Real-World Use of Highly Sensitive Liquid Biopsy Monitoring in Metastatic Breast Cancer Patients Treated with Endocrine Agents after Exposure to Aromatase Inhibitors
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of the Study Population
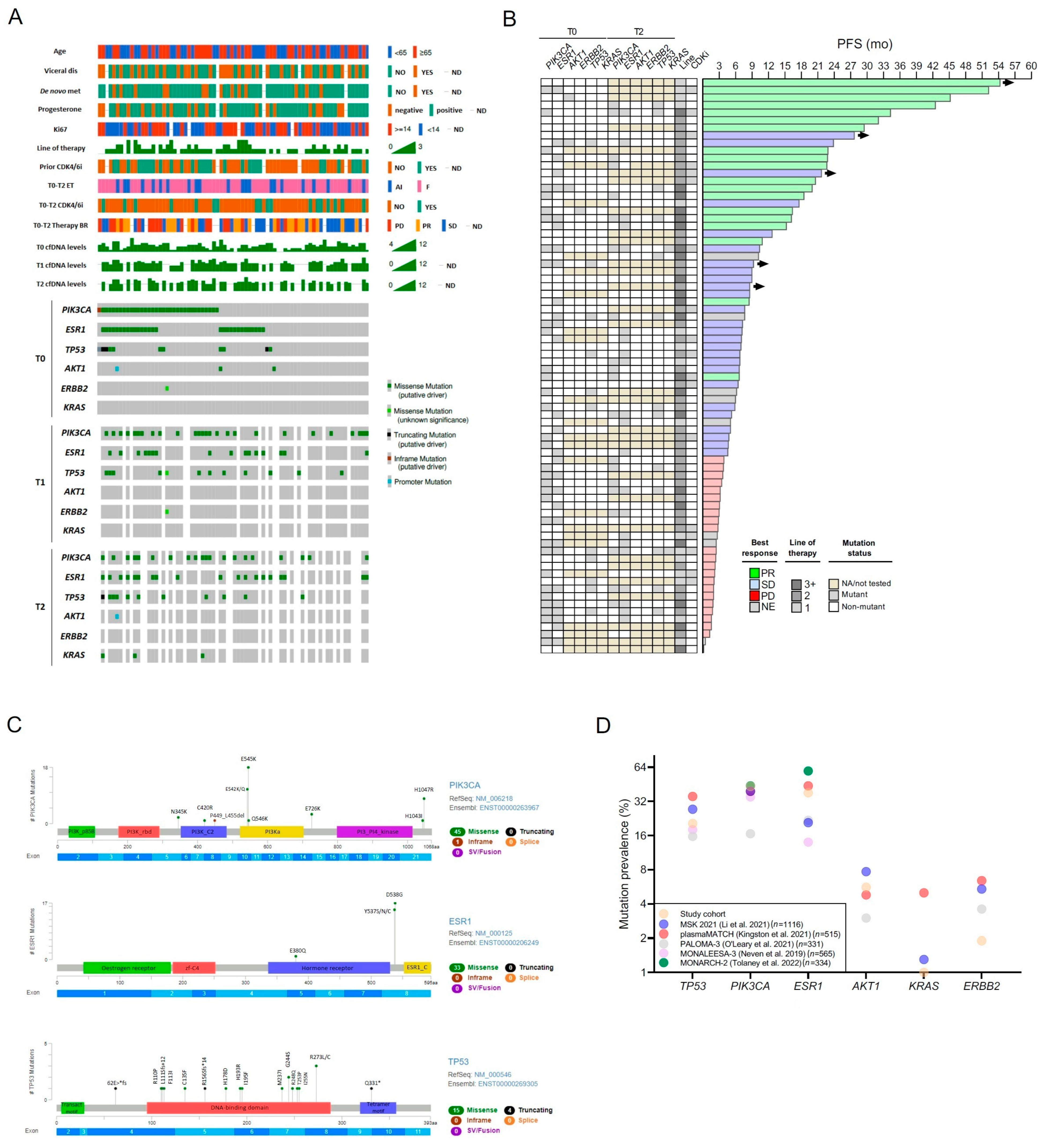
2.2. Mutational Landscape in Baseline ctDNA
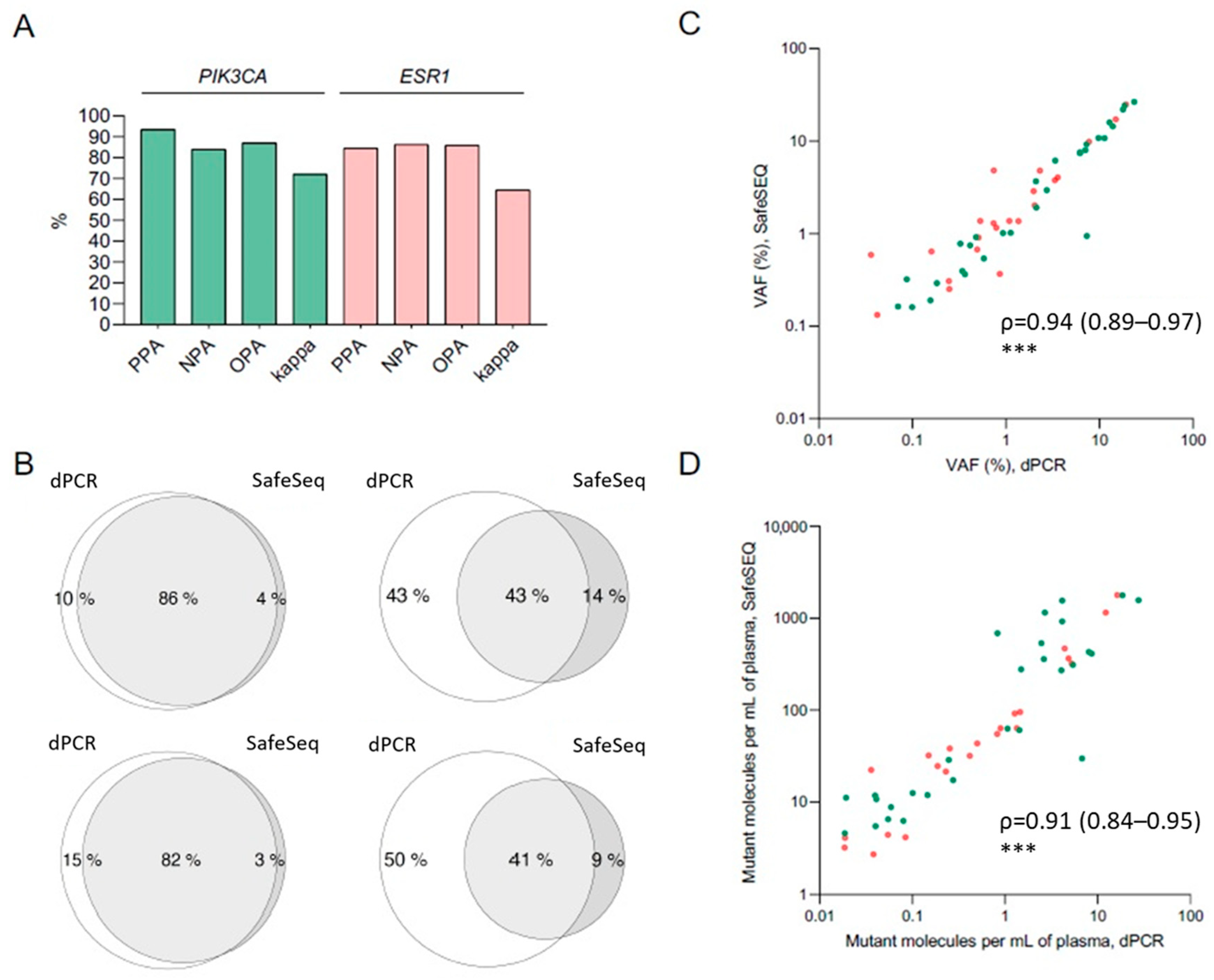
2.3. Concordance between dPCR and SafeSEQ NGS
2.4. Clonal Evolution from Archival Tissue to Liquid Biopsy
2.5. Cell-Free DNA at Baseline and Early Dynamics
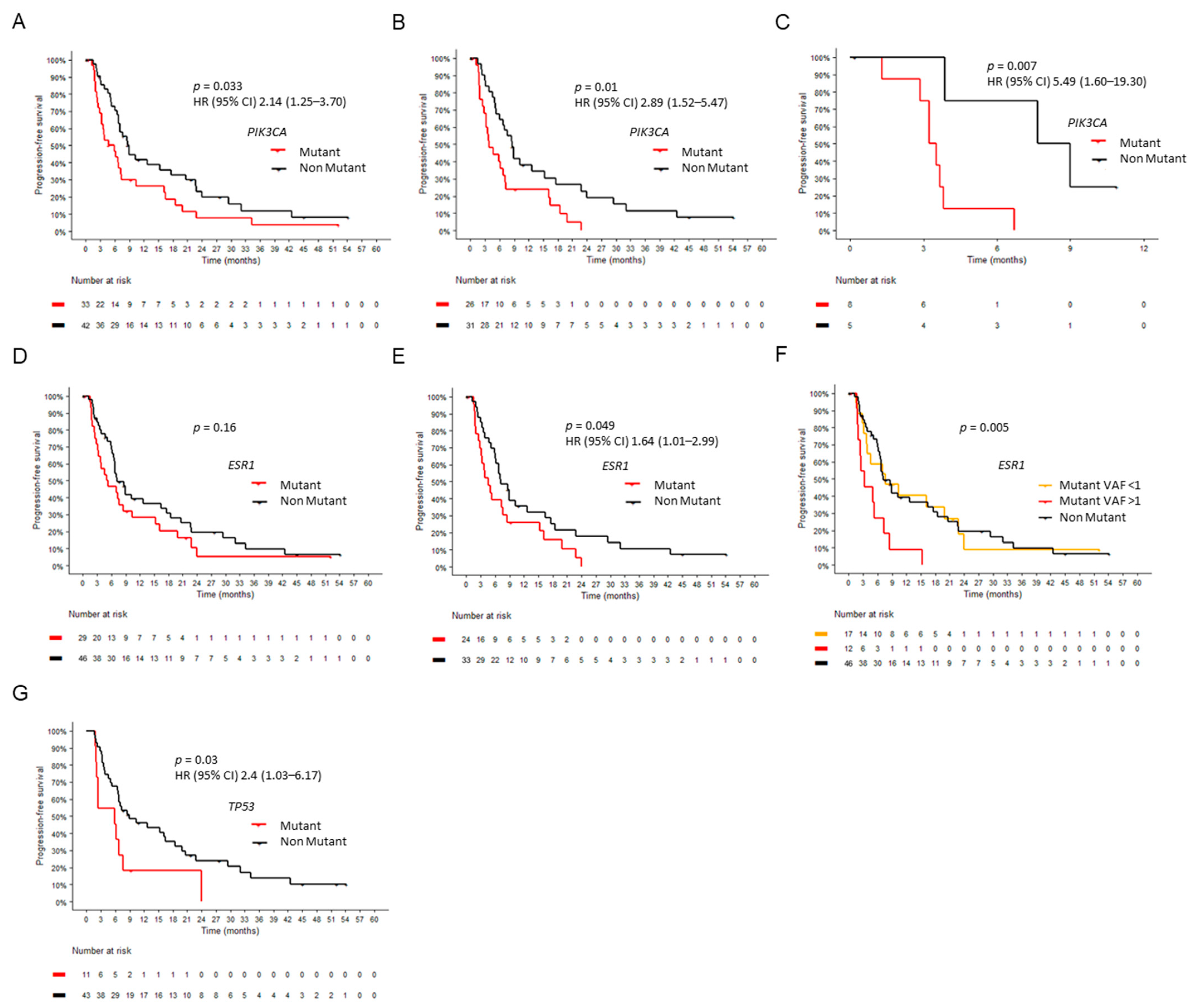
2.6. Circulating Tumor DNA at Baseline
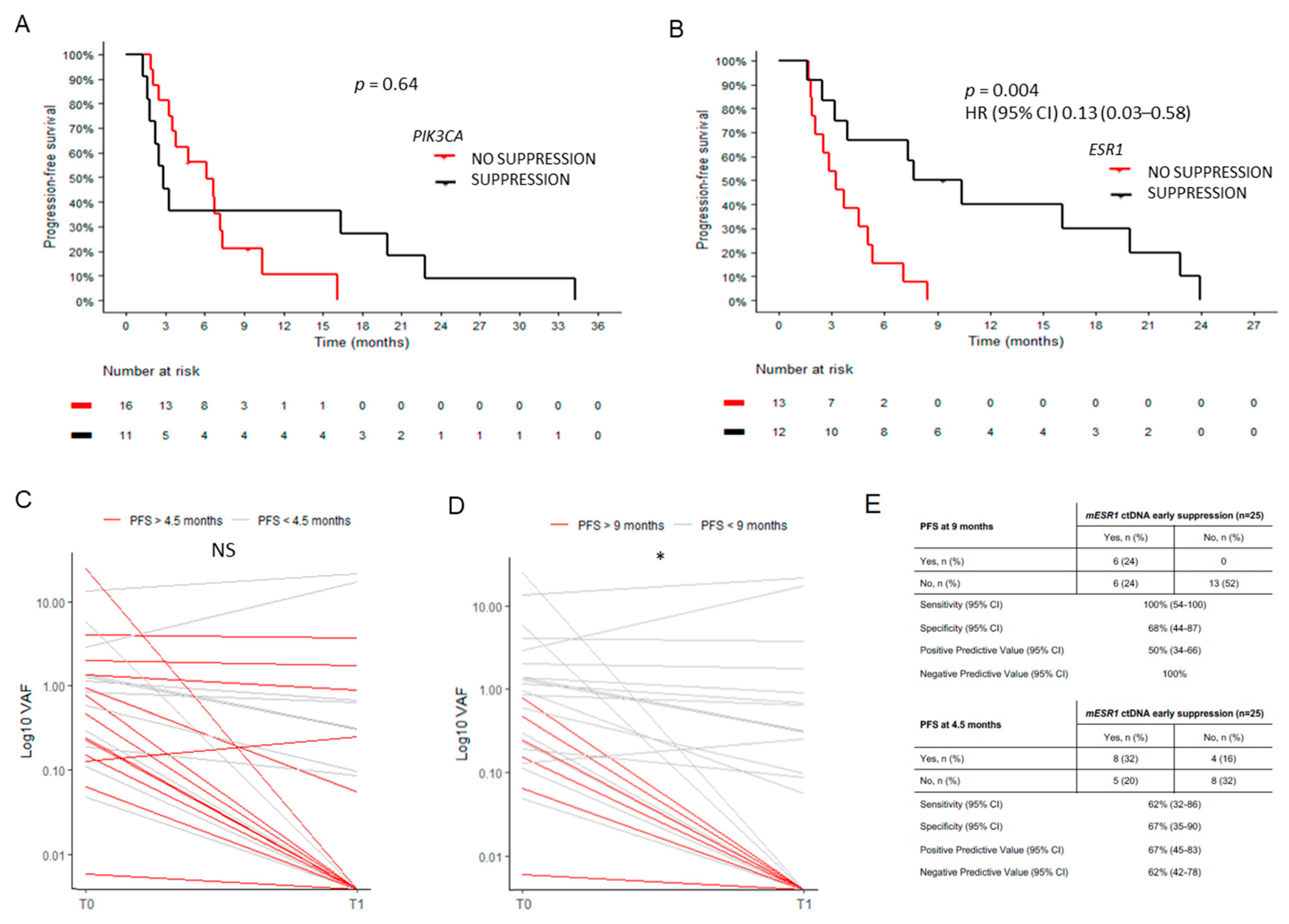
2.7. Circulating Tumor DNA Dynamics
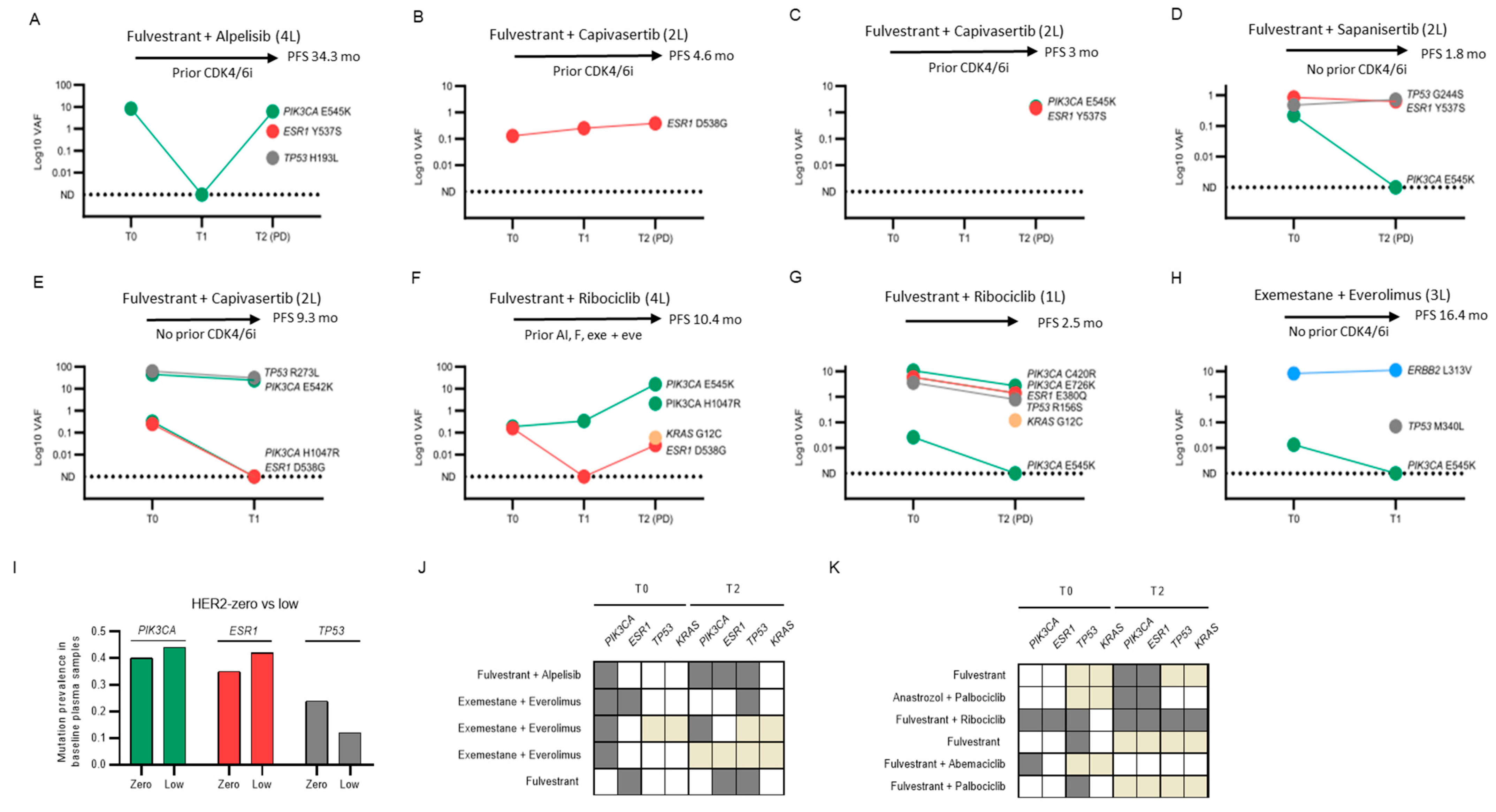
2.8. Individual Cases and Subgroups of Clinical Interest
- PI3K pathway targeting:
- PIK3CA, ESR1 and TP53 triple mutants:
- Emerging KRAS mutations:
- ERBB2 mutation:
- HER2-low versus HER2-zero:
- Clinical outliers:
3. Discussion
4. Materials and Methods
4.1. Study Design and Patient Cohort
4.2. DNA Extraction
4.3. Detection of ESR1 and PIK3CA Mutations by Digital PCR (dPCR)
4.4. NGS SafeSEQ Targeted Panel
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cescon, D.W.; Bratman, S.V.; Chan, S.M.; Siu, L.L. Circulating tumor DNA and liquid biopsy in oncology. Nat. Cancer 2020, 1, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Attard, G.; Bidard, F.-C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: A report from the ESMO Precision Medicine Working Group. Ann Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Kingston, B.; Kilburn, L.S.; Kernaghan, S.; Wardley, A.M.; Macpherson, I.R.; Baird, R.D.; Roylance, R.; Stephens, P.; Oikonomidou, O.; et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): A multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020, 21, 1296–1308. [Google Scholar] [CrossRef]
- Howell, S.J.; Casbard, A.; Carucci, M.; Ingarfield, K.; Butler, R.; Morgan, S.; Meissner, M.; Bale, C.; Bezecny, P.; Moon, S.; et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive, HER2-negative breast cancer (FAKTION): Overall survival, updated progression-free survival, and expanded biomarker analysis from a randomised, phase 2 trial. Lancet Oncol. 2022, 23, 851–864. [Google Scholar]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Ruiz-Borrego, M.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021, 22, 489–498. [Google Scholar] [CrossRef]
- De Laurentiis, M.; Costa, L.; Gligorov, J.; Knop, A.; Senkus-Konefka, E.; García-Sáenz, J.A.; Schmid, P.; Heniquez, A.; Serra, P.; Reising, A.; et al. EPIK-B5: A phase III, randomized study of alpelisib (ALP) plus fulvestrant (FUL) in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-), PIK3CA-mutated advanced breast cancer (ABC) progressing on/after an aromatase inhibitor (AI) with a cyclin-dependent kinase 4/6 inhibitor (CDK4/6i). J. Clin. Oncol. 2022, 40, TPS1109. [Google Scholar]
