Antigens from the Helminth Fasciola hepatica Exert Antiviral Effects against SARS-CoV-2 In Vitro
Abstract
1. Introduction
2. Results
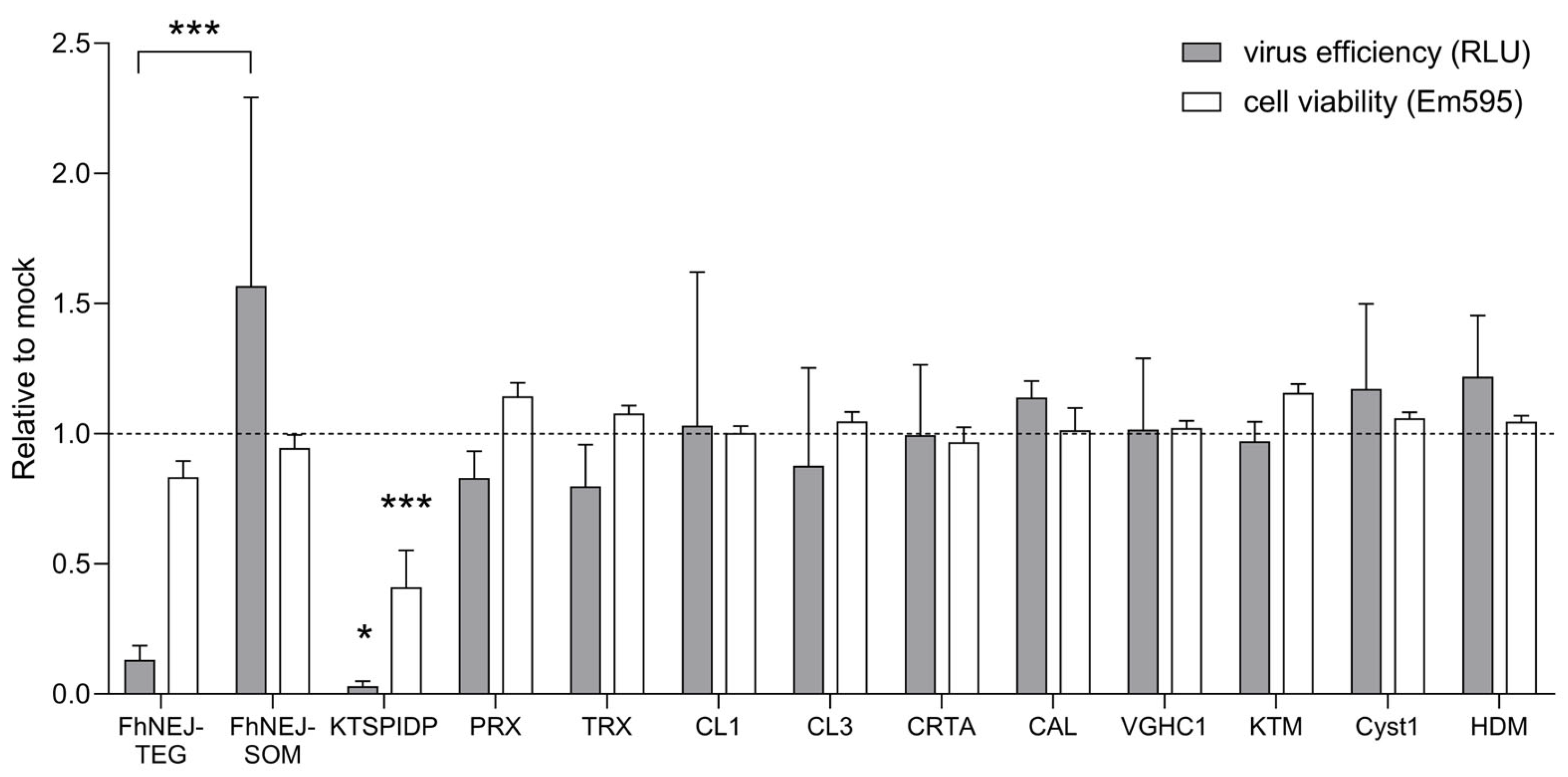
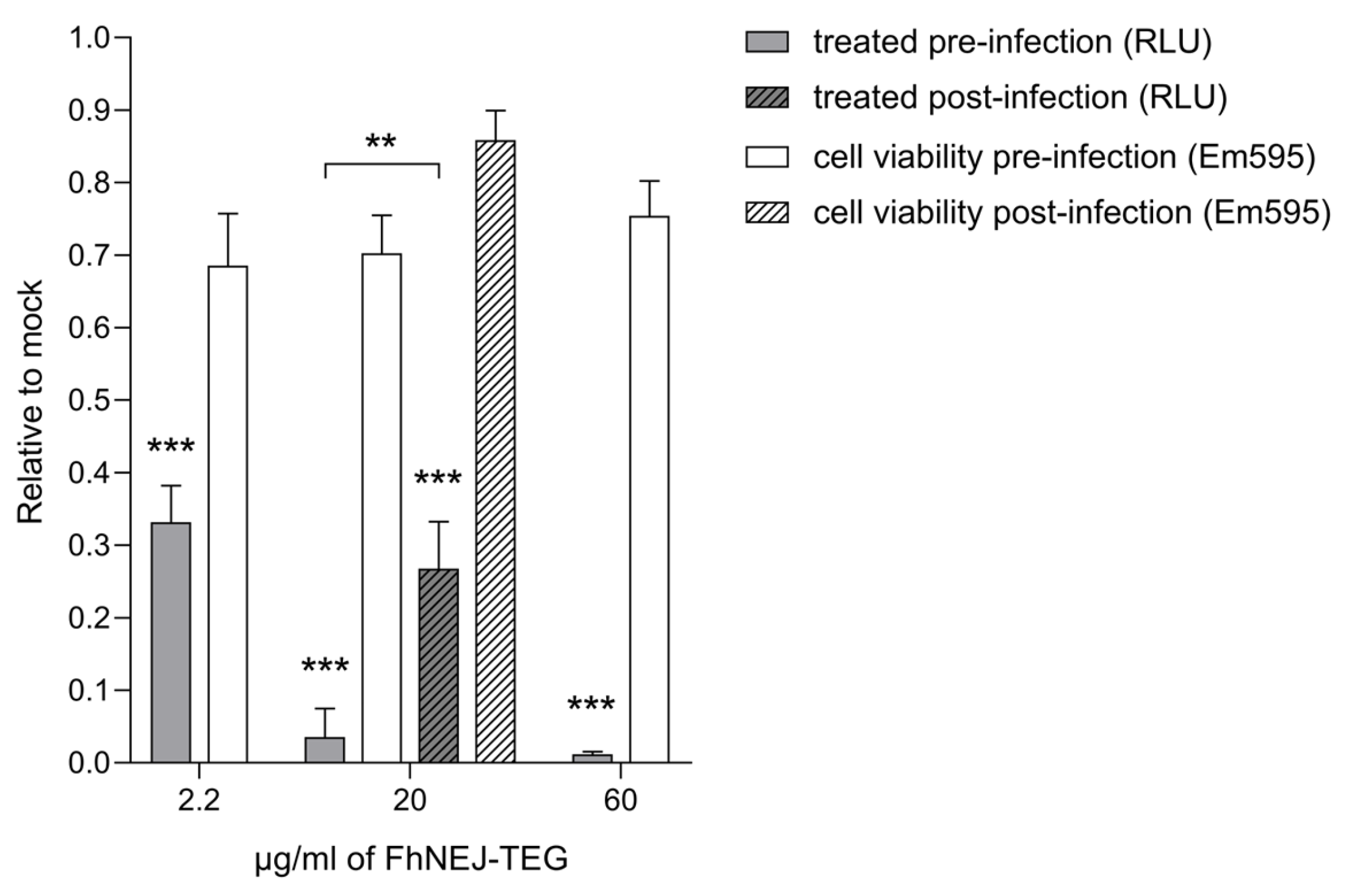
