Inhibitory Effect of Adsorption of Streptococcus mutans onto Scallop-Derived Hydroxyapatite
Abstract
1. Introduction
2. Results
2.1. Adsorption of S. mutans onto Scallop-Derived Hydroxyapatite
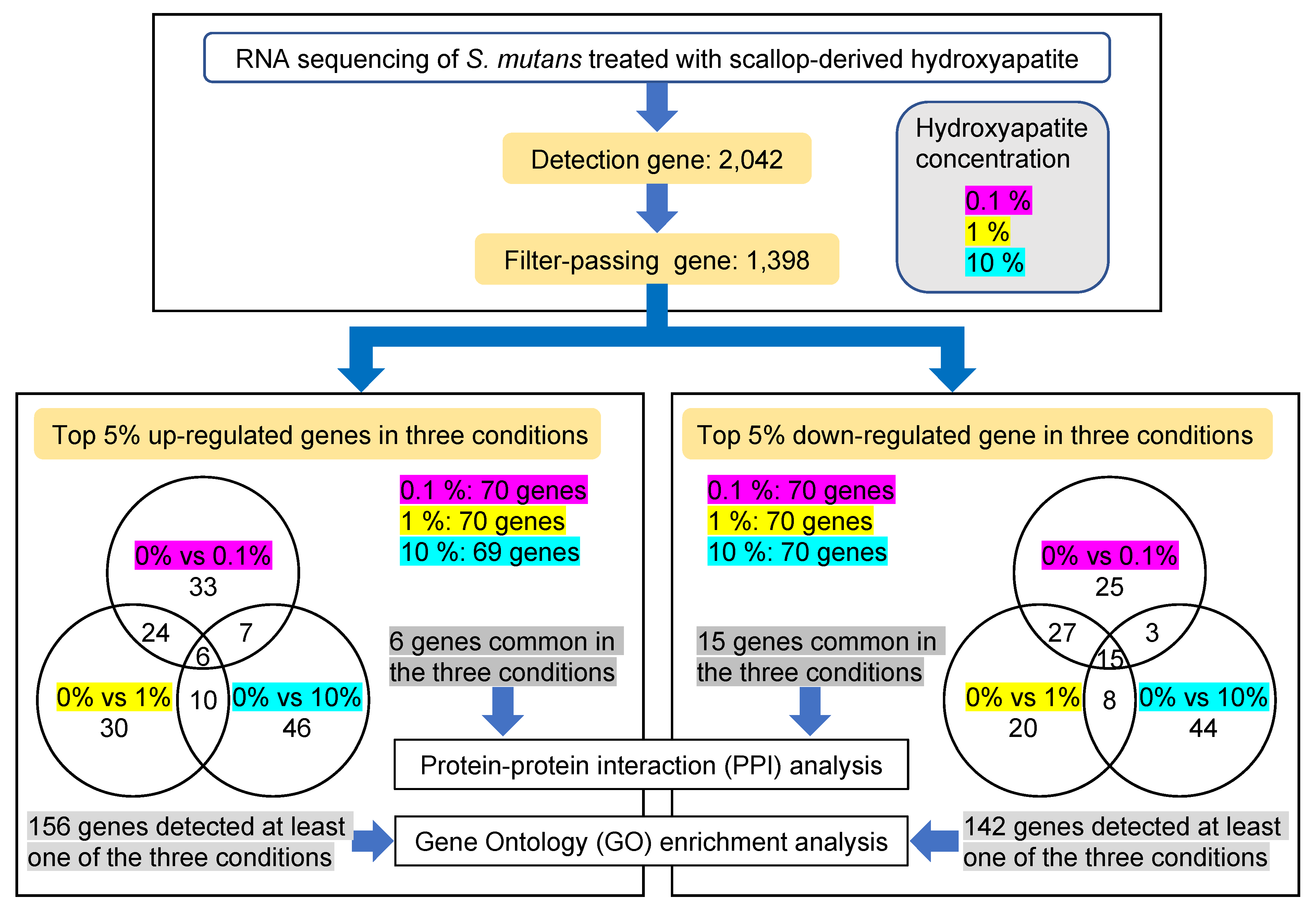
2.2. RNA Sequencing Analysis of S. mutans Treated with Scallop-Derived Hydroxyapatite
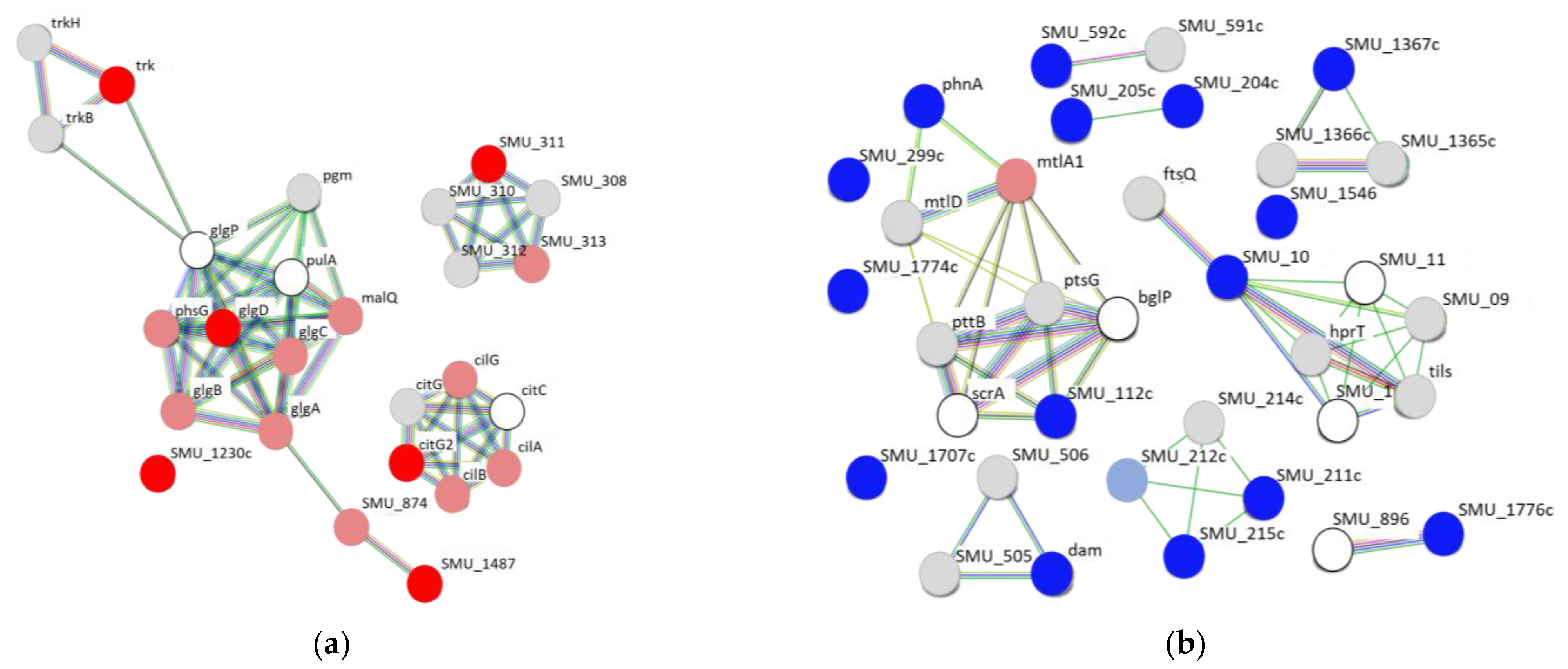
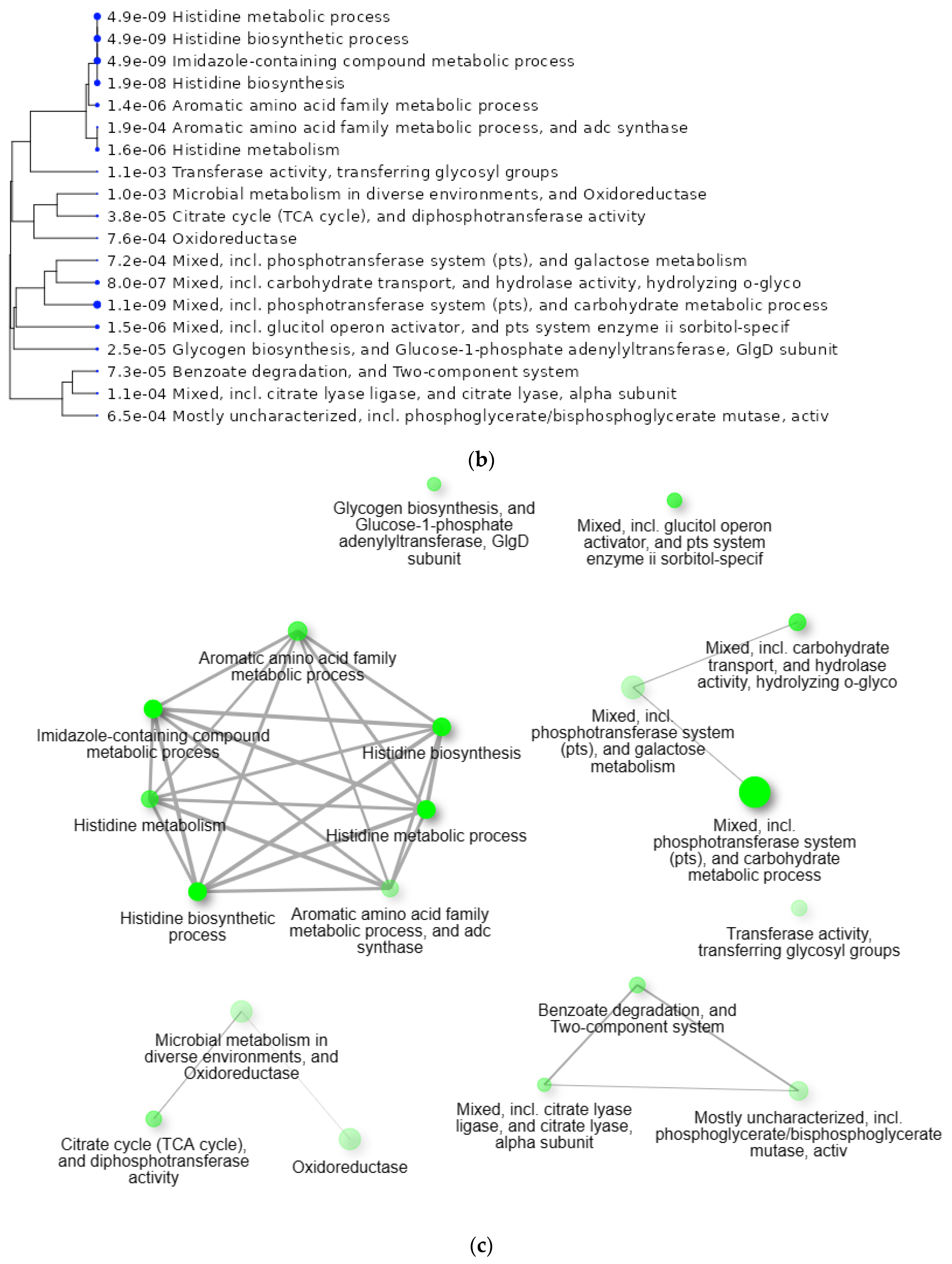
2.3. Protein–Protein Interaction Network Analysis of Upregulated and Downregulated Genes of S. mutans Treated with Scallop-Derived Hydroxyapatite
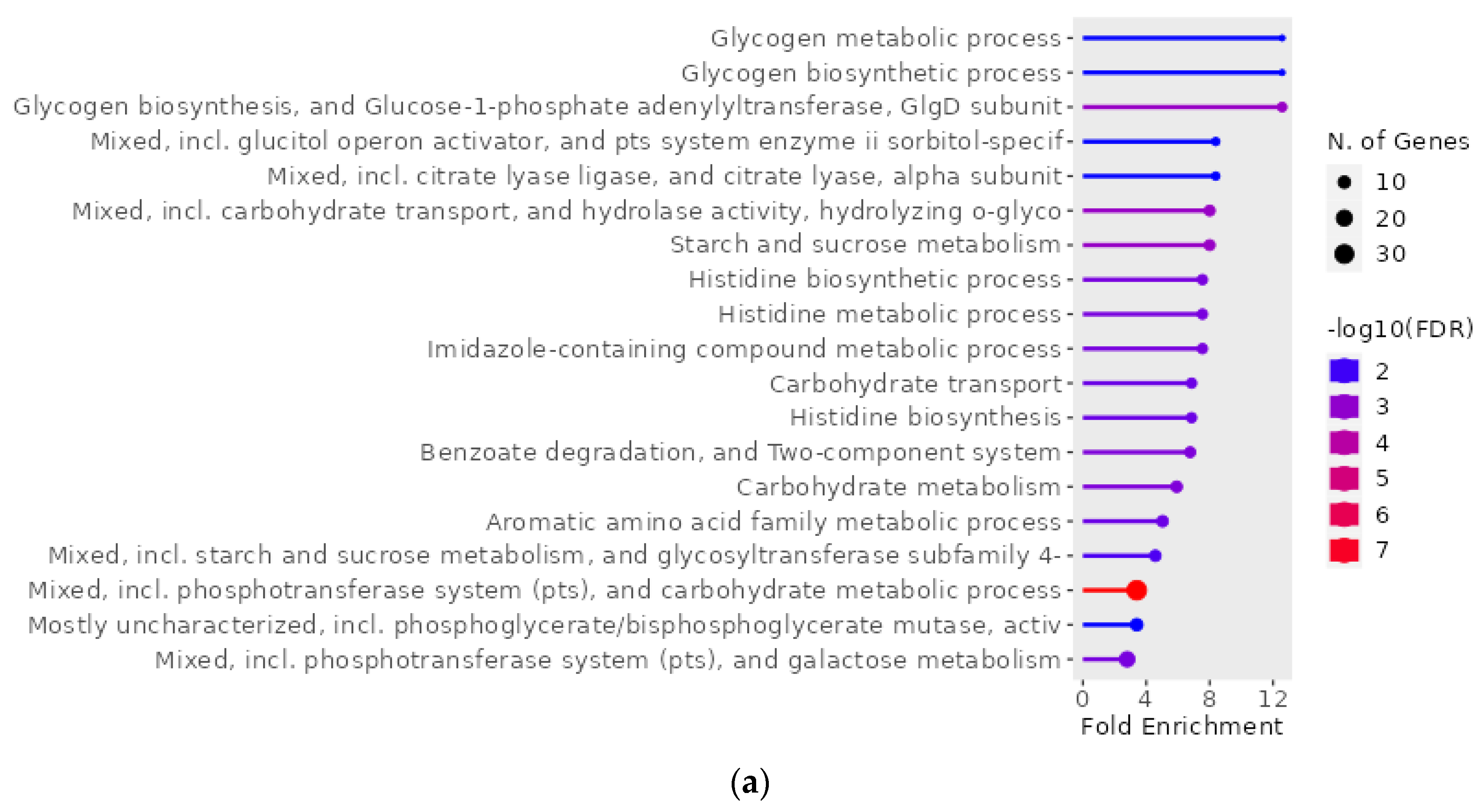
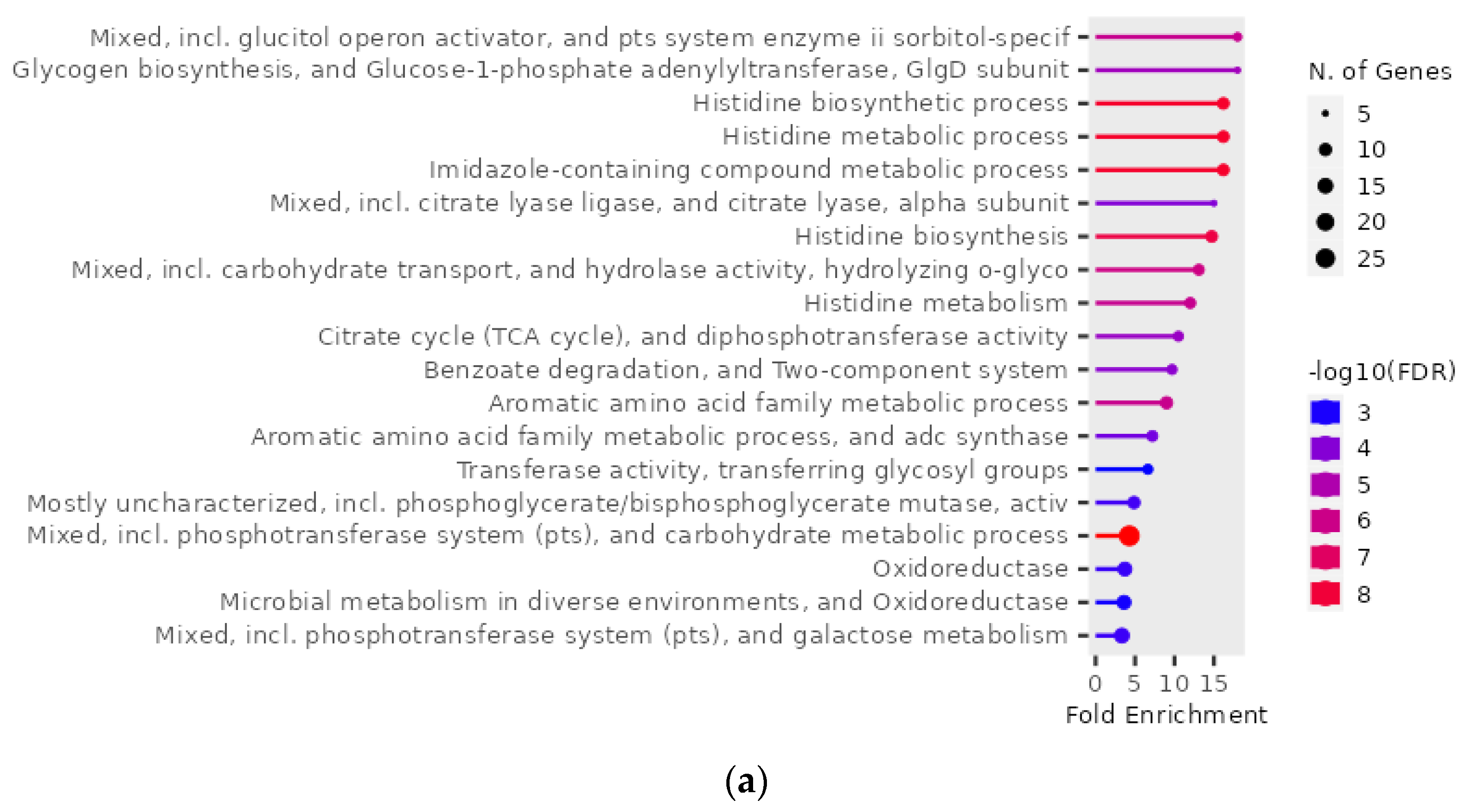
2.4. Gene Ontology Enrichment Analysis of Genes of S. mutans Upregulated on Treatment with Scallop-Derived Hydroxyapatite
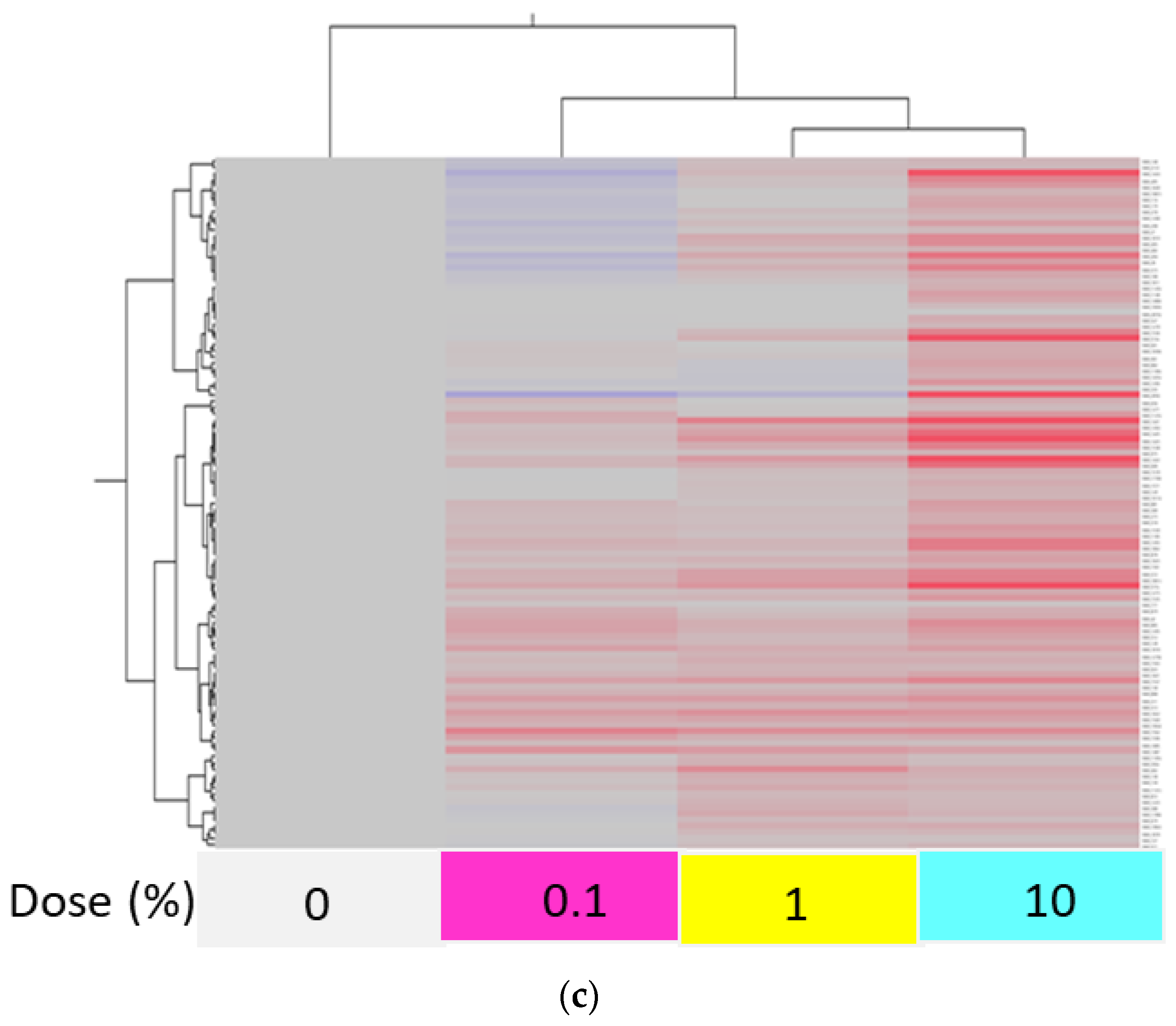
2.5. Principal Component Analysis and Heat Map Constructed Using Upregulated and Downregulated Genes of S. mutans Treated with Scallop-Derived Hydroxyapatite
2.6. GO Enrichment Analysis of 109 Genes of S. mutans Upregulated in the Presence of 10% Scallop-Derived Hydroxyapatite
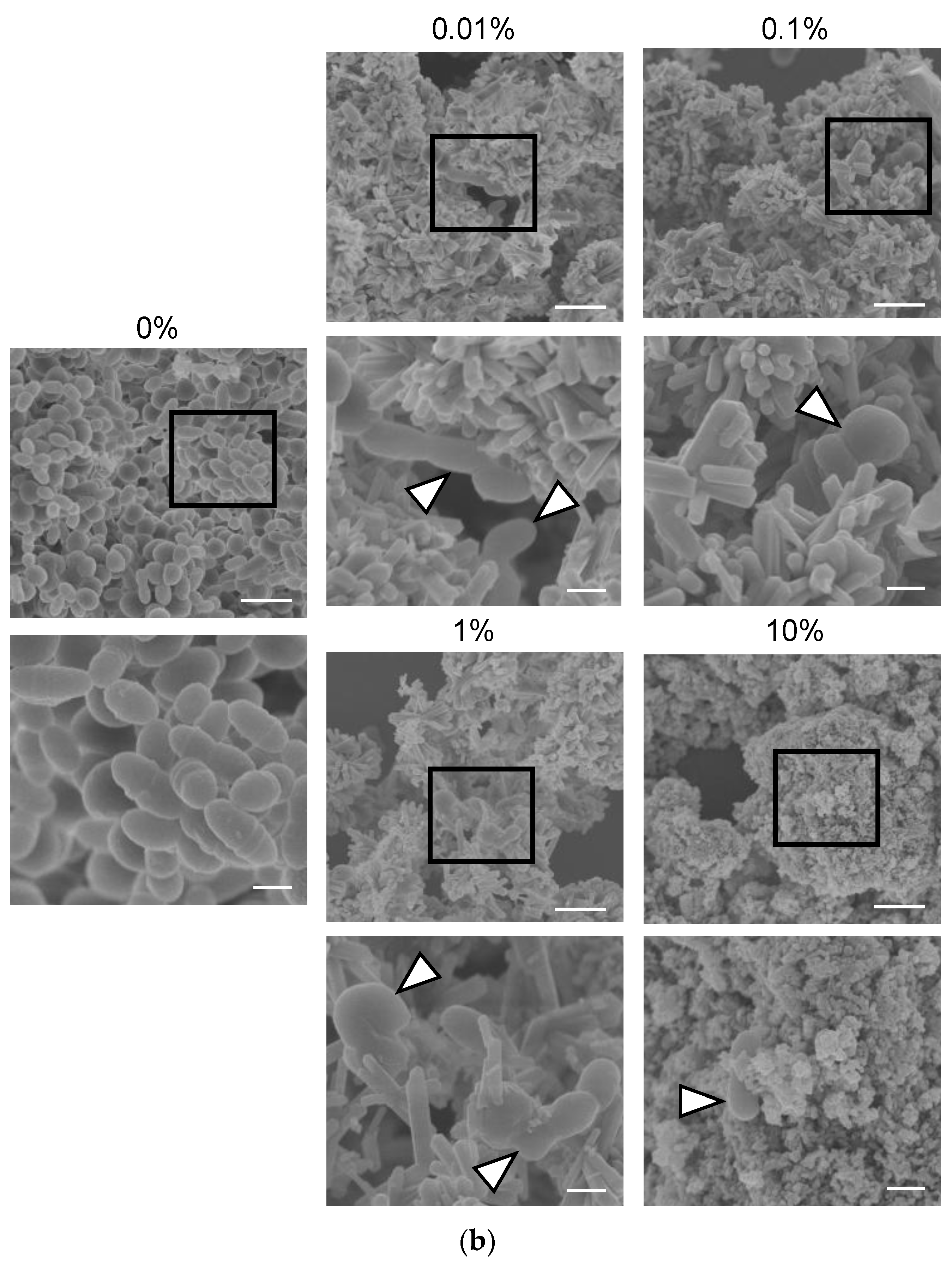
2.7. Analysis of Bacterial Growth and Morphology of S. mutans in the Presence of Scallop-Derived Hydroxyapatite
3. Discussion
4. Materials and Methods
4.1. S. mutans Strain and Culture Conditions
4.2. Scallop-Derived Hydroxyapatite
4.3. The Adsorption of S. mutans onto Scallop-Derived Hydroxyapatite
4.4. RNA Sequencing and FASTQ File Processing
4.5. Analysis of RNA Sequencing Count Data
4.6. Bioinformatic Analysis
4.7. Bacterial Growth Assay
4.8. Electron Microscopy
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149 Pt 2, 279–294. [Google Scholar] [CrossRef]
