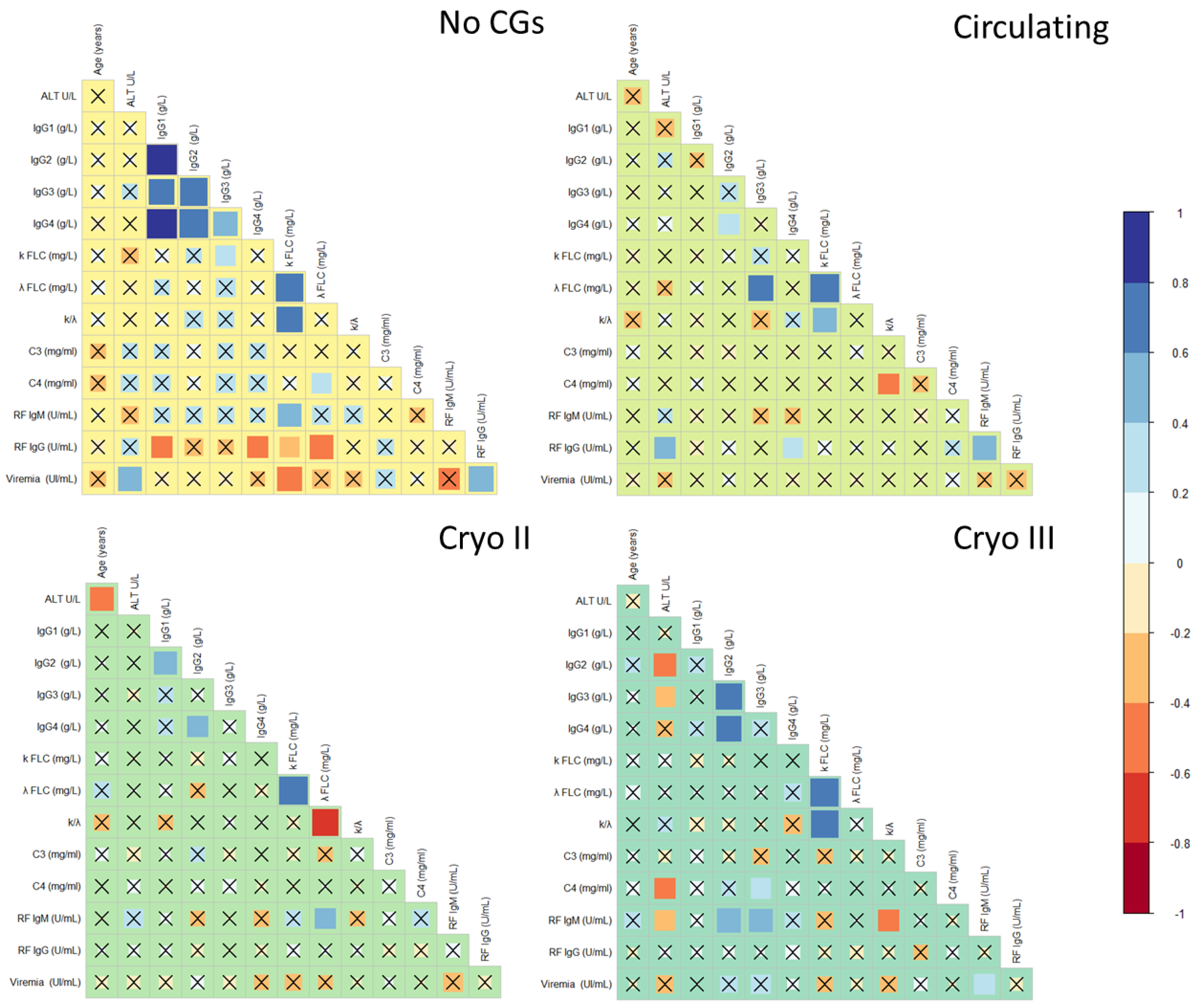
2.2. Comparative Analysis of Serological Correlates of HCV-RNA-Positive Patients
2.3. Analysis of TCR and IGH Clonal Rearrangements
3. Discussion
3.1. B-Cell Proliferation and Clonal Selection in HCV-Related Mixed Cryoglobulinemia
3.2. Symptomatic Similarity in HCV-Positive Patients with Circulating and Type II Cryoglobulins
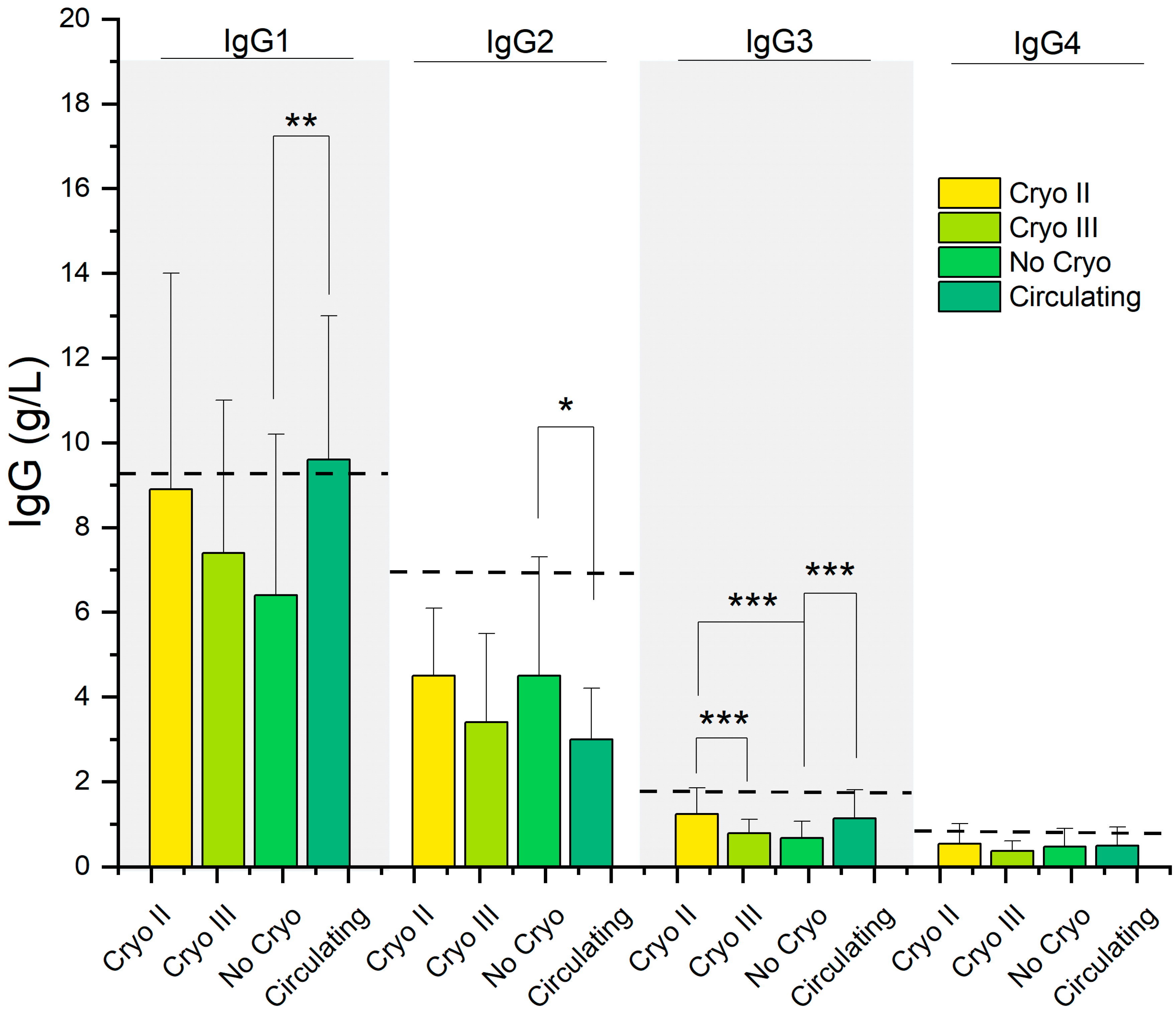
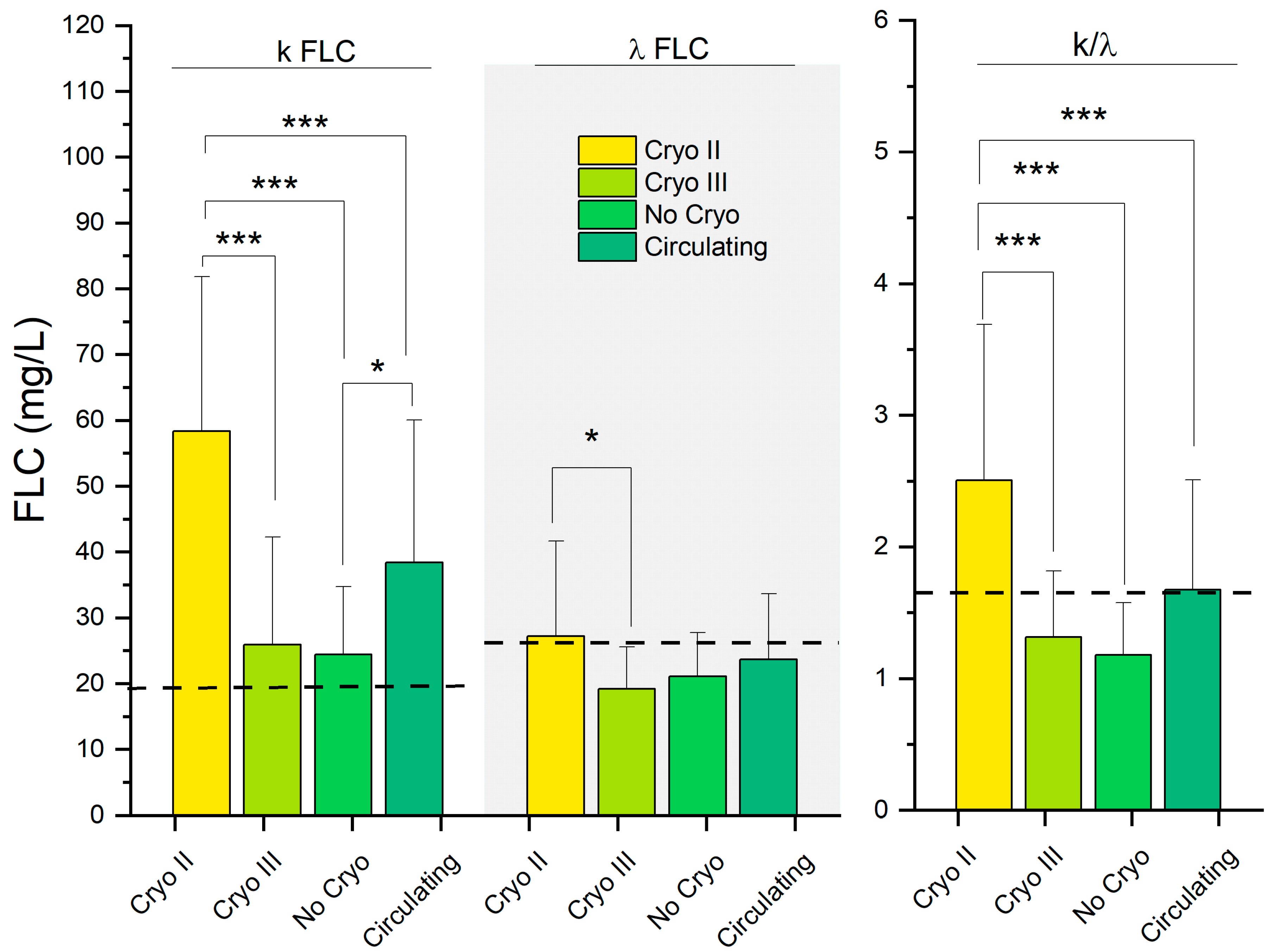
3.3. Beyond Clinical Symptoms: Similarities in Serological Parameters Highlight the Relationship between Patients with Circulating and Type II Cryoglobulins
3.4. Comparative Analysis of Clonal Rearrangements Reveals Similar Patterns in Patients with Circulating and Type II Cryoglobulins
4. Materials and Methods
4.1. Patients
4.2. Laboratory Testing
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Napodano, C.; Gulli, F.; Rapaccini, G.L.; Marino, M.; Basile, U. Cryoglobulins: Identification, classification, and novel biomarkers of mysterious proteins. Adv. Clin. Chem. 2021, 104, 299–340. [Google Scholar] [CrossRef]
- Basile, U.; Napodano, C.; Marino, M.; Gulli, F.; Colantuono, S.; Casato, M.; Pocino, K.; Basile, V.; Todi, L.; Rapaccini, G.L.; et al. Cryoglobulins: Putative effectors of adaptive immune response. Clin. Exp. Rheumatol. 2021, 39 (Suppl. S129), 171–179. [Google Scholar] [CrossRef] [PubMed]
- Gragnani, L.; Lorini, S.; Marri, S.; Basile, U.; Santarlasci, V.; Monti, M.; Madia, F.; Petraccia, L.; Stasi, C.; Marello, N.; et al. Hematological and Genetic Markers in the Rational Approach to Patients with HCV Sustained Virological Response with or without Persisting Cryoglobulinemic Vasculitis. Hepatology 2021, 74, 1164–1173. [Google Scholar] [CrossRef]
- Petrovic, D.; Dempsey, E.; Doherty, D.G.; Kelleher, D.; Long, A. Hepatitis C virus—T-cell responses and viral escape mutations: Highlights. Eur. J. Immunol. 2012, 42, 17–26. [Google Scholar] [CrossRef]
