Suppression of Indoxyl Sulfate Accumulation Reduces Renal Fibrosis in Sulfotransferase 1a1-Deficient Mice
Abstract
1. Introduction
2. Results
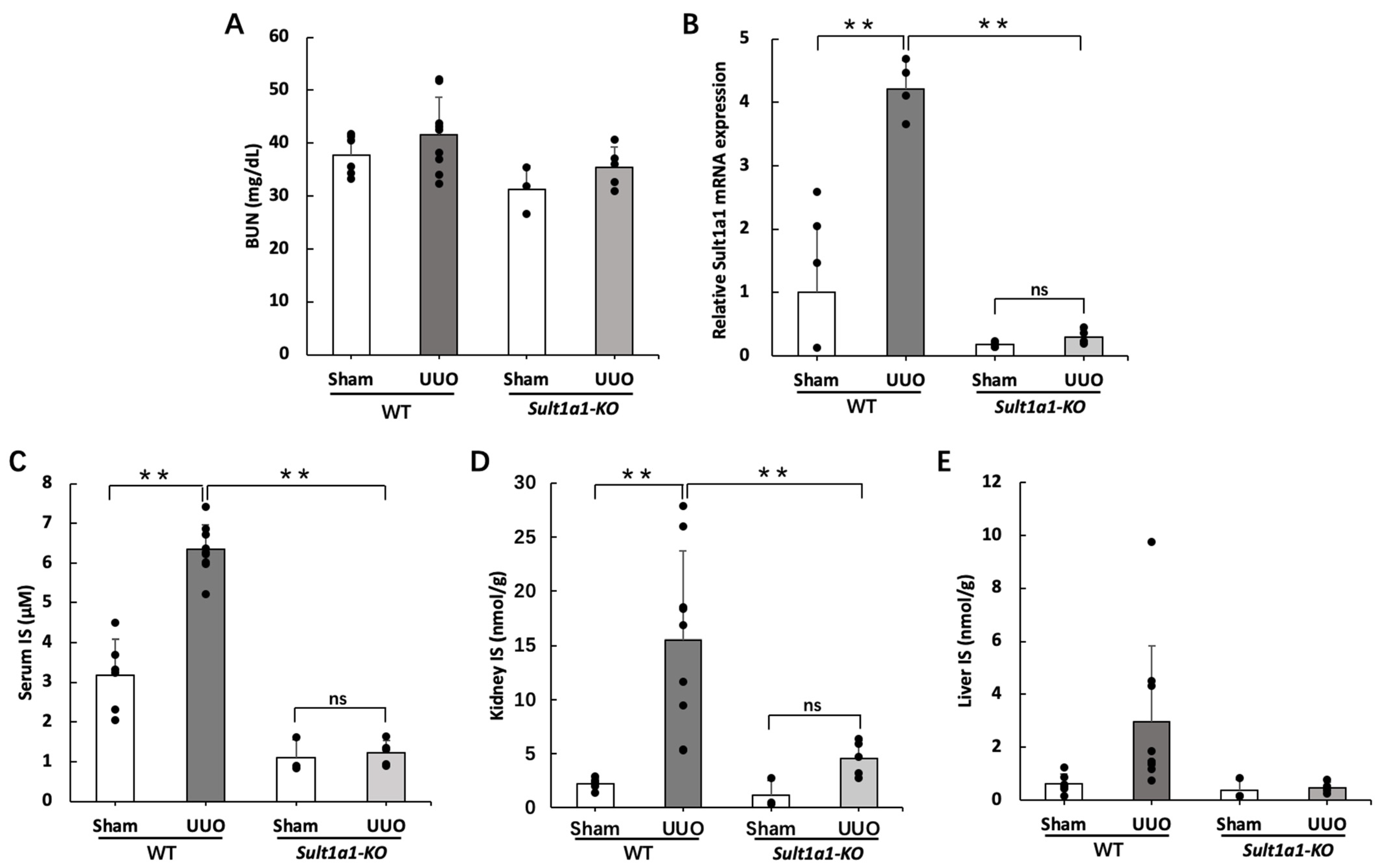
2.1. IS Accumulation Was Reduced in the UUO Model Using Sult1a1-KO Mice
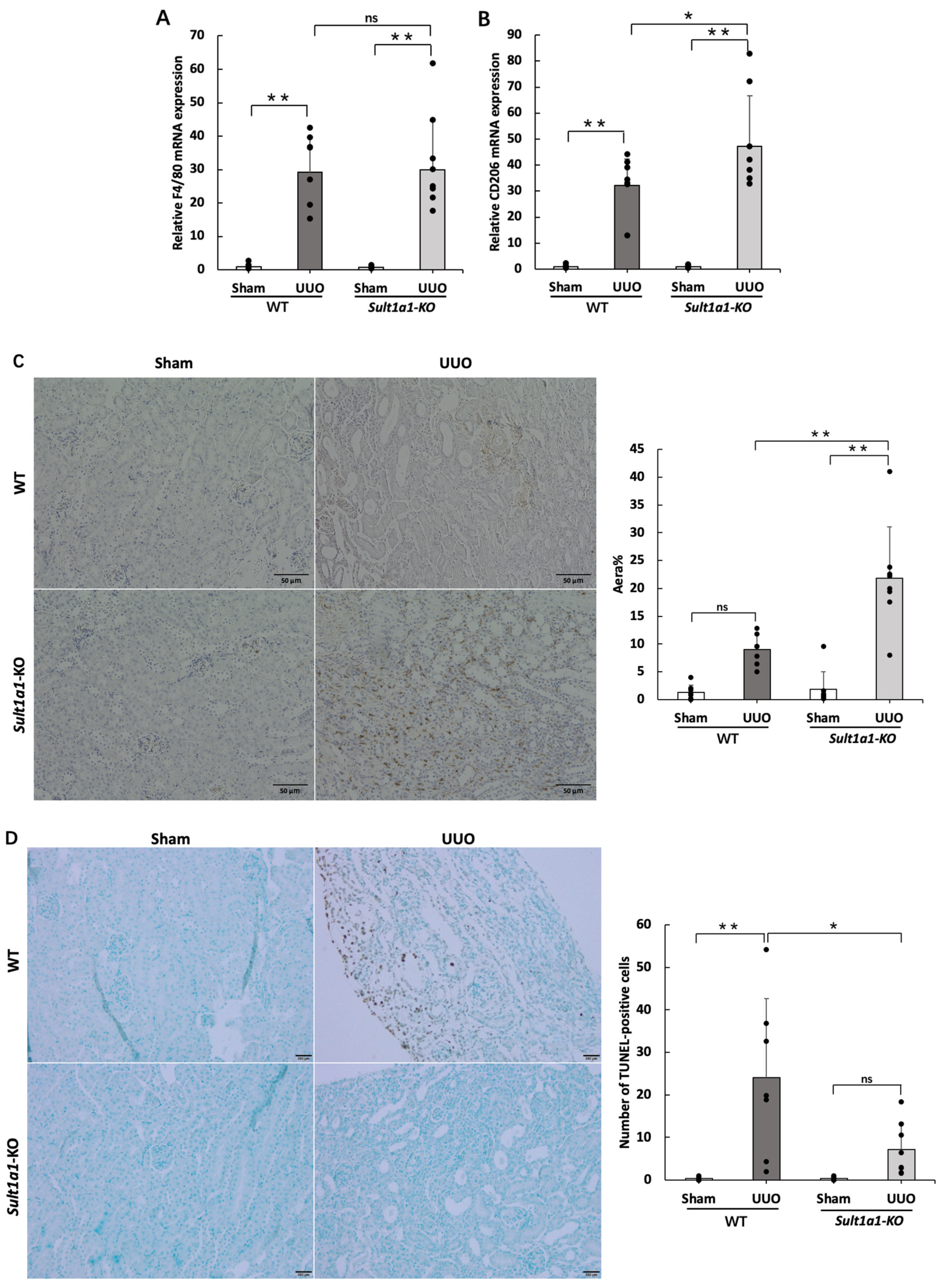
2.2. Gene Expression of Inflammation and Renal Fibrosis Improved in the Sult1a1-KO Mice UUO Model
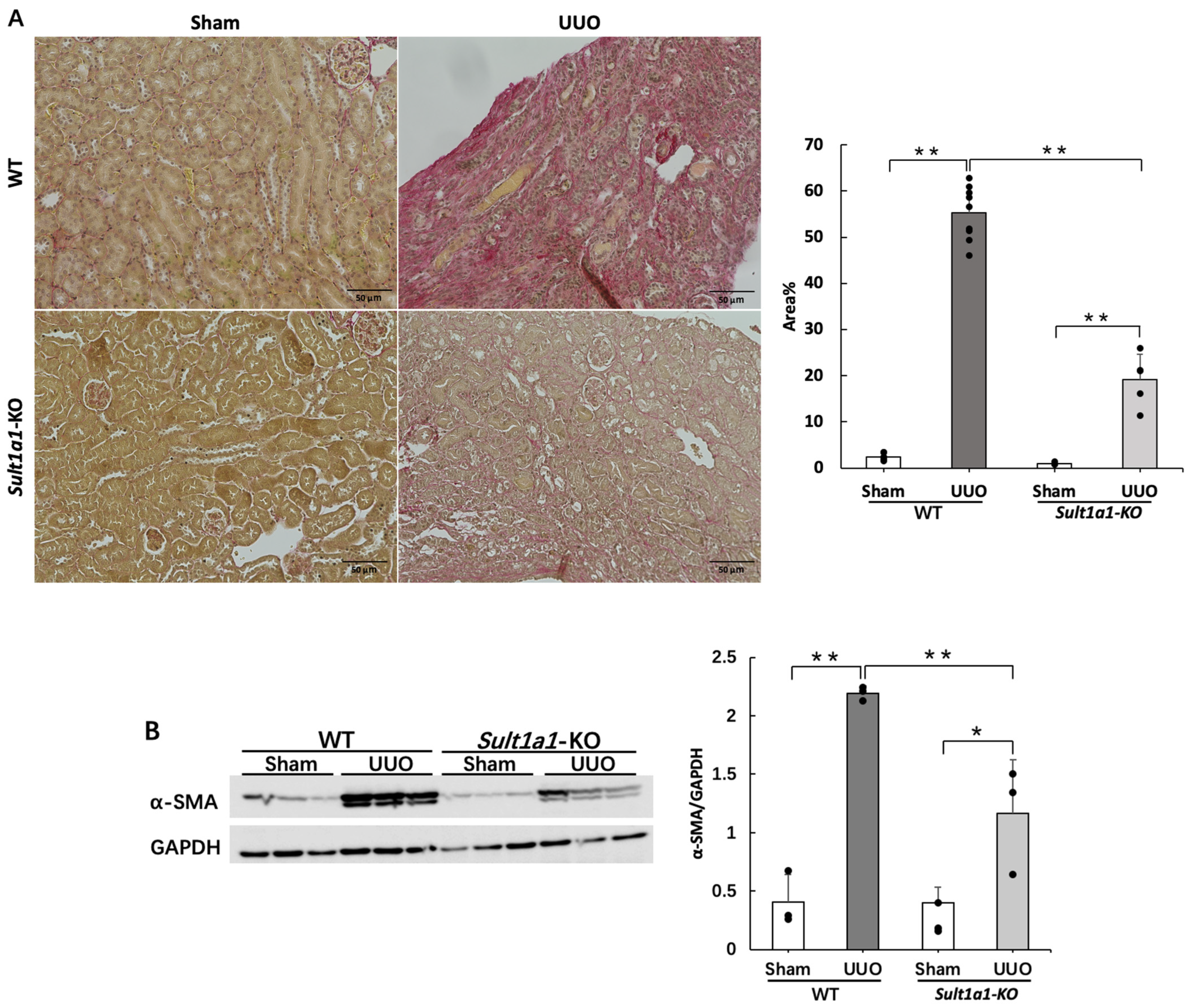
2.3. Renal Fibrosis Is Suppressed in the UUO Model Using Sult1a1-KO Mice
2.4. The UUO Model Using Sult1a1-KO Mice Allowed CD206+ Macrophage Infiltration and Reduced Apoptosis
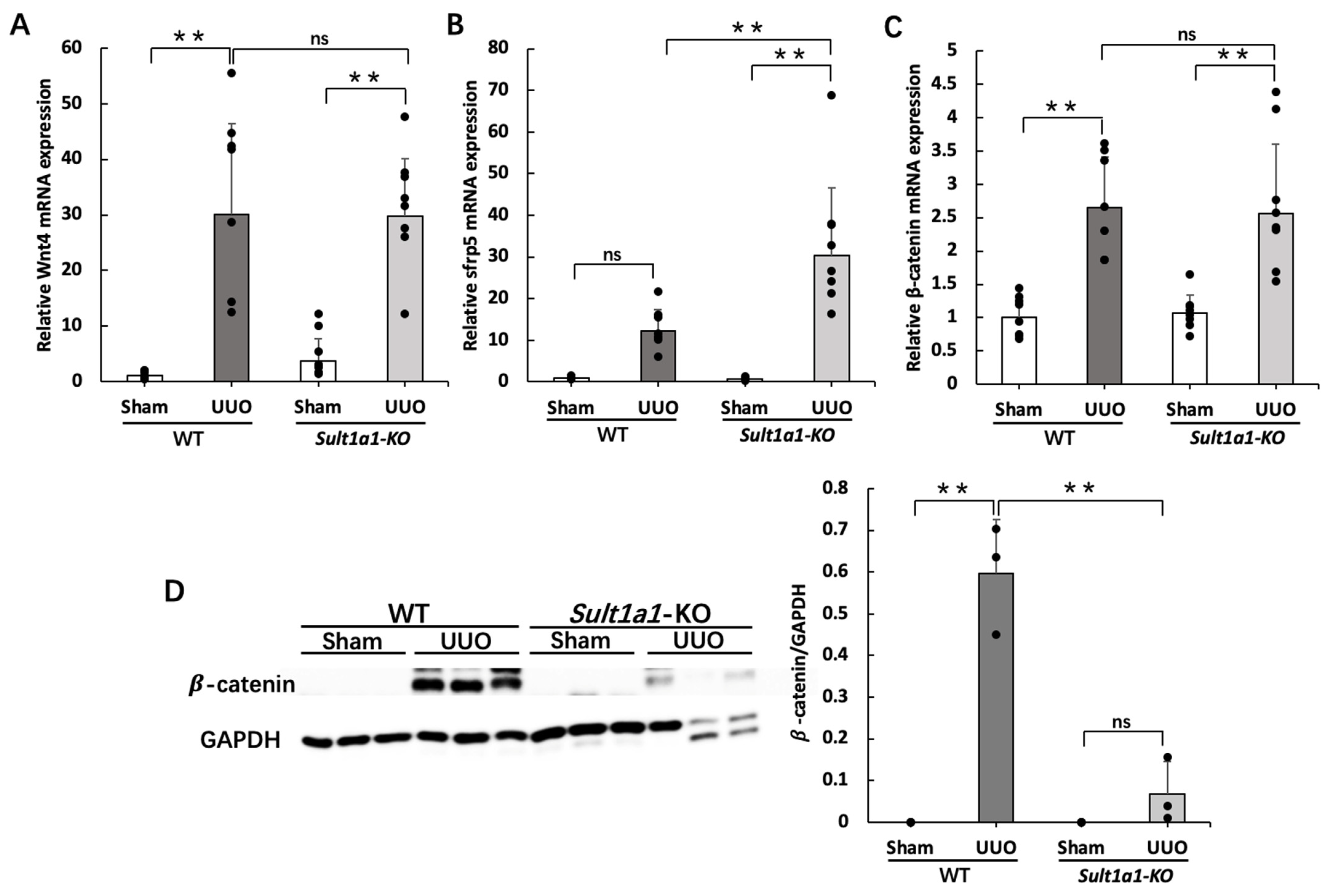
2.5. The UUO Model Using Sult1a1-KO Mice Activated Wnt/β-Catenin Signaling
2.6. Administration of rhEPO Attenuated Renal Fibrosis in the UUO Model Using Sult1a1-KO Mice
3. Discussion
4. Materials and Methods
4.1. Sult1a1-KO Mice
4.2. Animal Experiments
4.3. Liquid Chromatography/Mass Spectrometry/MS (LC-MS/MS) Assay of IS Concentration
4.4. Sirius Red Staining
4.5. TUNEL Staining
4.6. CD206 Immunostaining
4.7. Western Blot Analysis
4.8. Real-Time PCR
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Zhang, P.; Wang, F.; Zuo, L.; Zhou, Y.; Shi, Y.; Li, G.; Jiao, S.; Liu, Z.; Liang, W.; et al. Prevalence and factors associated with CKD: A population study from Beijing. Am. J. Kidney Dis. 2008, 51, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Zou, H.; Togtokh, A.; Ene-Iordache, B.; Carminati, S.; Remuzzi, A.; Wiebe, N.; Ayyalasomayajula, B.; Perico, N.; Remuzzi, G.; et al. Burden of CKD, proteinuria, and cardiovascular risk among Chinese, Mongolian, and