- Bidard, F.C.; Hardy-Bessard, A.-C.; Dalenc, F.; Bachelot, T.; Pierga, J.Y.; de la Motte Rouge, T.; Sabatier, R.; Dubotc, C.; Frenel, J.S.; Ferrero, J.M.; et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 1367–1377. [Google Scholar] [CrossRef]
- Jhaveri, K.L.; Goldman, J.W.; Hurvitz, S.A.; Guerrero-Zotano, A.; Unni, N.; Brufsky, A.; Park, H.; Wasiman, J.R.; Yang, E.S.; Spanggaard, I.; et al. Neratinib plus fulvestrant plus trastzuzumab (N+F+T) for hormone receptor-positive (HR+), HER2-negative, HER2-mutant metastatic breast cancer (MBC): Outcomes and biomarker analysis from the SUMMIT trial. J. Clin. Oncol. 2022, 40, 1028. [Google Scholar] [CrossRef]
- Asghar, U.S.; Kanani, R.; Roylance, R.; Mittnacht, S. Systematic Review of Molecular Biomarkers Predictive of Resistance to CDK4/6 Inhibition in Metastatic Breast Cancer. JCO Precis Oncol. 2022, 6, e2100002. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Hardy-Bessard, A.-C.; Bachelot, T.; Pierga, J.Y.; Canon, J.L.; Clatot, F.; Andre, F.; De La Motte Rouge, T.; Pistilli, B.; Dalencet, F.; et al. Abstract GS3-05: Fulvestrant-palbociclib vs continuing aromatase inhibitor-palbociclib upon detection of circulating ESR1 mutation in HR+ HER2- metastatic breast cancer patients: Results of PADA-1, a UCBG-GINECO randomized phase 3 trial. Cancer Res. 2022, 82, GS3–GS05. [Google Scholar] [CrossRef]
- Lipsyc-Sharf, M.; de Bruin, E.C.; Santos, K.; McEwen, R.; Stetson, D.; Patel, A.; Kirkner, G.J.; Hughes, M.E.; Tolaney, S.M.; Partridge, A.H.; et al. Circulating Tumor DNA and Late Recurrence in High-Risk Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer. J. Clin. Oncol. 2022, 40, 2408–2419. [Google Scholar] [CrossRef] [PubMed]
- Parsons, H.A.; Blewett, T.; Chu, X.; Sridhar, S.; Santos, K.; Xiong, K.; Abramson, V.G.; Patel, A.; Cheng, J.; Brufsky, A.; et al. Circulating tumor DNA association with residual cancer burden after neoadjuvant chemotherapy in triple-negative breast cancer in TBCRC 030. medRxiv. 2023. [Google Scholar] [CrossRef]
- Prat, A.; Brasó-Maristany, F.; Martínez-Sáez, O.; Sanfeliu, E.; Xia, Y.; Bellet, M.; Galván, P.; Martínez, D.; Pascual, T.; Marín-Aguilera, M.; et al. Circulating tumor DNA reveals complex biological features with clinical relevance in metastatic breast cancer. Nat Commun. 2023, 14, 1157. [Google Scholar] [CrossRef] [PubMed]
