An Engineered Heat-Inducible Expression System for the Production of Casbene in Nicotiana benthamiana
Abstract
1. Introduction
2. Results
2.1. Design of Chimeric Heat-Shock Promoters
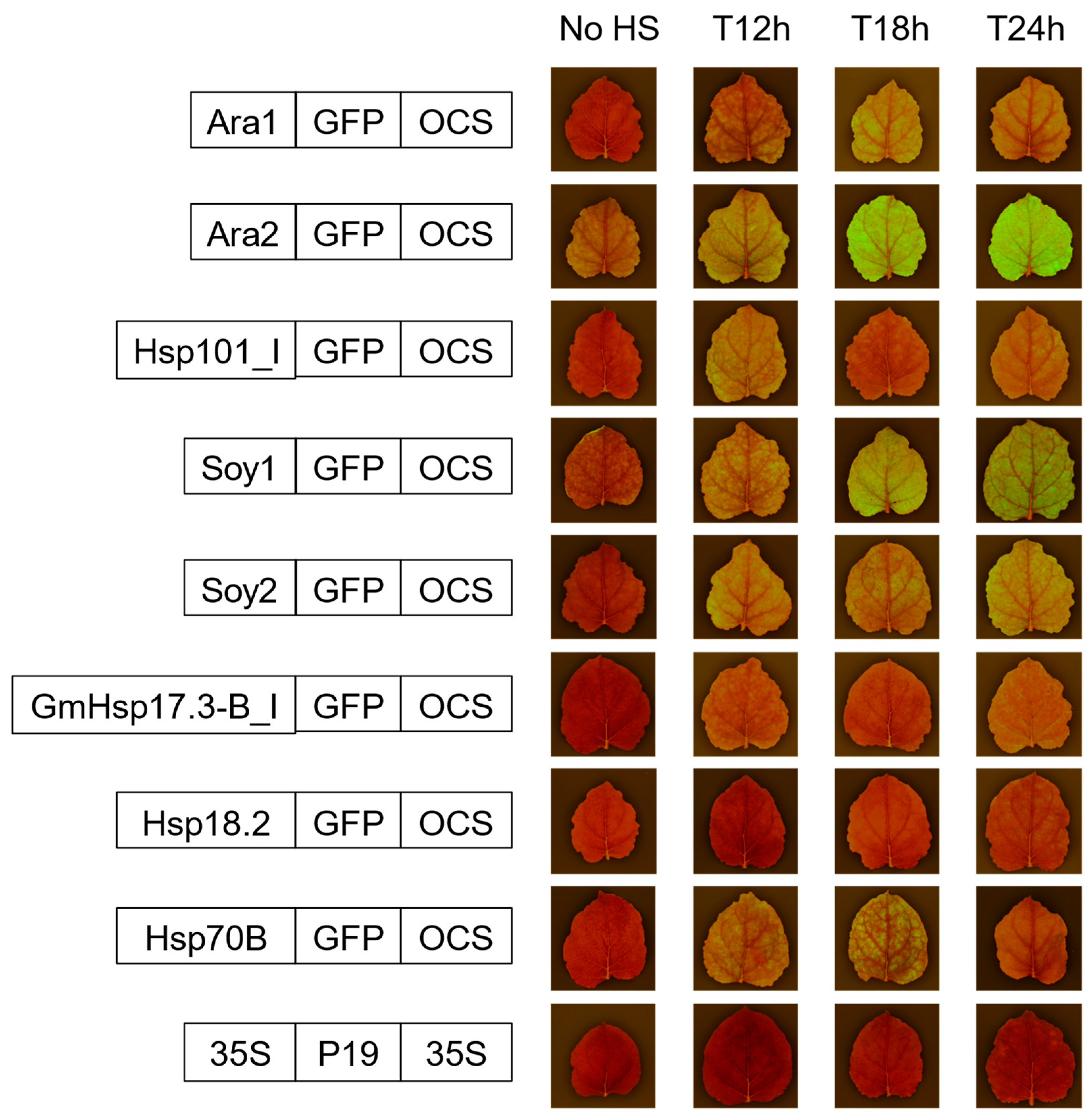
2.2. Transient Expression Test of GFP Constructs Driven by Heat-Shock Promoters in Nicotiana benthamiana
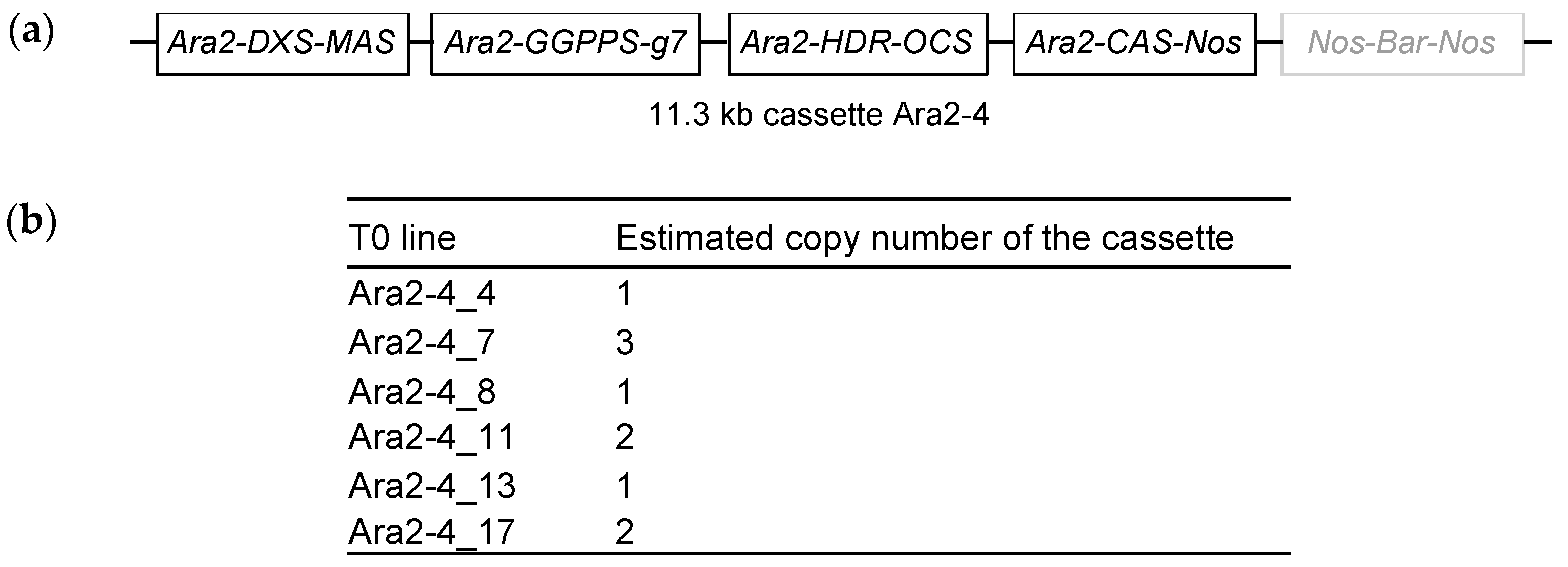
2.3. Stable Transformation of a Multigene Construct Driven by the Ara2 Promoter and Analysis of Casbene Production in the Associated Transgenic Lines
3. Discussion
4. Materials and Methods
4.1. Cloning of GFP Constructs and Transient Expression of GFP Driven by Heat Inducible Promoters in N. benthamiana
4.2. Heat-Shock and Visualization of GFP in Nicotiana benthamiana
4.3. Stable Expression in Nicotiana benthamiana of the Ara2-4 Cassette and Heating Treatment
4.4. Isolation and Quantification of Casbene in the Transgenic Lines
4.5. RNA Isolation and Real-Time Quantitative PCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritossa, F. A New Puffing Pattern Induced by Temperature Shock and DNP in Drosophila. Experientia 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Tissiéres, A.; Mitchell, H.K.; Tracy, U.M. Protein Synthesis in Salivary Glands of Drosophila Melanogaster: Relation to Chromosome Puffs. J. Mol. Biol. 1974, 84, 389–398. [Google Scholar] [CrossRef]
- Key, J.L.; Lin, C.Y.; Chen, Y.M. Heat Shock Proteins of Higher Plants. Proc. Natl. Acad. Sci. USA 1981, 78, 3526–3530. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, J.-H.; Ma, X.; Luo, D.-X.; Gong, Z.-H.; Lu, M.-H. The Plant Heat Stress Transcription Factors (HSFs): Structure, Regulation, and Function in Response to Abiotic Stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Pelham, H.R.B. A Regulatory Upstream Promoter Element in the Drosophila Hsp 70 Heat-Shock Gene. Cell 1982, 30, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Amin, J.; Ananthan, J.; Voellmy, R. Key Features of Heat Shock Regulatory Elements. Mol. Cell Biol. 1988, 8, 3761–3769. [Google Scholar]
- Saidi, Y.; Finka, A.; Chakhporanian, M.; Zrÿd, J.-P.; Schaefer, D.G.; Goloubinoff, P. Controlled Expression of Recombinant Proteins in Physcomitrella Patens by a Conditional Heat-Shock Promoter: A Tool for Plant Research and Biotechnology. Plant Mol. Biol. 2005, 59, 697–711. [Google Scholar] [CrossRef]