- Visentini, M.; Cagliuso, M.; Conti, V.; Carbonari, M.; Cibati, M.; Siciliano, G.; Cristofoletti, C.; Russo, G.; Casato, M.; Fiorilli, M. Clonal B cells of HCV-associated mixed cryoglobulinemia patients contain exhausted marginal zone-like and CD21 low cells overexpressing Stra13. Eur. J. Immunol. 2012, 42, 1468–1476. [Google Scholar] [CrossRef]
- Gragnani, L.; Lorini, S.; Marri, S.; Vacchi, C.; Madia, F.; Monti, M.; Ferri, C.; Zignego, A.L. Predictors of long-term cryoglobulinemic vasculitis outcomes after HCV eradication with direct-acting antivirals in the real-life. Autoimmun. Rev. 2022, 21, 102923. [Google Scholar] [CrossRef] [PubMed]
- Basile, U.; Marino, M.; Gragnani, L.; Napodano, C.; Gulli, F.; Pocino, K.; Lorini, S.; Santini, S.A.; Basile, V.; Miele, L.; et al. Sentinel biomarkers in HCV positive patients with mixed cryoglobulinemia. J. Immunol. Methods 2020, 476, 112687. [Google Scholar] [CrossRef]
- Basile, U.; Napodano, C.; Pocino, K.; Gulli, F.; Santini, S.A.; Todi, L.; Marino, M.; Rapaccini, G.L. Serological profile of asymptomatic HCV positive patients with low level of cryoglobulins. BioFactors 2019, 45, 318–325. [Google Scholar] [CrossRef]
- Gulli, F.; Marino, M.; Napodano, C.; Pocino, K.; Pandolfi, F.; Gasbarrini, A.; Rapaccini, G.L.; Basile, U. Biomarkers in HCV-related mixed cryoglobulinemia patients withnon-Hodgkin lymphoma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8067–8074. [Google Scholar] [CrossRef]
- Basile, U.; Gulli, F.; Napodano, C.; Pocino, K.; Basile, V.; Marrapodi, R.; Colantuono, S.; Todi, L.; Marino, M.; Rapaccini, G.L.; et al. Biomarkers of minimal residual disease in rituximab-treated patients with mixed cryoglobulinemia. Biotechnol. Appl. Biochem. 2021, 68, 319–329. [Google Scholar] [CrossRef]
- Kolopp-Sarda, M.N.; Azevedo, P.M.; Miossec, P. IgG subclasses in cryoglobulins: Link to composition and clinical manifestations. Arthritis Res. Ther. 2020, 22, 267. [Google Scholar] [CrossRef]