2.1. FhNEJ-TEG Reduces Infection Efficiency of VSV-S2 in Vero Cells In Vitro
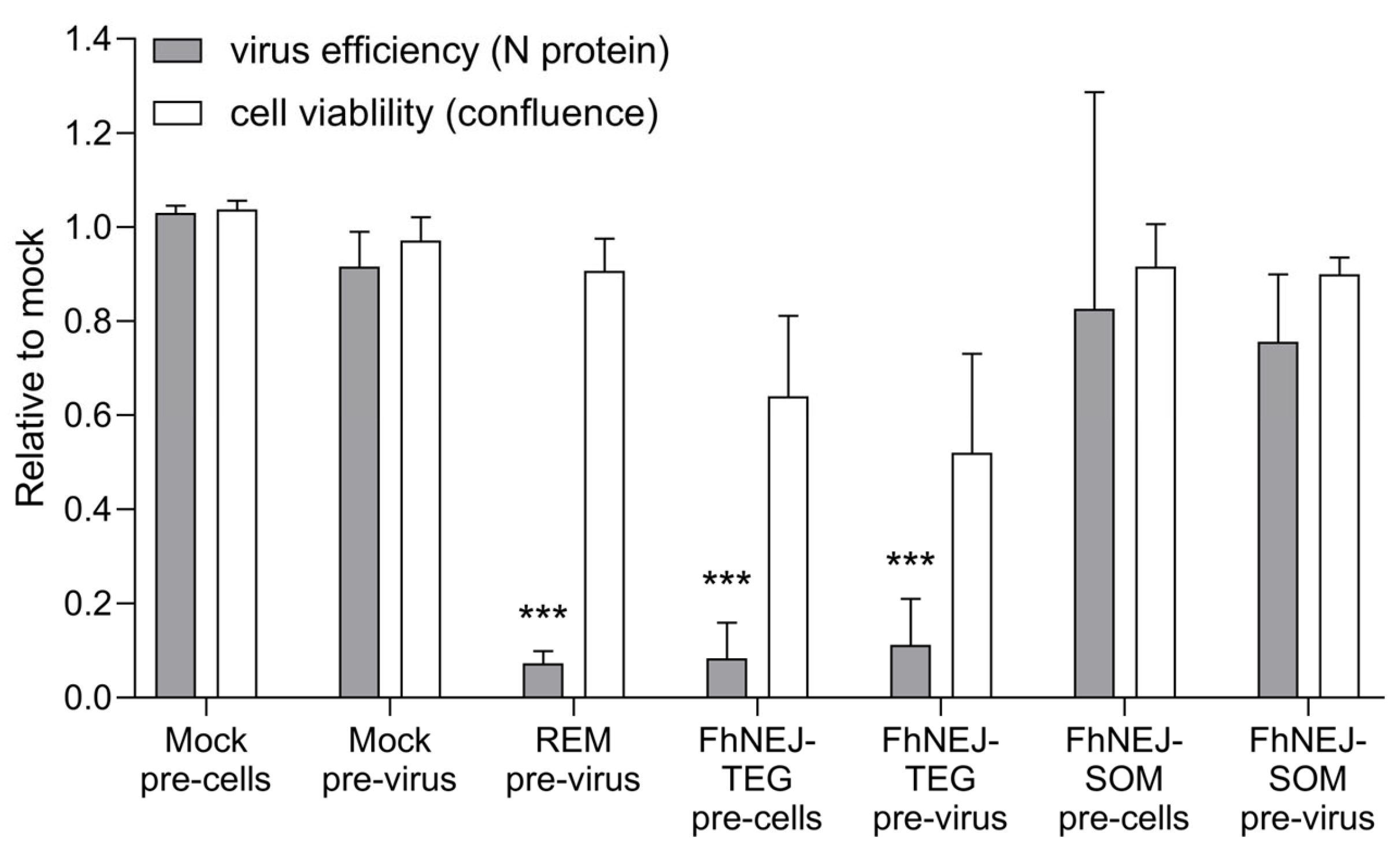
2.2. FhNEJ-TEG Inhibits SARS-CoV-2 Infection of Vero Cells In Vitro
3. Discussion
4. Materials and Methods
4.1. F. hepatica Compounds
4.2. Pseudotyped VSV-S2 Infection Assay
4.3. SARS-CoV-2 Infection Assay
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 29 May 2023).