- Navarre, C.; Sallets, A.; Gauthy, E.; Maîtrejean, M.; Magy, B.; Nader, J.; Pety de Thozée, C.; Crouzet, J.; Batoko, H.; Boutry, M. Isolation of Heat Shock-Induced Nicotiana Tabacum Transcription Promoters and Their Potential as a Tool for Plant Research and Biotechnology. Transgenic Res. 2011, 20, 799–810. [Google Scholar] [CrossRef]
- Harrington, S.A.; Backhaus, A.E.; Fox, S.; Rogers, C.; Borrill, P.; Uauy, C.; Richardson, A. A Heat-Shock Inducible System for Flexible Gene Expression in Cereals. Plant Methods 2020, 16, 137. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Rodrigues, L.R. Potential Applications of the Escherichia Coli Heat Shock Response in Synthetic Biology. Trends Biotechnol. 2018, 36, 186–198. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Couto, M.R.; Araújo, R.G.; Prather, K.L.J.; Kluskens, L.; Rodrigues, L.R. Hydroxycinnamic Acids and Curcumin Production in Engineered Escherichia Coli Using Heat Shock Promoters. Biochem. Eng. J. 2017, 125, 41–49. [Google Scholar] [CrossRef]
- Forestier, E.C.F.; Czechowski, T.; Cording, A.C.; Gilday, A.D.; King, A.J.; Brown, G.D.; Graham, I.A. Developing a Nicotiana Benthamiana Transgenic Platform for High-value Diterpene Production and Candidate Gene Evaluation. Plant Biotechnol. J 2021, 19, 1614–1623. [Google Scholar] [CrossRef]
- De Ridder, T.; Reddell, P.; Jones, P.; Brown, G.; Campbell, J. Tigilanol Tiglate-Mediated Margins: A Comparison With Surgical Margins in Successful Treatment of Canine Mast Cell Tumours. Front. Vet. Sci. 2021, 8, 764800. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.W.; Conroy, G.C.; Reddell, P.; Ogbourne, S.M. Population Genetic Analysis of a Medicinally Significant Australian Rainforest Tree, Fontainea Picrosperma, C.T. White (Euphorbiaceae): Biogeographic Patterns and Implications for Species Domestication and Plantation Establishment. BMC Plant Biol. 2016, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Brown, G.D.; Gilday, A.D.; Larson, T.R.; Graham, I.A. Production of Bioactive Diterpenoids in the Euphorbiaceae Depends on Evolutionarily Conserved Gene Clusters. Plant Cell 2014, 26, 3286–3298. [Google Scholar] [CrossRef]
- King, A.J.; Brown, G.D.; Gilday, A.D.; Forestier, E.; Larson, T.R.; Graham, I.A. A Cytochrome P450-Mediated Intramolecular Carbon-Carbon Ring Closure in the Biosynthesis of Multidrug-Resistance-Reversing Lathyrane Diterpenoids. ChemBioChem 2016, 17, 1593–1597. [Google Scholar] [CrossRef]