Nepalese participants in the International Society of Nephrology screening programs. Am. J. Kidney Dis. 2010, 56, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Ise, M.; Hirata, M.; Endo, K.; Ito, Y.; Seo, H.; Niwa, T. Indoxyl sulfate stimulates renal synthesis of transforming growth factor-beta 1 and progression of renal failure. Kidney Int. Suppl. 1997, 63, S211–S214. [Google Scholar] [PubMed]
- Niwa, T.; Ise, M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J. Lab. Clin. Med. 1994, 124, 96–104. [Google Scholar]
- Shimoishi, K.; Anraku, M.; Kitamura, K.; Tasaki, Y.; Taguchi, K.; Hashimoto, M.; Fukunaga, E.; Maruyama, T.; Otagiri, M. An oral adsorbent, AST-120 protects against the progression of oxidative stress by reducing the accumulation of indoxyl sulfate in the systemic circulation in renal failure. Pharm. Res. 2007, 24, 1283–1289. [Google Scholar] [CrossRef]
- Saito, H.; Yoshimura, M.; Saigo, C.; Komori, M.; Nomura, Y.; Yamamoto, Y.; Sagata, M.; Wakida, A.; Chuman, E.; Nishi, K.; et al. Hepatic sulfotransferase as a nephropreventing target by suppression of the uremic toxin indoxyl sulfate accumulation in ischemic acute kidney injury. Toxicol. Sci. 2014, 141, 206–217. [Google Scholar] [CrossRef]
- Saigo, C.; Nomura, Y.; Yamamoto, Y.; Sagata, M.; Matsunaga, R.; Jono, H.; Nishi, K.; Saito, H. Meclofenamate elicits a nephropreventing effect in a rat model of ischemic acute kidney injury by suppressing indoxyl sulfate production and restoring renal organic anion transporters. Drug Des. Devel. Ther. 2014, 8, 1073–1082. [Google Scholar] [CrossRef]