- Basile, U.; Gulli, F.; Gragnani, L.; Pocino, K.; Napodano, C.; Miele, L.; Santini, S.A.; Marino, M.; Zignego, A.L.; Rapaccini, G.L. Different biochemical patterns in type II and type III mixed cryoglobulinemia in HCV positive patients. Dig. Liver Dis. 2018, 50, 938–943. [Google Scholar] [CrossRef]
- Lorini, S.; Gragnani, L.; Zignego, A.L. The Relevance of MicroRNAs in the Pathogenesis and Prognosis of HCV-Disease: The Emergent Role of miR-17-92 in Cryoglobulinemic Vasculitis. Viruses 2020, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Ferri, C.; Zignego, A.L.; Pileri, S.A. Cryoglobulins. J. Clin. Pathol. 2002, 55, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Gragnani, L.; Fognani, E.; De Re, V.; Libra, M.; Garozzo, A.; Caini, P.; Cerretelli, G.; Giovannelli, A.; Lorini, S.; Monti, M.; et al. Notch4 and mhc class II polymorphisms are associated with hcv-related benign and malignant lymphoproliferative diseases. Oncotarget 2017, 8, 71528–71535. [Google Scholar] [CrossRef] [PubMed]
- Gragnani, L.; Lorini, S.; Marri, S.; Zignego, A.L. Role of Notch Receptors in Hematologic Malignancies. Cells 2020, 10, 16. [Google Scholar] [CrossRef]
- Gragnani, L.; Lorini, S.; Marri, S.; Rattotti, S.; Madia, F.; Zibellini, S.; Monti, M.; Basile, U.; Di Stasio, E.; Libra, M.; et al. B-cell activating factor (BAFF), BAFF promoter and BAFF receptor allelic variants in hepatitis C virus related Cryoglobulinemic Vasculitis and Non-Hodgkin’s Lymphoma. Hematol. Oncol. 2022, 40, 658–666. [Google Scholar] [CrossRef]
- Marino, M.; Bartoccioni, E.; Alboini, P.E.; Evoli, A. Rituximab in myasthenia gravis: A “to be or not to be” inhibitor of T cell function. Ann. N. Y. Acad. Sci. 2018, 1413, 41–48. [Google Scholar] [CrossRef]
- Monroe, J.G.; Dorshkind, K. Fate decisions regulating bone marrow and peripheral B lymphocyte development. Adv. Immunol. 2007, 95, 1–50. [Google Scholar] [CrossRef]