- Luo, D.; Callari, R.; Hamberger, B.; Wubshet, S.G.; Nielsen, M.T.; Andersen-Ranberg, J.; Hallström, B.M.; Cozzi, F.; Heider, H.; Lindberg Møller, B.; et al. Oxidation and Cyclization of Casbene in the Biosynthesis of Euphorbia Factors from Mature Seeds of Euphorbia Lathyris L. Proc. Natl. Acad. Sci. USA 2016, 113, E5082–E5089. [Google Scholar] [CrossRef]
- Gelvin, S.B. Agrobacterium-Mediated Plant Transformation: The Biology behind the “Gene-Jockeying” Tool. Microbiol. Mol. Biol. Rev. 2003, 67, 16–37. [Google Scholar] [CrossRef]
- Felenbok, B. The Ethanol Utilization Regulon of Aspergillus Nidulans: The AlcA-AlcR System as a Tool for the Expression of Recombinant Proteins. J. Biotechnol. 1991, 17, 11–17. [Google Scholar] [CrossRef]
- Samalova, M.; Brzobohaty, B.; Moore, I. POp6/LhGR: A Stringently Regulated and Highly Responsive Dexamethasone-Inducible Gene Expression System for Tobacco: POp6/LhGR in Tobacco. Plant J. 2005, 41, 919–935. [Google Scholar] [CrossRef]
- Zuo, J.; Niu, Q.-W.; Chua, N.-H. An Estrogen Receptor-Based Transactivator XVE Mediates Highly Inducible Gene Expression in Transgenic Plants. Plant J 2000, 24, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Schöffl, F.; Raschke, E.; Nagao, R.T. The DNA Sequence Analysis of Soybean Heat-shock Genes and Identification of Possible Regulatory Promoter Elements. EMBO J. 1984, 3, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Baumann, G.; Raschke, E.; Bevan, M.; Schöffl, F. Functional Analysis of Sequences Required for Transcriptional Activation of a Soybean Heat Shock Gene in Transgenic Tobacco Plants. EMBO J. 1987, 6, 1161. [Google Scholar] [CrossRef]
- Schöffl, F.; Rieping, M.; Baumann, G.; Bevan, M.; Angermüller, S. The Function of Plant Heat Shock Promoter Elements in the Regulated Expression of Chimaeric Genes in Transgenic Tobacco. Mol. Gen. Genet. MGG 1989, 217, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Rieping, M.; Schöffl, F. Synergistic Effect of Upstream Sequences, CCAAT Box Elements, and HSE Sequences for Enhanced Expression of Chimaeric Heat Shock Genes in Transgenic Tobacco. Molec. Gen. Genet. 1992, 231, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ow, D.W.; Jacobs, J.D.; Howell, S.H. Functional Regions of the Cauliflower Mosaic Virus 35S RNA Promoter Determined by Use of the Firefly Luciferase Gene as a Reporter of Promoter Activity. Proc. Natl. Acad. Sci. USA 1987, 84, 4870–4874. [Google Scholar] [CrossRef]