- Lin, C.J.; Chen, H.H.; Pan, C.F.; Chuang, C.K.; Wang, T.J.; Sun, F.J.; Wu, C.J. p-Cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J. Clin. Lab. Anal. 2011, 25, 191–197. [Google Scholar] [CrossRef]
- Takkavatakarn, K.; Phannajit, J.; Udomkarnjananun, S.; Tangchitthavorngul, S.; Chariyavilaskul, P.; Sitticharoenchai, P.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. Association between indoxyl sulfate and dialysis initiation and cardiac outcomes in chronic kidney disease patients. Int. J. Nephrol. Renovasc. Dis. 2022, 15, 115–126. [Google Scholar] [CrossRef]
- Niwa, T. Removal of protein-bound uraemic toxins by haemodialysis. Blood Purif. 2013, 35 (Suppl. S2), 20–25. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T. Role of indoxyl sulfate in the progression of chronic kidney disease and cardiovascular disease: Experimental and clinical effects of oral sorbent AST-120. Ther. Apher. Dial. 2011, 15, 120–124. [Google Scholar] [CrossRef]
- Gelasco, A.K.; Raymond, J.R. Indoxyl sulfate induces complex redox alterations in mesangial cells. Am. J. Physiol. Ren. Physiol. 2006, 290, F1551–F1558. [Google Scholar] [CrossRef]
- Fukuda, Y.; Takazoe, M.; Sugita, A.; Kosaka, T.; Kinjo, F.; Otani, Y.; Fujii, H.; Koganei, K.; Makiyama, K.; Nakamura, T.; et al. Oral spherical adsorptive carbon for the treatment of intractable anal fistulas in Crohn’s disease: A multicenter, randomized, double-blind, placebo-controlled trial. Am. J. Gastroenterol. 2008, 103, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, N.; Hou, H.; Gunda, N.; Narita, Y.; Jono, H.; Saito, H. Suppressed hepatic production of indoxyl sulfate attenuates cisplatin-induced acute kidney injury in sulfotransferase 1a1-deficient mice. Int. J. Mol. Sci. 2021, 22, 1764. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Watanabe, H.; Morisaki, T.; Matsuzaki, T.; Ohmura, T.; Hamada, A.; Saito, H. Involvement of indoxyl sulfate in renal and central nervous system toxicities during cisplatin-induced acute renal failure. Pharm. Res. 2007, 24, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, T.; Matsuzaki, T.; Yokoo, K.; Kusumoto, M.; Iwata, K.; Hamada, A.; Saito, H. Regulation of renal organic ion transporters in cisplatin-induced acute kidney injury and uremia in rats. Pharm. Res. 2008, 25, 2526–2533. [Google Scholar] [CrossRef]
- Luo, C.; Zhou, S.; Zhou, Z.; Liu, Y.; Yang, L.; Liu, J.; Zhang, Y.; Li, H.; Liu, Y.; Hou, F.F.; et al. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J. Am. Soc. Nephrol. 2018, 29, 1238–1256. [Google Scholar] [CrossRef]
- Souma, T.; Yamazaki, S.; Moriguchi, T.; Suzuki, N.; Hirano, I.; Pan, X.; Minegishi, N.; Abe, M.; Kiyomoto, H.; Ito, S.; et al. Plasticity of renal erythropoietin-producing cells governs fibrosis. J. Am. Soc. Nephrol. 2013, 24, 1599–1616. [Google Scholar] [CrossRef]
- Farris, A.B.; Colvin, R.B. Renal interstitial fibrosis: Mechanisms and evaluation. Curr. Opin. Nephrol. Hypertens 2012, 21, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Watanabe, H.; Imafuku, T.; Tokumaru, K.; Fujita, I.; Arimura, N.; Maeda, H.; Tanaka, M.; Matsushita, K.; Fukagawa, M.; et al. Indoxyl sulfate contributes to mTORC1-induced renal fibrosis via the OAT/NADPH oxidase/ROS pathway. Toxins 2021, 13, 909. [Google Scholar] [CrossRef] [PubMed]