- Padhan, R.; Prabheesh, K.P. The economics of COVID-19 pandemic: A Survey. Econ. Anal. Policy 2021, 70, 220–237. [Google Scholar] [CrossRef] [PubMed]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Bayati, A.; Kumar, R.; Francis, V.; McPherson, P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021, 296, 100306. [Google Scholar] [CrossRef]
- Singh, D.K.; Aladyeva, E.; Das, S.; Singh, B.; Esaulova, E.; Swain, A.; Ahmed, M.; Cole, J.; Moodley, C.; Mehra, S.; et al. Myeloid cell interferon responses correlate with clearance of SARS-CoV-2. Nat. Commun. 2022, 13, 679. [Google Scholar] [CrossRef]
- Cao, X. COVID-19: Immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Hays, R.; Pierce, D.; Giacomin, P.; Loukas, A.; Bourke, P.; McDermott, R. Helminth coinfection and COVID-19: An alternate hypothesis. PLoS Negl. Trop. Dis. 2020, 14, e0008628. [Google Scholar] [CrossRef] [PubMed]
- Siles-Lucas, M.; González-Miguel, J.; Geller, R.; Sanjuan, R.; Pérez-Arévalo, J.; Martínez-Moreno, Á. Potential influence of helminth molecules on COVID-19 pathology. Trends Parasitol. 2021, 37, 11–14. [Google Scholar] [CrossRef]
- Whitehead, B.; Christiansen, S.; Østergaard, L.; Nejsum, P. Helminths and COVID-19 susceptibility, disease progression, and vaccination efficacy. Trends Parasitol. 2022, 38, 277–279. [Google Scholar] [CrossRef]
- Adjobimey, T.; Meyer, J.; Terkeš, V.; Parcina, M.; Hoerauf, A. Helminth antigens differentially modulate the activation of CD4(+) and CD8(+) T lymphocytes of convalescent COVID-19 patients in vitro. BMC Med. 2022, 20, 241. [Google Scholar] [CrossRef]
- Desai, P.; Diamond, M.S.; Thackray, L.B. Helminth–virus interactions: Determinants of coinfection outcomes. Gut Microbes 2021, 13, 1961202. [Google Scholar] [CrossRef]
- Bullington, B.W.; Klemperer, K.; Mages, K.; Chalem, A.; Mazigo, H.D.; Changalucha, J.; Kapiga, S.; Wright, P.F.; Yazdanbakhsh, M.M.; Downs, J.A. Effects of Schistosomes on host anti-viral immune response and the acquisition, virulence, and prevention of viral infections: A systematic review. PLoS Pathog. 2021, 17, e1009555. [Google Scholar] [CrossRef] [PubMed]
- Petrellis, G.; Piedfort, O.; Katsandegwaza, B.; Dewals, B.G. Parasitic worms affect virus coinfection: A mechanistic overview. Trends Parasitol. 2023, 39, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pradhan, M.; Gorshkov, K.; Petersen, J.D.; Shen, M.; Guo, H.; Zhu, W.; Klumpp-Thomas, C.; Michael, S.; Itkin, M.; et al. A high throughput screening assay for inhibitors of SARS-CoV-2 pseudotyped particle entry. SLAS Discov. 2022, 27, 86–94. [Google Scholar] [CrossRef]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Bonilla, H.; Jagannathan, P.; Shafer, R.W. SARS-CoV-2 Antiviral Therapy. Clin. Microbiol. Rev. 2021, 34, e0010921. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Fascioliasis. In Digenetic Trematodes; Toledo, R., Fried, B., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 1154, pp. 71–103. ISBN 978-3-030-18615-9. [Google Scholar]
- Siles-Lucas, M.; Becerro-Recio, D.; Serrat, J.; González-Miguel, J. Fascioliasis and fasciolopsiasis: Current knowledge and future trends. Res. Vet. Sci. 2021, 134, 27–35. [Google Scholar] [CrossRef]