- Marsh, P.D. Microbial ecology of dental plaque and its significance in health and disease. Adv. Dent. Res. 1994, 8, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranche, J.; Brady, L.J. The biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Tay, F.R.; Niu, L.N.; Chen, J.H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral Sci. 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Segura-Egea, J.J.; Gould, K.; Sen, B.H.; Jonasson, P.; Cotti, E.; Mazzoni, A.; Sunay, H.; Tjäderhane, L.; Dummer, P.M.H. Antibiotics in endodontics: A review. Int. Endod. J. 2017, 50, 1169–1184. [Google Scholar] [CrossRef]
- Supová, M. Isolation and preparation of nanoscale bioapatites from natural sources: A review. J. Nanosci. Nanotechnol. 2014, 14, 546–563. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, Y. Defect induced charge redistribution and enhanced adsorption of lysozyme on hydroxyapatite for efficient antibacterial activity. Langmuir 2021, 37, 10786–10796. [Google Scholar] [CrossRef]
- Shang, B.; Wang, S.; Lu, L.; Ma, H.; Liu, A.; Zupanic, A.; Jiang, L.; Elnawawy, A.S.; Yu, Y. Poultry eggshell-derived antimicrobial materials: Current status and future perspectives. J. Environ. Manag. 2022, 314, 115096. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Różycka, D. Substituted hydroxyapatites with antibacterial properties. Biomed. Res. Int. 2014, 2014, 178123. [Google Scholar] [CrossRef]
- Dorcioman, G.; Grumezescu, V.; Stan, G.E.; Chifiriuc, M.C.; Gradisteanu, G.P.; Miculescu, F.; Matei, E.; Popescu-Pelin, G.; Zgura, I.; Craciun, V.; et al. Hydroxyapatite thin films of marine origin as sustainable candidates for dental implants. Pharmaceutics 2023, 15, 1294. [Google Scholar] [CrossRef]
- Devine, D.A.; Marsh, P.D.; Meade, J. Modulation of host responses by oral commensal bacteria. J. Oral Microbiol. 2015, 7, 26941. [Google Scholar] [CrossRef]