- Hempel, N.; Wang, H.; LeCluyse, E.L.; McManus, M.E.; Negishi, M. The human sulfotransferase SULT1A1 gene is regulated in a synergistic manner by Sp1 and GA binding protein. Mol. Pharmacol. 2004, 66, 1690–1701. [Google Scholar] [CrossRef]
- Granados, J.C.; Bhatnagar, V.; Nigam, S.K. Blockade of organic anion transport in humans after treatment with the drug probenecid leads to major metabolic alterations in plasma and urine. Clin. Pharmacol. Ther. 2022, 112, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, I.; Shimokata, K.; Niwa, T. Combination therapy with benazepril and oral adsorbent ameliorates progressive renal fibrosis in uremic rats. Nephron 2002, 90, 297–312. [Google Scholar] [CrossRef]
- Tecklenborg, J.; Clayton, D.; Siebert, S.; Coley, S.M. The role of the immune system in kidney disease. Clin. Exp. Immunol. 2018, 192, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Jiang, H.; Pan, J.; Huang, X.R.; Wang, Y.C.; Huang, H.F.; To, K.F.; Nikolic-Paterson, D.J.; Lan, H.Y.; Chen, J.H. Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J. Am. Soc. Nephrol. 2017, 28, 2053–2067. [Google Scholar] [CrossRef]
- Edeling, M.; Ragi, G.; Huang, S.; Pavenstädt, H.; Susztak, K. Developmental signalling pathways in renal fibrosis: The roles of Notch, Wnt and Hedgehog. Nat. Rev. Nephrol. 2016, 12, 426–439. [Google Scholar] [CrossRef]
- Kawakami, T.; Ren, S.; Duffield, J.S. Wnt signalling in kidney diseases: Dual roles in renal injury and repair. J. Pathol. 2013, 229, 221–231. [Google Scholar] [CrossRef]
