Photomorphogenesis and Photosynthetic Traits Changes in Rice Seedlings Responding to Red and Blue Light
Abstract
1. Introduction
2. Results
2.1. Plant Height, First Leaf Sheath Length, and Stem Width
2.2. Leaf Growth
2.3. Root Growth
2.4. Root Activity
2.5. Fresh Weight, Dry Weight
2.6. Pigment Content, SPAD
2.7. Chlorophyll Fluorescence
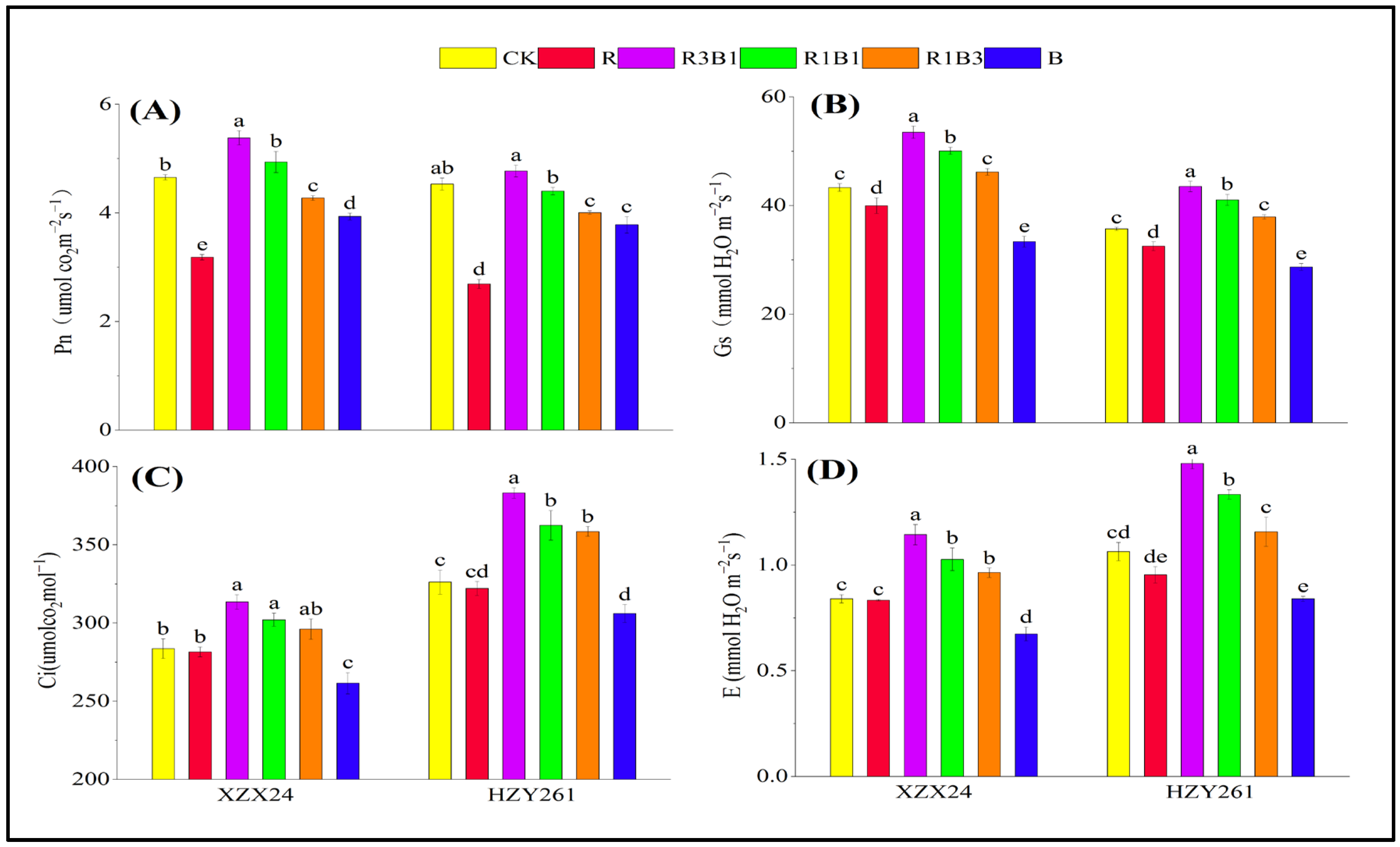
2.8. Photosynthesis
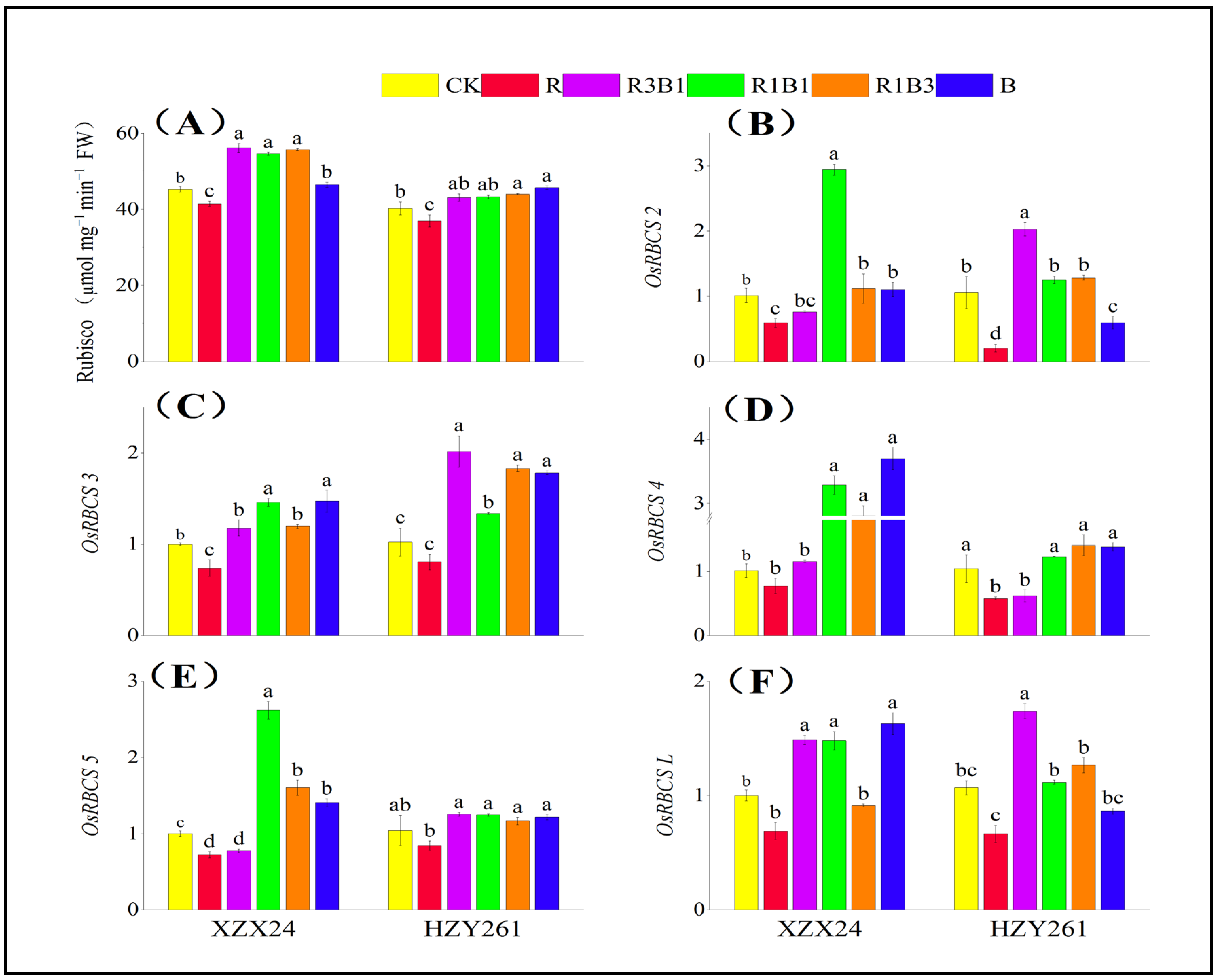
2.9. Rubisco Activity, Gene Expression
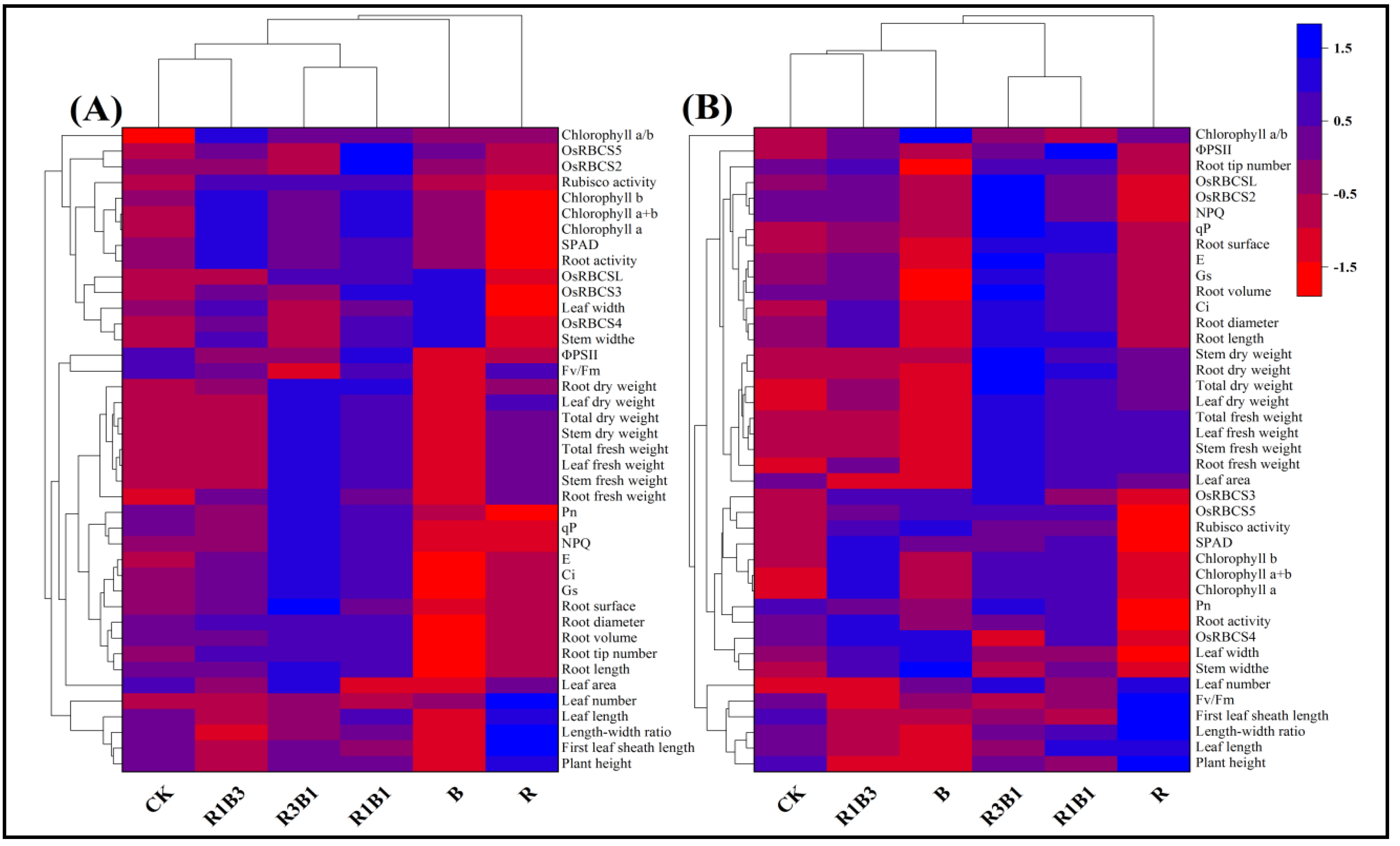
2.10. Heat Map Analysis
3. Discussion
3.1. Red and Blue Lights Can Regulate the Early Photomorphogenesis of Rice
3.2. Effects of Red and Blue Lights on Photosynthetic Pigments and Chlorophyll Fluorescence
3.3. Effects of Red and Blue Lights on Photosynthesis, Rubisco Activity, and Gene Expression
4. Materials and Methods
4.1. Plant Materials and a Description of the Experiment
4.2. Morphological Indicators and Root Activity Analysis
4.3. Chlorophyll Content and SPAD of Leaves
4.4. Chlorophyll Fluorescence
4.5. Photosynthetic Parameter Analysis
4.6. Rubisco Activity and Gene Expression
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Z.; Feng, S.; Gai, S.; Gao, P.; Xu, C.; Xia, M.; Lu, X. Affordable phosphor-converted LEDs with specific light quality facilitate the tobacco seedling growth with low energy consumption in industrial seedling raising. J. Photochem. Photobiol. B Biol. 2022, 235, 112564. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Ji, H.; Yang, Y.; Chu, Q.; Yang, Y.; Liu, H.; Jiang, X. Analysis on transporting methods of cultivation unit for vertical cultivation in plant factory. Agriculture 2021, 11, 989. [Google Scholar] [CrossRef]
- Shamshiri, R.R.; Kalantari, F.; Ting, K.C.; Thorp, K.R.; Hameed, I.A.; Weltzien, C.; Shad, Z.M. Advances in greenhouse automation and controlled environment agriculture: A transition to plant factories and urban agriculture. Int. J. Agric. Biol. Eng. 2018, 11, 1–22. [Google Scholar] [CrossRef]
- Vu, P.L.; Lee, M.H.; Park, J.S. Optimization of indole-3-acetic acid concentration in a nutrient solution for increasing bioactive compound accumulation and production of agastache rugosa in a plant factory. Agriculture 2020, 10, 343. [Google Scholar] [CrossRef]
- Yuan, W.; Zha, L.; Liu, W. Dynamic responses of ascorbate pool and metabolism in lettuce to light intensity at night time under continuous light provided by red and blue LEDs. Plants 2021, 10, 214. [Google Scholar] [CrossRef]