- Von Boehmer, H.; Melchers, F. Checkpoints in lymphocyte development and autoimmune disease. Nat. Immunol. 2010, 11, 14–20. [Google Scholar] [CrossRef]
- Langerak, A.W.; Groenen, P.J.T.A.; Brüggemann, M.; Beldjord, K.; Bellan, C.; Bonello, L.; Boone, E.; Carter, G.I.; Catherwood, M.; Davi, F.; et al. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia 2012, 26, 2159–2171. [Google Scholar] [CrossRef]
- Brüggemann, M.; Kotrová, M.; Knecht, H.; Bartram, J.; Boudjogrha, M.; Bystry, V.; Fazio, G.; Froňková, E.; Giraud, M.; on behalf of the EuroClonality-NGS Working Group; et al. Standardized next-generation sequencing of immunoglobulin and T-cell receptor gene recombinations for MRD marker identification in acute lymphoblastic leukaemia: A EuroClonality-NGS validation study. Leukemia 2019, 33, 2241–2253. [Google Scholar] [CrossRef] [PubMed]
- Ciccozzi, M.; Lo Presti, A.; Ciccaglione, A.R.; Zehender, G.; Ciotti, M. Phylogeny and phylodinamic of Hepatitis C in Italy. BMC Infect. Dis. 2012, 12, S5. [Google Scholar] [CrossRef] [PubMed]
- Dustin, L.B. Innate and Adaptive Immune Responses in Chronic HCV Infection. Curr. Drug Targets 2017, 18, 826–843. [Google Scholar] [CrossRef]
- Gragnani, L.; Fognani, E.; Piluso, A.; Zignego, A.L. Hepatitis C virus-related mixed cryoglobulinemia: Is genetics to blame? World J. Gastroenterol. 2013, 19, 8910–8915. [Google Scholar] [CrossRef] [PubMed]
- Basile, U.; Gulli, F.; Gragnani, L.; Fognani, E.; Napodano, C.; Pocino, K.; Zignego, A.L.; Rapaccini, G.L. IgG3 subclass: A possible trigger of mixed cryoglobulin cascade in hepatitis C virus chronic infection. Dig. Liver Dis. 2017, 49, 1233–1239. [Google Scholar] [CrossRef]
- Retamozo, S.; Quartuccio, L.; Ramos-Casals, M. Cryoglobulinemia. Med. Clin. 2022, 158, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Napodano, C.; Marino, M.; Stefanile, A.; Pocino, K.; Scatena, R.; Gulli, F.; Rapaccini, G.L.; Delli Noci, S.; Capozio, G.; Rigante, D.; et al. Immunological Role of IgG Subclasses. Immunol. Investig. 2021, 50, 427–444. [Google Scholar] [CrossRef]
