Synthesis, In Vitro, and In Vivo Investigations of Pterostilbene-Tethered Analogues as Anti-Breast Cancer Candidates
Abstract
1. Introduction
2. Results and Discussion
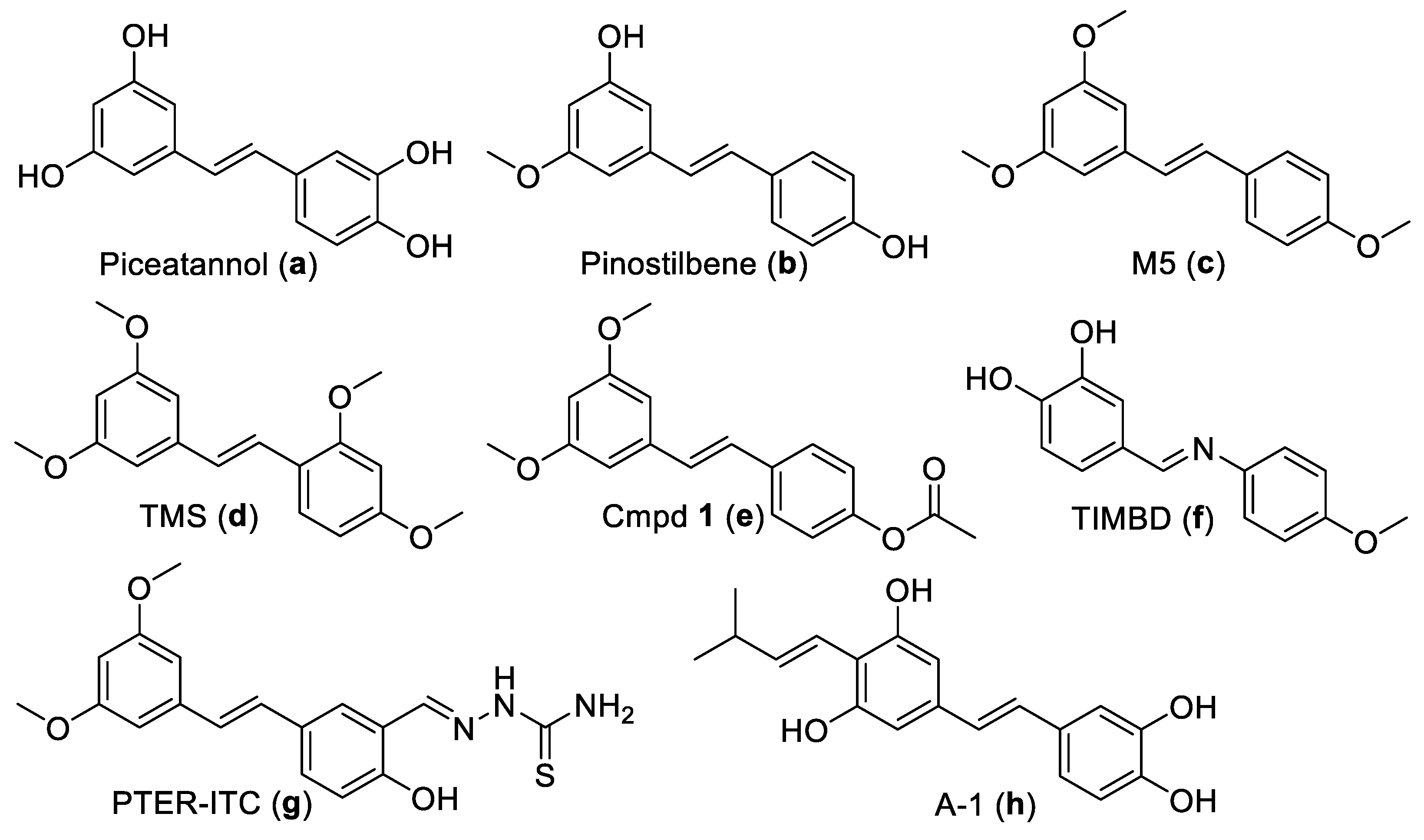
2.1. Chemistry
2.2. Evaluation of Anti-BC Activity
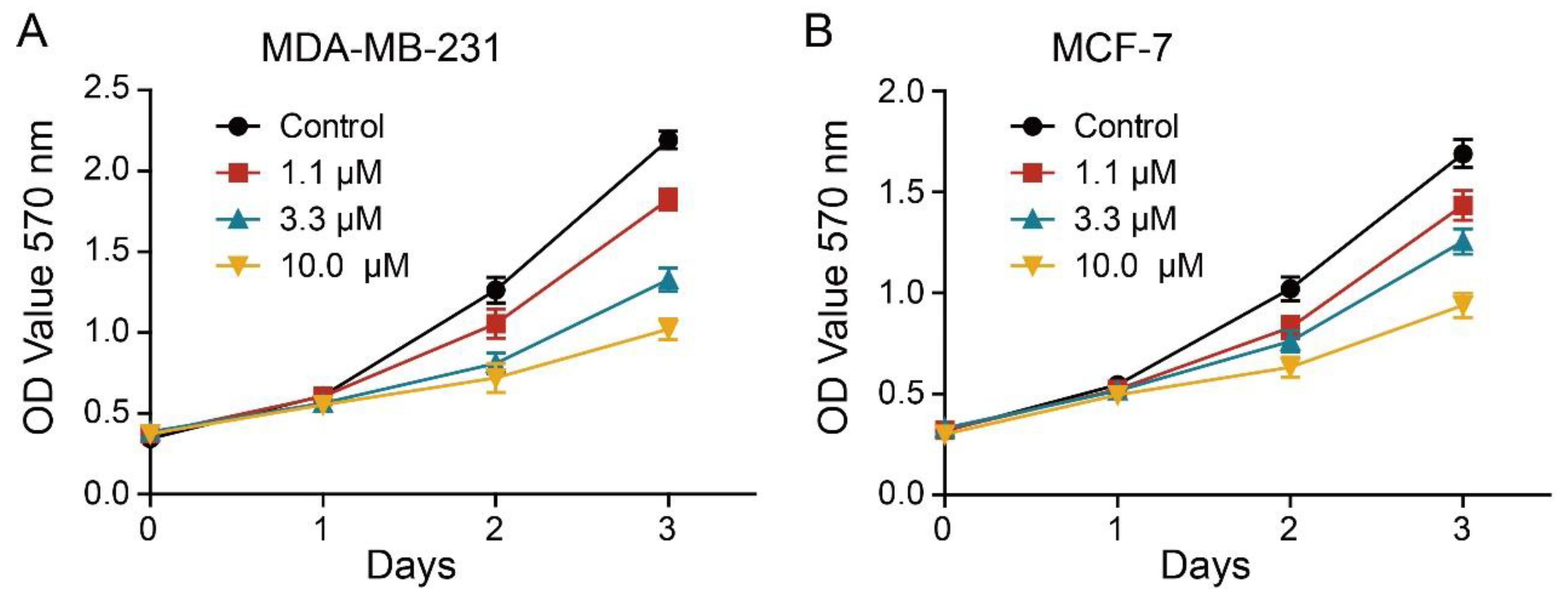
2.3. Effect of 2L on BC Cells Proliferation
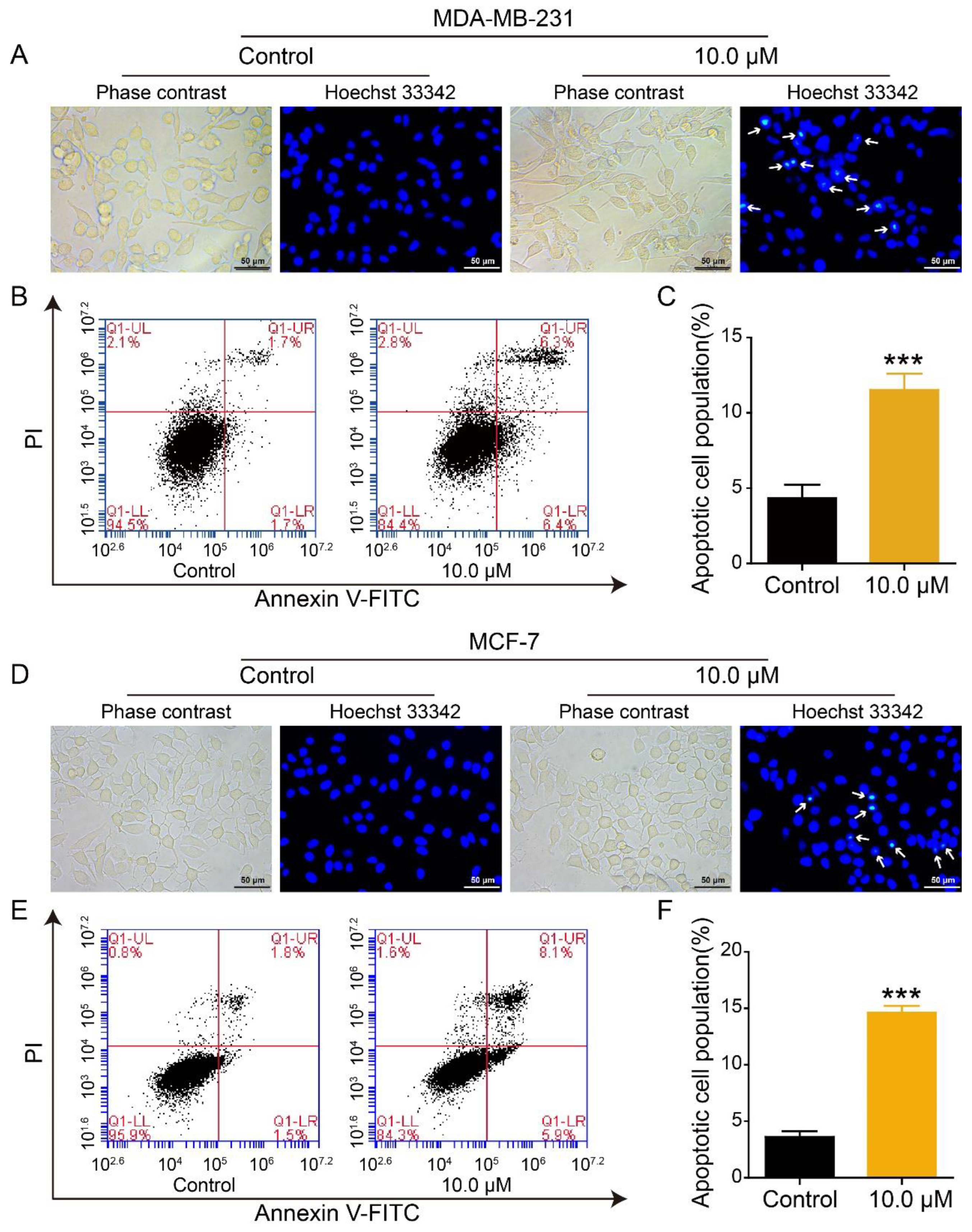
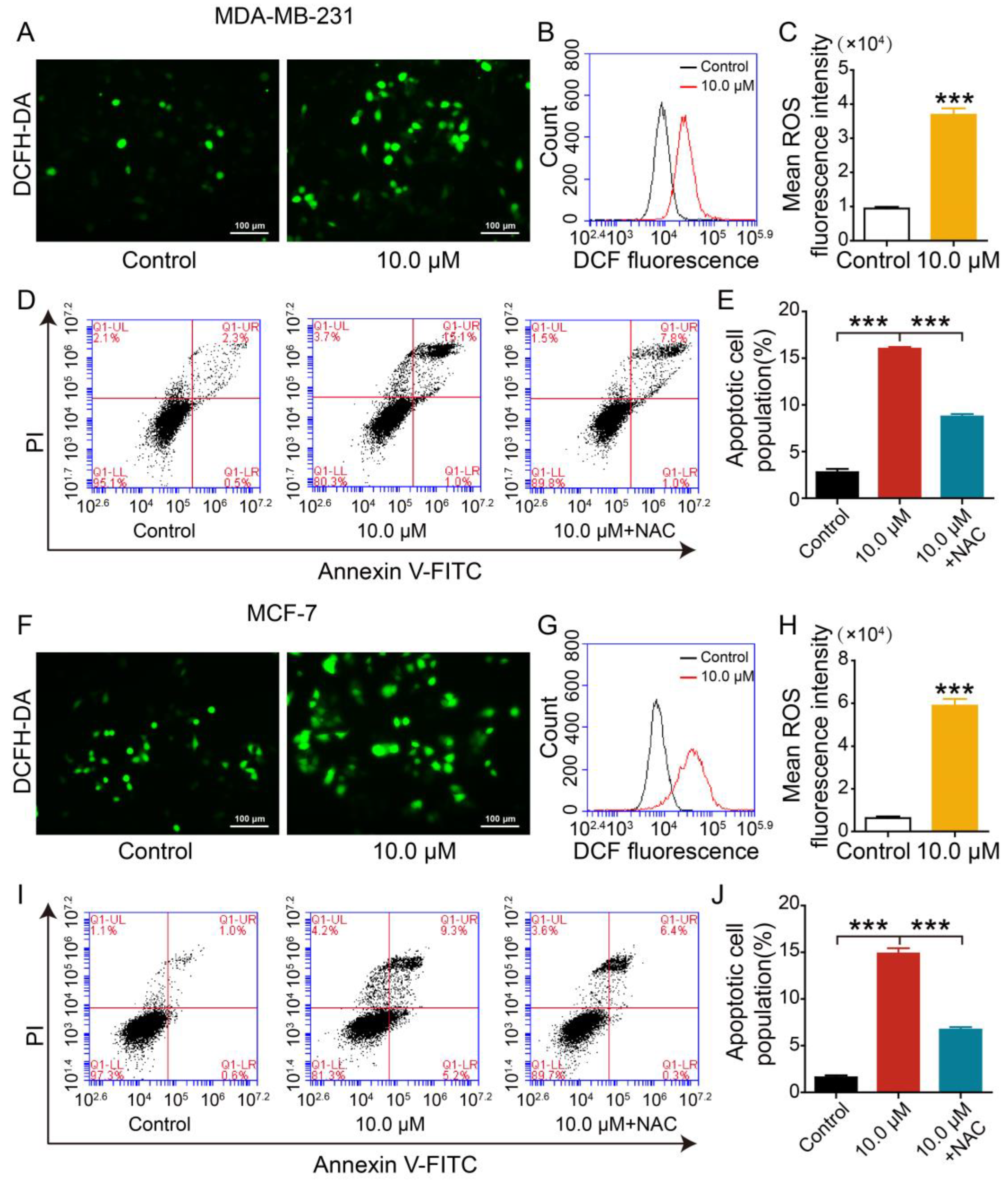
2.4. Effect of 2L on Apoptosis of BC Cells
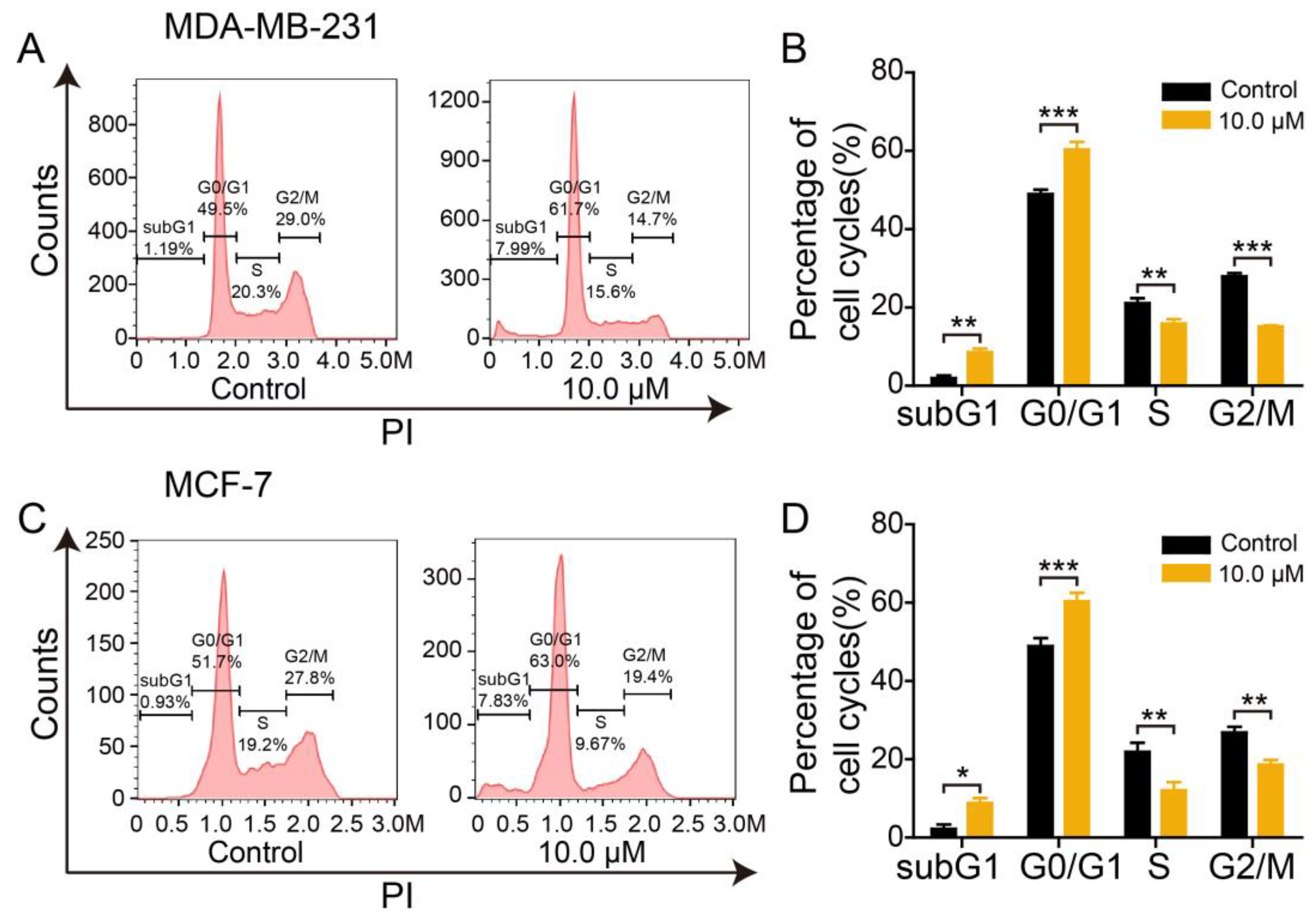
2.5. Effect of 2L on DNA Content of BC Cells
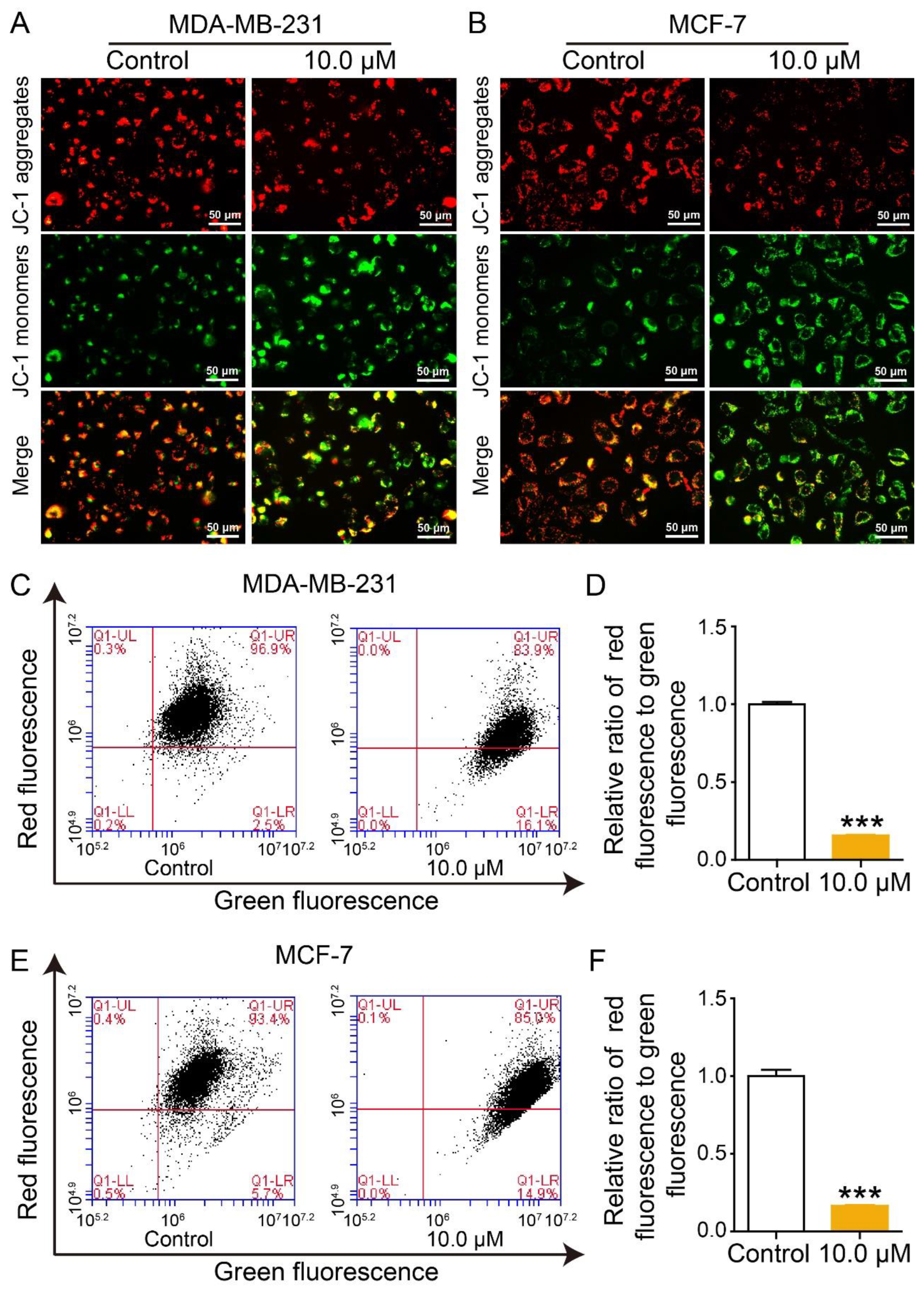
2.6. Effect of 2L on Mitochondrial Membrane Potential
2.7. Effect of 2L on Reactive Oxygen Species
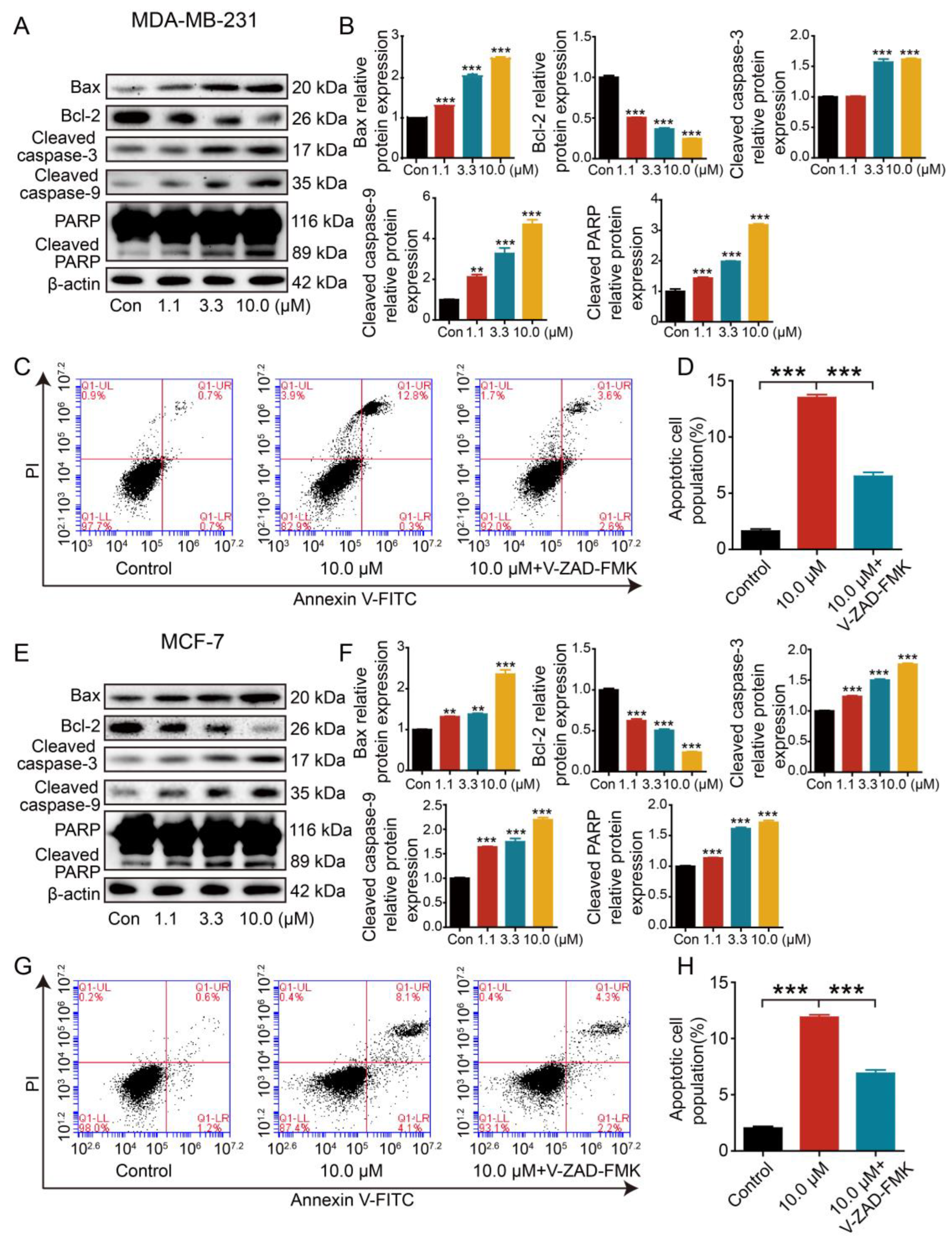
2.8. Effect of 2L on Apoptosis-Associated Proteins
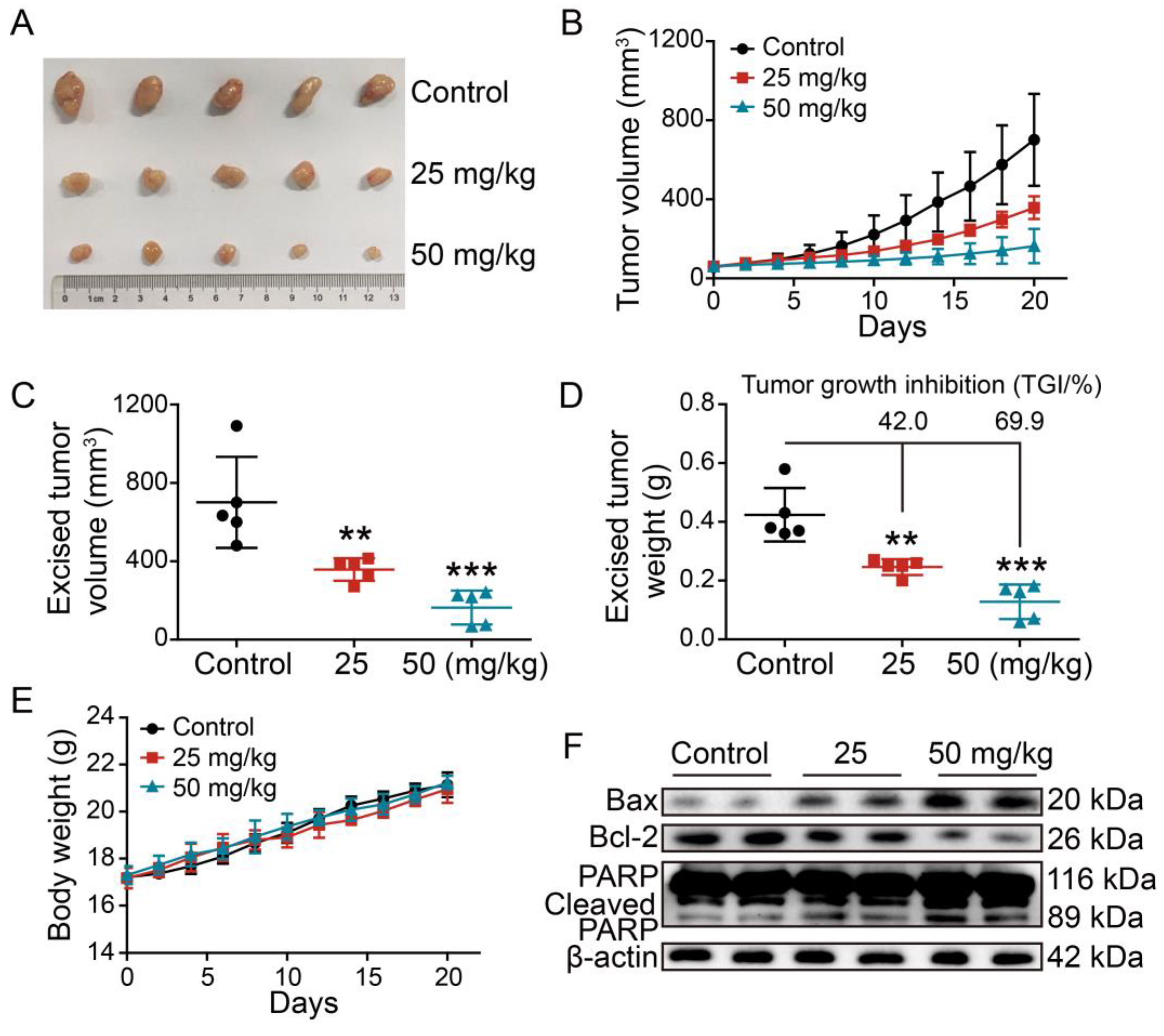
2.9. Evaluation of the Anti-Tumor Efficacy of 2L In Vivo
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedures
3.1.2. General Procedure for the Synthesis of the Title Compounds 2A–2N
3.2. Biological Evaluation
3.2.1. Cell Culture and Cell Proliferation Assay
3.2.2. Hoechst Staining
3.2.3. Cell Apoptosis Analysis
3.2.4. Mitochondrial Membrane Potential (MMP) Assay
3.2.5. Measurement of Intracellular ROS Levels
3.2.6. In Vivo Models
3.2.7. Western Blot Assay
3.2.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wang, B.; Wang, Z.; He, J.; Ma, X. Common multiple primary cancers associated with breast and gynecologic cancers and their risk factors, pathogenesis, treatment and prognosis: A review. Front. Oncol. 2022, 12, 840431. [Google Scholar] [CrossRef] [PubMed]
- Israel, B.B.; Tilghman, S.L.; Parker-Lemieux, K.; Payton-Stewart, F. Phytochemicals: Current strategies for treating breast cancer. Oncol. Lett. 2018, 15, 7471–7478. [Google Scholar] [CrossRef] [PubMed]