- Kharshiing, E.V.; Mawphlang, O.I.L.; Lama, V.; Bhattacharjee, R.; Sahoo, L. Manipulation of light environment for optimising photoreceptor activity towards enhancing plant traits of agronomic and horticultural importance in crops. J. Hortic. Sci. Biotechnol. 2022, 97, 535–551. [Google Scholar] [CrossRef]
- Liu, T.; Yuan, Q.; Wang, Y. Hierarchical optimization control based on crop growth model for greenhouse light environment. Comput. Electron. Agric. 2021, 180, 105854. [Google Scholar] [CrossRef]
- Zheng, J.; Meng, S.; Zhang, X.; Zhao, H.; Ning, X.; Chen, F.; Liu, W. Increasing the comprehensive economic benefits of farmland with even-lighting agrivoltaic systems. PLoS ONE 2021, 16, e0254482. [Google Scholar] [CrossRef]
- Paradiso, R.; Simona, P. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Li, J.; Zhangzhong, L.; Zhang, X.; Wei, X.; Zhang, S.; Wang, L.; Zheng, W. Effects of light conversion film on the growth of leafy vegetables in facilities under haze weather. Agronomy 2022, 12, 2391. [Google Scholar] [CrossRef]
- Di Qinghua Li, J.; Yufen, D.; Wei, M.; Shi, Q.; Li, Y.; Fengjuan, Y. Combination of Red and Blue Lights Improved the Growth and Development of Eggplant (Solanum melongena L.) Seedlings by Regulating Photosynthesis. J. Plant Growth Regul. 2021, 40, 1477–1492. [Google Scholar] [CrossRef]
- DeChaine, J.M.; Gardner, G.; Weinig, C. Phytochromes differentially regulate seed germination responses to light quality and temperature cues during seed maturation. Plant Cell Environ. 2009, 32, 1297–1309. [Google Scholar] [CrossRef]
- Vieira, B.C.; Bianca MARodrigues Garcia, Q.S. Light exposure time and light quality on seed germination of vellozia species (velloziaceae) from brazilian campo rupestre. Flora 2018, 238, 94–101. [Google Scholar] [CrossRef]
- Lee, H.; Gyu, H.I.; Cheong, E.J. Effect of different treatments and light quality on Ulmus pumila L. germination and seedling growth. For. Sci. Technol. 2021, 17, 162–168. [Google Scholar] [CrossRef]
- Yu, Y.; Qin, W.; Li, Y.; Zhang, C.; Wang, Y.; Yang, Z.; Li, F. Red light promotes cotton embryogenic callus formation by influencing endogenous hormones, polyamines and antioxidative enzyme activities. Plant Growth Regul. 2019, 87, 187–199. [Google Scholar] [CrossRef]
- Cioć, M.; Dziurka, M.; Pawłowska, B. Changes in Endogenous Phytohormones of Gerbera jamesonii Axillary Shoots Multiplied under Different Light Emitting Diodes Light Quality. Molecules 2022, 27, 1804. [Google Scholar] [CrossRef]
- Mao, R.; Guo, S. Performance of the mixed LED light quality on the growth and energy efficiency of arthrospira platensis. Appl. Microbiol. Biotechnol. 2018, 102, 5245–5254. [Google Scholar] [CrossRef]