- Gulli, F.; Basile, U.; Gragnani, L.; Napodano, C.; Pocino, K.; Miele, L.; Santini, S.A.; Zignego, A.L.; Gasbarrini, A.; Rapaccini, G.L. IgG cryoglobulinemia. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6057–6062. [Google Scholar] [CrossRef]
- Napodano, C.; Pocino, K.; Rigante, D.; Stefanile, A.; Gulli, F.; Marino, M.; Basile, V.; Rapaccini, G.L.; Basile, U. Free light chains and autoimmunity. Autoimmun. Rev. 2019, 18, 484–492. [Google Scholar] [CrossRef]
- Basile, U.; Gulli, F.; Gragnani, L.; Napodano, C.; Pocino, K.; Rapaccini, G.L.; Mussap, M.; Zignego, A.L. Free light chains: Eclectic multipurpose biomarker. J. Immunol. Methods 2017, 451, 11–19. [Google Scholar] [CrossRef]
- Gulli, F.; Napodano, C.; Marino, M.; Ciasca, G.; Pocino, K.; Basile, V.; Visentini, M.; Stefanile, A.; Todi, L.; De Spirito, M.; et al. Serum immunoglobulin free light chain levels in systemic autoimmune rheumatic diseases. Clin. Exp. Immunol. 2020, 199, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Cigliana, G.; Gulli, F.; Napodano, C.; Pocino, K.; De Santis, E.; Colacicco, L.; Cordone, I.; Conti, L.; Basile, U. Serum free light chain quantitative assays: Dilemma of a biomarker. J. Clin. Lab. Anal. 2018, 32, e22243. [Google Scholar] [CrossRef]
- Basile, U.; Torti, E.; Dell’Abate, M.T.; Colacicco, L.; Gulli, F.; Zuppi, C.; Rapaccini, G.L. Pre-analytical phase in cryoglobulin (CRG) detection: An alternative method for sample transport. Clin. Chem. Lab. Med. 2016, 54, e123–e126. [Google Scholar] [CrossRef]