- Vito, A.; Rathmann, S.; Mercanti, N.; El-Sayes, N.; Mossman, K.; Valliant, J. Combined radionuclide therapy and immunotherapy for treatment of triple negative breast cancer. Int. J. Mol. Sci. 2021, 22, 4843. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Olov, N.; Bagheri-Khoulenjani, S.; Mirzadeh, H. Combinational drug delivery using nanocarriers for breast cancer treatments: A review. J. Biomed. Mater. Res. A 2018, 106, 2272–2283. [Google Scholar] [CrossRef]
- Du, M.; Ouyang, Y.; Meng, F.; Ma, Q.; Liu, H.; Zhuang, Y.; Pang, M.; Cai, T.; Cai, Y. Nanotargeted agents: An emerging therapeutic strategy for breast cancer. Nanomedicine 2019, 14, 1771–1786. [Google Scholar] [CrossRef]
- Hawash, M. Recent advances of tubulin inhibitors targeting the colchicine binding site forcancer therapy. Biomolecules 2022, 12, 1843. [Google Scholar] [CrossRef]
- Rimando, A.M.; Cuendet, M.; Desmarchelier, C.; Mehta, R.G.; Pezzuto, J.M.; Duke, S.O. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J. Agric. Food Chem. 2002, 50, 3453–3457. [Google Scholar] [CrossRef]
- Estrela, J.M.; Ortega, A.; Mena, S.; Rodriguez, M.L.; Asensi, M. Pterostilbene: Biomedical applications. Crit. Rev. Clin. Lab. Sci. 2013, 50, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.J.; Fernández, M.; Picó, Y.; Mañes, J.; Asensi, M.; Carda, C.; Asensio, G.; Estrela, J.M. Dietary administration of high doses of pterostilbene and quercetin to mice is not toxic. J. Agric. Food Chem. 2009, 57, 3180–3186. [Google Scholar] [CrossRef]
- Riche, D.M.; McEwen, C.L.; Riche, K.D.; Sherman, J.J.; Wofford, M.R.; Deschamp, D.; Griswold, M. Analysis of safety from a human clinical trial with pterostilbene. J. Toxicol. 2013, 2013, 463595. [Google Scholar] [CrossRef]