- Frąszczak, B.; Kula-Maximenko, M. The biometric parameters of microgreen crops grown under various light conditions. Agriculture 2022, 12, 576. [Google Scholar] [CrossRef]
- Rahman, M.H.; Md Obyedul, K.A.; Islam, M.J.; Rana, M.S.; Li, K.; Lim, Y.S. Production of Potato (Solanum tuberosum L.) Seed Tuber under Artificial LED Light Irradiation in Plant Factory. Plants 2021, 10, 297. [Google Scholar] [CrossRef]
- Jin, D.; Su, X.; Li, Y.; Shi, M.; Bobo, Y.; Wan, W.; Zou, J. Effect of red and blue light on cucumber seedlings grown in a plant factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Huang, K.; Liu, Y.; Liu, M.; Wang, J. Effect of LED spectrum on the quality and nitrogen metabolism of lettuce under recycled hydroponics. Front. Plant Sci. 2021, 12, 678197. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liao, Q.; Li, Q.; Yang, Q.; Wang, F.; Li, J. Effects of LED red and blue light component on growth and photosynthetic characteristics of coriander in plant factory. Horticulturae 2022, 8, 1165. [Google Scholar] [CrossRef]
- Nguyen, T.P.D.; Jang, D.C.; Tran, T.T.H.; Nguyen, Q.T.; Kim, I.S.; Hoang, T.L.H.; Vu, N.T. Influence of Green Light Added with Red and Blue LEDs on the Growth, Leaf Microstructure and Quality of Spinach (Spinacia oleracea L.). Agronomy 2021, 11, 1724. [Google Scholar] [CrossRef]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F.M. Adding blue to red supplemental light increases biomass and yield of greenhouse-grown tomatoes, but only to an optimum. Front. Plant Sci. 2018, 9, 2002. [Google Scholar] [CrossRef] [PubMed]
- Jae, W.S.; Shiva, R.B.; Shin, Y.K.; Lee, J.G. The Influence of Red and Blue Light Ratios on Growth Performance, Secondary Metabolites, and Antioxidant Activities of Centella asiatica (L.) Urban. Horticulturae 2022, 8, 601. [Google Scholar] [CrossRef]
- Alrajhi, A.A.; Alsahli, A.S.; Alhelal, I.M.; Rihan, H.Z.; Fuller, M.P.; Alsadon, A.A.; Ibrahim, A.A. The Effect of LED Light Spectra on the Growth, Yield and Nutritional Value of Red and Green Lettuce (Lactuca sativa). Plants 2023, 12, 463. [Google Scholar] [CrossRef]
- Graham, T.; Yorio, N.; Zhang, P.; Massa, G.; Wheeler, R. Early seedling response of six candidate crop species to increasing levels of blue light. Life Sci. Space Res. 2019, 21, 40–48. [Google Scholar] [CrossRef]
- Fantini, E.; Facella, P. Cryptochromes in the field: How blue light influences crop development. Physiol. Plant. 2020, 169, 336–346. [Google Scholar] [CrossRef]