- Van Dongen, J.J.M.; Langerak, A.W.; Brüggemann, M.; Evans, P.A.S.; Hummel, M.; Lavender, F.L.; Delabesse, E.; Davi, F.; Schuuring, E.; García-Sanz, R.; et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003, 17, 2257–2317. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, R.; Vaccaro, M.; Romanò, S.; Di Giacinto, F.; Papi, M.; Rapaccini, G.L.; De Spirito, M.; Miele, L.; Basile, U.; Ciasca, G. Machine Learning-Assisted FTIR Analysis of Circulating Extracellular Vesicles for Cancer Liquid Biopsy. J. Pers. Med. 2022, 12, 949. [Google Scholar] [CrossRef]
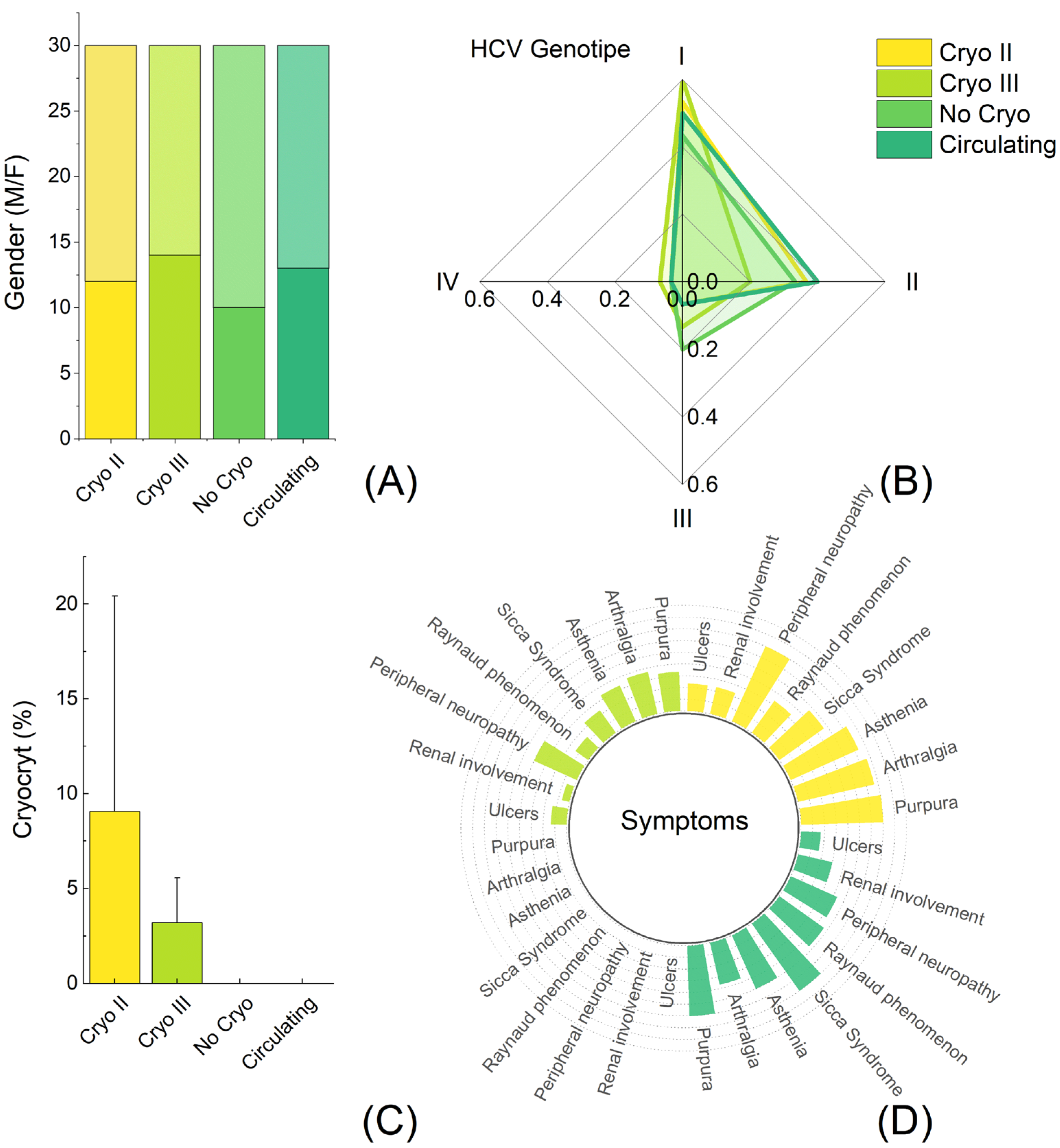


| 1° Group (30) | 2° Group (30) | 3° Group (30) | 4° Group (30) | |
| Sex (M/F) | 10/20 | 13/17 | 12/18 | 14/16 |
| HCV-RNA | Yes | Yes | Yes | Yes |
| HCV genotype | ||||
| 1 (%) | 13 | 15 | 16 | 18 |
| 2 (%) | 10 | 12 | 11 | 6 |
| 3 (%) | 6 | 2 | 2 | 4 |
| 4 (%) | 1 | 1 | 1 | 2 |
| CG | No | Circulating | Type II | Type III |
| Cryocrit% | No | No | 9.06 ± 11.36 | 3.21 ± 2.35 |
| SYMPTOMS | ||||
| Purpura | No | 18 | 21 | 10 |
| Arthalgia | No | 11 | 20 | 11 |
| Asthenia | No | 15 | 19 | 10 |
| Sicca Syndrome | No | 21 | 14 | 7 |
| Raynaud phenomenon | No | 14 | 10 | 4 |
| Peripheral neuropathy | No | 13 | 21 | 12 |
| Renal involvment | No | 9 | 7 | 2 |
| Ulcers | No | 5 | 7 | 4 |
| Treatment | No | No | No | No |
| Features 1 | Circulating n = 30 | Cryo II n = 30 | Cryo III n = 30 | No Cryo n = 30 | p2 | Circulating vs. Cryo II 3 | Circulating vs. Cryo III 3 | Cryo II vs. Cryo III 3 | Circulating vs. No Cryo 3 | Cryo II vs. No Cryo 3 | Cryo III vs. No Cryo 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALT U/L | 55.4 (27.5) | 47.3 (37.3) | 33.5 (14.9) | 41.1 (22.7) | 0.016 | >0.9 | 0.012 | 0.3 | 0.2 | >0.9 | >0.9 |
| IgG1 (g/L) | 9.63 (3.40) | 8.92 (5.12) | 7.44 (3.57) | 6.15 (3.76) | 0.005 | >0.9 | 0.2 | >0.9 | 0.006 | 0.051 | >0.9 |
| IgG2 (g/L) | 3.05 (1.22) | 4.48 (1.93) | 3.35 (2.11) | 4.54 (2.80) | 0.009 | 0.054 | >0.9 | 0.2 | 0.041 | >0.9 | 0.2 |
| IgG3 (g/L) | 1.16 (0.67) | 1.23 (0.61) | 0.79 (0.33) | 0.58 (0.38) | <0.001 | >0.9 | 0.073 | 0.008 | <0.001 | <0.001 | 0.7 |