- González-Miguel, J.; Becerro-Recio, D.; Sotillo, J.; Simón, F.; Siles-Lucas, M. Set up of an in Vitro model to study early host-parasite interactions between newly excysted juveniles of Fasciola hepatica and host intestinal cells using a quantitative proteomics approach. Vet. Parasitol. 2020, 278, 109028. [Google Scholar] [CrossRef] [PubMed]
- Becerro-Recio, D.; Serrat, J.; López-García, M.; Sotillo, J.; Simón, F.; González-Miguel, J.; Siles-Lucas, M. Proteomics coupled with in vitro model to study the early crosstalk occurring between newly excysted juveniles of Fasciola hepatica and host intestinal cells. PLoS Negl. Trop. Dis. 2022, 16, e0010811. [Google Scholar] [CrossRef]
- Wuerthner, V.P.; Hua, J.; Hoverman, J.T. The benefits of coinfection: Trematodes alter disease outcomes associated with virus infection. J. Anim. Ecol. 2017, 86, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.J.; Mulcahy, G. The roles of IL-10 and TGF-beta in controlling IL-4 and IFN-gamma production during experimental Fasciola hepatica infection. Int. J. Parasitol. 2008, 38, 1673–1680. [Google Scholar] [CrossRef]
- Ramshaw, I.A.; Ramsay, A.J.; Karupiah, G.; Rolph, M.S.; Mahalingam, S.; Ruby, J.C. Cytokines and immunity to viral infections. Immunol. Rev. 1997, 159, 119–135. [Google Scholar] [CrossRef]
- McSorley, H.J.; Smyth, D.J. IL-33: A central cytokine in helminth infections. Semin. Immunol. 2021, 53, 101532. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, W.V.; Fröhlich, A.; Senn, K.; Kallert, S.; Fernandez, M.; Johnson, S.; Kreutzfeldt, M.; Hegazy, A.N.; Schrick, C.; Fallon, P.G.; et al. The alarmin interleukin-33 drives protective antiviral CD8+ T cell responses. Science 2012, 335, 984–989. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, A.J.; McSorley, H.J.; Davidson, D.J.; Fitch, P.M.; Errington, C.; Mackenzie, K.J.; Gollwitzer, E.S.; Johnston, C.J.C.; MacDonald, A.S.; Edwards, M.R.; et al. Enteric helminth-induced type I interferon signaling protects against pulmonary virus infection through interaction with the microbiota. J. Allergy Clin. Immunol. 2017, 140, 1068–1078.e6. [Google Scholar] [CrossRef]
- Koch, J.; Uckeley, Z.M.; Doldan, P.; Stanifer, M.; Boulant, S.; Lozach, P. TMPRSS2 expression dictates the entry route used by SARS-CoV-2 to infect host cells. EMBO J. 2021, 40, e107821. [Google Scholar] [CrossRef]
- Chen, C.Z.; Xu, M.; Pradhan, M.; Gorshkov, K.; Petersen, J.D.; Straus, M.R.; Zhu, W.; Shinn, P.; Guo, H.; Shen, M.; et al. Identifying SARS-CoV-2 entry inhibitors through drug repurposing screens of SARS-S and MERS-S pseudotyped particles. ACS Pharmacol. Transl. Sci. 2020, 3, 1165–1175. [Google Scholar] [CrossRef]
- Robinson, M.W.; Donnelly, S.; Hutchinson, A.T.; To, J.; Taylor, N.L.; Norton, R.S.; Perugini, M.A.; Dalton, J.P. A family of helminth molecules that modulate innate cell responses via molecular mimicry of host antimicrobial peptides. PLoS Pathog. 2011, 7, e1002042. [Google Scholar] [CrossRef]
- Dorey, A.; Cwiklinski, K.; Rooney, J.; De Marco Verissimo, C.; López Corrales, J.; Jewhurst, H.; Fazekas, B.; Calvani, N.E.D.; Hamon, S.; Gaughan, S.; et al. Autonomous non antioxidant roles for Fasciola hepatica secreted thioredoxin-1 and peroxiredoxin-1. Front. Cell. Infect. Microbiol. 2021, 11, 667272. [Google Scholar] [CrossRef]
