The Shear Stress–Regulated Expression of Glypican-4 in Endothelial Dysfunction In Vitro and Its Clinical Significance in Atherosclerosis
Abstract
1. Introduction
2. Results
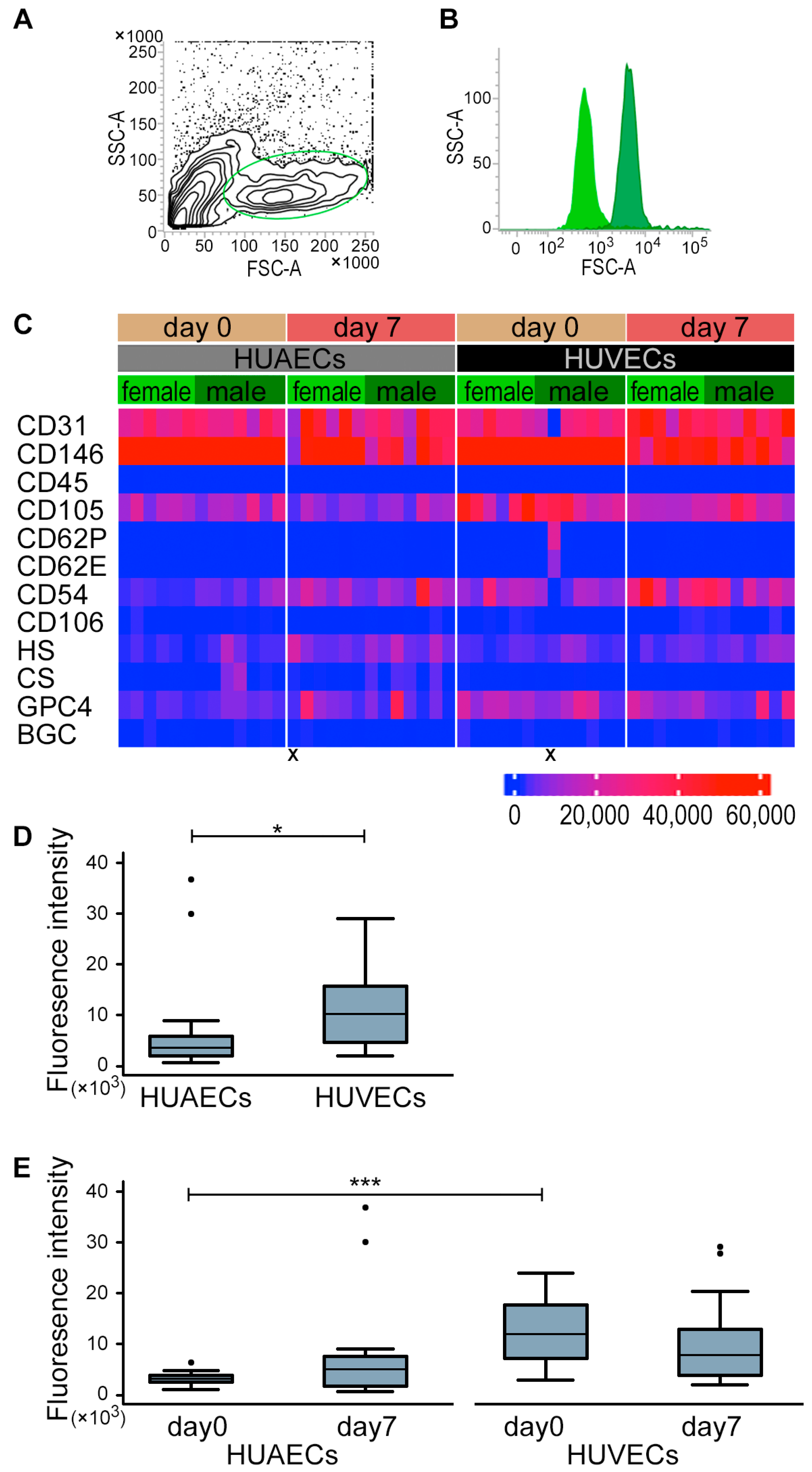
2.1. HUVECs and HUAECs Expressed GPC4
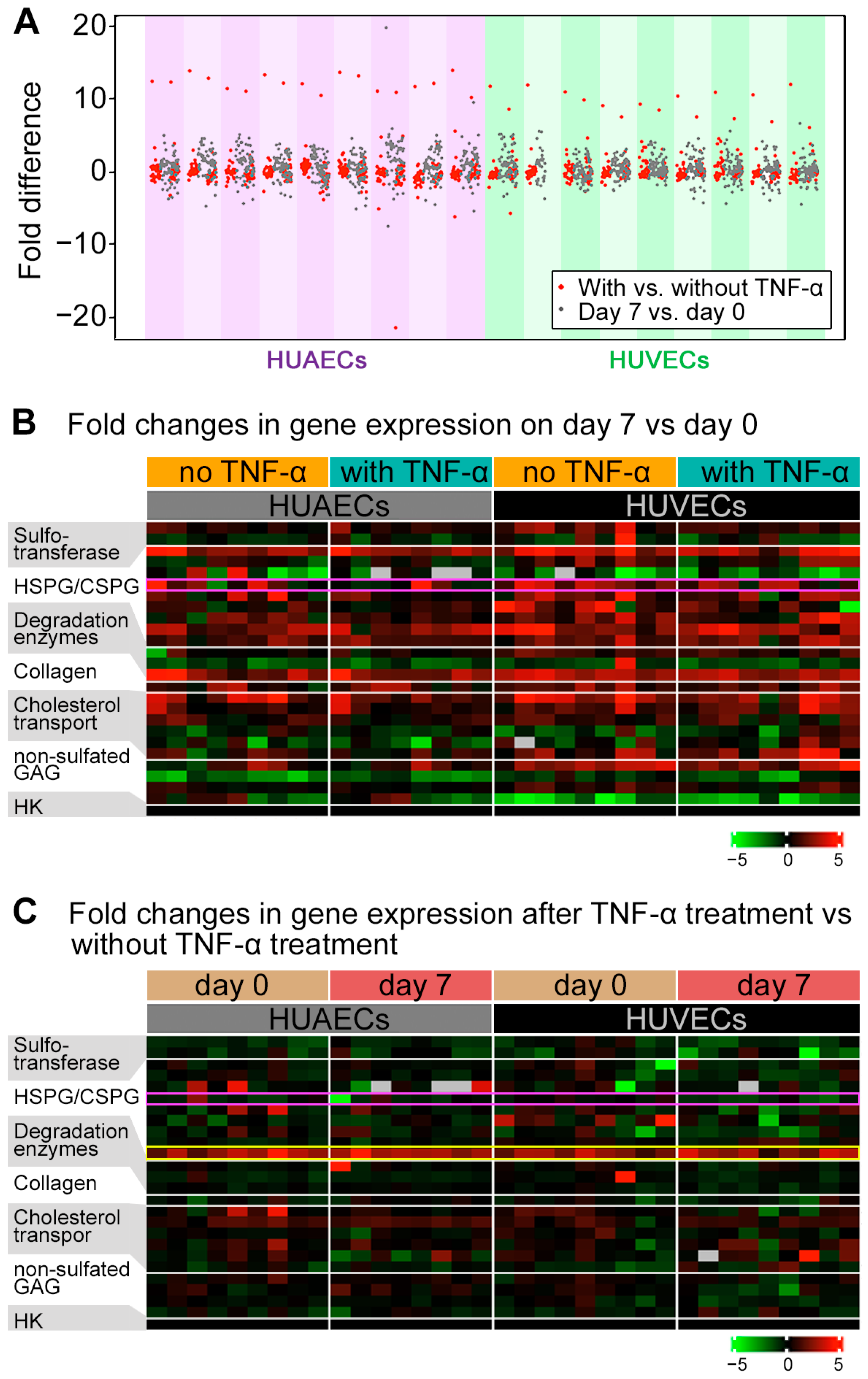
2.2. GPC4 Expression Was Regulated by Shear Stress
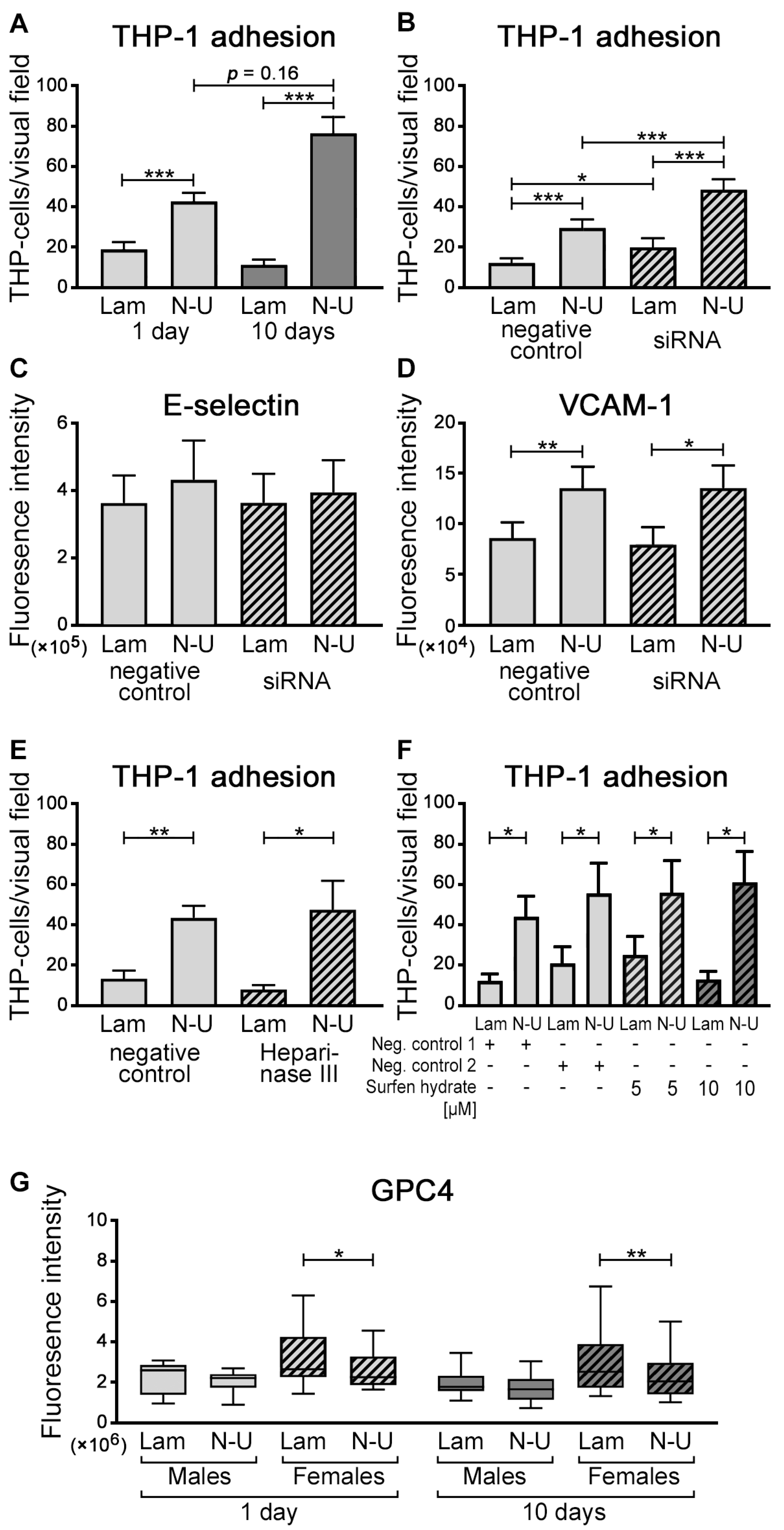
2.3. GPC4 Knockdown in HUVECs Facilitated THP-1 Cell Adhesion
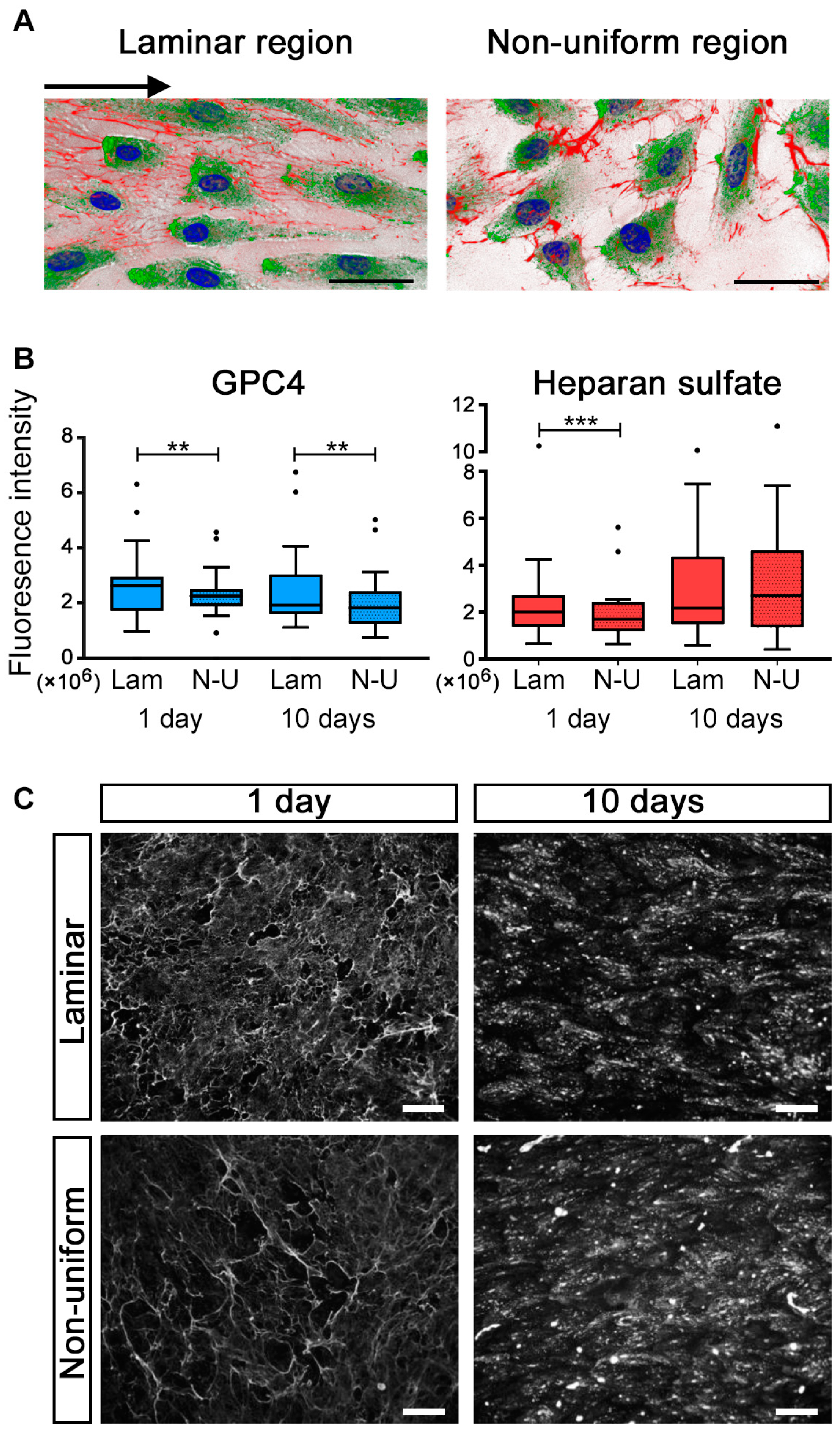
2.4. Sex Differences in GPC4
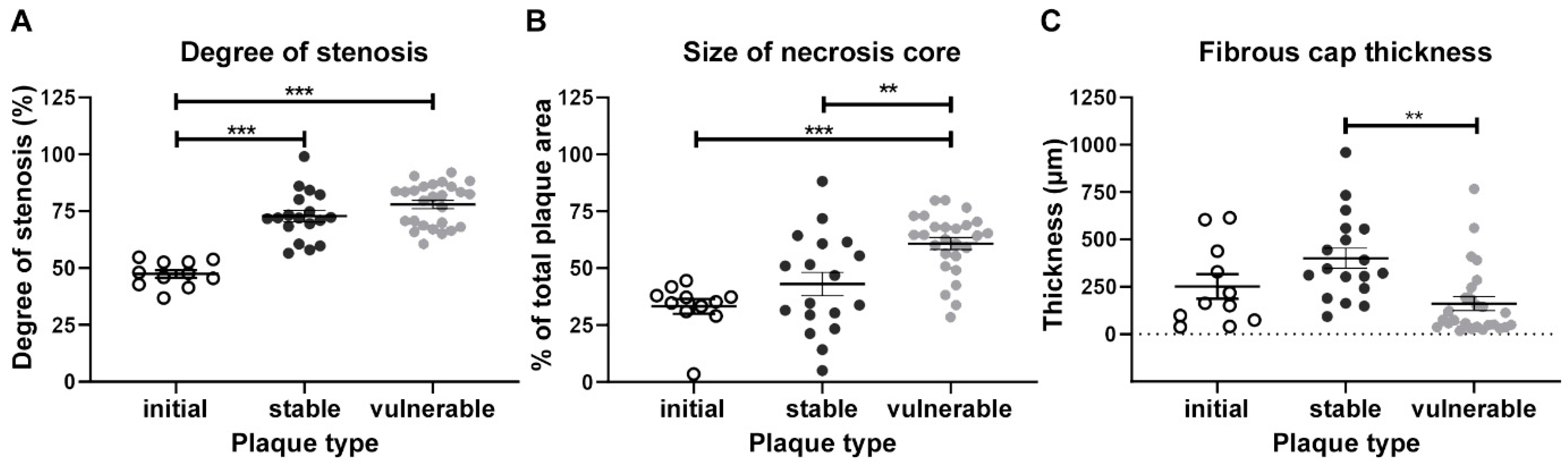
2.5. Human Atherosclerotic Plaques Lesions—General Characteristics
2.6. Distribution of Glycocalyx Components in Human Atherosclerotic Lesions
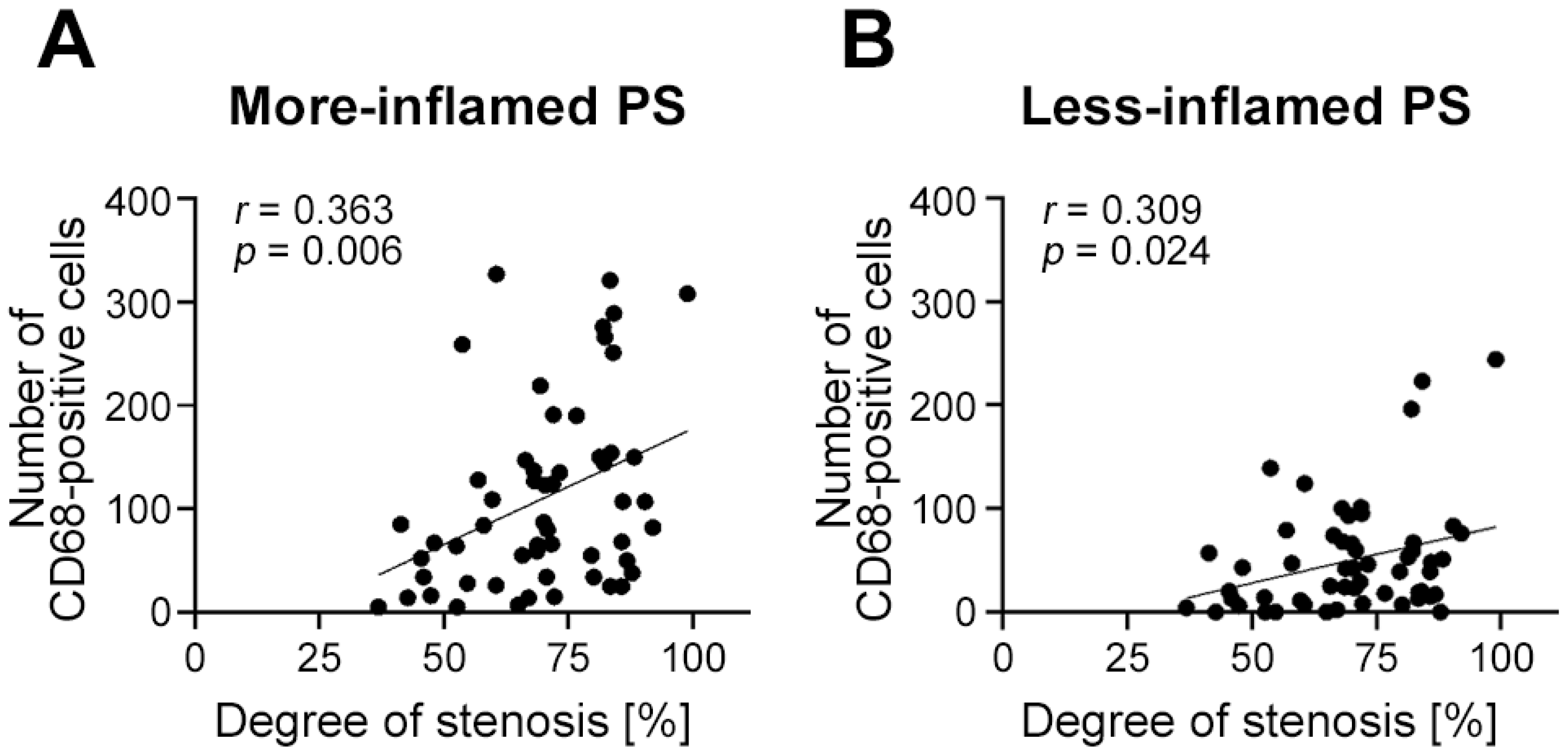
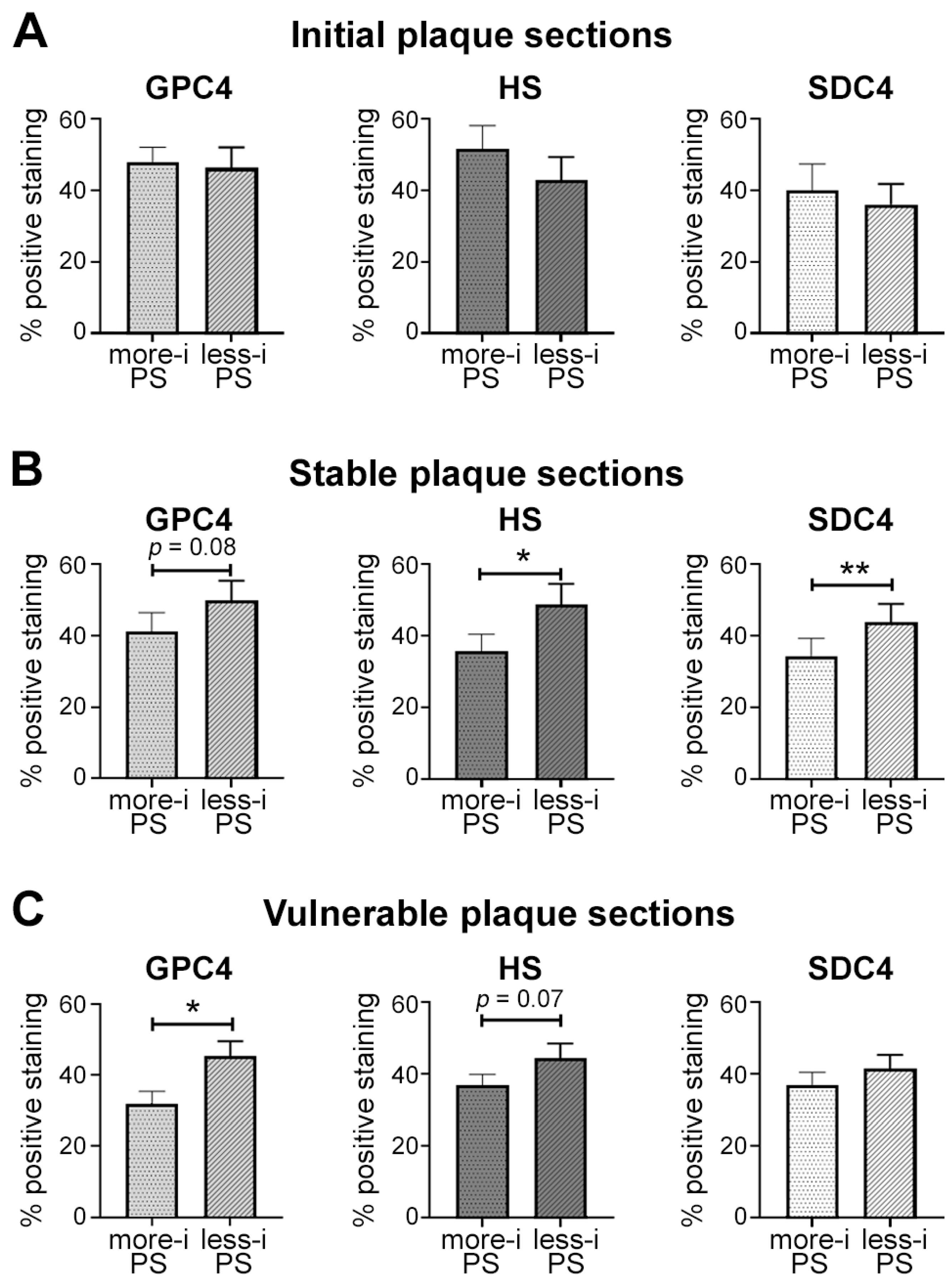
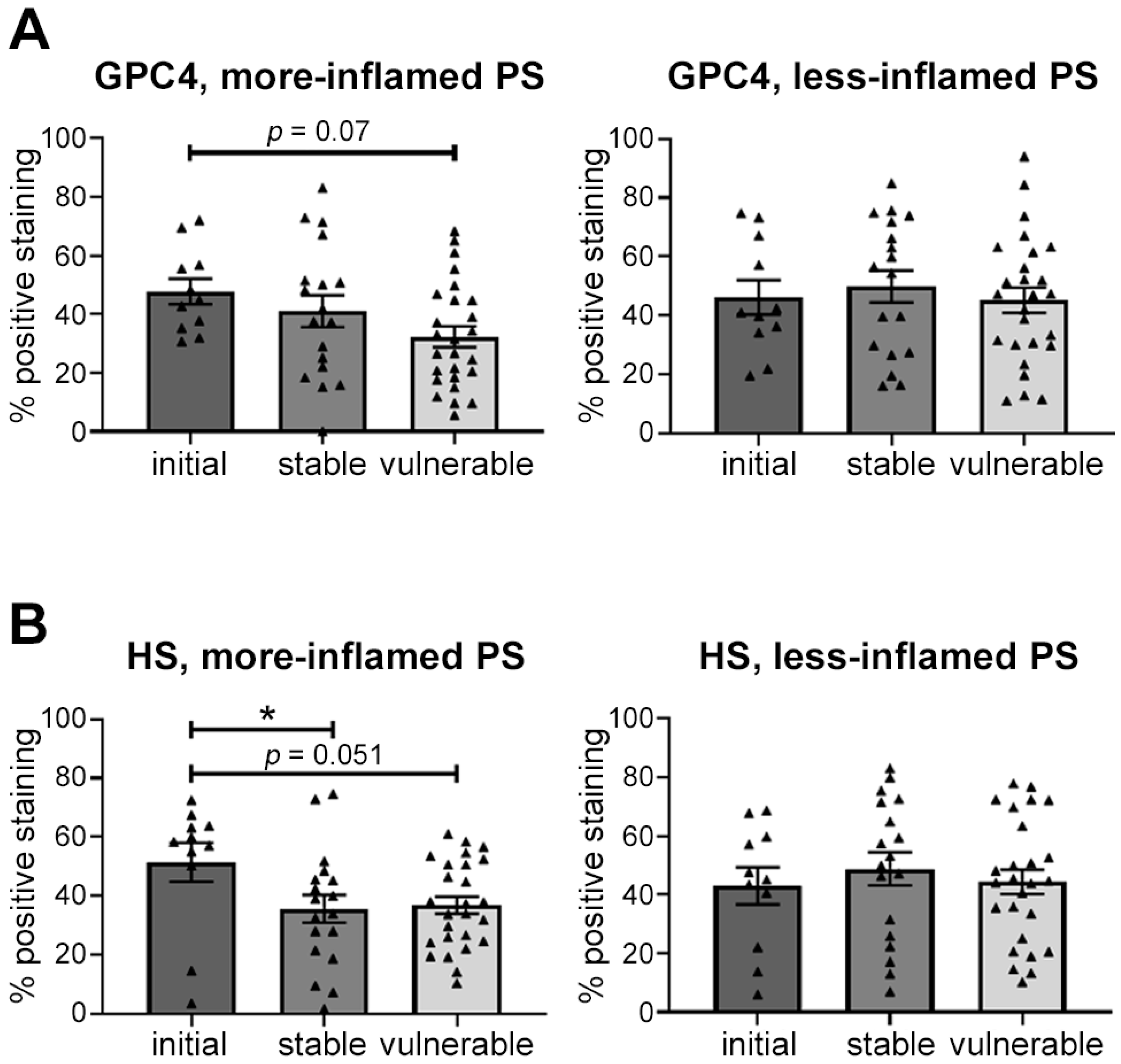
2.7. Endothelial Expression of Glycocalyx Components Depends on Plaque Vulnerability and Local Inflammation
3. Discussion
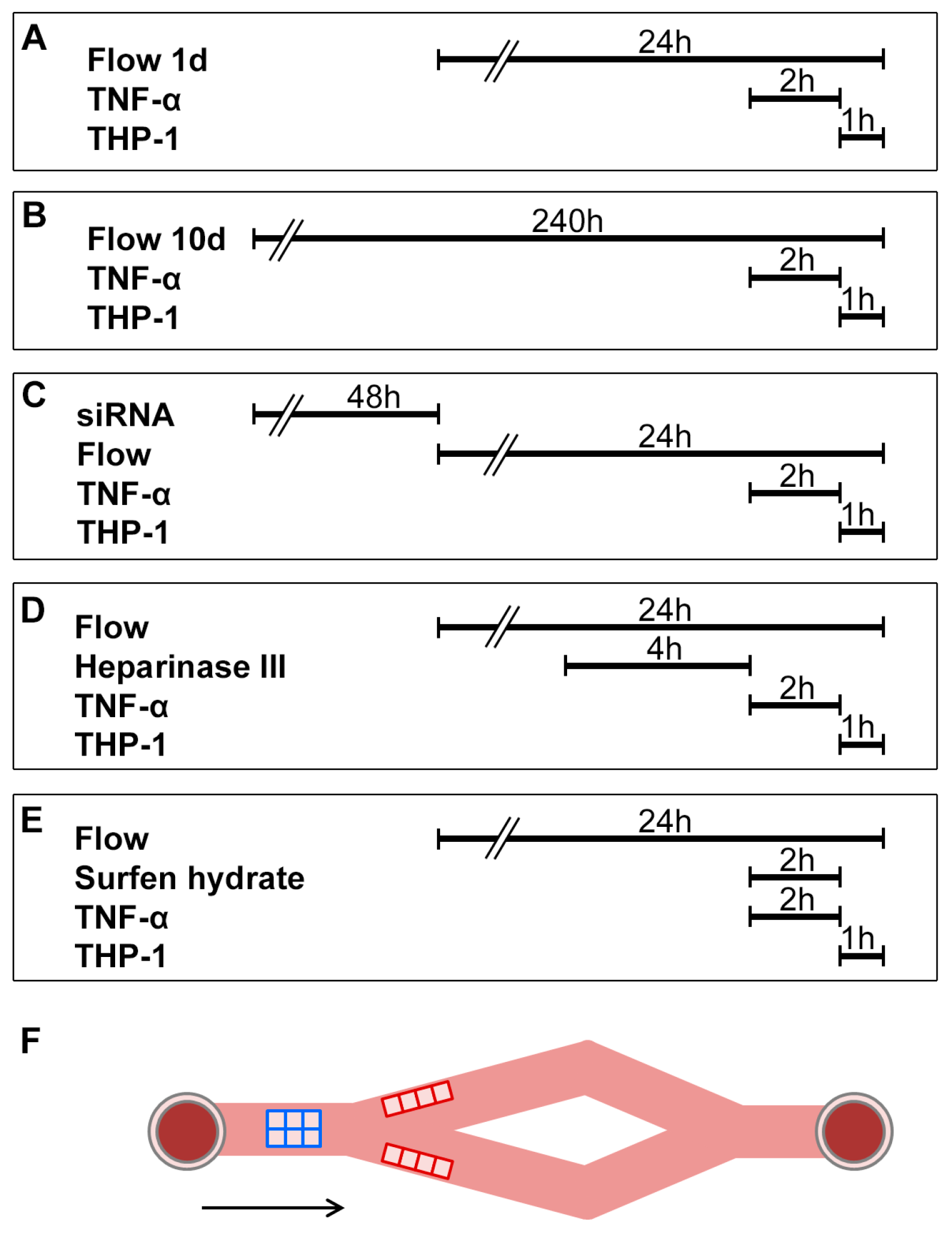
4. Materials and Methods
4.1. Cell Culture and Expression Analyses
4.2. Dynamic Flow Assay
4.3. Dynamic Adhesion Assays
4.4. Immunofluorescent Staining
4.5. Patients and Arterial Specimen Collection
4.6. Classification into Initial, Vulnerable, or StablePlaque Sections
4.7. Classification into More- or Less-Inflamed PSs
4.8. Immunohistochemical Staining
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, K.J.; Tabas, I. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Tannock, L.R.; King, V.L. Proteoglycan mediated lipoprotein retention: A mechanism of diabetic atherosclerosis. Rev. Endocr. Metab. Disord. 2008, 9, 289–300. [Google Scholar] [CrossRef]
- Stancu, C.S.; Toma, L.; Sima, A.V. Dual role of lipoproteins in endothelial cell dysfunction in atherosclerosis. Cell Tissue Res. 2012, 349, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef]