- Füllgrabe, J.; Gosal, W.S.; Creed, P.; Liu, S.; Lumby, C.K.; Morley, D.J.; Ost, T.W.B.; Vilella, A.J.; Yu, S.; Bignell, H.; et al. Simultaneous sequencing of genetic and epigenetic bases in DNA. Nat. Biotechnol. 2023, 1–8. [Google Scholar] [CrossRef]
- Weymann, D.; Pataky, R.; Regier, D.A. Economic Evaluations of Next-Generation Precision Oncology: A Critical Review. JCO Precis. Oncol. 2018, 2, 1–23. [Google Scholar] [CrossRef]
- Spencer, D.H.; Sehn, J.K.; Abel, H.J.; Watson, M.A.; Pfeifer, J.D.; Duncavage, E.J. Comparison of Clinical Targeted Next-Generation Sequence Data from Formalin-Fixed and Fresh-Frozen Tissue Specimens. J. Mol. Diagn. 2013, 15, 623–633. [Google Scholar] [CrossRef]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef]
- O’Leary, B.; Cutts, R.J.; Huang, X.; Hrebien, S.; Liu, Y.; André, F.; Loibl, S.; Loi, S.; Garcia-Murillas, I.; Cristofanilli, M.; et al. Circulating Tumor DNA Markers for Early Progression on Fulvestrant With or Without Palbociclib in ER+ Advanced Breast Cancer. J. Natl. Cancer Inst. 2021, 113, 309–317. [Google Scholar] [CrossRef]
- Kingston, B.; Cutts, R.J.; Bye, H.; Beaney, M.; Walsh-Crestani, G.; Hrebien, S.; Swift, C.; Kilburn, L.S.; Kernaghan, S.; Moretti, L.; et al. Genomic profile of advanced breast cancer in circulating tumour DNA. Nat. Commun. 2021, 12, 2423. [Google Scholar] [CrossRef]
- Neven, P.; Petrakova, K.; Val Bianchi, G.; De la Cruz-Merino, L.; Jerusalem, G.; Sonke, G.; Nusch, A.; Beck, J.T.; Chia, S.; Solovieff, N.; et al. Abstract PD2-05: Biomarker analysis by baseline circulating tumor DNA alterations in the MONALEESA-3 study. Cancer Res. 2019, 79, PD2–PD5. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Toi, M.; Neven, P.; Sohn, J.; Grischke, E.M.; Llombart-Cussac, A.; Soliman, H.; Wang, H.; Wijayawardana, S.; Jansen, V.M.; et al. Clinical Significance of PIK3CA and ESR1 Mutations in Circulating Tumor DNA: Analysis from the MONARCH 2 Study of Abemaciclib plus Fulvestrant. Clin. Cancer Res. 2022, 28, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, B.; Guo, J.; Shao, H.; Del Priore, I.S.; Chang, Q.; Kudo, R.; Li, Z.; Razavi, P.; Liu, B.; et al. INK4 tumor suppressor proteins mediate resistance to CDK4/6 kinase inhibitors. Cancer Discov. 2021, 12, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; McShane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO-College of American Pathologists Guideline Update. J. Clin. Oncol. 2023, JCO2202864. [Google Scholar] [CrossRef]
- Li, Z.; Spoelstra, N.S.; Sikora, M.J.; Sams, S.B.; Elias, A.; Richer, J.K.; Lee, A.V.; Oesterreich, S. Mutual exclusivity of ESR1 and TP53 mutations in endocrine resistant metastatic breast cancer. Npj Breast Cancer 2022, 8, 62. [Google Scholar] [CrossRef]