- Hyeon, E.O.; Yoon, A.; Yoo, G.P. Red Light Enhances the Antioxidant Properties and Growth of Rubus hongnoensis. Plants 2021, 10, 2589. [Google Scholar] [CrossRef]
- Gao, S.; Chen, Z.; Xu, K.; Kong, Y.; Lv, Y.; Cao, B. Effect of different LED light quality combination on the content of vitamin C, soluble sugar, organic acids, amino acids, antioxidant capacity and mineral elements in green onion (Allium fistulosum L.). Food Res. Int. 2022, 156, 111329. [Google Scholar] [CrossRef]
- Li, X.; Jacob, M.; Crunkleton, D.W.; Johannes, T.W. Effect of blue and red-orange LEDs on the growth and biochemical profile of Chlamydomonas reinhardtii. J. Appl. Phycol. 2021, 33, 1367–1377. [Google Scholar] [CrossRef]
- Simlat, M.; Ślęzak, P.; Moś, M.; Warchoł, M.; Skrzypek, E.; Ptak, A. The effect of light quality on seed germination, seedling growth and selected biochemical properties of stevia rebaudiana bertoni. Sci. Hortic. 2016, 211, 295–304. [Google Scholar] [CrossRef]
- Gao, S.; Xuena, L.; Liu, Y.; Bili, C.; Zijing, C.; Xu, K. Comparison of the effects of LED light quality combination on growth and nutrient accumulation in green onion (Allium fistulosum L.). Protoplasma 2021, 258, 753–763. [Google Scholar] [CrossRef]
- Stefański, P.; Siedlarz, P.; Matysik, P.; Rybka, K. Usefulness of LED lightings in cereal breeding on example of wheat, barley and oat seedlings. Int. J. Agric. Biol. Eng. 2019, 12, 32–37. [Google Scholar] [CrossRef]
- Muthusamy, M.; Kim, J.A.; Mi-Jeong, J.; Lee, S.I. Blue and red light upregulate α-expansin 1 (EXPA1) in transgenic Brassica rapa and its overexpression promotes leaf and root growth in Arabidopsis. Plant Growth Regul. 2020, 91, 75–87. [Google Scholar] [CrossRef]
- Fan, C.; Manivannan, A.; Hao, W. Light quality-mediated influence of morphogenesis in micropropagated horticultural crops: A comprehensive overview. BioMed Res. Int. 2022, 11, 4615079. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. Both the quality and positioning of the night interruption light are important for flowering and plant extension growth. J. Plant Growth Regul. 2020, 39, 583–593. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, Y.; Yi, X.; Zhang, X.; Fan, D.; Chow, W.S.; Zhang, W. Diaheliotropic leaf movement enhances leaf photosynthetic capacity and photosynthetic light and nitrogen use efficiency via optimising nitrogen partitioning among photosynthetic components in cotton (Gossypium hirsutum L.). Plant Biol. 2018, 20, 213–222. [Google Scholar] [CrossRef]