- Terada, Y.; Tanaka, H.; Okado, T.; Shimamura, H.; Inoshita, S.; Kuwahara, M.; Sasaki, S. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J. Am. Soc. Nephrol. 2003, 14, 1223–1233. [Google Scholar] [CrossRef]
- Yu, Y.; Guan, X.; Nie, L.; Liu, Y.; He, T.; Xiong, J.; Xu, X.; Li, Y.; Yang, K.; Wang, Y.; et al. DNA hypermethylation of sFRP5 contributes to indoxyl sulfate-induced renal fibrosis. J. Mol. Med. 2017, 95, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Bhoopalan, S.V.; Huang, L.J.; Weiss, M.J. Erythropoietin regulation of red blood cell production: From bench to bedside and back. F1000Research 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Bellomo, R. Erythropoietin (EPO) in acute kidney injury. Ann. Intensive Care 2011, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Duangchan, T.; Rattanasompattikul, M.; Chitchongyingcharoen, N.; Mas-Oodi, S.; Promkan, M.; Rongkiettechakorn, N.; Korpraphong, S.; Supokawej, A. Indoxyl sulfate impairs in vitro erythropoiesis by triggering apoptosis and senescence. Exp. Biol. Med. 2022, 247, 1350–1363. [Google Scholar] [CrossRef]
- Hamza, E.; Metzinger, L.; Metzinger-Le Meuth, V. Uremic toxins affect erythropoiesis during the course of chronic kidney disease: A review. Cells 2020, 9, 2039. [Google Scholar] [CrossRef]
- Spandou, E.; Tsouchnikas, I.; Karkavelas, G.; Dounousi, E.; Simeonidou, C.; Guiba-Tziampiri, O.; Tsakiris, D. Erythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion model. Nephrol. Dial. Transpl. 2006, 21, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Dodds, A.; Nicholls, M. Haematological aspects of renal disease. Anaesth. Intensive Care 1983, 11, 361–368. [Google Scholar] [CrossRef]