- Mira, A. Oral microbiome studies: Potential diagnostic and therapeutic implications. Adv. Dent. Res. 2018, 29, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Meto, A.; Colombari, B.; Odorici, A.; Giva, L.B.; Pericolini, E.; Regina, A.L.; Blasi, E. Antibacterial effects of MicroRepair ®BIOMA-Based toothpaste and chewing gum on orthodontic elastics contaminated in vitro with saliva from healthy donors: A pilot study. Appl. Sci. 2020, 10, 6721. [Google Scholar] [CrossRef]
- Odorici, A.; Colombari, B.; Bellini, P.; Meto, A.; Venturelli, I.; Blasi, E. Novel options to counteract oral biofilm formation: In vitro evidence. Int. J. Environ. Res. Public Health 2022, 19, 8056. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, T.; Sohier, J.; Rosset, P.; Layrolle, P. Biomimetic materials for bone tissue engineering—State of art and future trends. Adv. Eng. Mater. 2011, 13, 135–150. [Google Scholar] [CrossRef]
- Mucalo, M.R. Special issue: Novel advances and approaches in biomedical materials based on calcium phosphates. Materials 2019, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Mitran, V.; Ion, R.; Miculescu, F.; Necula, M.G.; Mocanu, A.-C.; Stan, G.E.; Antoniac, I.V.; Cimpean, A. Osteoblast cell response to naturally derived calcium phosphate-based materials. Materials 2018, 11, 1097. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- DileepKumar, V.G.; Sridhar, M.S.; Aramwit, P.; Krut’ko, V.K.; Musskaya, O.N.; Glazov, I.E.; Reddy, N. A review on the synthesis and properties of hydroxyapatite for biomedical applications. J. Biomater. Sci. Polym. Ed. 2022, 33, 229–261. [Google Scholar] [CrossRef]
- Anil, A.; Ibraheem, W.I.; Meshni, A.A.; Preethanath, R.S.; Anil, S. Nano-hydroxyapatite (nHAp) in the remineralization of early dental caries: A scoping review. Int. J. Environ. Res. Public Health 2022, 19, 5629. [Google Scholar] [CrossRef] [PubMed]