- Williams, M.M.; Spoelstra, N.S.; Arnesen, S.; O’Neill, K.I.; Christenson, J.L.; Reese, J.; Torkko, K.C.; Goodspeed, A.; Rosas, E.; Hanamura, T.; et al. Steroid Hormone Receptor and Infiltrating Immune Cell Status Reveals Therapeutic Vulnerabilities of ESR1-Mutant Breast Cancer. Cancer Res. 2021, 81, 732–746. [Google Scholar] [CrossRef]
- Moreno, F.; Gayarre, J.; López-Tarruella, S.; Del Monte-Millán, M.; Picornell, A.C.; Álvarez, E.; García-Saenz, J.A.; Jerez, Y.; Márquez-Rodas, I.; Echavarría, I.; et al. Concordance of Genomic Variants in Matched Primary Breast Cancer, Metastatic Tumor, and Circulating Tumor DNA: The MIRROR Study. JCO Precis. Oncol. 2019, 3, 1–16. [Google Scholar] [CrossRef]
- Galvano, A.; Castellana, L.; Gristina, V.; La Mantia, M.; Insalaco, L.; Barraco, N.; Perez, A.; Cutaia, S.; Calò, V.; Bazan Russo, T.D.; et al. The diagnostic accuracy of PIK3CA mutations by circulating tumor DNA in breast cancer: An individual patient data meta-analysis. Ther. Adv. Med. Oncol. 2022, 14, 17588359221110162. [Google Scholar] [CrossRef]
- Suppan, C.; Graf, R.; Jahn, S.; Zhou, Q.; Klocker, E.V.; Bartsch, R.; Terbuch, A.; Kashofer, K.; Regitnig, P.; Lindenmann, J.; et al. Sensitive and robust liquid biopsy-based detection of PIK3CA mutations in hormone-receptor-positive metastatic breast cancer patients. Br. J. Cancer 2022, 126, 456–463. [Google Scholar] [CrossRef]
- Ahn, S.G.; Bae, S.J.; Kim, Y.; Ji, J.H.; Chu, C.; Kim, D.; Lee, J.; Cha, Y.J.; Lee, K.A.; Jeong, J. Primary endocrine resistance of ER+ breast cancer with ESR1 mutations interrogated by droplet digital PCR. Npj Breast Cancer 2022, 8, 58. [Google Scholar] [CrossRef]
- Wang, P.; Bahreini, A.; Gyanchandani, R.; Lucas, P.C.; Hartmaier, R.J.; Watters, R.J.; Jonnalagadda, A.R.; Trejo Bittar, H.E.; Berg, A.; Hamilton, R.L.; et al. Sensitive Detection of Mono- and Polyclonal ESR1 Mutations in Primary Tumors, Metastatic Lesions, and Cell-Free DNA of Breast Cancer Patients. Clin. Cancer Res. 2016, 22, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, M.; George, A.M.; Brueffer, C.; Gladchuk, S.; Chen, Y.; Vallon-Christersson, J.; Hegardt, C.; Häkkinen, J.; Rydén, L.; Malmberg, M.; et al. Preexisting Somatic Mutations of Estrogen Receptor Alpha (ESR1) in Early-Stage Primary Breast Cancer. NCI Cancer Spectr. 2021, 5, pkab028. [Google Scholar] [CrossRef]
- Fernandez-Garcia, D.; Hills, A.; Page, K.; Hastings, R.K.; Toghill, B.; Goddard, K.S.; Ion, C.; Ogle, O.; Boydell, A.R.; Gleason, K.; et al. Plasma cell-free DNA (cfDNA) as a predictive and prognostic marker in patients with metastatic breast cancer. Breast Cancer Res. 2019, 21, 149. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Surowy, H.; Wallwiener, M.; Surowy, H.; Cuk, K.; Schott, S.; Trumpp, A.; Pantel, K.; Sohn, C.; Schneeweiss, A.; et al. Cell-free circulating DNA as independent prognostic markers in metastatic breast cancer. Ann. Oncol. 2017, 28, v93. [Google Scholar] [CrossRef]