- Gobe, G.C.; Bennett, N.C.; West, M.; Colditz, P.; Brown, L.; Vesey, D.A.; Johnson, D.W. Increased progression to kidney fibrosis after erythropoietin is used as a treatment for acute kidney injury. Am. J. Physiol. Renal. Physiol. 2014, 306, F681–F692. [Google Scholar] [CrossRef]
- Fujii, H.; Nakai, K.; Fukagawa, M. Role of oxidative stress and indoxyl sulfate in progression of cardiovascular disease in chronic kidney disease. Ther. Apher. Dial. 2011, 15, 125–128. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Rong, Y.; Kiang, T.K.L. Characterization of human sulfotransferases catalyzing the formation of p-cresol sulfate and identification of mefenamic acid as a potent metabolism inhibitor and potential therapeutic agent for detoxification. Toxicol. Appl. Pharmacol. 2021, 425, 115553. [Google Scholar] [CrossRef] [PubMed]



| Target Protein | The Primary Antibody | The Secondary Antibody |
|---|---|---|
| α-SMA | Dilution: 5000× | Dilution: 20,000× |
| β-catenin | Dilution: 5000× | Dilution: 10,000× |
| GAPDH | Dilution: 5000× | Dilution: 5000× |
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Sult1a1 | TGAGACGCACTCACCCTGTTCT | TCCACAGTCTCCTCAGGTAGAG |
| Col1a1 | GACAAATGAATGGGGCAAG | CAATGTCCAGAGGTGCAATG |
| IL-6 | TACCACTTCACAAGTCGGAGGC | CTGCAAGTGCATCATCGTTGTTC |
| TNF-α | CTACCTTGTTGCCTCCTCTTT | GAGCAGAGGTTCAGTGATGTAG |
| IL-1β | AGTTGACGGACCCCAAAAG | AGCTGGATGCTCTCATCAGG |
| F4/80 | CGTGTTGTTGGTGGCACTGTGA | CCACATCAGTGTTCCAGGAGAC |
| CD206 | GTTCACCTGGAGTGATGGTTCTC | AGGACATGCCAGGGTCACCTTT |
| Wnt4 | GCGTAGCCTTCTCACAGTCC | CGCATGTGTGTCAAGATGG |
| sFRP5 | GATCTGTGCCCAGTGTGAGA | TATGCAGGACCAGCTTCTTGGTGT |