- Cicha, I.; Wörner, A.; Urschel, K.; Beronov, K.; Goppelt-Struebe, M.; Verhoeven, E.; Daniel, W.G.; Garlichs, C.D. Carotid plaque vulnerability-a positive feedback between hemodynamic and biochemical mechanisms. Stroke 2011, 42, 3502–3510. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Kuhlmann, M.T.; Eligehausen, S.; Kiefer, F.; Hermann, S.; Schäfers, M. Molecular imaging of MMP activity discriminates unstable from stable plaque phenotypes in shear-stress induced murine atherosclerosis. PLoS ONE 2018, 13, e0204305. [Google Scholar] [CrossRef]
- Paritala, P.K.; Yarlagadda, T.; Mendieta, J.B.; Wang, J.; McGahan, T.; Lloyd, T.; Yarlagadda, P.; Li, Z. Plaque Longitudinal Heterogeneity in Morphology, Property, and Mechanobiology. Cerebrovasc. Dis. 2021, 50, 510–519. [Google Scholar] [CrossRef]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef]
- Ebong, E.E.; Macaluso, F.P.; Spray, D.C.; Tarbell, J.M. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1908–1915. [Google Scholar] [CrossRef]
- Springer, T.A. Adhesion receptors of the immune system. Nature 1990, 346, 425–434. [Google Scholar] [CrossRef]
- Florian, J.A.; Kosky, J.R.; Ainslie, K.; Pang, Z.; Dull, R.O.; Tarbell, J.M. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res. 2003, 93, e136–e142. [Google Scholar] [CrossRef] [PubMed]
- Yayon, A.; Klagsbrun, M.; Esko, J.D.; Leder, P.; Ornitz, D.M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 1991, 64, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Okigaki, M.; Takahashi, T.; Katsume, A.; Adachi, Y.; Yamaguchi, S.; Matsunaga, S.; Takeda, M.; Matsui, A.; Kishita, E.; et al. Endothelial FGF receptor signaling accelerates atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H154–H161. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetti, S.; Paka, L.; Obunike, J.C.; Berglund, L.; Goldberg, I.J. Subendothelial retention of lipoprotein (a). Evidence that reduced heparan sulfate promotes lipoprotein binding to subendothelial matrix. J. Clin. Investig. 1997, 100, 867–874. [Google Scholar] [CrossRef]
- Wilsie, L.C.; Orlando, R.A. The low density lipoprotein receptor-related protein complexes with cell surface heparan sulfate proteoglycans to regulate proteoglycan-mediated lipoprotein catabolism. J. Biol. Chem. 2003, 278, 15758–15764. [Google Scholar] [CrossRef]