| IgG4 (g/L) | 0.49 (0.45) | 0.54 (0.47) | 0.37 (0.24) | 0.47 (0.43) | 0.4 | >0.9 | >0.9 | 0.7 | >0.9 | >0.9 | >0.9 |
| k FLC (mg/L) | 38.5 (21.7) | 58.4 (23.5) | 25.9 (16.3) | 24.4 (10.4) | <0.001 | <0.001 | 0.063 | <0.001 | 0.025 | <0.001 | >0.9 |
| λ FLC (mg/L) | 23.7 (10.0) | 27.2 (14.4) | 19.2 (6.4) | 21.1 (6.7) | 0.014 | >0.9 | 0.5 | 0.013 | >0.9 | 0.11 | >0.9 |
| k/λ | 1.67 (0.84) | 2.51 (1.18) | 1.32 (0.50) | 1.18 (0.40) | <0.001 | <0.001 | 0.5 | <0.001 | 0.10 | <0.001 | >0.9 |
| C3 (mg/mL) | 94.0 (15.3) | 89.4 (26.2) | 104.7 (9.7) | 119 (16) | <0.001 | >0.9 | 0.13 | 0.007 | <0.001 | <0.001 | 0.013 |
| C4 (mg/mL) | 15.0 (4.2) | 12.7 (6.5) | 15.3 (3.1) | 28.9 (5.8) | <0.001 | 0.5 | >0.9 | 0.3 | <0.001 | <0.001 | <0.001 |
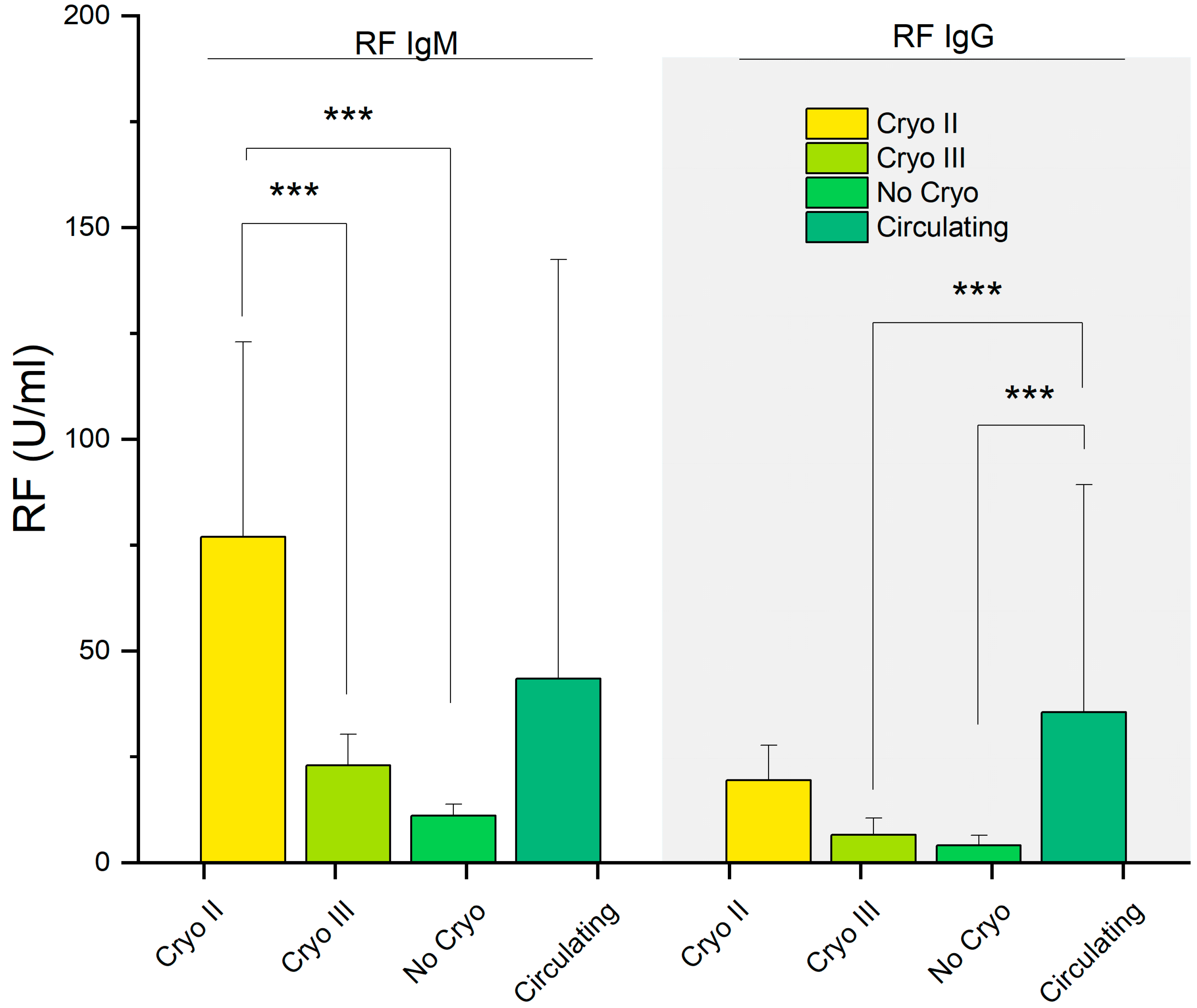
| RF IgM (U/mL) | 43.4 (99.0) | 76.9 (46.1) | 22.9 (7.4) | 11.1 (2.7) | <0.001 | 0.12 | >0.9 | 0.001 | 0.15 | <0.001 | >0.9 |
| RF IgG (U/mL) | 35.5 (53.8) | 19.5 (8.2) | 6.6 (3.9) | 4.13 (2.29) | <0.001 | 0.15 | <0.001 | 0.4 | <0.001 | 0.2 | >0.9 |
| Viremia (UI/mL) | 25,011 (30,753) | 3,429,521 (462,606) | 55,999 (41,320) | 34,729 (42,468) | <0.001 | <0.001 | >0.9 | <0.001 | >0.9 | <0.001 | >0.9 |
| Features | Circulating, n = 30 | Cryo II, n = 30 | HCV-RNA Neg, n = 10 | p 1 | Circulating vs. Cryo II 2 | Circulating vs. No Cryo 2 | Cryo II vs. HCV-RNA Neg 2 |
|---|---|---|---|---|---|---|---|
| TCRγ A | 0.023 | ns | ns | 0.021 | |||
| Monoclonal | 17% | 6.9% | 0% | ||||
| Oligoclonal | 27% | 45% | 0% | ||||
| Polyclonal | 57% | 48% | 100% | ||||
| TCRγ B | 0.010 | ns | 0.033 | 0.009 | |||
| Monoclonal | 6.7% | 18% | 0% | ||||
| Oligoclonal | 43% | 43% | 0% | ||||
| Polyclonal | 50% | 39% | 100% | ||||
| IGH FR1 | 0.003 | ns | 0.051 | <0.001 | |||
| Monoclonal | 20% | 34% | 0% | ||||
| Oligoclonal | 30% | 38% | 0% | ||||
| Polyclonal | 50% | 28% | 100% | ||||
| IGHFR2 | <0.001 | ns | <0.001 | <0.001 | |||
| Monoclonal | 27% | 38% | 0% | ||||
| Oligoclonal | 47% | 34% | 0% | ||||