- Sun, C.; Li, Y.; Zhang, Y.; Huang, H.; Chen, H.; Chen, J.; Han, L.; Chen, X.; Chen, X.; Zhang, Y. Subacute oral toxicology and toxicokinetics of pterostilbene, a novel Top1/Tdp1 inhibiting anti-tumor reagent. Drug Chem. Toxicol. 2023, 46, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Xue, E.X.; Lin, J.P.; Zhang, Y.; Sheng, S.R.; Liu, H.X.; Zhou, Y.L.; Xu, H. Pterostilbene inhibits inflammation and ROS production in chondrocytes by activating Nrf2 pathway. Oncotarget 2017, 8, 41988–42000. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Liu, X.; Li, X.; Cao, Y.; Bai, Y.; Qi, F. Pterostilbene inhibits amyloid-β-induced neuroinflammation in a microglia cell line by inactivating the NLRP3/caspase-1 inflammasome pathway. J. Cell Biochem. 2018, 119, 7053–7062. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zorita, S.; Milton-Laskíbar, I.; Aguirre, L.; Fernández-Quintela, A.; Xiao, J.; Portillo, M.P. Effects of pterostilbene on diabetes, liver steatosis and serum lipids. Curr. Med. Chem. 2021, 28, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.C.; Tsai, Y.C.; Lin, J.N.; Fan, L.L.; Pan, M.H.; Ho, C.T.; Wu, J.-Y.; Way, T.-D. Activation of AMPK by pterostilbene suppresses lipogenesis and cell-cycle progression in p53 positive and negative human prostate cancer cells. J. Agric. Food Chem. 2012, 60, 6399–6407. [Google Scholar] [CrossRef]
- Yu, C.L.; Yang, S.F.; Hung, T.W.; Lin, C.L.; Hsieh, Y.H.; Chiou, H.L. Inhibition of eIF2α dephosphorylation accelerates pterostilbene-induced cell death in human hepatocellular carcinoma cells in an ER stress and autophagy-dependent manner. Cell Death Dis. 2019, 10, 418. [Google Scholar] [CrossRef]
- Bracht, J.W.P.; Karachaliou, N.; Berenguer, J.; Pedraz-Valdunciel, C.; Filipska, M.; Codony-Servat, C.; Codony-Servat, J.; Rosell, R. Osimertinib and pterostilbene in EGFR-mutation-positive non-small cell lung cancer (NSCLC). Int. J. Biol. Sci. 2019, 15, 2607–2614. [Google Scholar] [CrossRef]