- Osterholm, C.; Folkersen, L.; Lengquist, M.; Ponten, F.; Renne, T.; Li, J.; Hedin, U. Increased expression of heparanase in symptomatic carotid atherosclerosis. Atherosclerosis 2013, 226, 67–73. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflug. Arch. Eur. J. Physiol. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Voyvodic, P.L.; Min, D.; Liu, R.; Williams, E.; Chitalia, V.; Dunn, A.K.; Baker, A.B. Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J. Biol. Chem. 2014, 289, 9547–9559. [Google Scholar] [CrossRef]
- Baeyens, N.; Mulligan-Kehoe, M.J.; Corti, F.; Simon, D.D.; Ross, T.D.; Rhodes, J.M.; Wang, T.Z.; Mejean, C.O.; Simons, M.; Humphrey, J.; et al. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 17308–17313. [Google Scholar] [CrossRef]
- Patterson, A.M.; Cartwright, A.; David, G.; Fitzgerald, O.; Bresnihan, B.; Ashton, B.A.; Middleton, J. Differential expression of syndecans and glypicans in chronically inflamed synovium. Ann. Rheum. Dis. 2008, 67, 592–601. [Google Scholar] [CrossRef]
- Ussar, S.; Bezy, O.; Blüher, M.; Kahn, C.R. Glypican-4 enhances insulin signaling via interaction with the insulin receptor and serves as a novel adipokine. Diabetes 2012, 61, 2289–2298. [Google Scholar] [CrossRef]
- Berletch, J.B.; Yang, F.; Xu, J.; Carrel, L.; Disteche, C.M. Genes that escape from X inactivation. Hum. Genet. 2011, 130, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Carrel, L.; Willard, H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005, 434, 400–404. [Google Scholar] [CrossRef]
- Koo, A.; Dewey, C.F., Jr.; Garcia-Cardena, G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am. J. Physiol. Cell Physiol. 2013, 304, C137–C146. [Google Scholar] [CrossRef]
- Urschel, K.; Worner, A.; Daniel, W.G.; Garlichs, C.D.; Cicha, I. Role of shear stress patterns in the TNF-alpha-induced atherogenic protein expression and monocytic cell adhesion to endothelium. Clin. Hemorheol. Microcirc. 2010, 46, 203–210. [Google Scholar] [CrossRef]