| Polyclonal | 27% | 28% | 100% | ||||
| IGHFR3 | 0.009 | ns | 0.015 | 0.003 | |||
| Monoclonal | 17% | 24% | 0% | ||||
| Oligoclonal | 40% | 41% | 0% | ||||
| Polyclonal | 43% | 34% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napodano, C.; Ciasca, G.; Chiusolo, P.; Pocino, K.; Gragnani, L.; Stefanile, A.; Gulli, F.; Lorini, S.; Minnella, G.; Fosso, F.; et al. Serological and Molecular Characterization of Hepatitis C Virus-Related Cryoglobulinemic Vasculitis in Patients without Cryoprecipitate. Int. J. Mol. Sci. 2023, 24, 11602. https://doi.org/10.3390/ijms241411602
Napodano C, Ciasca G, Chiusolo P, Pocino K, Gragnani L, Stefanile A, Gulli F, Lorini S, Minnella G, Fosso F, et al. Serological and Molecular Characterization of Hepatitis C Virus-Related Cryoglobulinemic Vasculitis in Patients without Cryoprecipitate. International Journal of Molecular Sciences. 2023; 24(14):11602. https://doi.org/10.3390/ijms241411602
Chicago/Turabian StyleNapodano, Cecilia, Gabriele Ciasca, Patrizia Chiusolo, Krizia Pocino, Laura Gragnani, Annunziata Stefanile, Francesca Gulli, Serena Lorini, Gessica Minnella, Federica Fosso, and et al. 2023. "Serological and Molecular Characterization of Hepatitis C Virus-Related Cryoglobulinemic Vasculitis in Patients without Cryoprecipitate" International Journal of Molecular Sciences 24, no. 14: 11602. https://doi.org/10.3390/ijms241411602
APA StyleNapodano, C., Ciasca, G., Chiusolo, P., Pocino, K., Gragnani, L., Stefanile, A., Gulli, F., Lorini, S., Minnella, G., Fosso, F., Di Santo, R., Romanò, S., Basile, V., De Stefano, V., Rapaccini, G. L., Zignego, A. L., Di Stasio, E., Marino, M., & Basile, U. (2023). Serological and Molecular Characterization of Hepatitis C Virus-Related Cryoglobulinemic Vasculitis in Patients without Cryoprecipitate. International Journal of Molecular Sciences, 24(14), 11602. https://doi.org/10.3390/ijms241411602