- Pan, M.H.; Chang, Y.H.; Badmaev, V.; Nagabhushanam, K.; Ho, C.T. Pterostilbene induces apoptosis and cell cycle arrest in human gastric carcinoma cells. J. Agric. Food Chem. 2007, 55, 7777–7785. [Google Scholar] [CrossRef] [PubMed]
- Wawszczyk, J.; Jesse, K.; Smolik, S.; Kapral, M. Mechanism of pterostilbene-induced cell death in HT-29 colon cancer cells. Molecules 2022, 27, 369. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Hsu, K.Y.; Hung, C.M.; Lin, Y.C.; Yang, N.S.; Ho, C.T.; Kuo, S.-C.; Way, T.-D. The anti-tumor efficiency of pterostilbene is promoted with a combined treatment of Fas signaling or autophagy inhibitors in triple negative breast cancer cells. Food Funct. 2014, 5, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Wawszczyk, J.; Jesse, K.; Kapral, M. Pterostilbene-mediated inhibition of cell proliferation and cell death induction in amelanotic and melanotic melanoma. Int. J. Mol. Sci. 2023, 24, 1115. [Google Scholar] [CrossRef]
- Li, Y.; Sun, C.; Zhang, Y.; Chen, X.; Huang, H.; Han, L.; Xing, L.; Zhao, D.; Chen, X.; Zhang, Y. Phase I metabolism of pterostilbene, a dietary resveratrol derivative: Metabolite identification, species differences, isozyme contribution, and further bioactivation. J. Agric. Food Chem. 2023, 71, 331–346. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Sun, C.; Chen, X.; Han, L.; Wang, T.; Liu, J.; Chen, X.; Zhao, D. Effect of pterostilbene, a natural derivative of resveratrol, in the treatment of colorectal cancer through Top1/Tdp1-mediated DNA repair pathway. Cancers 2021, 13, 4002. [Google Scholar] [CrossRef]
- Kumar, V.; Haldar, S.; Das, N.S.; Ghosh, S.; Dhankhar, P.; Sircar, D.; Roy, P. Pterostilbene-isothiocyanate inhibits breast cancer metastasis by selectively blocking IKK-β/NEMO interaction in cancer cells. Biochem. Pharmacol. 2021, 192, 114717. [Google Scholar] [CrossRef]
