The Pitaya Flower Tissue’s Gene Differential Expression Analysis between Self-Incompatible and Self-Compatible Varieties for the Identification of Genes Involved in Self-Incompatibility Regulation
Abstract
1. Introduction
2. Results
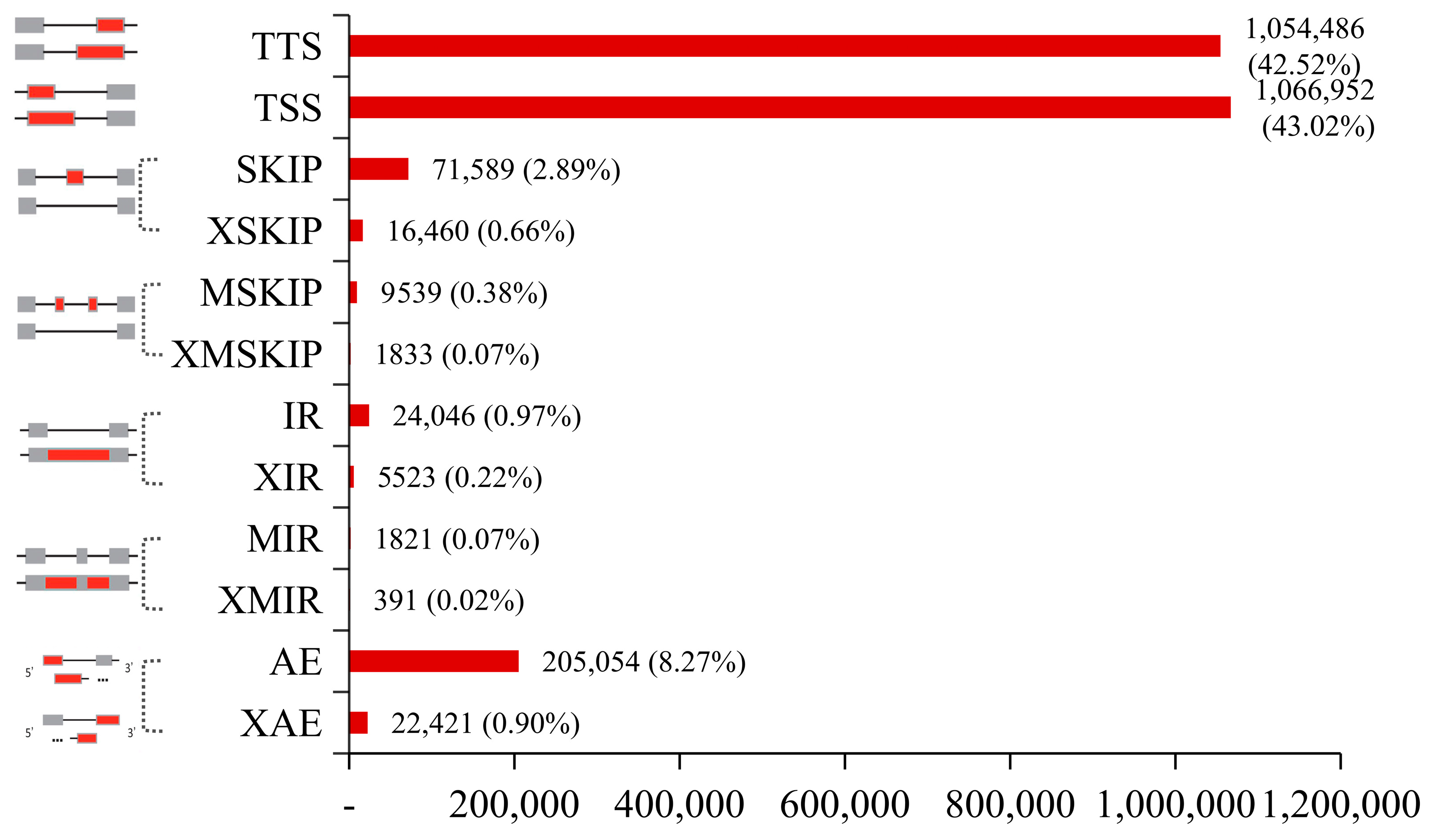
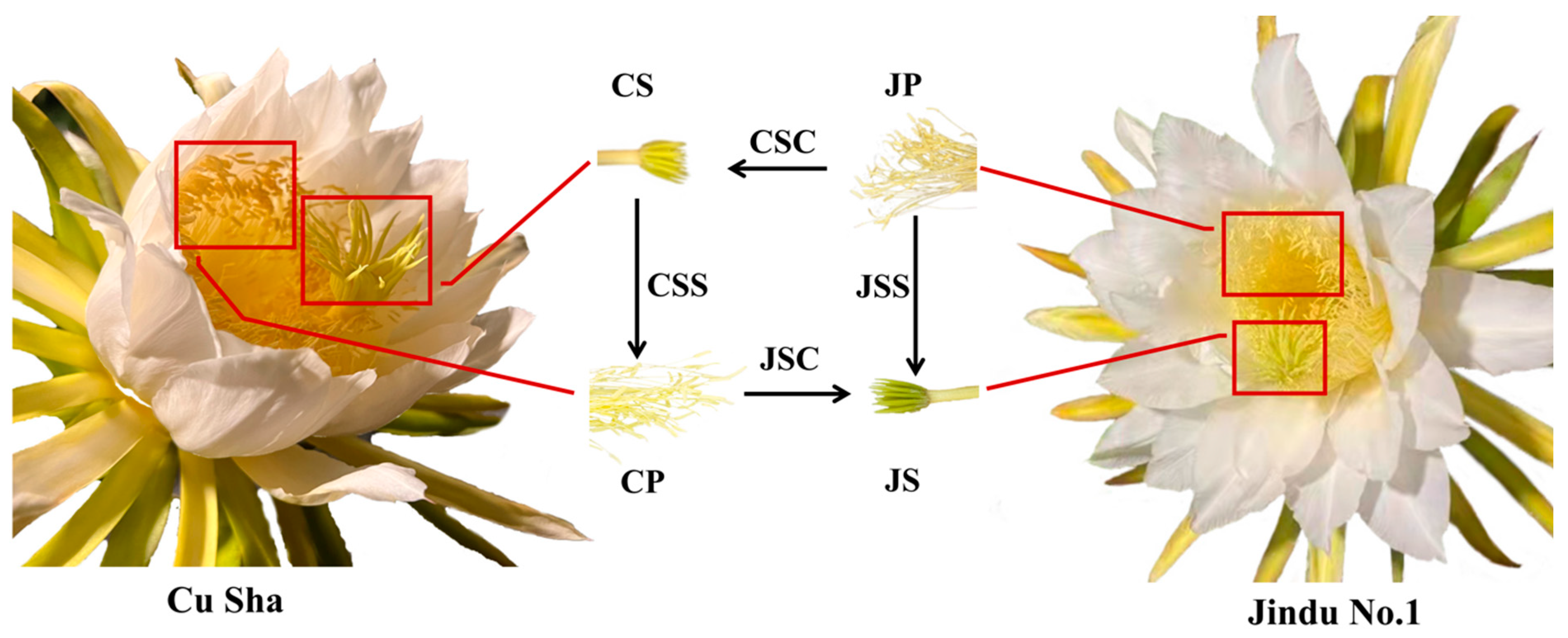
2.1. Overview of Transcriptome Sequence Analysis and Alternative Splicing (AS) Events
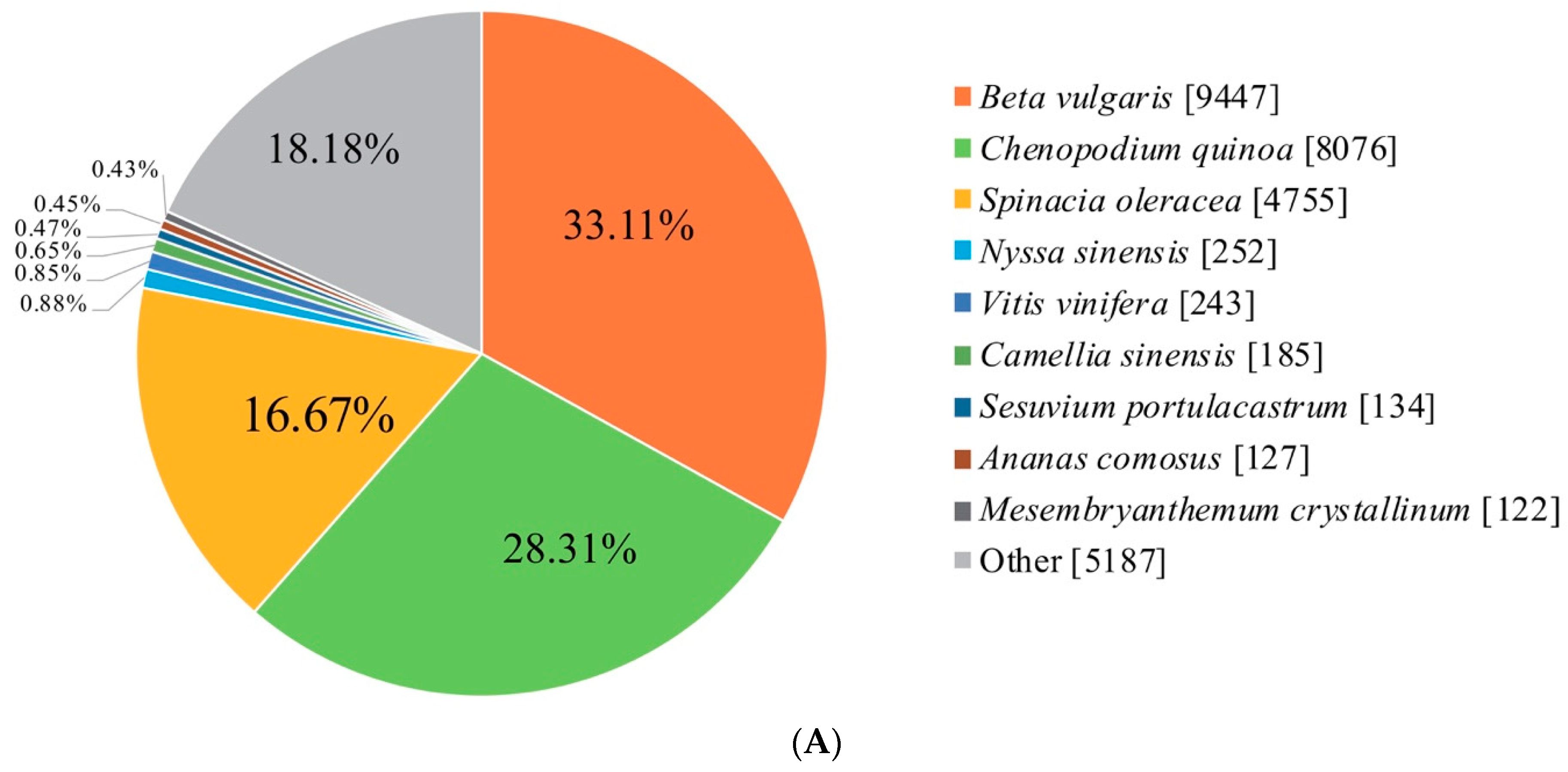
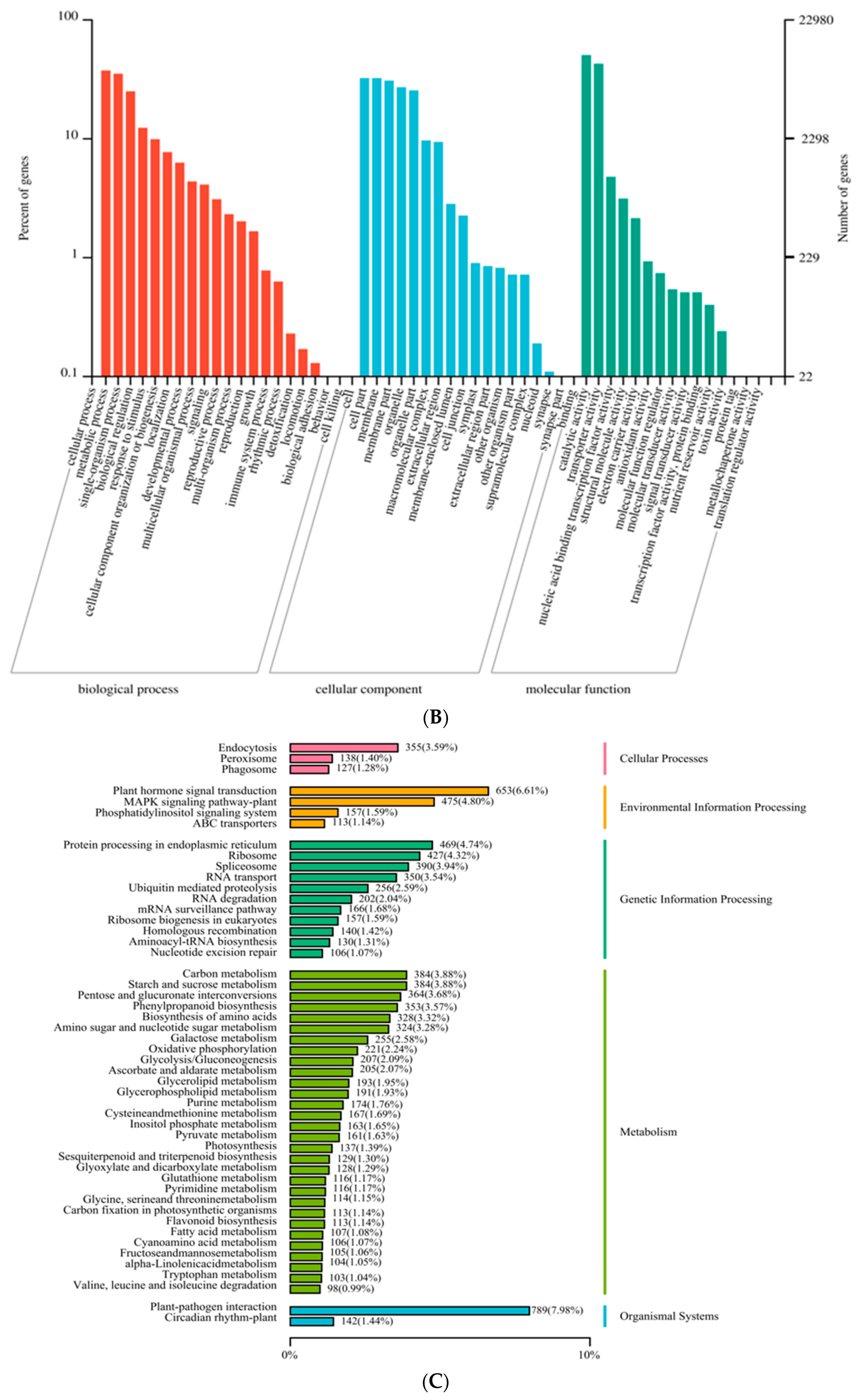
2.2. Identification of Novel Genes and Functional Annotation
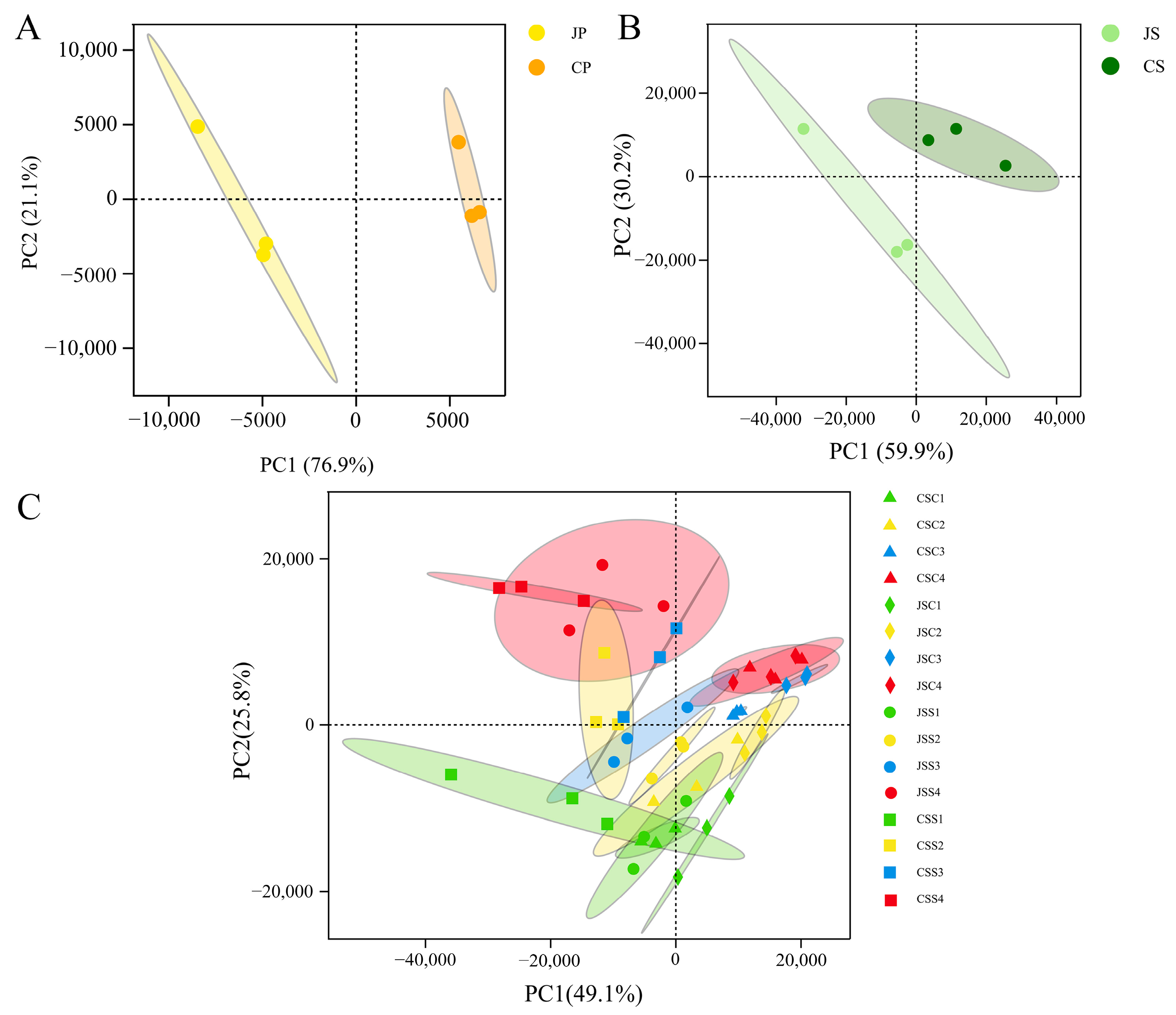
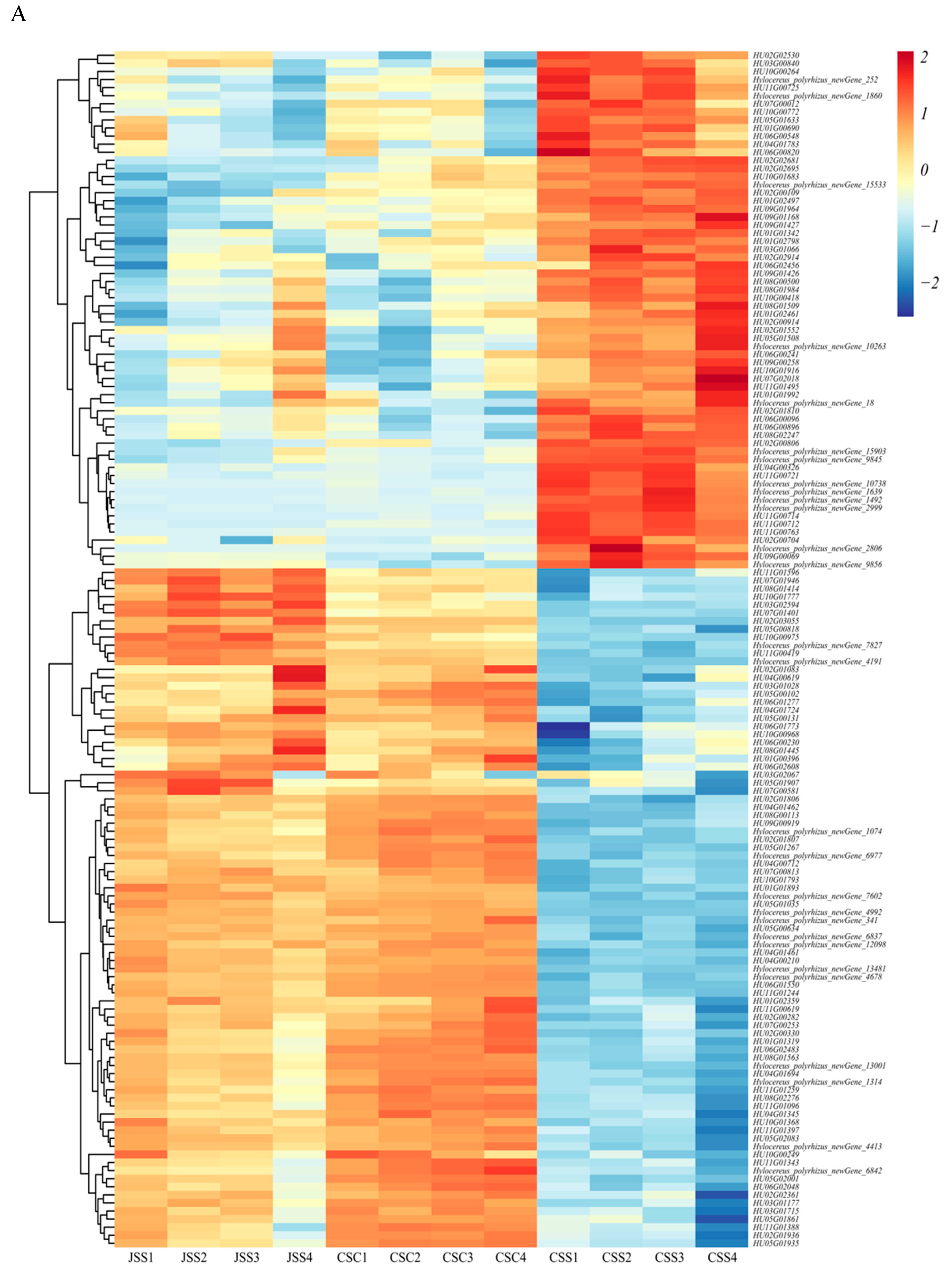
2.3. Gene Expression Profile of Different Tissues
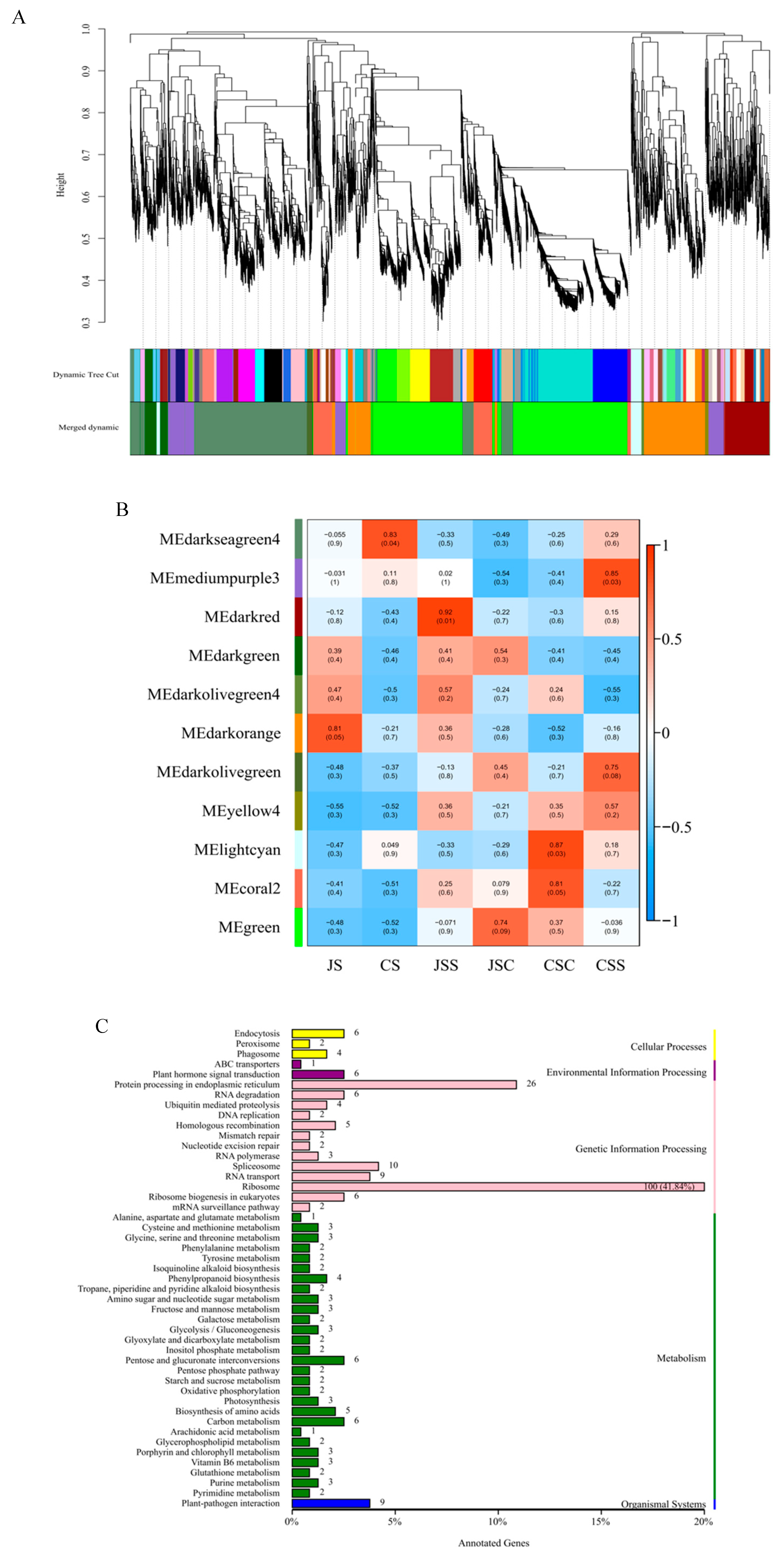
2.4. Gene Co-Expression Analysis for Different Pollination Types
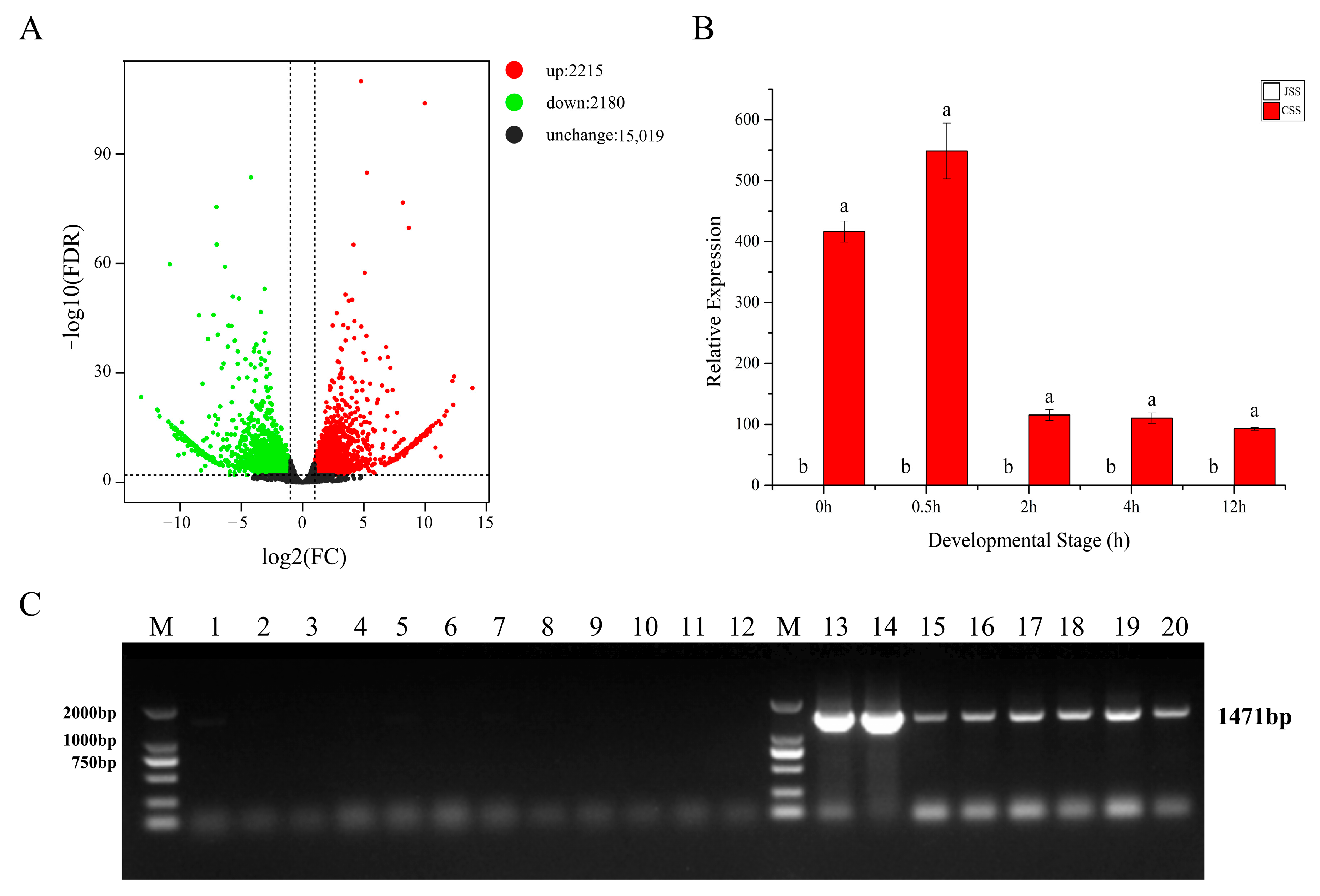
2.5. Effect of Self-Pollination and Cross-Pollination on Stigma Gene Expression
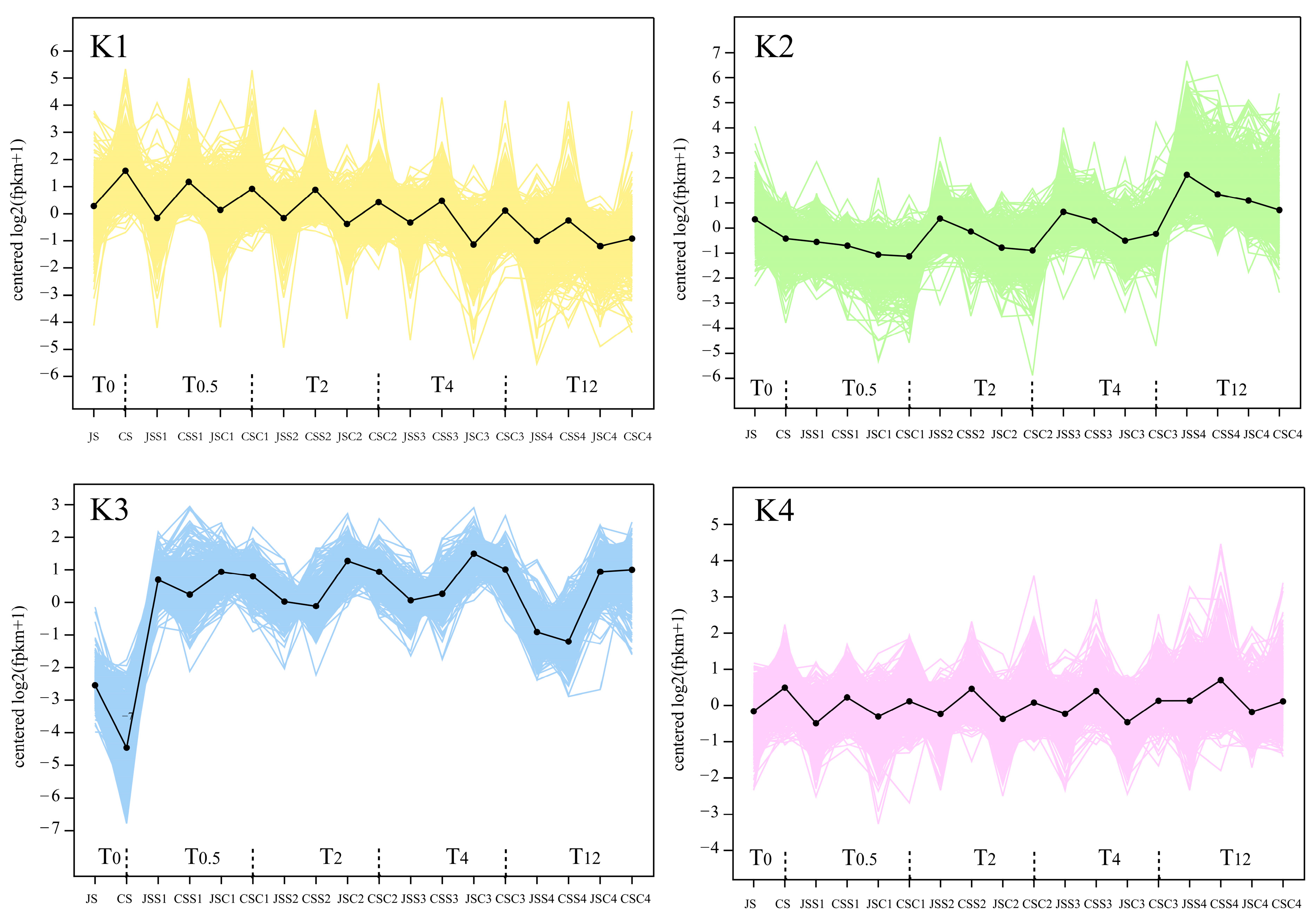
2.6. Stigma Gene Expression Patterns at Different Time Points Post Pollination
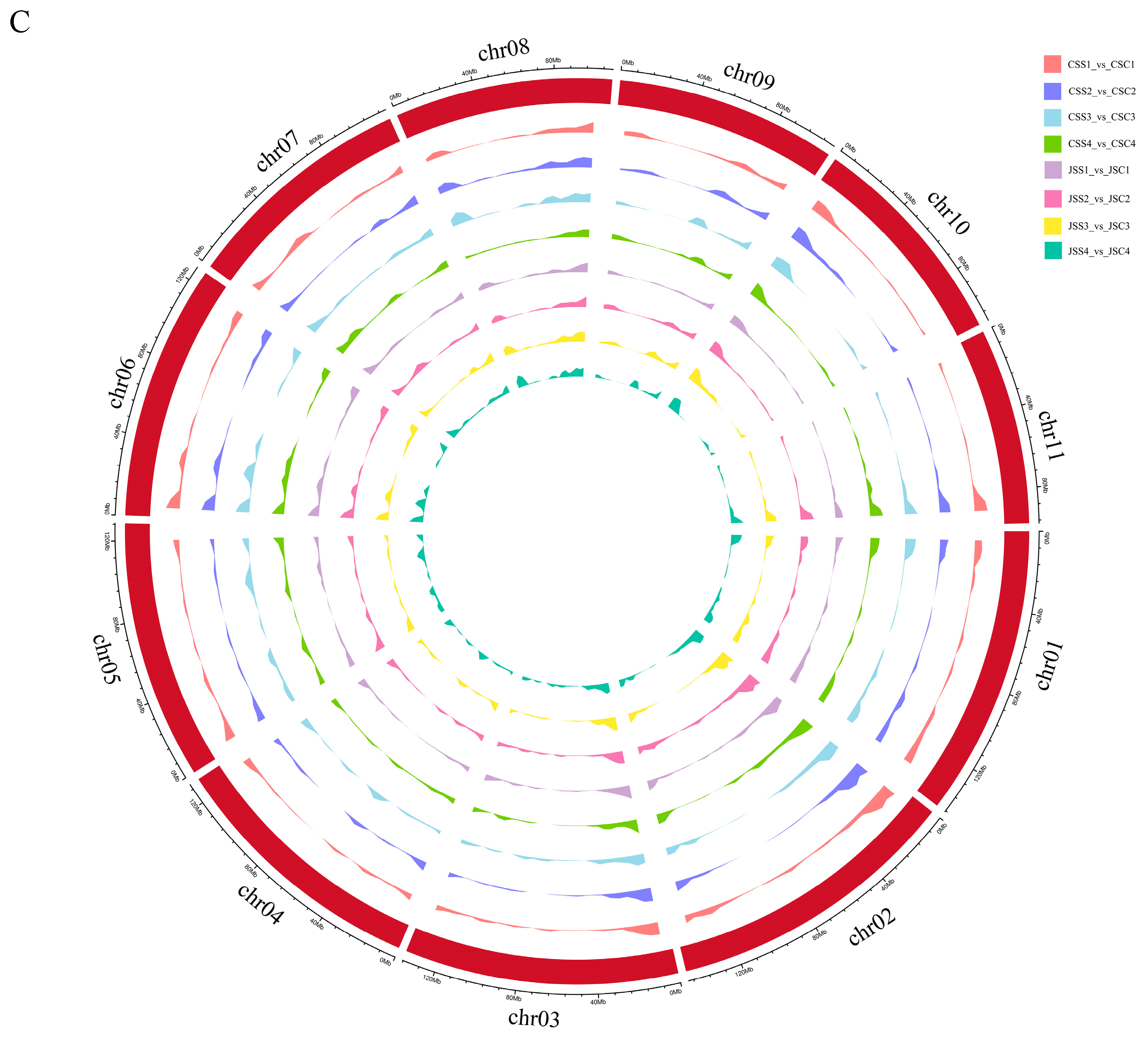
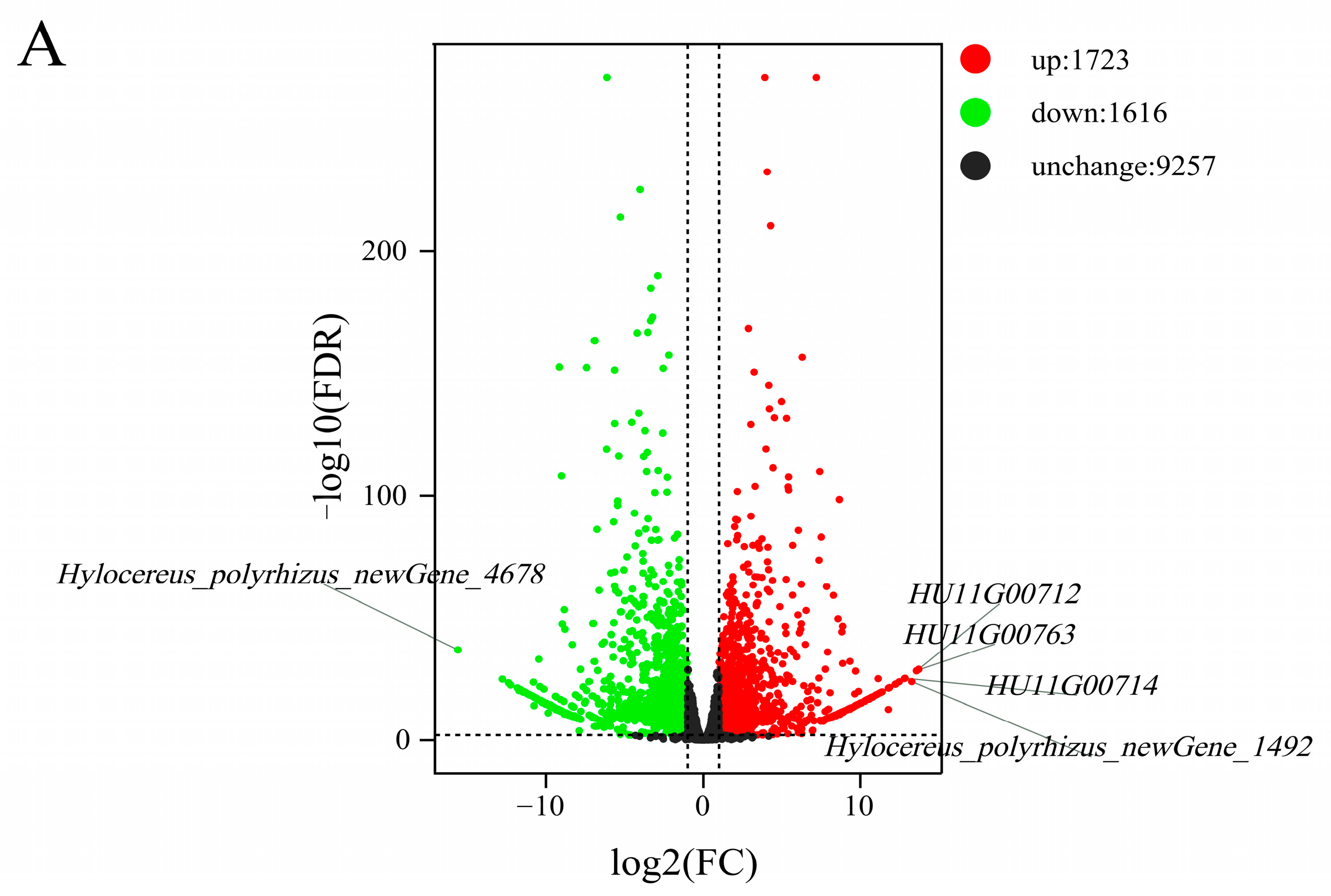
2.7. Analysis of the DEGs Associated with the SI Response in Stigmas
2.8. Selection and Identification of the Pistil S-RNases Related to SI Traits
2.9. Screening for the DEGs Involved in Pollen–Stigma Interactions in Pollen
2.10. Screening and Analysis of the F-Box Genes Associated with SI
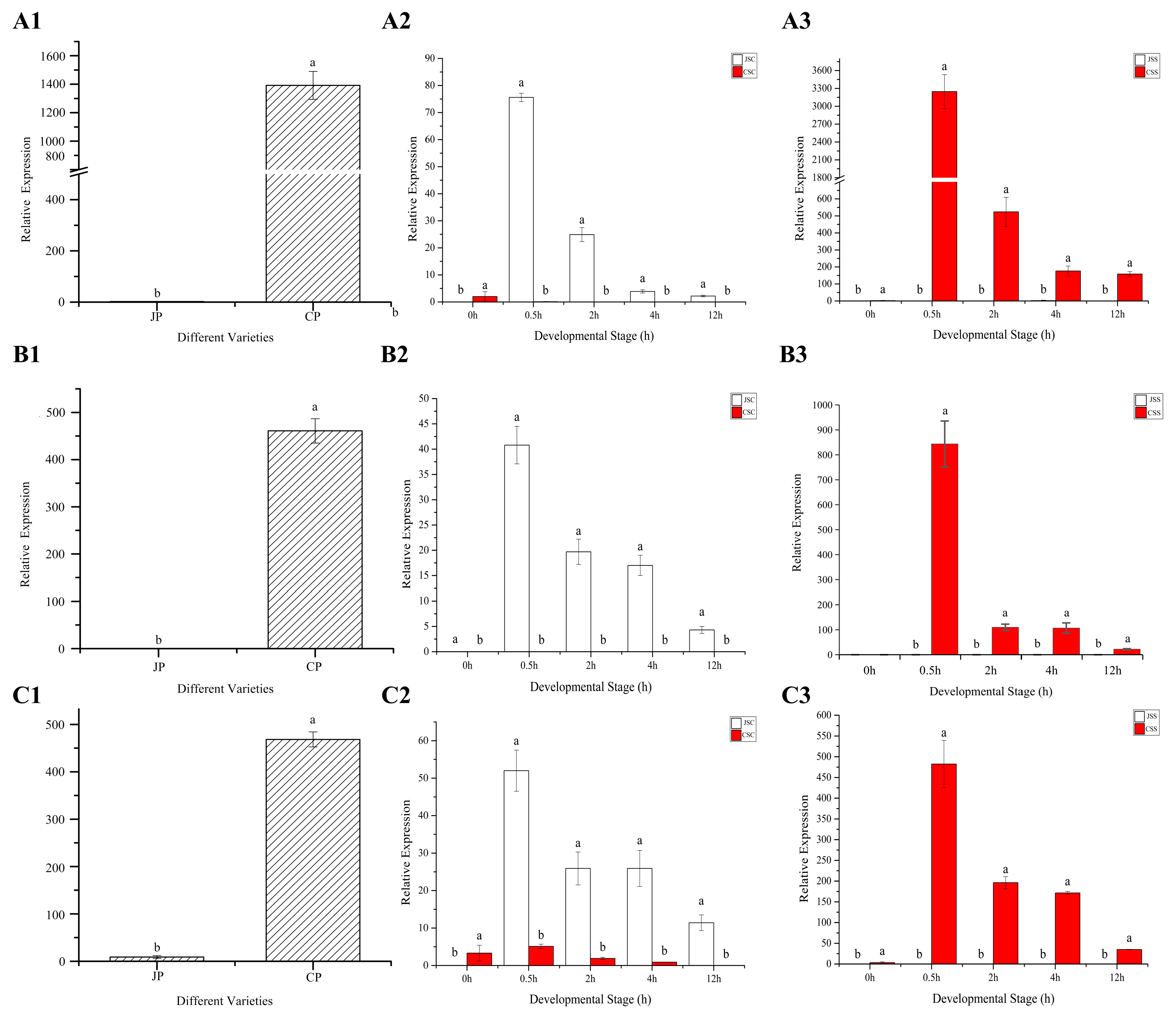
2.11. qRT-PCR Validation of SI-Related DEG Genes
3. Discussion
3.1. HuS-RNase2 May Be a Candidate Stamen S Gene Associated with the SI Trait of Pitaya
3.2. Possible Association of F-box with S-RNase in SI Reactions of Pitaya