- Marr, A.K.; Joseph, B.; Mertins, S.; Ecke, R.; Müller-Altrock, S.; Goebel, W. Overexpression of PrfA leads to growth inhibition of Listeria monocytogenes in glucose-containing culture media by interfering with glucose uptake. J. Bacteriol. 2006, 188, 3887–3901. [Google Scholar] [CrossRef]
- de Young, K.D.; Stankeviciute, G.; Klein, E.A. Sugar-phosphate metabolism regulates stationary-phase entry and stalk elongation in Caulobacter crescentus. J. Bacteriol. 2020, 202, e00468-19. [Google Scholar] [CrossRef]
- Wang, S.; Long, L.; Yang, X.; Qiu, Y.; Tao, T.; Peng, X.; Li, Y.; Han, A.; Senadheera, D.B.; Downey, J.S.; et al. Dissecting the role of VicK phosphatase in aggregation and biofilm formation of Streptococcus mutans. J. Dent. Res. 2021, 100, 631–638. [Google Scholar] [CrossRef]
- Gerhard, E.M.; Wang, W.; Li, C.; Guo, J.; Ozbolat, I.T.; Rahn, K.M.; Armstrong, A.D.; Xia, J.; Qian, G.; Yang, J. Design strategies and applications of nacre-based biomaterials. Acta Biomater. 2017, 54, 21–34. [Google Scholar] [CrossRef]
- Liu, C.; Chen, S.H.; Yang-Zhou, C.H.; Zhang, Q.G.; Michael, R.N. Application of nano-hydroxyapatite derived from oyster shell in fabricating superhydrophobic sponge for efficient oil/water separation. Molecules 2021, 26, 3703. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Z.; Zhang, H.; Hu, Q.; Zou, Y. Potential uses of scallop shell powder as a substrate for the cultivation of king oyster mushroom (Pleurotus eryngii). Horticulturae 2022, 8, 333. [Google Scholar] [CrossRef]
- Limeback, H.; Meyer, F.; Enax, J. Tooth whitening with hydroxyapatite: A systematic review. Dent. J. 2023, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ishihara, M.; Nakamura, S.; Fukuda, K.; Takayama, T.; Hiruma, S.; Murakami, K.; Fujita, M.; Yokoe, H. Preparation and application of bioshell calcium oxide (BiSCaO) nanoparticle-dispersions with bactericidal activity. Molecules 2019, 24, 3415. [Google Scholar] [CrossRef] [PubMed]