- Lee-Kuo, K.; Yan-Jie, H.; Wui-Ting, L.; Pang-Hung, H.; Pai-An, H. Growth, pigment content, antioxidant activity, and phytoene desaturase gene expression in Caulerpa lentillifera grown under different combinations of blue and red light-emitting diodes. J. Appl. Phycol. 2020, 32, 1971–1982. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.; Tang, Z.; Wu, Y.; Xiao, X.; Zhang, G.; Yu, J. Red and Blue LED Light Supplementation in the Morning Pre-activates the Photosynthetic System of Tomato (Solanum lycopersicum L.) Leaves and Promotes Plant Growth. Agronomy 2022, 12, 897. [Google Scholar] [CrossRef]
- Rahman, M.M.; David, L.F.; Soyed, M.A.; Hasan, M.T.; Mohammad, K.B.; Alameh, K. LED illumination for high-quality high-yield crop growth in protected cropping environments. Plants 2021, 10, 2470. [Google Scholar] [CrossRef] [PubMed]
- Marečková, M.; Barták, M.; Hájek, J. Temperature effects on photosynthetic performance of Antarctic lichen Dermatocarpon polyphyllizum: A chlorophyll fluorescence study. Polar Biol. 2019, 42, 685–701. [Google Scholar] [CrossRef]
- Guimarães, Z.T.M.; dos Santos, V.A.H.F.; Ferreira, M.J. Chlorophyll a fluorescence parameters are related to the leaf economics spectrum of tropical tree species in a mixed plantation. Trees 2022, 36, 763–775. [Google Scholar] [CrossRef]
- Polzella, A.; Terzaghi, M.; Trupiano, D.; Baronti, S.; Scippa, G.S.; Chiatante, D.; Montagnoli, A. Morpho-physiological responses of pisum sativum L. to different light-emitting diode (LED) light spectra in combination with biochar amendment. Agronomy 2020, 10, 398. [Google Scholar] [CrossRef]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.; Govindjee Zhu, X.G. Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynth. Res. 2019, 139, 107–121. [Google Scholar] [CrossRef]
- Vitale, E.; Luigi, G.I.; Amitrano, C.; Velikova, V.; Tsonev, T.; Simoniello, P.; Arena, C. Light Quality Modulates Photosynthesis and Antioxidant Properties of B. vulgaris L. Plants from Seeds Irradiated with High-Energy Heavy Ions: Implications for Cultivation in Space. Plants 2022, 11, 1816. [Google Scholar] [CrossRef]
- Borlongan, I.A.; Suzuki, S.; Nishihara, G.N.; Jumpei, K.; Ryuta, T. Effects of light quality and temperature on the photosynthesis and pigment content of a subtidal edible red alga Meristotheca papulosa (Solieriaceae, Gigartinales) from Japan. J. Appl. Phycol. 2020, 32, 1329–1340. [Google Scholar] [CrossRef]