- Fribbens, C.; O’Leary, B.; Kilburn, L.; Hrebien, S.; Garcia-Murillas, I.; Beaney, M.; Cristofanilli, M.; Andre, F.; Loi, S.; Loibl, S.; et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. J. Clin. Oncol. 2016, 34, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Cutts, R.J.; Liu, Y.; Hrebien, S.; Huang, X.; Fenwick, K.; André, F.; Loibl, S.; Loi, S.; Garcia-Murillas, I.; et al. The Genetic Landscape and Clonal Evolution of Breast Cancer Resistance to Palbociclib plus Fulvestrant in the PALOMA-3 Trial. Cancer Discov. 2018, 8, 1390–1403. [Google Scholar] [CrossRef] [PubMed]
- Jeselsohn, R.; Buchwalter, G.; De Angelis, C.; Brown, M.; Schiff, R. ESR1 mutations as a mechanism for acquired endocrine resistance in breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Chandarlapaty, S.; Chen, D.; He, W.; Sung, P.; Samoila, A.; You, D.; Bhatt, T.; Patel, P.; Voi, M.; Gnant, M.; et al. Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer: A Secondary Analysis of the BOLERO-2 Clinical Trial. JAMA Oncol. 2016, 2, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.-C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 2022, 40, 3246. [Google Scholar] [CrossRef]
- Bardia, A.; Chandarlapaty, S.; Linden, H.M.; Ulaner, G.A.; Gosselin, A.; Cartot-Cotton, S.; Cohen, P.; Doroumian, S.; Paux, G.; Celanovic, M.; et al. AMEERA-1 phase 1/2 study of amcenestrant, SAR439859, in postmenopausal women with ER-positive/HER2-negative advanced breast cancer. Nat. Commun. 2022, 13, 4116. [Google Scholar] [CrossRef]
- André, F.; Hurvitz, S.; Fasolo, A.; Tseng, L.M.; Jerusalem, G.; Wilks, S.; O’Regan, R.; Isaacs, C.; Toi, M.; Burris, H.; et al. Molecular Alterations and Everolimus Efficacy in Human Epidermal Growth Factor Receptor 2-Overexpressing Metastatic Breast Cancers: Combined Exploratory Biomarker Analysis From BOLERO-1 and BOLERO-3. J. Clin. Oncol. 2016, 34, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Ma, F.; Liu, B.; Guan, X.; Li, L.; Li, C.; Qian, H.; Xu, B. Everolimus in hormone receptor-positive metastatic breast cancer: PIK3CA mutation H1047R was a potential efficacy biomarker in a retrospective study. BMC Cancer 2019, 19, 442. [Google Scholar] [CrossRef] [PubMed]