- Harandi-Zadeh, S.; Boycott, C.; Beetch, M.; Yang, T.; Martin, B.J.E.; Ren, K.; Kwasniak, A.; Dupuis, J.H.; Lubecka, K.; Yada, R.Y.; et al. Pterostilbene changes epigenetic marks at enhancer regions of oncogenes in breast cancer cells. Antioxidants 2021, 10, 1232. [Google Scholar] [CrossRef]
- Hung, C.M.; Liu, L.C.; Ho, C.T.; Lin, Y.C.; Way, T.D. Pterostilbene enhances TRAIL-induced apoptosis through the induction of death receptors and downregulation of cell survival proteins in TRAIL-resistance triple negative breast cancer cells. J. Agric. Food Chem. 2017, 65, 11179–11191. [Google Scholar] [CrossRef]
- van den Brand, A.D.; Villevoye, J.; Nijmeijer, S.M.; van den Berg, M.; van Duursen, M.B.M. Anti-tumor properties of methoxylated analogues of resveratrol in malignant MCF-7 but not in non-tumorigenic MCF-10A mammary epithelial cell lines. Toxicology 2019, 422, 35–43. [Google Scholar] [CrossRef]
- Bader, Y.; Madlener, S.; Strasser, S.; Maier, S.; Saiko, P.; Stark, N.; Popescu, R.; Huber, D.; Gollinger, M.; Erker, T.; et al. Stilbene analogues affect cell cycle progression and apoptosis independently of each other in an MCF-7 array of clones with distinct genetic and chemoresistant backgrounds. Oncol. Rep. 2008, 19, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseinpour, S.; Weaver, A.; Sudhakaran, M.; Ho, L.C.; Le, T.; Doseff, A.I.; Medina-Bolivar, F. Arachidin-1, a prenylated stilbenoid from peanut, enhances the anticancer effects of paclitaxel in triple-negative breast cancer cells. Cancers 2023, 15, 399. [Google Scholar] [CrossRef]
- Komorowska, D.; Gajewska, A.; Hikisz, P.; Bartosz, G.; Rodacka, A. Comparison of the effects of resveratrol and its derivatives on the radiation response of MCF-7 breast cancer cells. Int. J. Mol. Sci. 2021, 22, 9511. [Google Scholar] [CrossRef]