| β-catenin | GCAGCAGCAGTCTTACTTGG | CCCTCATCTAGCGTCTCAGG |
| Fibronectin | AGACCATACCTGCCGAATGTAG | GAGAGCTTCCTGTCCTGTAGAG |
| EPO | CACAACCCATCGTGACATTTTC | CATCTGCGACAGTCGAGTTCTG |
| GAPDH | CGACTTCAACAGCAACTCCCACTCTTCC | TGGGTGGTCCAGGGTTTCTTACTCCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, H.; Horikawa, M.; Narita, Y.; Jono, H.; Kakizoe, Y.; Izumi, Y.; Kuwabara, T.; Mukoyama, M.; Saito, H. Suppression of Indoxyl Sulfate Accumulation Reduces Renal Fibrosis in Sulfotransferase 1a1-Deficient Mice. Int. J. Mol. Sci. 2023, 24, 11329. https://doi.org/10.3390/ijms241411329
Hou H, Horikawa M, Narita Y, Jono H, Kakizoe Y, Izumi Y, Kuwabara T, Mukoyama M, Saito H. Suppression of Indoxyl Sulfate Accumulation Reduces Renal Fibrosis in Sulfotransferase 1a1-Deficient Mice. International Journal of Molecular Sciences. 2023; 24(14):11329. https://doi.org/10.3390/ijms241411329
Chicago/Turabian StyleHou, Huixian, Mai Horikawa, Yuki Narita, Hirofumi Jono, Yutaka Kakizoe, Yuichiro Izumi, Takashige Kuwabara, Masashi Mukoyama, and Hideyuki Saito. 2023. "Suppression of Indoxyl Sulfate Accumulation Reduces Renal Fibrosis in Sulfotransferase 1a1-Deficient Mice" International Journal of Molecular Sciences 24, no. 14: 11329. https://doi.org/10.3390/ijms241411329
APA StyleHou, H., Horikawa, M., Narita, Y., Jono, H., Kakizoe, Y., Izumi, Y., Kuwabara, T., Mukoyama, M., & Saito, H. (2023). Suppression of Indoxyl Sulfate Accumulation Reduces Renal Fibrosis in Sulfotransferase 1a1-Deficient Mice. International Journal of Molecular Sciences, 24(14), 11329. https://doi.org/10.3390/ijms241411329