3.3. Physiological Changes in Signal Transduction and Phytohormones in Pitaya
3.4. SI Reaction Develops in a Short Period of Time
4. Materials and Methods
4.1. Total RNA Extraction cDNA Library Construction
4.2. Transcriptome Sequencing
4.3. Identification of Gene Expression Level and DEGs
4.4. Clustering Analysis and Construction of the Gene Co-Expression Network
4.5. Enrichment Analysis of DEGs Sets in Different Pollination Modes
4.6. Co-Expression Trend of Genes at Different Transcription Stages
4.7. Interacting with SI-Response-Related Gene Protein Networks
4.8. Functional Identification and Population Validation of HuS-RNase2
4.9. qRT-PCR Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, M.; Cao, Z.H.; Zhu, A.D.; Liu, Y.L.; Tao, M.Q.; Yang, H.Y.; Xu, Q.; Wang, S.H.; Liu, J.J.; Li, Y.P.; et al. Evolution of self-compatibility by a mutant S-m-RNase in citrus. Nat. Plants 2020, 6, 131–142. [Google Scholar] [CrossRef] [PubMed]
- de Nettancourt, D. Incompatibility and Incongruity in Wild and Cultivated Plants; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar]
- Fujii, S.; Kubo, K.; Takayama, S. Non-self- and self-recognition models in plant self-incompatibility. Nat. Plants 2016, 2, 16056. [Google Scholar] [CrossRef] [PubMed]
- Busch, J.W.; Witthuhn, T.; Joseph, M. Fewer S-alleles are maintained in plant populations with sporophytic as opposed to gametophytic self-incompatibility. Plant Species Biol. 2014, 29, 34–46. [Google Scholar] [CrossRef]
- Riday, H.; Krohn, A.L. Genetic map-based location of the red clover (Trifolium pratense L.) gametophytic self-incompatibility locus. Theor. Appl. Genet. 2010, 121, 761–767. [Google Scholar] [CrossRef]