- Odell, J.T.; Nagy, F.; Chua, N.-H. Identification of DNA Sequences Required for Activity of the Cauliflower Mosaic Virus 35S Promoter. Nature 1985, 313, 810–812. [Google Scholar] [CrossRef]
- Fang, R.X.; Nagy, F.; Sivasubramaniam, S.; Chua, N.H. Multiple Cis Regulatory Elements for Maximal Expression of the Cauliflower Mosaic Virus 35S Promoter in Transgenic Plants. Plant Cell 1989, 1, 141–150. [Google Scholar] [CrossRef]
- Benfey, P.N.; Ren, L.; Chua, N.H. The CaMV 35S Enhancer Contains at Least Two Domains Which Can Confer Different Developmental and Tissue-Specific Expression Patterns. EMBO J. 1989, 8, 2195–2202. [Google Scholar] [CrossRef]
- Hong, S.-W.; Vierling, E. Hsp101 Is Necessary for Heat Tolerance but Dispensable for Development and Germination in the Absence of Stress. Plant J. 2001, 27, 25–35. [Google Scholar] [CrossRef]
- Doğramacı, M.; Horvath, D.P.; Chao, W.S.; Foley, M.E.; Christoffers, M.J.; Anderson, J.V. Low Temperatures Impact Dormancy Status, Flowering Competence, and Transcript Profiles in Crown Buds of Leafy Spurge. Plant Mol. Biol. 2010, 73, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Padidam, M. Chemically Regulated Gene Expression in Plants. Curr. Opin. Plant Biol. 2003, 6, 169–177. [Google Scholar] [CrossRef]
- Engler, C.; Youles, M.; Gruetzner, R.; Ehnert, T.-M.; Werner, S.; Jones, J.D.G.; Patron, N.J.; Marillonnet, S. A Golden Gate Modular Cloning Toolbox for Plants. ACS Synth. Biol. 2014, 3, 839–843. [Google Scholar] [CrossRef]
- Gallie, D.R.; Sleat, D.E.; Watts, J.W.; Turner, P.C.; Wilson, T.M.A. The 5′-Leader Sequence of Tobacco Mosaic Virus RNA Enhances the Expression of Foreign Gene Transcripts in Vitro and in Vivo. Nucl. Acids Res. 1987, 15, 3257–3273. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Komeda, Y. Characterization of Two Genes Encoding Small Heat-Shock Proteins in Arabidopsis ThMinnn. Mol. Gen. Genet. 1989, 219, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.Y.; Vierling, E.; Guy, C.L. Comprehensive Expression Profile Analysis of the Arabidopsis Hsp70 Gene Family. Plant Physiol. 2001, 126, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Sarrion-Perdigones, A.; Vazquez-Vilar, M.; Palaci, J.; Castelijns, B.; Forment, J.; Ziarsolo, P.; Blanca, J.; Granell, A.; Orzaez, D. GoldenBraid 2.0: A Comprehensive DNA Assembly Framework for Plant Synthetic Biology. Plant Physiol. 2013, 162, 1618–1631. [Google Scholar] [CrossRef]