- Athanasios, A.; Charalampos, V.; Vasileios, T.; Ashraf, G.M. Protein-protein interaction (PPI) network: Recent advances in drug discovery. Curr. Drug Metab. 2017, 18, 5–10. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.H.; Wang, S.; Zhang, Y.; Huang, T.; Cai, Y.D. Prediction and analysis of essential genes using the enrichments of gene ontology and KEGG pathways. PLoS ONE 2017, 12, e0184129. [Google Scholar] [CrossRef] [PubMed]
- Yost, S.; Duran-Pinedo, A.E.; Teles, R.; Krishnan, K.; Frias-Lopez, J. Functional signatures of oral dysbiosis during periodontitis progression revealed by microbial metatranscriptome analysis. Genome Med. 2015, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zou, Z.; Anderson, D.; Sang, Y.; Higashi, D.; Kreth, J.; Merritt, J. The transcription regulator BrsR serves as a network hub of natural competence protein-protein interactions in Streptococcus mutans. Proc. Natl. Acad. Sci. USA 2021, 118, e2106048118. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Kujala, U.; Edlund, M.B. Pyruvate dehydrogenase activity in Streptococcus mutans. Infect. Immun. 1985, 49, 674–678. [Google Scholar] [CrossRef]
- Busuioc, M.; Mackiewicz, K.; Buttaro, B.A.; Piggot, P.J. Role of intracellular polysaccharide in persistence of Streptococcus mutans. J. Bacteriol. 2009, 191, 7315–7322. [Google Scholar] [CrossRef]
- David, C.C.; Jacobs, D.J. Principal component analysis: A method for determining the essential dynamics of proteins. Methods Mol. Biol. 2014, 1084, 193–226. [Google Scholar]