- Qu, H.Y.; Zhang, Z.; Wu, F.F.; Wang, Y.Z. The role of Ca2+ and Ca2+ channels in the gametophytic self-incompatibility of Pyrus pyrifolia. Cell Calcium 2016, 60, 299–308. [Google Scholar] [CrossRef] [PubMed]
- East, E.M.; Yarnell, S.H. Studies on self-sterility. VIII. Self-sterility allelomorphs. Genetics 1929, 14, 455–487. [Google Scholar] [CrossRef]
- Murfett, J.; Atherton, T.L.; Mou, B.; Gasser, C.S.; McClure, B.A. S-RNase expressed in transgenic Nicotiana causes S-allele-specific pollen rejection. Nature 1994, 367, 563–566. [Google Scholar] [CrossRef]
- Ma, C.H.; Qu, H.Y. Gametophytic self-incompatibility in Rosaceae fruit trees. Acta Sci. Pol. Hortorum Cultus 2019, 18, 149–156. [Google Scholar] [CrossRef]
- Sijacic, P.; Wang, X.; Skirpan, A.L.; Wang, Y.; Dowd, P.E.; McCubbin, A.G.; Huang, S.; Kao, T.H. Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature 2004, 429, 302–305. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Liang, M.; Wang, N.; Xu, Q.; Deng, X.X.; Chai, L.J. Reproduction in woody perennial citrus: An update on nucellar embryony and self-incompatibility. Plant Reprod. 2018, 31, 43–57. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, F.; He, X.; Luo, C.; Huang, G.; Hu, Y. Characterization of the ‘Xiangshui’ lemon transcriptome by de novo assembly to discover genes associated with self-incompatibility. Mol. Genet. Genom. 2015, 290, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.A.; Shankar, V. Ribonucleases from T2 family. Crit. Rev. Microbiol. 2002, 28, 79–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, P.; de Graaf, B.H.J.; Zhang, H.; Jiao, H.; Tang, C.; Zhang, S.; Wu, J. Phosphatidic acid counteracts S-RNase signaling in pollen by stabilizing the actin cytoskeleton. Plant Cell 2018, 30, 1023–1039. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, W.; Han, B.; Liang, L.; Zhang, Y.; Hong, G.; Xue, Y. An F-box gene linked to the self-incompatibility (S) locus of Antirrhinum is expressed specifically in pollen and tapetum. Plant Mol. Biol. 2002, 50, 29–42. [Google Scholar]
- Kakui, H.; Kato, M.; Ushijima, K.; Kitaguchi, M.; Kato, S.; Sassa, H. Sequence divergence and loss-of-function phenotypes of S locus F-box brothers genes are consistent with non-self recognition by multiple pollen determinants in self-incompatibility of Japanese pear (Pyrus pyrifolia). Plant J. 2011, 68, 1028–1038. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Rao, M.J.; Hu, J.B.; Xu, Q.; Liu, C.C.; Cao, Z.H.; Larkin, R.M.; Deng, X.X.; Bosch, M.; Chai, L.J. Systems and breakdown of self-incompatibility. Crit. Rev. Plant Sci. 2022, 41, 209–239. [Google Scholar] [CrossRef]
- Chen, C.B.; Li, F.P.; Xie, F.F.; Chen, J.X.; Hua, Q.Z.; Chen, J.Y.; Wu, Z.J.; Zhang, Z.K.; Zhang, R.; Zhao, J.T.; et al. Pitaya Genome and Multiomics Database (PGMD): A comprehensive and integrative resource of Selenicereus undatus. Genes 2022, 13, 745. [Google Scholar] [CrossRef]
- Chen, J.Y.; Xie, F.F.; Cui, Y.Z.; Chen, C.B.; Lu, W.J.; Hu, X.D.; Hua, Q.Z.; Zhao, J.; Wu, Z.J.; Gao, D.; et al. A chromosome-scale genome sequence of pitaya (Hylocereus undatus) provides novel insights into the genome evolution and regulation of betalain biosynthesis. Hortic. Res. 2021, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Lichtenzveig, J.; Abbo, S.; Nerd, A.; Tel-Zur, N.; Mizrahi, Y. Cytology and mating systems in the climbing cacti Hylocereus and Selenicereus. Am. J. Bot. 2000, 87, 1058–1065. [Google Scholar] [CrossRef]