- Zeng, Y.; Tarbell, J.M. The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS ONE 2014, 9, e86249. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, X.; Wang, Z.; Gao, X.; Ge, Z.; Gu, Y.; Ye, P.; Chao, Y.; Zhu, L.; Li, X.; et al. AMP-activated protein kinase regulates glycocalyx impairment and macrophage recruitment in response to low shear stress. Faseb J. 2019, 33, 7202–7212. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Mayer, M.; Cancel, L.M.; Bartosch, A.M.; Mathews, R.; Tarbell, J.M. The Glycocalyx core protein Glypican 1 protects vessel wall endothelial cells from stiffness-mediated dysfunction and disease. Cardiovasc. Res. 2020, 117, 1592–1605. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.D.; Shworak, N.W.; Liu, J.; Schwartz, J.J.; Zhang, L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J. Clin. Investig. 1997, 100, S67–S75. [Google Scholar] [CrossRef] [PubMed]
- Ebong, E.E.; Lopez-Quintero, S.V.; Rizzo, V.; Spray, D.C.; Tarbell, J.M. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr. Biol. Quant. Biosci. Nano Macro 2014, 6, 338–347. [Google Scholar] [CrossRef]
- Bartosch, A.M.W.; Mathews, R.; Tarbell, J.M. Endothelial Glycocalyx-Mediated Nitric Oxide Production in Response to Selective AFM Pulling. Biophys. J. 2017, 113, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, L.F.; Lomakina, E.B.; Kuebel, J.; Waugh, R.E. Changes in endothelial glycocalyx layer protective ability after inflammatory stimulus. Am. J. Physiol. Cell Physiol. 2021, 320, C216–C224. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, A.M.W.; Mathews, R.; Mahmoud, M.M.; Cancel, L.M.; Haq, Z.S.; Tarbell, J.M. Heparan sulfate proteoglycan glypican-1 and PECAM-1 cooperate in shear-induced endothelial nitric oxide production. Sci. Rep. 2021, 11, 11386. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Beronov, K.; Ramirez, E.L.; Osterode, K.; Goppelt-Struebe, M.; Raaz, D.; Yilmaz, A.; Daniel, W.G.; Garlichs, C.D. Shear stress preconditioning modulates endothelial susceptibility to circulating TNF-alpha and monocytic cell recruitment in a simplified model of arterial bifurcations. Atherosclerosis 2009, 207, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.P.; Yang, Y.; Janssen, W.J.; Gandjeva, A.; Perez, M.J.; Barthel, L.; Zemans, R.L.; Bowman, J.C.; Koyanagi, D.E.; Yunt, Z.X.; et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012, 18, 1217–1223. [Google Scholar] [CrossRef]
- Allen, S.; Khan, S.; Tam, S.; Koschinsky, M.; Taylor, P.; Yacoub, M. Expression of adhesion molecules by lp(a): A potential novel mechanism for its atherogenicity. Faseb J. 1998, 12, 1765–1776. [Google Scholar] [CrossRef]
- Giuffre, L.; Cordey, A.S.; Monai, N.; Tardy, Y.; Schapira, M.; Spertini, O. Monocyte adhesion to activated aortic endothelium: Role of L-selectin and heparan sulfate proteoglycans. J. Cell Biol. 1997, 136, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Schuksz, M.; Fuster, M.M.; Brown, J.R.; Crawford, B.E.; Ditto, D.P.; Lawrence, R.; Glass, C.A.; Wang, L.; Tor, Y.; Esko, J.D. Surfen, a small molecule antagonist of heparan sulfate. Proc. Natl. Acad. Sci. USA 2008, 105, 13075–13080. [Google Scholar] [CrossRef] [PubMed]
- Cancel, L.M.; Ebong, E.E.; Mensah, S.; Hirschberg, C.; Tarbell, J.M. Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis 2016, 252, 136–146. [Google Scholar] [CrossRef]
- Bar, A.; Targosz-Korecka, M.; Suraj, J.; Proniewski, B.; Jasztal, A.; Marczyk, B.; Sternak, M.; Przybylo, M.; Kurpinska, A.; Walczak, M.; et al. Degradation of Glycocalyx and Multiple Manifestations of Endothelial Dysfunction Coincide in the Early Phase of Endothelial Dysfunction Before Atherosclerotic Plaque Development in Apolipoprotein E/Low-Density Lipoprotein Receptor-Deficient Mice. J. Am. Heart Assoc. 2019, 8, e011171. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.K.; Cooper, S.; Danielzak, L.; Leask, R.L. Glycocalyx Degradation Induces a Proinflammatory Phenotype and Increased Leukocyte Adhesion in Cultured Endothelial Cells under Flow. PLoS ONE 2016, 11, e0167576. [Google Scholar] [CrossRef]