- Moynahan, M.E.; Chen, D.; He, W.; Sung, P.; Samoila, A.; You, D.; Bhatt, T.; Patel, P.; Ringeisen, F.; Hortobagyi, G.N.; et al. Correlation between PIK3CA mutations in cell-free DNA and everolimus efficacy in HR+, HER2− advanced breast cancer: Results from BOLERO-2. Br. J. Cancer 2017, 116, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lindsay, D.; Chen, Q.B.; Garrett, A.L.; Tan, X.M.; Anders, C.K.; Carey, L.A.; Gupta, G.P. Tracking plasma DNA mutation dynamics in estrogen receptor positive metastatic breast cancer with dPCR-SEQ. npj Breast Cancer 2018, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Clatot, F.; Perdrix, A.; Augusto, L.; Beaussire, L.; Delacour, J.; Calbrix, C.; Sefrioui, D.; Viailly, P.J.; Bubenheim, M.; Moldovan, C.; et al. Kinetics, prognostic and predictive values of ESR1 circulating mutations in metastatic breast cancer patients progressing on aromatase inhibitor. Oncotarget 2016, 7, 74448–74459. [Google Scholar] [CrossRef]
- Clatot, F.; Perdrix, A.; Beaussire, L.; Lequesne, J.; Lévy, C.; Emile, G.; Bubenheim, M.; Lacaille, S.; Calbrix, C.; Augusto, L.; et al. Risk of early progression according to circulating ESR1 mutation, CA-15.3 and cfDNA increases under first-line anti-aromatase treatment in metastatic breast cancer. Breast Cancer Res. 2020, 22, 56. [Google Scholar] [CrossRef]
- SABCS 2022: Capivasertib and Fulvestrant for Patients with Aromatase Inhibitor-Resistant Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results from the Phase III CAPItello-291 Trial. Available online: https://clin.larvol.com/abstract-detail/SABCS%202022/60535045 (accessed on 16 March 2023).
- Wander, S.A.; Cohen, O.; Gong, X.; Johnson, G.N.; Buendia-Buendia, J.E.; Lloyd, M.R.; Kim, D.; Luo, F.; Mao, P.; Helvie, K.; et al. The Genomic Landscape of Intrinsic and Acquired Resistance to Cyclin-Dependent Kinase 4/6 Inhibitors in Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Cancer Discov. 2020, 10, 1174–1193. [Google Scholar] [CrossRef]
- Jänne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef]
- Kinde, I.; Wu, J.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B. Detection and quantification of rare mutations with massively parallel sequencing. Proc. Natl. Acad. Sci. USA 2011, 108, 9530–9535. [Google Scholar] [CrossRef]
- de Andrade, K.C.; Lee, E.E.; Tookmanian, E.M.; Kesserwan, C.A.; Manfredi, J.J.; Hatton, J.N.; Loukissas, J.K.; Zavadil, J.; Zhou, L.; Olivier, M.; et al. The TP53 Database: Transition from the International Agency for Research on Cancer to the US National Cancer Institute. Cell Death Differ. 2022, 29, 1071–1073. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, l1. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 75) | PIK3CA Mutant (n = 33) | PIK3CA Wild-Type (n = 42) | p Value | ESR1 Mutant (n = 29) | ESR1 Wild-Type (n = 46) | p Value | |