- Chelsky, Z.L.; Yue, P.; Kondratyuk, T.P.; Paladino, D.; Pezzuto, J.M.; Cushman, M.; Turkson, J. A resveratrol analogue promotes ERKMAPK-dependent Stat3 serine and tyrosine phosphorylation alterations and antitumor effects in vitro against human tumor cells. Mol. Pharmacol. 2015, 88, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Ronghe, A.; Chatterjee, A.; Singh, B.; Dandawate, P.; Abdalla, F.; Bhat, N.K.; Padhye, S.; Bhat, H.K. 4-(E)-{(p-tolylimino)-methylbenzene-1,2-diol}, 1 a novel resveratrol analog, differentially regulates estrogen receptors α and β in breast cancer cells. Toxicol. Appl. Pharmacol. 2016, 301, 1–13. [Google Scholar] [CrossRef]
- Hu, C.J.; Li, G.X.; Mu, Y.; Wu, W.X.; Cao, B.X.; Wang, Z.X.; Yu, H.; Guan, P.; Han, L.; Li, L.; et al. Discovery of anti-TNBC agents targeting PTP1B: Total synthesis, structure-activity relationship, in vitro and in vivo investigations of jamunones. J. Med. Chem. 2021, 64, 6008–6020. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M.; Jaradat, N.; Eid, A.M.; Abubaker, A.; Mufleh, O.; Al-Hroub, Q.; Sobuh, S. Synthesis of novel isoxazole-carboxamide derivatives as promising agents for melanoma and targeted nano-emulgel conjugate for improved cellular permeability. BMC Chem. 2022, 16, 47. [Google Scholar] [CrossRef]
- Hawash, M.; Qaoud, M.T.; Jaradat, N.; Abdallah, S.; Issa, S.; Adnan, N.; Hoshya, M.; Sobuh, S.; Hawash, Z. Anticancer activity of thiophene carboxamide derivatives as CA-4 biomimetics: Synthesis, biological potency, 3D spheroid model, and molecular dynamics simulation. Biomimetics 2022, 7, 247. [Google Scholar] [CrossRef]
- Wyllie, A.H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980, 284, 555–556. [Google Scholar] [CrossRef]
- He, Z.; Chen, Q.; He, L.; Xiong, J.X.; Gao, K.; Lai, B.L.; Zheng, L.; Pu, Y.; Jiao, Y.; Ma, Z.; et al. Cyt-C mediated mitochondrial pathway plays an important role in Oocyte apoptosis in Ricefield Eel (Monopterus albus). Int. J. Mol. Sci. 2022, 23, 10555. [Google Scholar] [CrossRef]