- González-Miguel, J.; Becerro-Recio, D.; Siles-Lucas, M. Insights into Fasciola hepatica Juveniles: Crossing the fasciolosis rubicon. Trends Parasitol. 2021, 37, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Falcón, C.R.; Masih, D.; Gatti, G.; Sanchez, M.C.; Motrán, C.C.; Cervi, L. Fasciola hepatica kunitz type molecule decreases dendritic cell activation and their ability to induce inflammatory responses. PLoS ONE 2014, 9, e114505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, Y.; Zhang, G.; Wang, X.; Li, Z.; Shang, Y.; Ning, C.; Ji, C.; Cai, X.; Xia, X.; et al. Molecular characteristics and potent immunomodulatory activity of Fasciola hepatica cystatin. Korean J. Parasitol. 2022, 60, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, S.L.; McManus, D.P. Protease inhibitors of parasitic flukes: Emerging roles in parasite survival and immune defence. Trends Parasitol. 2017, 33, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Scarcella, M.; d’Angelo, D.; Ciampa, M.; Tafuri, S.; Avallone, L.; Pavone, L.M.; De Pasquale, V. The key role of lysosomal protease cathepsins in viral infections. Int. J. Mol. Sci. 2022, 23, 9089. [Google Scholar] [CrossRef]
- Cwiklinski, K.; Dalton, J.P. Advances in Fasciola hepatica research using ‘omics’ technologies. Int. J. Parasitol. 2018, 48, 321–331. [Google Scholar] [CrossRef]
- EMA COVID-19 Treatments. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-treatments (accessed on 29 May 2023).
- Coronavirus (COVID-19) Drugs. Available online: https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs (accessed on 29 May 2023).
- Desmyter, J.; Melnick, J.L.; Rawls, W.E. Defectiveness of Interferon Production and of Rubella Virus Vnterference in a Line of African Green Monkey Kidney Cells (Vero). J. Virol. 1968, 2, 955–961. [Google Scholar] [CrossRef]
- Emeny, J.M.; Morgan, M.J. Regulation of the Interferon System: Evidence That Vero Cells Have a Genetic Defect in Interferon Production. J. Gen. Virol. 1979, 43, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.O.; Ziemin, S.; Le Beau, M.M.; Pitha, P.; Smith, S.D.; Chilcote, R.R.; Rowley, J.D. Homozygous Deletion of the Alpha- and Beta 1-Interferon Genes in Human Leukemia and Derived Cell Lines. Proc. Natl. Acad. Sci. USA 1988, 85, 5259–5263. [Google Scholar] [CrossRef]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef]
- Wang, C.; Shan, L.; Qu, S.; Xue, M.; Wang, K.; Fu, F.; Wang, L.; Wang, Z.; Feng, L.; Xu, W.; et al. The Coronavirus PEDV Evades Type III Interferon Response Through the MiR-30c-5p/SOCS1 Axis. Front. Microbiol. 2020, 11, 1180. [Google Scholar] [CrossRef]
- Weber, F. Antiviral Innate Immunity: Introduction. In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 577–583. ISBN 978-0-12-814516-6. [Google Scholar]
- Pervolaraki, K.; Guo, C.; Albrecht, D.; Boulant, S.; Stanifer, M.L. Type-Specific Crosstalk Modulates Interferon Signaling in Intestinal Epithelial Cells. J. Interferon Cytokine Res. 2019, 39, 650–660. [Google Scholar] [CrossRef]