|---|---|---|---|---|---|---|---|
| Age—median (range) yr | 65.0 (39.2–87.7) | 66.6 (41.7–87.7) | 64.3 (39.2–86.6) | 0.87 | 60.2 (39.2–87.7) | 65.5 (39.5–87.1) | 0.34 |
| ≤65 yr—no. (%) | 37 (49) | 16 (48) | 21 (50) | 16 (55) | 21 (46) | ||
| >65 yr—no. (%) | 38 (51) | 17 (52) | 21 (50) | 0.89 | 13 (45) | 25 (54) | 0.42 |
| Line of therapy—no. (%) | |||||||
| First | 16 (21) | 6 (18) | 10 (24) | 4 (14) | 12 (26) | ||
| Second | 38 (51) | 16 (49) | 22 (52) | 13 (45) | 25 (54) | ||
| Third or higher | 21 (28) | 11 (33) | 10 (24) | 0.63 | 12 (41) | 9 (20) | 0.1 |
| Previous line of chemotherapy—no. (%) | |||||||
| Yes | 9 (12) | 1 (3) | 8 (19) | 5 (17) | 4 (9) | ||
| No | 66 (88) | 32 (97) | 34 (81) | 0.07 | 24 (83) | 42 (91) | 0.27 |
| Previous CDK4/6 inhibitor | |||||||
| Yes | 29 (39) | 16 (48) | 13 (31) | 14 (48) | 15 (33) | ||
| No | 46 (61) | 17 (52) | 29 (69) | 0.15 | 15 (52) | 31 (67) | 0.18 |
| ECOG-PS—no. (%) | |||||||
| 0 | 65 (87) | 28 (85) | 37 (88) | 25 (86) | 40 (87) | ||
| 1 | 10 (23) | 5 (15) | 5 (12) | 0.74 | 4 (14) | 6 (13) | 0.99 |
| Endocrine therapy—no. (%) | |||||||
| Aromatase inhibitor | 22 (29) | 10 (30) | 12 (29) | 9 (31) | 13 (28) | ||
| Fulvestrant | 53 (71) | 23 (70) | 30 (71) | 0.87 | 20 (69) | 33 (72) | 0.79 |
| CDK4/6 inhibitor—no. (%) | |||||||
| Palbociclib | 11 (15) | 2 (6) | 9 (21) | 2 (7) | 9 (19) | ||
| Ribociclib | 5 (7) | 3 (9) | 2 (5) | 4 (14) | 1 (2) | ||
| Abemaciclib | 2 (3) | 2 (6) | 0 (0) | 0.05 | 0 (0) | 2 (4) | 0.03 |
| Metastatic sites—no. (%) | |||||||
| Visceral | 39 (52) | 13 (39) | 26 (62) | 19 (65) | 20 (43) | ||
| Non visceral | 36 (48) | 20 (61) | 16 (38) | 10 (35) | 26 (57) | ||
| Bone-only | 16 (21) | 9 (27) | 5 (12) | 0.06 | 5 (17) | 5 (11) | 0.14 |
| De novo metastases—no. (%) | |||||||
| Yes | 14 (19) | 7 (21) | 7 (17) | 7 (24) | 7 (15) | ||
| No | 61 (81) | 26 (79) | 35 (83) | 0.77 | 22 (76) | 39 (85) | 0.33 |
| Adjuvant endocrine resistance—no. (%) * | |||||||
| Sensitive | 24 (32) | 10 (30) | 14 (33) | 7 (24) | 17 (37) | ||
| Primary | 16 (21) | 6 (18) | 10 (24) | 5 (17) | 11 (24) | ||
| Secondary | 16 (21) | 7 (21) | 9 (21) | 10 (35) | 6 (13) | ||
| N/A | 14 (19) | 7 (21) | 7 (17) | 7 (24) | 7 (15) | ||
| Unknown | 5 (7) | 3 (10) | 2 (5) | 0.93 | 0(0) | 5 (11) | 0.08 |
| Primary tumor histology—no. (%) | |||||||
| Ductal | 34 (45) | 10 (30) | 24 (57) | 12 (41) | 22 (48) | ||
| Lobular | 9 (12) | 5 (15) | 4 (10) | 5 (17) | 4 (9) | ||
| Other (mixed, NOS) | 17 (23) | 10 (30) | 7 (17) | 4 (14) | 13 (28) | ||
| Unknown | 15 (20) | 8 (24) | 7 (17) | 0.09 | 8 (28) | 7 (15) | 0.27 |
| Primary tumor grade—no. (%) | |||||||
| Low | 4 (5) | 3 (10) | 1 (2) | 1 (3) | 3 (7) | ||