- Liao, J.C.; Chao, Y.P.; Patnaik, R. Alteration of the biochemical valves in the central metabolism of Escherichia coli. Ann. N. Y. Acad. Sci. 1994, 745, 21–34. [Google Scholar] [CrossRef]
- Shleeva, M.O.; Kaprelyants, A.S. Hypobiosis of mycobacteria: Biochemical aspects. Biochemistry 2023, 88, 52–74. [Google Scholar] [CrossRef]
- Lu, J.; Carter, D.A.; Turnbull, L.; Rosendale, D.; Hedderley, D.; Stephens, J.; Gannabathula, S.; Steinhorn, G.; Schlothauer, R.C.; Whitchurch, C.B. The effect of New Zealand kanuka, manuka and clover honeys on bacterial growth dynamics and cellular morphology varies according to the species. PLoS ONE 2013, 8, e55898. [Google Scholar] [CrossRef]
- Serbanescu, D.; Ojkic, N.; Banerjee, S. Cellular resource allocation strategies for cell size and shape control in bacteria. FEBS J. 2022, 289, 7891–7906. [Google Scholar] [CrossRef]
- Mitsuhata, C.; Kado, N.; Hamada, M.; Nomura, R.; Kozai, K. Characterization of the unique oral microbiome of children with Down syndrome. Sci. Rep. 2022, 12, 14150. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.C.; Wong, A. Effect of adsorbed protein on hydroxyapatite zeta potential and Streptococcus mutans adherence. Infect. Immun. 1983, 39, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Shimabayashi, S.; Hoshino, M.; Hino, T. Zeta potential of hydroxyapatite particles in the presence of polyethylene glycol mono-p-isooctylphenyl ether and sodium dodecylsulfate in an aqueous phase. Phosphorus Res. Bull. 2004, 17, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Silingardi, F.; Bonvicini, F.; Cassani, M.C.; Mazzaro, R.; Rubini, K.; Gentilomi, G.A.; Bigi, A.; Boanini, E. Hydroxyapatite decorated with tungsten oxide nanoparticles: New composite materials against bacterial growth. J. Funct. Biomater. 2022, 3, 88. [Google Scholar] [CrossRef] [PubMed]