- Muendlein, A.; Brandtner, E.M.; Leiherer, A.; Geiger, K.; Heinzle, C.; Gaenger, S.; Fraunberger, P.; Mader, A.; Saely, C.H.; Drexel, H. Serum glypican-4 is a marker of future vascular risk and mortality in coronary angiography patients. Atherosclerosis 2022, 345, 33–38. [Google Scholar] [CrossRef]
- Muendlein, A.; Brandtner, E.M.; Leiherer, A.; Geiger, K.; Heinzle, C.; Gaenger, S.; Fraunberger, P.; Mader, A.; Saely, C.H.; Drexel, H. Data on the association of serum glypican-4 with future major adverse cardiovascular events and mortality in patients undergoing coronary angiography. Data Brief 2022, 42, 108142. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, T.; Minami, Y.; Yamaoka-Tojo, M.; Kato, A.; Katsura, A.; Sato, T.; Muramatsu, Y.; Kakizaki, R.; Fujiyoshi, K.; Hashimoto, T.; et al. Endothelial glycocalyx and severity and vulnerability of coronary plaque in patients with coronary artery disease. Atherosclerosis 2020, 302, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.H.; de Carvalho Borges, M.; Schmidt, A.; Marin-Neto, J.A.; Pazin-Filho, A. Evaluation of the endothelial glycocalyx damage in patients with acute coronary syndrome. Atherosclerosis 2016, 247, 184–188. [Google Scholar] [CrossRef]
- Valerio, L.; Peters, R.J.; Zwinderman, A.H.; Pinto-Sietsma, S.J. Sublingual endothelial glycocalyx and atherosclerosis. A cross-sectional study. PLoS ONE 2019, 14, e0213097. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Lambadiari, V.; Rafouli-Stergiou, P.; Makavos, G.; Thymis, J.; Kostelli, G.; Varoudi, M.; Katogiannis, K.; Theodoropoulos, K.; et al. Endothelial glycocalyx and microvascular perfusion are associated with carotid intima-media thickness and impaired myocardial deformation in psoriatic disease. J. Hum. Hypertens. 2022, 36, 1113–1120. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Wenner, M.M.; Stachenfeld, N.S. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1569–H1588. [Google Scholar] [CrossRef]