- Tetsuyuki, E.; Ken-ichi, K.; Shin, I.; Yoichiro, F.; Masahiro, S.; Akira, I.; Seiji, T. Ubiquitin-proteasome-mediated degradation of S-RNase in a solanaceous cross-compatibility reaction. Plant J. 2014, 78, 1014–1021. [Google Scholar]
- Suwabe, K.; Nagasaka, K.; Windari, E.A.; Hoshiai, C.; Ota, T.; Takada, M.; Kitazumi, A.; Masuko-Suzuki, H.; Kagaya, Y.; Yano, K.; et al. Double-locking mechanism of self-compatibility in Arabidopsis thaliana: The synergistic effect of transcriptional depression and disruption of coding region in the male specificity gene. Front. Plant Sci. 2020, 11, 576140. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Shi, H.H.; Dai, H.F.; Wang, Y.L.; Zhao, J.S.; Nguyen, C.D.; Huang, X.L.; Sun, Q.M. Pollen germination and hand pollination in pitaya (Selenicereus spp.). Emir. J. Food Agric. 2022, 34, 369–387. [Google Scholar] [CrossRef]
- Chu, Y.-C.; Chang, J.-C. High temperature suppresses fruit/seed set and weight, and cladode regreening in red-fleshed ‘Da Hong’ Pitaya (Hylocereus polyrhizus) under controlled conditions. HortScience 2020, 55, 1259–1264. [Google Scholar] [CrossRef]
- Kishore, K. Phenological growth stages of dragon fruit (Hylocereus undatus) according to the extended BBCH-scale. Sci. Hortic. 2016, 213, 294–302. [Google Scholar] [CrossRef]
- Shihao, S.; Won, P.J.; Zhi-xiang, L.; Lan, L.; Henry, M.D.; Nian, W.Y.; Qing, Z.; Yi, X. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. USA 2014, 111, E5593–E5601. [Google Scholar]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fornes, O.; Castro-Mondragon, J.A.; Khan, A.; van der Lee, R.; Zhang, X.; Richmond, P.A.; Modi, B.P.; Correard, S.; Gheorghe, M.; Baranašić, D. JASPAR 2020: Update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2020, 48, D87–D92. [Google Scholar] [CrossRef]