- Weber, E.; Engler, C.; Gruetzner, R.; Werner, S.; Marillonnet, S. A Modular Cloning System for Standardized Assembly of Multigene Constructs. PLoS ONE 2011, 6, e16765. [Google Scholar] [CrossRef]
- Werner, S.; Engler, C.; Weber, E.; Gruetzner, R.; Marillonnet, S. Fast Track Assembly of Multigene Constructs Using Golden Gate Cloning and the MoClo System. Bioengineered 2012, 3, 38–43. [Google Scholar] [CrossRef]
- Norkunas, K.; Harding, R.; Dale, J.; Dugdale, B. Improving Agroinfiltration-Based Transient Gene Expression in Nicotiana Benthamiana. Plant Methods 2018, 14, 71. [Google Scholar] [CrossRef]
- Rohmer, M. The Discovery of a Mevalonate-Independent Pathway for Isoprenoid Biosynthesis in Bacteria, Algae and Higher Plants†. Nat. Prod. Rep. 1999, 16, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-D.; Papp, I.; Moscone, E.A.; Iglesias, V.A.; Vaucheret, H.; Matzke, A.J.M.; Matzke, M.A. Gene Silencing Mediated by Promoter Homology Occurs at the Level of Transcription and Results in Meiotically Heritable Alterations in Methylation and Gene Activity. Plant J. 1996, 9, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Tzfira, T.; Li, J.; Lacroix, B.; Citovsky, V. Agrobacterium T-DNA Integration: Molecules and Models. Trends Genet. 2004, 20, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Kim, W.-C. A Fruitful Decade Using Synthetic Promoters in the Improvement of Transgenic Plants. Front. Plant Sci. 2019, 10, 1433. [Google Scholar] [CrossRef]
- Yoshida, K.; Kasai, T.; Garcia, M.R.C.; Sawada, S.; Shoji, T.; Shimizu, S.; Yamazaki, K.; Komeda, Y.; Shinmyo, A. Heat-Inducible Expression System for a Foreign Gene in Cultured Tobacco Cells Using the HSP18. 2 Promoter of Arabidopsis Thaliana. Appl. Microbiol. Biotechnol. 1995, 44, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Alsamir, M.; Mahmood, T.; Trethowan, R.; Ahmad, N. An Overview of Heat Stress in Tomato (Solanum lycopersicum L.). Saudi J. Biol. Sci. 2021, 28, 1654–1663. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility Nicotiana Benthamiana Domin. Available online: https://www.gbif.org/species/3800423 (accessed on 8 November 2021).