- Parys, E.; Krupnik, T.; Kułak, I.; Kania, K.; Romanowska, E. Photosynthesis of the Cyanidioschyzon merolae cells in blue, red, and white light. Photosynth. Res. 2021, 147, 61–73. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, M.; Cheng, F.; Liu, S.; Liang, Y. Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of cunninghamia lanceolata. BMC Plant Biol. 2020, 20, 269. [Google Scholar] [CrossRef]
- Khattak, A.M.; Pearson, S. Spectral Filters and Temperature Effects on the Growth and Development of Chrysanthemums under Low Light Integral. Plant Growth Regul. 2006, 49, 61–68. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, S.; Asghar, S.; Zhang, L.; Qin, Q.; Chen, K.; Xu, C. Regeneration mechanism of toyonoka strawberry under different color plastic films. Plant Sci. 2005, 168, 1425–1431. [Google Scholar] [CrossRef]
- Lim, S. Light quality affects water use of sweet basil by changing its stomatal development. Agronomy 2021, 11, 303. [Google Scholar] [CrossRef]
- Dong, F.; Wang, C.; Sun, X.; Bao, Z.; Chen, D.; Sun, C.; Liu, S. Sugar metabolic changes in protein expression associated with different light quality combinations in tomato fruit. Plant Growth Regul. 2019, 88, 267–282. [Google Scholar] [CrossRef]
- Choi, H.G.; Byoung, Y.M.; Nam, J.K. Effects of LED light on the production of strawberry during cultivation in a plastic greenhouse and in a growth chamber. Sci. Hortic. 2015, 189, 22–31. [Google Scholar] [CrossRef]
- He, C.; Zeng, Y.; Fu, Y.; Wu, J.; Liang, Q. Light quality affects the proliferation of in vitro cultured plantlets of camellia oleifera huajin. PeerJ 2020, 8, e10016. [Google Scholar] [CrossRef]
- Košvancová-Zitová, M.; Urban, O.; Navrátil, M.; Špunda, V.; Robson, T.M.; Marek, M.V. Blue radiation stimulates photosynthetic induction in Fagus sylvatica L. Photosynthetica 2009, 47, 388–398. [Google Scholar] [CrossRef]
- Li, J.; Wei, Z.; Min, X.; Zhao, P.; Yang, L.; Liu, N. Physiological and Biochemical Changes in the Seeds of Naturally Aged Wenling Medic (Medicago polymorpha) with Its Recovery of Viability. Agronomy 2023, 13, 787. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photo-chemical and non-photochemical components-calculation of qP and fv’/Fm’ without measuring fo’. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Ren, M.; Mao, G.; Zheng, H.; Wang, W.; Tang, Q. Growth changes of tomato seedlings responding to sodium salt of α-naphthalene acetic acid and potassium salt of fulvic acid. Sci. Rep. 2023, 13, 4024. [Google Scholar] [CrossRef]
- Song, Y.; Shang, W.; Ma, D.; Wang, Z.; He, S.; Shi, L.; Shen, Y.; He, D.; Wang, E.; Wang, X. Effect on the Growth and Photosynthetic Characteristics of Anthurium andreanum (‘Pink Champion’, ‘Alabama’) under Hydroponic Culture by Different LED Light Spectra. Horticulturae 2022, 8, 389. [Google Scholar] [CrossRef]