- Simon, A.; Alejandro, R.; Wolfgang, H. Detecting differential usage of exons from RNA-seq data. Genome Res. 2012, 22, 2008–2017. [Google Scholar]
- Kao, T.H.; Huang, S. Gametophytic self-incompatibility: A mechanism for self/nonself discrimination during sexual reproduction. Plant Physiol. 1994, 105, 461–466. [Google Scholar] [CrossRef]
- McClure, B.A.; Haring, V.; Ebert, P.R.; Anderson, M.A.; Simpson, R.J.; Sakiyama, F.; Clarke, A.E. Style self-incompatibility gene products of Nicotiana alata are ribonucleases. Nature 1989, 342, 955–957. [Google Scholar] [CrossRef]
- Qiao, H.; Wang, F.; Zhao, L.; Zhou, J.; Lai, Z.; Zhang, Y.; Robbins, T.P.; Xue, Y. The F-box protein AhSLF-S2 controls the pollen function of S-RNase–Based Self-Incompatibility. Plant Cell Online 2004, 16, 2307–2322. [Google Scholar] [CrossRef]
- Qiao, H.; Wang, H.; Zhao, L.; Zhou, J.; Huang, J.; Zhang, Y.; Xue, Y.T. The F-box protein AhSLF-S2 physically interacts with S-RNases that may be inhibited by the ubiquitin/26S proteasome pathway of protein degradation during compatible pollination in antirrhinum. Plant Cell 2004, 16, 582–595. [Google Scholar] [CrossRef]
- Balotf, S.; Wilson, R.; Tegg, R.S.; Nichols, D.S.; Wilson, C.R. Shotgun proteomics as a powerful tool for the study of the proteomes of plants, their pathogens, and plant-pathogen interactions. Proteomes 2022, 10, 5. [Google Scholar] [CrossRef]