- Grandoch, M.; Kohlmorgen, C.; Melchior-Becker, A.; Feldmann, K.; Homann, S.; Muller, J.; Kiene, L.S.; Zeng-Brouwers, J.; Schmitz, F.; Nagy, N.; et al. Loss of Biglycan Enhances Thrombin Generation in Apolipoprotein E-Deficient Mice: Implications for Inflammation and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, e41–e50. [Google Scholar] [CrossRef]
- Kunjathoor, V.V.; Chiu, D.S.; O’Brien, K.D.; LeBoeuf, R.C. Accumulation of biglycan and perlecan, but not versican, in lesions of murine models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 462–468. [Google Scholar] [CrossRef]
- Nakashima, Y.; Fujii, H.; Sumiyoshi, S.; Wight, T.N.; Sueishi, K. Early human atherosclerosis: Accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.C.; Tang, T.; Wilson, P.G.; Yoder, M.H.; Tannock, L.R. Increased atherosclerosis in mice with increased vascular biglycan content. Atherosclerosis 2014, 235, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Zyzynska-Granica, B.; Koziak, K. Identification of suitable reference genes for real-time PCR analysis of statin-treated human umbilical vein endothelial cells. PLoS ONE 2012, 7, e51547. [Google Scholar] [CrossRef] [PubMed]
- Schacher, N.M.; Raaz-Schrauder, D.; Pasutto, F.; Stumpfe, F.M.; Tauchi, M.; Dietel, B.; Achenbach, S.; Urschel, K. Impact of single nucleotide polymorphisms in the VEGFR2 gene on endothelial cell activation under nonuniform shear stress. Int. J. Mol. Med. 2019, 44, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Dalferes, E.R., Jr.; Radhakrishnamurthy, B.; Ruiz, H.A.; Berenson, G.S. Composition of proteoglycans from human atherosclerotic lesions. Exp. Mol. Pathol. 1987, 47, 363–376. [Google Scholar] [CrossRef]

| All (%) | Male (%) | Female (%) | Odds Ratio [95% CI] | p-Value | |
|---|---|---|---|---|---|
| Patients | 55 (100) | 38 (69) | 17 (31) | - | |
| Age (median ± SD) | 75 ± 8.9 | 75 ± 9.2 | 70 ± 8.2 | - | 0.385 a |
| BMI (mean ± SEM) | 27.7 ± 0.7 | 28.3 ± 0.8 | 26.4 ± 1.4 | - | 0.099 a |
| Obesity | 16 (29.1) | 11 (28.9) | 5 (29.4) | 1.02 [0.3126–3.259] | 1.0 b |