- Gerasymenko, I.M.; Sheludko, Y.V. Synthetic Cold-Inducible Promoter Enhances Recombinant Protein Accumulation during Agrobacterium-Mediated Transient Expression in Nicotiana Excelsior at Chilling Temperatures. Biotechnol. Lett. 2017, 39, 1059–1067. [Google Scholar] [CrossRef]
- Hamilton, C.M.; Frary, A.; Lewis, C.; Tanksley, S.D. Stable Transfer of Intact High Molecular Weight DNA into Plant Chromosomes. Proc. Natl. Acad. Sci. USA 1996, 93, 9975–9979. [Google Scholar] [CrossRef]
- Rajeevkumar, S.; Anunanthini, P.; Sathishkumar, R. Epigenetic Silencing in Transgenic Plants. Front. Plant Sci. 2015, 6, 693. [Google Scholar] [CrossRef]
- Höfgen, R.; Willmitzer, L. Storage of Competent Cells for Agrobacterium Transformation. Nucleic Acids Res. 1988, 16, 9877. [Google Scholar] [CrossRef]
- Sainsbury, F.; Thuenemann, E.C.; Lomonossoff, G.P. PEAQ: Versatile Expression Vectors for Easy and Quick Transient Expression of Heterologous Proteins in Plants. Plant Biotechnol. J. 2009, 7, 682–693. [Google Scholar] [CrossRef]
- Voinnet, O.; Rivas, S.; Mestre, P.; Baulcombe, D. An Enhanced Transient Expression System in Plants Based on Suppression of Gene Silencing by the P19 Protein of Tomato Bushy Stunt Virus. Plant J. 2003, 33, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Shagin, D.A.; Barsova, E.V.; Yanushevich, Y.G.; Fradkov, A.F.; Lukyanov, K.A.; Labas, Y.A.; Semenova, T.N.; Ugalde, J.A.; Meyers, A.; Nunez, J.M.; et al. GFP-like Proteins as Ubiquitous Metazoan Superfamily: Evolution of Functional Features and Structural Complexity. Mol. Biol. Evol. 2004, 21, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Beck, G.; Coman, D.; Herren, E.; Ruiz-Sola, M.Á.; Rodríguez-Concepción, M.; Gruissem, W.; Vranová, E. Characterization of the GGPP Synthase Gene Family in Arabidopsis Thaliana. Plant Mol. Biol. 2013, 82, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Leon, P.; Boronat, A.; Rodriguezconcepcion, M. The Plastidial MEP Pathway: Unified Nomenclature and Resources. Trends Plant Sci. 2008, 13, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Horsch, R.B.; Fry, J.; Hoffmann, N.; Neidermeyer, J.; Rogers, S.G.; Fraley, R.T. Leaf Disc Transformation. In Plant Molecular Biology Manual; Gelvin, S.B., Schilperoort, R.A., Verma, D.P.S., Eds.; Springer: Dordrecht, The Netherlands, 1989; pp. 63–71. ISBN 978-94-009-0951-9. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forestier, E.C.F.; Cording, A.C.; Loake, G.J.; Graham, I.A. An Engineered Heat-Inducible Expression System for the Production of Casbene in Nicotiana benthamiana. Int. J. Mol. Sci. 2023, 24, 11425. https://doi.org/10.3390/ijms241411425
Forestier ECF, Cording AC, Loake GJ, Graham IA. An Engineered Heat-Inducible Expression System for the Production of Casbene in Nicotiana benthamiana. International Journal of Molecular Sciences. 2023; 24(14):11425. https://doi.org/10.3390/ijms241411425
Chicago/Turabian StyleForestier, Edith C. F., Amy C. Cording, Gary J. Loake, and Ian A. Graham. 2023. "An Engineered Heat-Inducible Expression System for the Production of Casbene in Nicotiana benthamiana" International Journal of Molecular Sciences 24, no. 14: 11425. https://doi.org/10.3390/ijms241411425
APA StyleForestier, E. C. F., Cording, A. C., Loake, G. J., & Graham, I. A. (2023). An Engineered Heat-Inducible Expression System for the Production of Casbene in Nicotiana benthamiana. International Journal of Molecular Sciences, 24(14), 11425. https://doi.org/10.3390/ijms241411425