- Li, C.; Tao, R.F.; Li, Y.; Duan, M.H.; Xu, J.H. Transcriptome analysis of the thermosensitive genic male-sterile line provides new insights into fertility alteration in rice (Oryza sativa). Genomics 2020, 112, 2119–2129. [Google Scholar] [CrossRef]
- Xie, F.F.; Hua, Q.Z.; Chen, C.B.; Zhang, L.L.; Zhang, Z.K.; Chen, J.Y.; Zhang, R.; Zhao, J.S.; Hu, G.B.; Zhao, J.T.; et al. Transcriptomics-based identification and characterization of glucosyltransferases involved in betalain biosynthesis in Hylocereus megalanthus. Plant Physiol. Biochem. 2020, 152, 112–124. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Li, J.Q.; Wu, S.F.; Zhu, Y.; Chen, Y.W.; He, F.C. Integrated nr database in protein annotation system and its localization. Comput. Eng. 2006, 32, 71–72. [Google Scholar]
- Apweiler, R.; Bairoch, A.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M.; et al. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017, 45, D115–D119. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef]
- Punta, M.; Coggill, P.C.; Eberhardt, R.Y.; Mistry, J.; Tate, J.; Boursnell, C.; Pang, N.; Forslund, K.; Ceric, G.; Clements, J.; et al. The Pfam protein families database. Nucleic Acids Res. 2007, 36, D281–D288. [Google Scholar] [CrossRef]
- Minoru, K.; Susumu, G.; Shuichi, K.; Yasushi, O.; Masahiro, H. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar]
- Eddy, S.R. Profile hidden Markov models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

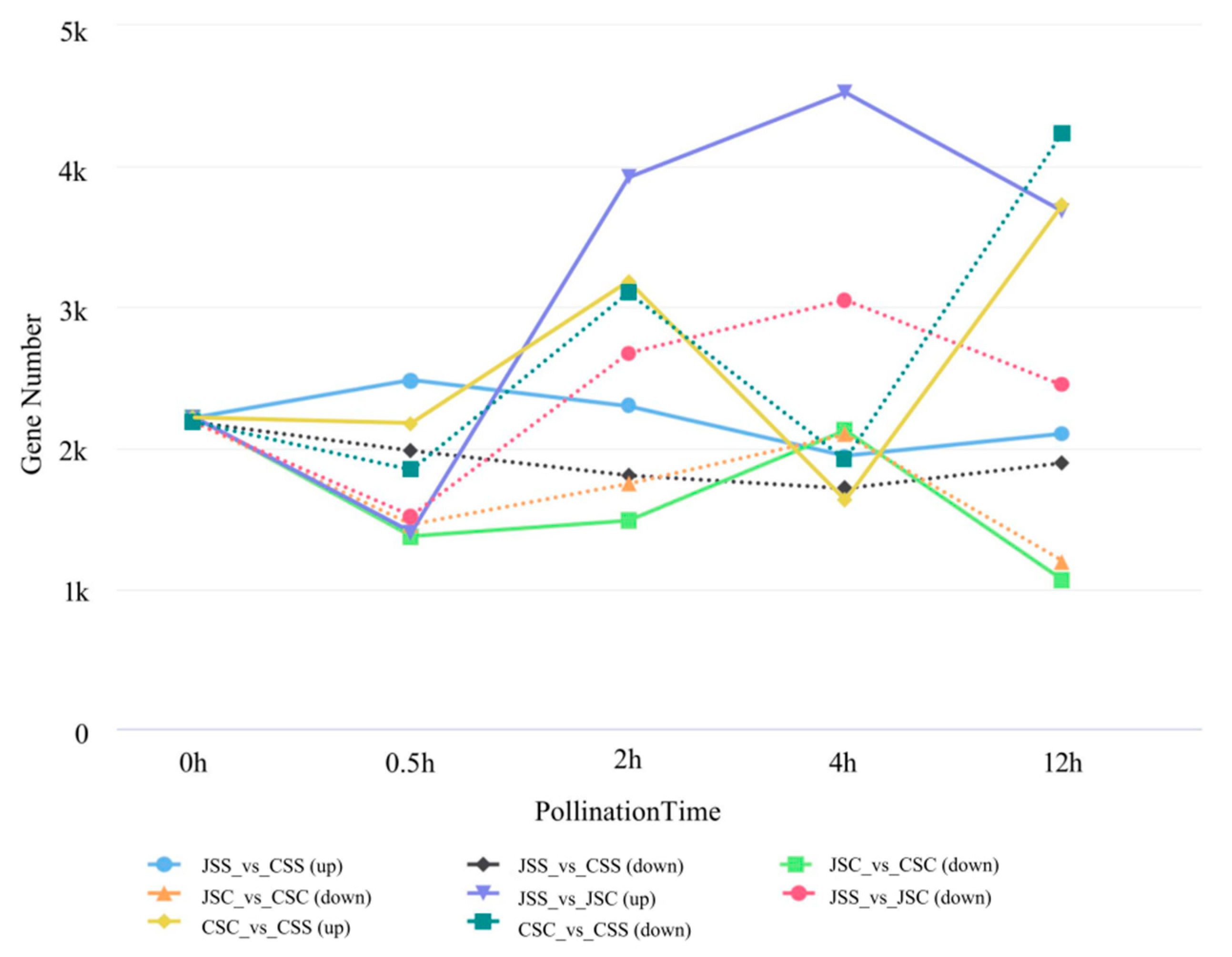





| Comparison Group | 0.5 h | 2 h | 4 h | 12 h |
|---|---|---|---|---|
| JSS_vs_CSS | 4463 | 4099 | 3649 | 3988 |
| JSS_vs_JSC | 2913 | 6597 | 7580 | 6129 |
| CSC_vs_CSS | 4016 | 6288 | 3540 | 7970 |