- Huang, K.B.; Wang, F.Y.; Tang, X.M.; Feng, H.W.; Chen, Z.F.; Liu, Y.C.; Liu, Y.-N.; Liang, H. Organometallic Gold(III) complexes similar to tetrahydroisoquinoline induce ER-stress-mediated apoptosis and pro-death autophagy in A549 cancer cells. J. Med. Chem. 2018, 61, 3478–3490. [Google Scholar] [CrossRef]
- Li, J.; Kwon, N.; Jeong, Y.; Lee, S.; Kim, G.; Yoon, J. Aggregation-induced fluorescence probe for monitoring membrane potential changes in mitochondria. ACS Appl. Mater. Interfaces 2018, 10, 12150–12154. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Yamazaki, H.; Socorro, M.; Monier, D.; Beniash, E.; Napierala, D. Reactive oxygen species (ROS) generation as an underlying mechanism of inorganic phosphate (Pi)-induced mineralization of osteogenic cells. Free Radic. Biol. Med. 2020, 153, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species-sources, functions, oxidative damage. Pol. Merkur. Lekarski. 2020, 48, 124–127. [Google Scholar] [PubMed]
- Jiang, X.; Li, G.; Zhu, B.; Zang, J.; Lan, T.; Jiang, R.; Wang, B. p20BAP31 induces cell apoptosis via both AIF caspase-independent and the ROS/JNK mitochondrial pathway in colorectal cancer. Cell Mol. Biol. Lett. 2023, 28, 25. [Google Scholar] [CrossRef]
- Asmarinah, A.; Paradowska-Dogan, A.; Kodariah, R.; Tanuhardja, B.; Waliszewski, P.; Mochtar, C.A.; Budiningsih, Y.; Weidner, W.; Hinsch, E. Expression of the Bcl-2 family genes and complexes involved in the mitochondrial transport in prostate cancer cells. Int. J. Oncol. 2014, 45, 1489–1496. [Google Scholar] [CrossRef]
- Ponder, K.G.; Boise, L.H. The prodomain of caspase-3 regulates its own removal and caspase activation. Cell Death Discov. 2019, 5, 56. [Google Scholar] [CrossRef]