- Sun, J.L.; Sui, X.L.; Huang, H.Y.; Wang, S.H.; Wei, Y.X.; Zhang, Z.X. Low light stress down-regulated rubisco gene expression and photosynthetic capacity during cucumber (Cucumis sativus L.) leaf development. J. Integr. Agric. 2014, 13, 997–1007. [Google Scholar] [CrossRef]
- Ren, M.; Liu, S.; Mao, G.; Tang, C.; Gai, P.; Guo, X.; Zheng, H.; Wang, W.; Tang, Q. Simultaneous Application of Red and Blue Light Regulate Carbon and Nitrogen Metabolism, Induces Antioxidant Defense System and Promote Growth in Rice Seedlings under Low Light Stress. Int. J. Mol. Sci. 2023, 24, 10706. [Google Scholar] [CrossRef]
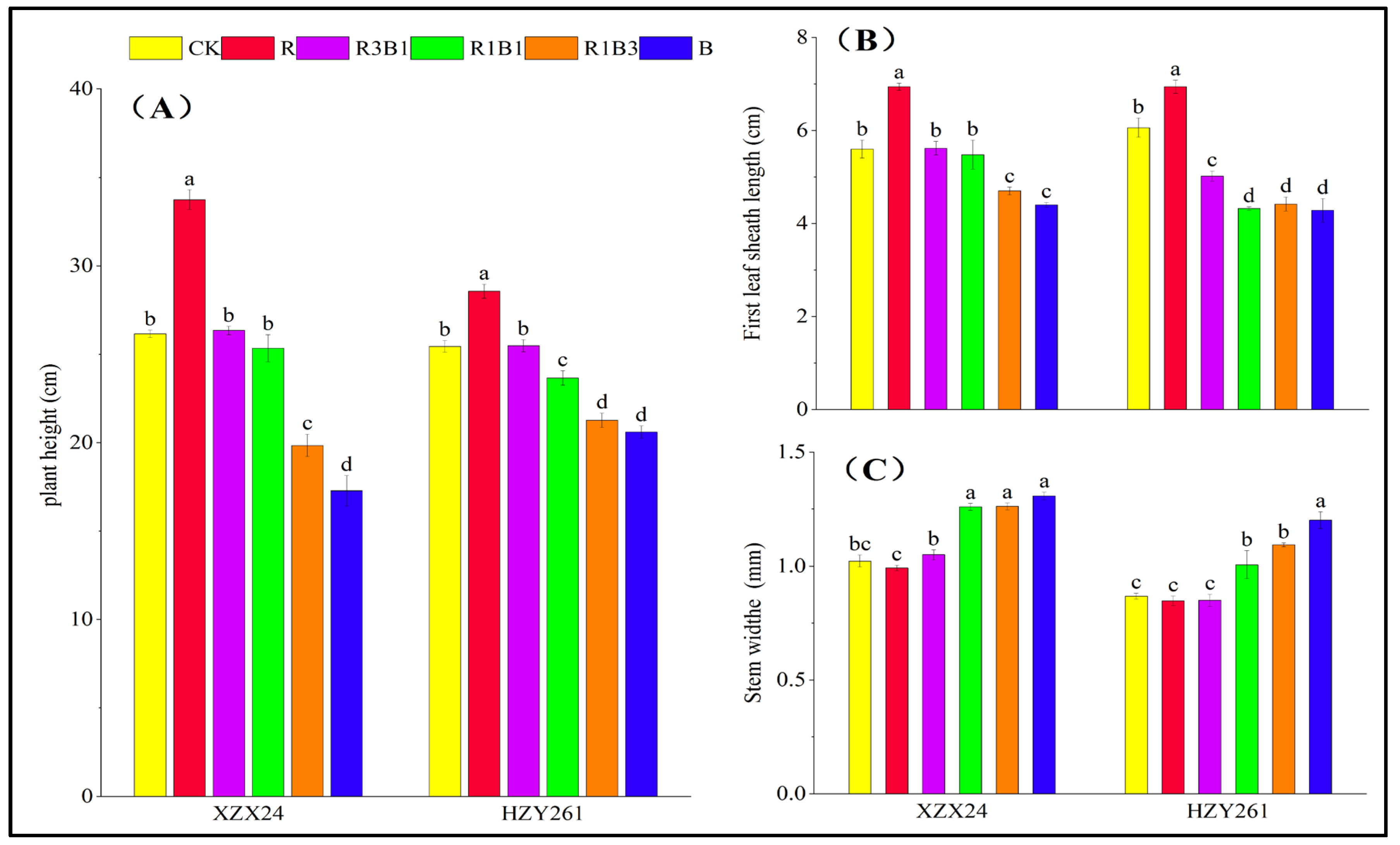
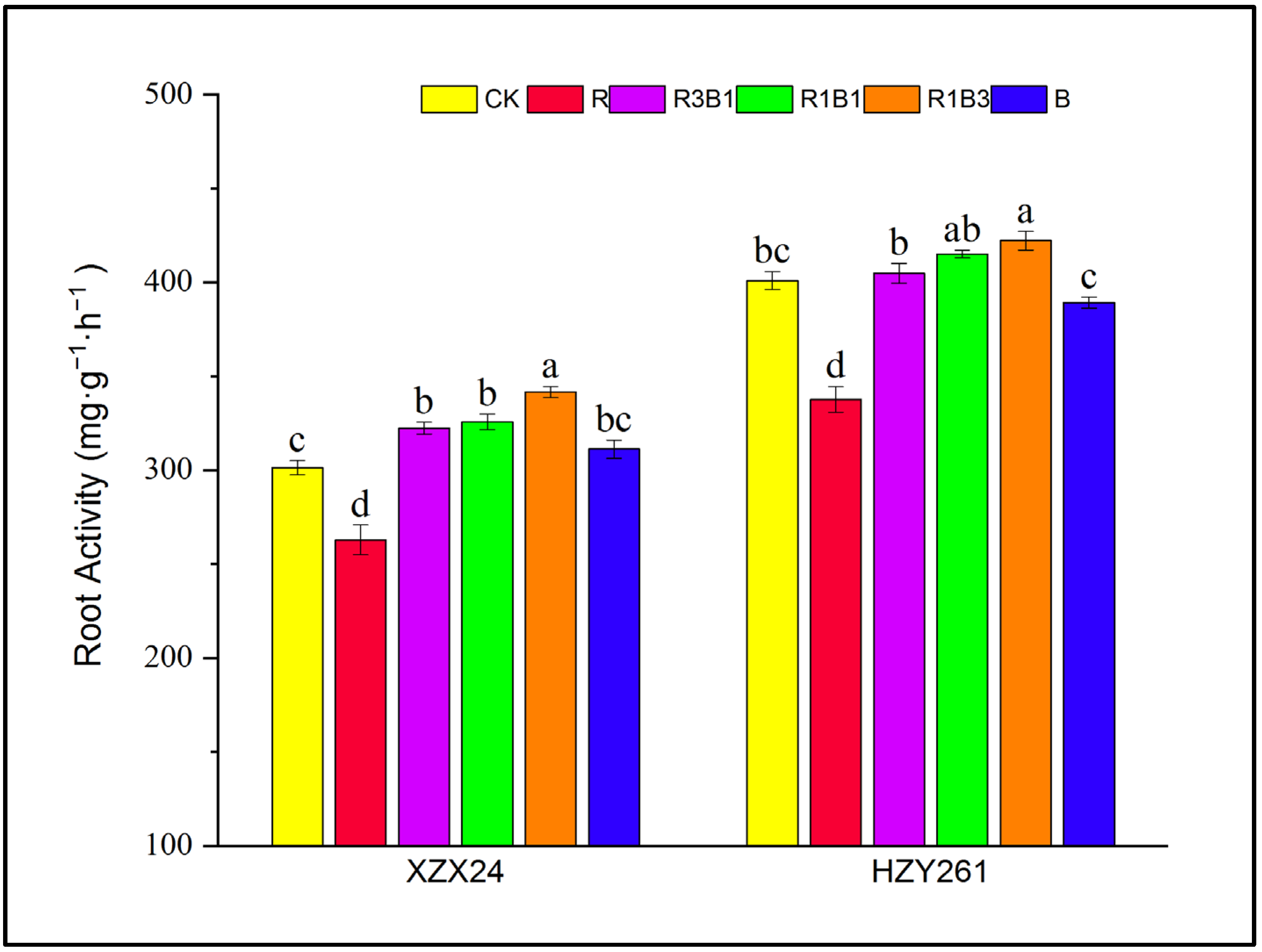

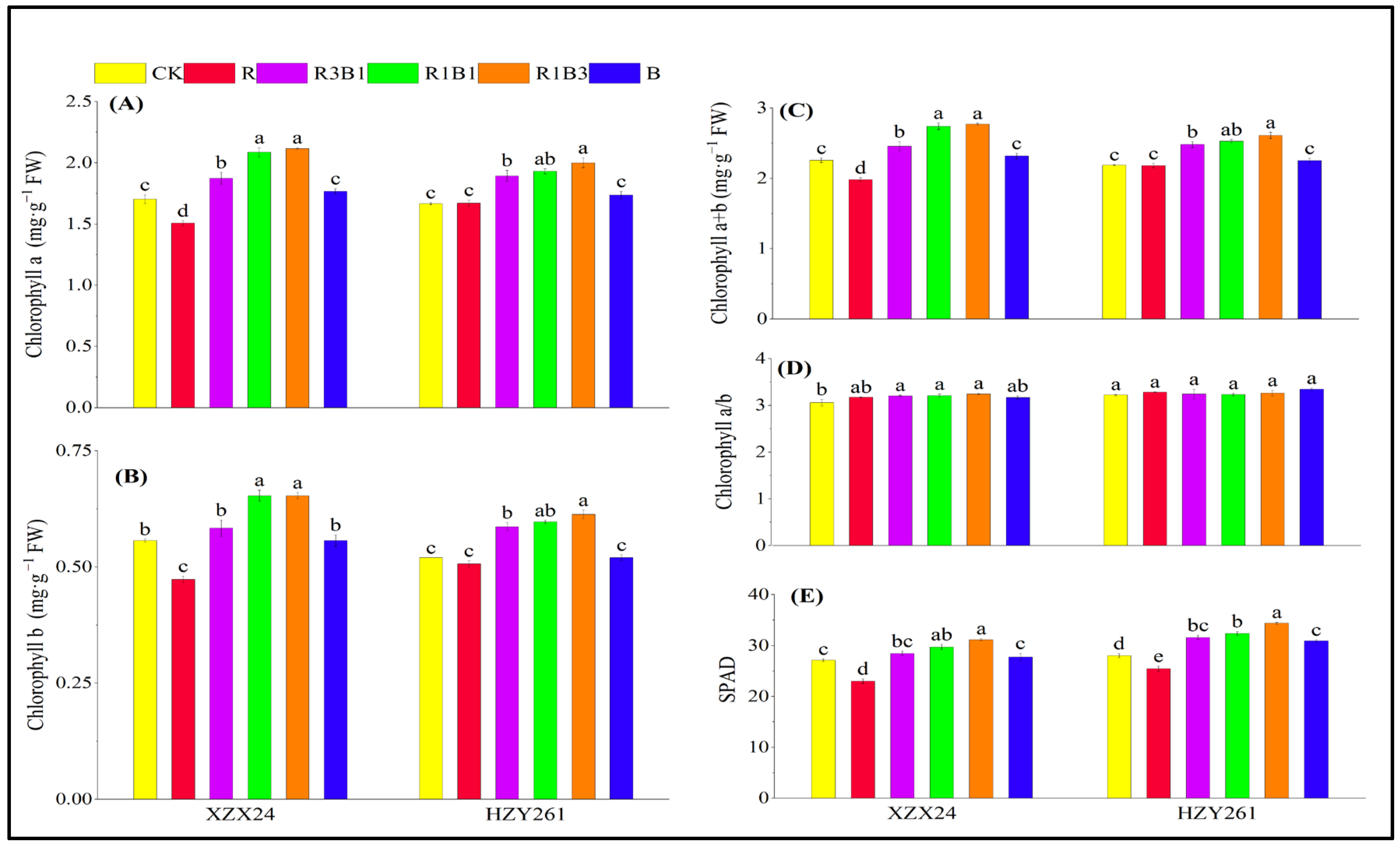
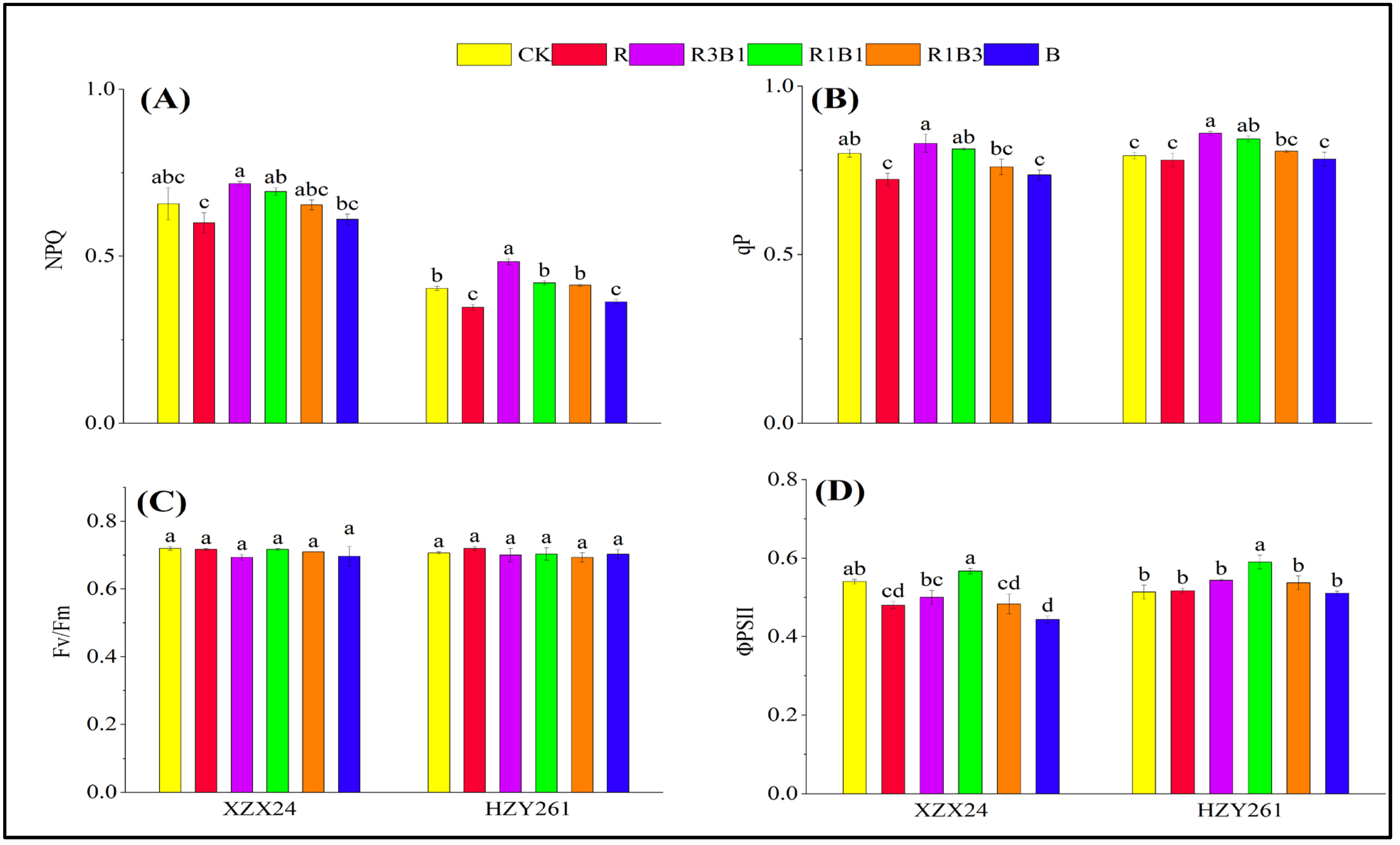
| Variety | Treatments | Leaf Length (cm) | Leaf Width (mm) | Length-Width Ratio | Leaf Area (cm3) | Leaf Number (No. Plant−1) |
|---|---|---|---|---|---|---|
| XZX24 | CK | 7.26 ± 0.11 c | 3.52 ± 0.03 c | 20.64 ± 0.51 b | 1.4 ± 0.03 b | 2.86 ± 0.04 b |
| R | 8.57 ± 0.06 a | 3.11 ± 0.04 d | 27.58 ± 0.40 a | 1.27 ± 0.02 c | 3.34 ± 0.07 a | |
| R3B1 | 6.45 ± 0.04 d | 3.42 ± 0.03 c | 18.86 ± 0.30 c | 1.52 ± 0.03 a | 2.90 ± 0.09 b | |
| R1B1 | 7.68 ± 0.06 b | 3.85 ± 0.07 b | 19.98 ± 0.25 b | 1.02 ± 0.02 e | 2.98 ± 0.10 b | |
| R1B3 | 5.78 ± 0.04 e | 4.05 ± 0.04 a | 14.27 ± 0.19 d | 1.16 ± 0.02 d | 2.90 ± 0.00 b | |
| B | 5.44 ± 0.06 f | 4.09 ± 0.04 a | 13.3 ± 0.20 d | 1.01 ± 0.02 e | 3.02 ± 0.08 b | |
| HZY261 | CK | 7.01 ± 0.05 b | 3.75 ± 0.04 b | 18.71 ± 0.11 c | 1.26 ± 0.02 ab | 2.74 ± 0.04 c |
| R | 8.33 ± 0.04 a | 3.26 ± 0.04 c | 25.53 ± 0.24 a | 1.41 ± 0.02 a | 3.06 ± 0.04 a | |
| R3B1 | 6.79 ± 0.14 b | 3.65 ± 0.04 b | 18.62 ± 0.52 c | 1.23 ± 0.12 b | 3.02 ± 0.05 ab | |
| R1B1 | 8.02 ± 0.09 a | 3.77 ± 0.10 b | 21.29 ± 0.49 b | 1.32 ± 0.02 ab | 2.90 ± 0.00 b | |
| R1B3 | 5.97 ± 0.48 c | 4.15 ± 0.16 a | 14.43 ± 1.20 d | 1.01 ± 0.01 c | 2.74 ± 0.04 c | |
| B | 5.53 ± 0.22 c | 4.34 ± 0.03 a | 12.75 ± 0.56 d | 0.95 ± 0.02 c | 3.02 ± 0.08 ab |
| Variety | Treatments | Root Length (cm) | Root Surface Area (cm2) | Root Volume (cm3) | Root Diameter (mm) | Root Tip Number (No. Plant−1) |
|---|---|---|---|---|---|---|
| XZX24 | CK | 62.89 ± 0.28 c | 8.58 ± 0.09 d | 0.28 ± 0.02 b | 1.18 ± 0.01 a | 82.17 ± 0.93 b |
| R | 54.80 ± 1.05 d | 7.39 ± 0.07 e | 0.24 ± 0.01 c | 0.96 ± 0.02 b | 69.17 ± 0.60 c | |
| R3B1 | 71.55 ± 1.69 a | 11.29 ± 0.17 a | 0.31 ± 0.01 a | 1.24 ± 0.01 a | 96.00 ± 1.04 a | |