| JSC_vs_CSC | 2821 | 3222 | 4214 | 2252 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, M.; Ding, Y.; Li, T.; Jiang, S.; Kang, S.; Wei, S.; Xie, J.; Huang, J.; Hu, W.; et al. The Pitaya Flower Tissue’s Gene Differential Expression Analysis between Self-Incompatible and Self-Compatible Varieties for the Identification of Genes Involved in Self-Incompatibility Regulation. Int. J. Mol. Sci. 2023, 24, 11406. https://doi.org/10.3390/ijms241411406
Wang Z, Wang M, Ding Y, Li T, Jiang S, Kang S, Wei S, Xie J, Huang J, Hu W, et al. The Pitaya Flower Tissue’s Gene Differential Expression Analysis between Self-Incompatible and Self-Compatible Varieties for the Identification of Genes Involved in Self-Incompatibility Regulation. International Journal of Molecular Sciences. 2023; 24(14):11406. https://doi.org/10.3390/ijms241411406
Chicago/Turabian StyleWang, Zhouwen, Meng Wang, Yi Ding, Tao Li, Senrong Jiang, Shaoling Kang, Shuangshuang Wei, Jun Xie, Jiaquan Huang, Wenbin Hu, and et al. 2023. "The Pitaya Flower Tissue’s Gene Differential Expression Analysis between Self-Incompatible and Self-Compatible Varieties for the Identification of Genes Involved in Self-Incompatibility Regulation" International Journal of Molecular Sciences 24, no. 14: 11406. https://doi.org/10.3390/ijms241411406
APA StyleWang, Z., Wang, M., Ding, Y., Li, T., Jiang, S., Kang, S., Wei, S., Xie, J., Huang, J., Hu, W., Li, H., & Tang, H. (2023). The Pitaya Flower Tissue’s Gene Differential Expression Analysis between Self-Incompatible and Self-Compatible Varieties for the Identification of Genes Involved in Self-Incompatibility Regulation. International Journal of Molecular Sciences, 24(14), 11406. https://doi.org/10.3390/ijms241411406