- Joza, N.; Susin, S.A.; Daugas, E.; Stanford, W.L.; Cho, S.K.; Li, C.Y.; Sasaki, T.; Elia, A.J.; Cheng, H.-Y.M.; Ravagnan, L.; et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 2001, 410, 549–554. [Google Scholar] [CrossRef]

| Compounds | BC Cell Lines (IC50: μM) | MCF-10A (IC50: μM) | |
|---|---|---|---|
| MDA-MB-231 | MCF-7 | ||
| 2K | 18.65 ± 0.71 *** | 21.01 ± 0.56 *** | 49.03 ± 1.31 |
| 2L | 10.39 ± 0.40 *** | 11.73 ± 0.55 *** | 68.87 ± 0.93 |
| 2M | 49.34 ± 2.48 * | 42.03 ± 0.79 | 70.39 ± 0.43 |
| 2N | 17.70 ± 0.81 *** | 18.06 ± 0.55 *** | 37.33 ± 0.96 |
| PTE | 63.59 ± 0.40 | 44.26 ± 0.48 | >100 |
| Dox b | 2.30 ± 0.32 | 3.57 ± 0.41 | 2.27 ± 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Li, J.; Wang, W.; Feng, X.; Yu, X.; Yuan, S.; Zhang, W.; Chen, J.; Hu, C. Synthesis, In Vitro, and In Vivo Investigations of Pterostilbene-Tethered Analogues as Anti-Breast Cancer Candidates. Int. J. Mol. Sci. 2023, 24, 11468. https://doi.org/10.3390/ijms241411468
Li G, Li J, Wang W, Feng X, Yu X, Yuan S, Zhang W, Chen J, Hu C. Synthesis, In Vitro, and In Vivo Investigations of Pterostilbene-Tethered Analogues as Anti-Breast Cancer Candidates. International Journal of Molecular Sciences. 2023; 24(14):11468. https://doi.org/10.3390/ijms241411468
Chicago/Turabian StyleLi, Guoxun, Jian Li, Wenqian Wang, Xiaoqing Feng, Xingkang Yu, Shuo Yuan, Wei Zhang, Jialing Chen, and Caijuan Hu. 2023. "Synthesis, In Vitro, and In Vivo Investigations of Pterostilbene-Tethered Analogues as Anti-Breast Cancer Candidates" International Journal of Molecular Sciences 24, no. 14: 11468. https://doi.org/10.3390/ijms241411468
APA StyleLi, G., Li, J., Wang, W., Feng, X., Yu, X., Yuan, S., Zhang, W., Chen, J., & Hu, C. (2023). Synthesis, In Vitro, and In Vivo Investigations of Pterostilbene-Tethered Analogues as Anti-Breast Cancer Candidates. International Journal of Molecular Sciences, 24(14), 11468. https://doi.org/10.3390/ijms241411468