| Smoker * | 25 (45.5) | 17 (44.7) | 8 (47.1) | 1.10 [0.3799–3.303] | 0.89 c |
| T2D | 19 (34.5) | 15 (39.5) | 4 (23.5) | 0.47 [0.5561–1.798] | 0.36 b |
| Hypertension | 52 (94.5) | 36 (94.7) | 16 (94.1) | 0.89 [0.0981–13.61] | 1.0 b |
| Increased lipid serum levels ** | 47 (85.5) | 32 (84.2) | 15 (88.2) | 1.41 [0.2876–7.450] | 1.0 b |
| Asymptomatic | 37 (67.3) | 26 (68.4) | 11 (64.8) | 0.85 [0.2739–2.911] | 0.97 c |
| Symptomatic | 18 (32.7) | 12 (31.6) | 6 (35.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urschel, K.; Hug, K.P.; Zuo, H.; Büttner, M.; Furtmair, R.; Kuehn, C.; Stumpfe, F.M.; Botos, B.; Achenbach, S.; Yuan, Y.; et al. The Shear Stress–Regulated Expression of Glypican-4 in Endothelial Dysfunction In Vitro and Its Clinical Significance in Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 11595. https://doi.org/10.3390/ijms241411595
Urschel K, Hug KP, Zuo H, Büttner M, Furtmair R, Kuehn C, Stumpfe FM, Botos B, Achenbach S, Yuan Y, et al. The Shear Stress–Regulated Expression of Glypican-4 in Endothelial Dysfunction In Vitro and Its Clinical Significance in Atherosclerosis. International Journal of Molecular Sciences. 2023; 24(14):11595. https://doi.org/10.3390/ijms241411595
Chicago/Turabian StyleUrschel, Katharina, Karsten P. Hug, Hanxiao Zuo, Michael Büttner, Roman Furtmair, Constanze Kuehn, Florian M. Stumpfe, Balaz Botos, Stephan Achenbach, Yan Yuan, and et al. 2023. "The Shear Stress–Regulated Expression of Glypican-4 in Endothelial Dysfunction In Vitro and Its Clinical Significance in Atherosclerosis" International Journal of Molecular Sciences 24, no. 14: 11595. https://doi.org/10.3390/ijms241411595
APA StyleUrschel, K., Hug, K. P., Zuo, H., Büttner, M., Furtmair, R., Kuehn, C., Stumpfe, F. M., Botos, B., Achenbach, S., Yuan, Y., Dietel, B., & Tauchi, M. (2023). The Shear Stress–Regulated Expression of Glypican-4 in Endothelial Dysfunction In Vitro and Its Clinical Significance in Atherosclerosis. International Journal of Molecular Sciences, 24(14), 11595. https://doi.org/10.3390/ijms241411595