| R1B1 | 68.29 ± 0.22 b | 9.60 ± 0.20 b | 0.29 ± 0.01 b | 1.22 ± 0.01 a | 93.33 ± 1.42 a | |
| R1B3 | 65.79 ± 0.48 b | 9.02 ± 0.10 c | 0.29 ± 0.00 b | 1.21 ± 0.04 a | 93.83 ± 0.60 a | |
| B | 49.90 ± 1.00 e | 6.86 ± 0.09 f | 0.19 ± 0.02 d | 0.83 ± 0.01 c | 60.33 ± 1.20 d | |
| HZY261 | CK | 42.86 ± 0.26 c | 6.54 ± 0.06 c | 0.21 ± 0.02 c | 0.98 ± 0.04 c | 83.17 ± 2.95 b |
| R | 40.44 ± 0.30 d | 6.41 ± 0.20 c | 0.19 ± 0.01 d | 0.85 ± 0.01 d | 66.17 ± 0.67 c | |
| R3B1 | 50.15 ± 0.34 a | 13.00 ± 0.31 a | 0.23 ± 0.02 b | 1.22 ± 0.02 b | 91.00 ± 0.58 a | |
| R1B1 | 51.19 ± 0.88 a | 13.46 ± 0.30 a | 0.26 ± 0.01 a | 1.32 ± 0.02 a | 92.17 ± 2.03 a | |
| R1B3 | 47.38 ± 0.82 b | 8.55 ± 0.16 b | 0.22 ± 0.02 bc | 1.19 ± 0.01 b | 93.17 ± 1.30 a | |
| B | 37.85 ± 0.12 e | 5.50 ± 0.09 d | 0.16 ± 0.00 e | 0.78 ± 0.01 d | 50.83 ± 1.42 d |
| Gene | Accession No. | Up-Primer (5′-3′) | Down-Primer (5′-3′) |
|---|---|---|---|
| OsActin3 | Os03g0718100 | CCACTATGTTCCCTGGCATT | GTACTCAGCCTTGGCAATCC |
| OsRBCS2 | AK121444 | CAGCAAGGTCGGATTCGTCT | CACACGAAACAAGGTGGGAG |
| OsRBCS3 | AK068555 | TTCCAAGGGCACAAGTCCAC | TAGGCAGCAATCCACCAGC |
| OsRBCS4 | AK068266 | GTGGATGAGTAAAGGGACAA | CAGGAGTAAATCAAGAGCGT |
| OsRBCS5 | AK099574 | TACCTGCTCCGGTCCAAATG | TCAAAGCTGCCAAAACCGAA |
| OsRBCSL | OrsajCp033 | CCCCGGAGTACGAAACCAAG | GAGGCGGACCTTGGAAAGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, M.; Liu, S.; Tang, C.; Mao, G.; Gai, P.; Guo, X.; Zheng, H.; Tang, Q. Photomorphogenesis and Photosynthetic Traits Changes in Rice Seedlings Responding to Red and Blue Light. Int. J. Mol. Sci. 2023, 24, 11333. https://doi.org/10.3390/ijms241411333
Ren M, Liu S, Tang C, Mao G, Gai P, Guo X, Zheng H, Tang Q. Photomorphogenesis and Photosynthetic Traits Changes in Rice Seedlings Responding to Red and Blue Light. International Journal of Molecular Sciences. 2023; 24(14):11333. https://doi.org/10.3390/ijms241411333
Chicago/Turabian StyleRen, Maofei, Shanzhen Liu, Chengzhu Tang, Guiling Mao, Panpan Gai, Xiaoli Guo, Huabin Zheng, and Qiyuan Tang. 2023. "Photomorphogenesis and Photosynthetic Traits Changes in Rice Seedlings Responding to Red and Blue Light" International Journal of Molecular Sciences 24, no. 14: 11333. https://doi.org/10.3390/ijms241411333
APA StyleRen, M., Liu, S., Tang, C., Mao, G., Gai, P., Guo, X., Zheng, H., & Tang, Q. (2023). Photomorphogenesis and Photosynthetic Traits Changes in Rice Seedlings Responding to Red and Blue Light. International Journal of Molecular Sciences, 24(14), 11333. https://doi.org/10.3390/ijms241411333