| Intermediate | 38 (51) | 15 (46) | 23 (55) | 16 (55) | 22 (48) | ||
| High | 14 (19) | 6 (18) | 8 (19) | 4 (14) | 10 (22) | ||
| Unknown | 19 (25) | 9 (27) | 10 (24) | 0.39 | 8 (28) | 11 (24) | 0.58 |
| Primary tumor Ki67 (%)—no. (%) | |||||||
| 1–14 | 31 (41) | 16 (48) | 15 (36) | 11 (38) | 20 (43) | ||
| 15–20 | 10 (13) | 5 (15) | 5 (12) | 5 (17) | 5 (11) | ||
| >20 | 17 (23) | 6 (18) | 11 (26) | 5 (17) | 12 (26) | ||
| Unknown | 17 (23) | 6 (18) | 11 (26) | 0.54 | 8 (28) | 9 (20) | 0.58 |
| HER2 IHC—no. (%) | |||||||
| Low | 47 (63) | 21 (63) | 26 (62) | 20 (69) | 27 (59) | ||
| Zero | 20 (26) | 8 (24) | 12 (28) | 7 (24) | 13 (28) | ||
| Unknown | 8 (11) | 4 (13) | 4 (9) | 0.72 | 2 (7) | 6 (13) | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes-Antrás, J.; Martínez-Rodríguez, A.; Guevara-Hoyer, K.; López-Cade, I.; Lorca, V.; Pascual, A.; de Luna, A.; Ramírez-Ruda, C.; Swindell, J.; Flores, P.; et al. Real-World Use of Highly Sensitive Liquid Biopsy Monitoring in Metastatic Breast Cancer Patients Treated with Endocrine Agents after Exposure to Aromatase Inhibitors. Int. J. Mol. Sci. 2023, 24, 11419. https://doi.org/10.3390/ijms241411419
Fuentes-Antrás J, Martínez-Rodríguez A, Guevara-Hoyer K, López-Cade I, Lorca V, Pascual A, de Luna A, Ramírez-Ruda C, Swindell J, Flores P, et al. Real-World Use of Highly Sensitive Liquid Biopsy Monitoring in Metastatic Breast Cancer Patients Treated with Endocrine Agents after Exposure to Aromatase Inhibitors. International Journal of Molecular Sciences. 2023; 24(14):11419. https://doi.org/10.3390/ijms241411419
Chicago/Turabian StyleFuentes-Antrás, Jesús, Ana Martínez-Rodríguez, Kissy Guevara-Hoyer, Igor López-Cade, Víctor Lorca, Alejandro Pascual, Alicia de Luna, Carmen Ramírez-Ruda, Jennifer Swindell, Paloma Flores, and et al. 2023. "Real-World Use of Highly Sensitive Liquid Biopsy Monitoring in Metastatic Breast Cancer Patients Treated with Endocrine Agents after Exposure to Aromatase Inhibitors" International Journal of Molecular Sciences 24, no. 14: 11419. https://doi.org/10.3390/ijms241411419
APA StyleFuentes-Antrás, J., Martínez-Rodríguez, A., Guevara-Hoyer, K., López-Cade, I., Lorca, V., Pascual, A., de Luna, A., Ramírez-Ruda, C., Swindell, J., Flores, P., Lluch, A., Cescon, D. W., Pérez-Segura, P., Ocaña, A., Jones, F., Moreno, F., García-Barberán, V., & García-Sáenz, J. Á. (2023). Real-World Use of Highly Sensitive Liquid Biopsy Monitoring in Metastatic Breast Cancer Patients Treated with Endocrine Agents after Exposure to Aromatase Inhibitors. International Journal of Molecular Sciences, 24(14), 11419. https://doi.org/10.3390/ijms241411419