- Beiranvand, M.; Farhadi, S.; Mohammadi-Gholami, A. Ag NPs decorated on the magnetic rod-like hydroxyapatite/MIL-101(Fe) nanocomposite as an efficient catalyst for the reduction of some nitroaromatic compounds and as an effective antimicrobial agent. RSC Adv. 2023, 13, 13683–13697. [Google Scholar] [CrossRef]
- Nomura, R.; Morita, Y.; Matayoshi, S.; Nakano, K. Inhibitory effect of surface pre-reacted glass-ionomer (S-PRG) eluate against adhesion and colonization by Streptococcus mutans. Sci. Rep. 2018, 8, 5056. [Google Scholar] [CrossRef]
- Ooshima, T.; Izumitani, A.; Sobue, S.; Hamada, S. Cariostatic effect of palatinose on experimental dental caries in rats. Jpn. J. Med. Sci. Biol. 1983, 36, 219–223. [Google Scholar] [CrossRef]
- Kien, P.T.; Phu, H.D.; Linh, N.V.V.; Quyen, T.N.; Hoa, N.T. Recent trends in hydroxyapatite (HA) synthesis and the synthesis report of nanostructure HA by hydrothermal reaction. Adv. Exp. Med. Biol. 2018, 1077, 343–354. [Google Scholar]
- Nomura, R.; Ohata, J.; Otsugu, M.; Okawa, R.; Naka, S.; Matsumoto-Nakano, M.; Nakano, K. Inhibitory effects of flavedo, albedo, fruits, and leaves of Citrus unshiu extracts on Streptococcus mutans. Arch. Oral Biol. 2021, 124, 105056. [Google Scholar] [CrossRef]
- Nomura, R.; Kitamura, T.; Matayoshi, S.; Ohata, J.; Suehiro, Y.; Iwashita, N.; Okawa, R.; Nakano, K. Inhibitory effect of a gel paste containing surface pre-reacted glass-ionomer (S-PRG) filler on the cariogenicity of Streptococcus mutans. Sci. Rep. 2021, 11, 23495. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Otsugu, M.; Naka, S.; Teramoto, N.; Kojima, A.; Muranaka, Y.; Matsumoto-Nakano, M.; Ooshima, T.; Nakano, K. Contribution of Streptococcus mutans serotype k strains interaction with fibrinogen to the pathogenicity of infective endocarditis. Infect Immun. 2014, 82, 5223–5234. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, Y.; Nomura, R.; Matayoshi, S.; Otsugu, M.; Iwashita, N.; Nakano, K. Evaluation of the collagen-binding properties and virulence of killed Streptococcus mutans in a silkworm model. Sci. Rep. 2022, 12, 2800. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usuda, M.; Kametani, M.; Hamada, M.; Suehiro, Y.; Matayoshi, S.; Okawa, R.; Naka, S.; Matsumoto-Nakano, M.; Akitomo, T.; Mitsuhata, C.; et al. Inhibitory Effect of Adsorption of Streptococcus mutans onto Scallop-Derived Hydroxyapatite. Int. J. Mol. Sci. 2023, 24, 11371. https://doi.org/10.3390/ijms241411371
Usuda M, Kametani M, Hamada M, Suehiro Y, Matayoshi S, Okawa R, Naka S, Matsumoto-Nakano M, Akitomo T, Mitsuhata C, et al. Inhibitory Effect of Adsorption of Streptococcus mutans onto Scallop-Derived Hydroxyapatite. International Journal of Molecular Sciences. 2023; 24(14):11371. https://doi.org/10.3390/ijms241411371
Chicago/Turabian StyleUsuda, Momoko, Mariko Kametani, Masakazu Hamada, Yuto Suehiro, Saaya Matayoshi, Rena Okawa, Shuhei Naka, Michiyo Matsumoto-Nakano, Tatsuya Akitomo, Chieko Mitsuhata, and et al. 2023. "Inhibitory Effect of Adsorption of Streptococcus mutans onto Scallop-Derived Hydroxyapatite" International Journal of Molecular Sciences 24, no. 14: 11371. https://doi.org/10.3390/ijms241411371
APA StyleUsuda, M., Kametani, M., Hamada, M., Suehiro, Y., Matayoshi, S., Okawa, R., Naka, S., Matsumoto-Nakano, M., Akitomo, T., Mitsuhata, C., Koumoto, K., Kawauchi, K., Nishikata, T., Yagi, M., Mizoguchi, T., Fujikawa, K., Taniguchi, T., Nakano, K., & Nomura, R. (2023). Inhibitory Effect of Adsorption of Streptococcus mutans onto Scallop-Derived Hydroxyapatite. International Journal of Molecular Sciences, 24(14), 11371. https://doi.org/10.3390/ijms241411371