- Felgenhauer, U.; Schoen, A.; Gad, H.H.; Hartmann, R.; Schaubmar, A.R.; Failing, K.; Drosten, C.; Weber, F. Inhibition of SARS-CoV-2 by Type I and Type III Interferons. J. Biol. Chem. 2020, 295, 13958–13964. [Google Scholar] [CrossRef]
- Banerjee, A.; El-Sayes, N.; Budylowski, P.; Jacob, R.A.; Richard, D.; Maan, H.; Aguiar, J.A.; Demian, W.L.; Baid, K.; D’Agostino, M.R.; et al. Experimental and Natural Evidence of SARS-CoV-2-Infection-Induced Activation of Type I Interferon Responses. iScience 2021, 24, 102477. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Glebov, O.O. Understanding SARS-CoV-2 Endocytosis for COVID-19 Drug Repurposing. FEBS J. 2020, 287, 3664–3671. [Google Scholar] [CrossRef] [PubMed]
- Hernández-González, A.; Valero, M.L.; del Pino, M.S.; Oleaga, A.; Siles-Lucas, M. Proteomic analysis of in vitro newly excysted juveniles from Fasciola hepatica. Mol. Biochem. Parasitol. 2010, 172, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Gozalbo-Rovira, R.; Gimenez, E.; Latorre, V.; Francés-Gómez, C.; Albert, E.; Buesa, J.; Marina, A.; Blasco, M.L.; Signes-Costa, J.; Rodríguez-Díaz, J.; et al. SARS-CoV-2 antibodies, serum inflammatory biomarkers and clinical severity of hospitalized COVID-19 patients. J. Clin. Virol. 2020, 131, 104611. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, P.; Francés-Gómez, C.; Chiner-Oms, Á.; López, M.G.; Jiménez-Serrano, S.; Cancino-Muñoz, I.; Ruiz-Hueso, P.; Torres-Puente, M.; Bracho, M.A.; D’Auria, G.; et al. Evolutionary and phenotypic characterization of two Spike mutations in European lineage 20E of SARS-CoV-2. mBio 2021, 12, e02315-21. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-NCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Bouchet-Valat, M. Rcmdr: R Commander. R Package Version 2.8-0. Available online: https://socialsciences.mcmaster.ca/jfox/Misc/Rcmdr/ (accessed on 17 September 2020).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrat, J.; Francés-Gómez, C.; Becerro-Recio, D.; González-Miguel, J.; Geller, R.; Siles-Lucas, M. Antigens from the Helminth Fasciola hepatica Exert Antiviral Effects against SARS-CoV-2 In Vitro. Int. J. Mol. Sci. 2023, 24, 11597. https://doi.org/10.3390/ijms241411597
Serrat J, Francés-Gómez C, Becerro-Recio D, González-Miguel J, Geller R, Siles-Lucas M. Antigens from the Helminth Fasciola hepatica Exert Antiviral Effects against SARS-CoV-2 In Vitro. International Journal of Molecular Sciences. 2023; 24(14):11597. https://doi.org/10.3390/ijms241411597
Chicago/Turabian StyleSerrat, Judit, Clara Francés-Gómez, David Becerro-Recio, Javier González-Miguel, Ron Geller, and Mar Siles-Lucas. 2023. "Antigens from the Helminth Fasciola hepatica Exert Antiviral Effects against SARS-CoV-2 In Vitro" International Journal of Molecular Sciences 24, no. 14: 11597. https://doi.org/10.3390/ijms241411597
APA StyleSerrat, J., Francés-Gómez, C., Becerro-Recio, D., González-Miguel, J., Geller, R., & Siles-Lucas, M. (2023). Antigens from the Helminth Fasciola hepatica Exert Antiviral Effects against SARS-CoV-2 In Vitro. International Journal of Molecular Sciences, 24(14), 11597. https://doi.org/10.3390/ijms241411597





