Potential Role of Glucagon-like Peptide-1 Receptor Agonists in the Treatment of Cognitive Decline and Dementia in Diabetes Mellitus
Abstract
1. Introduction
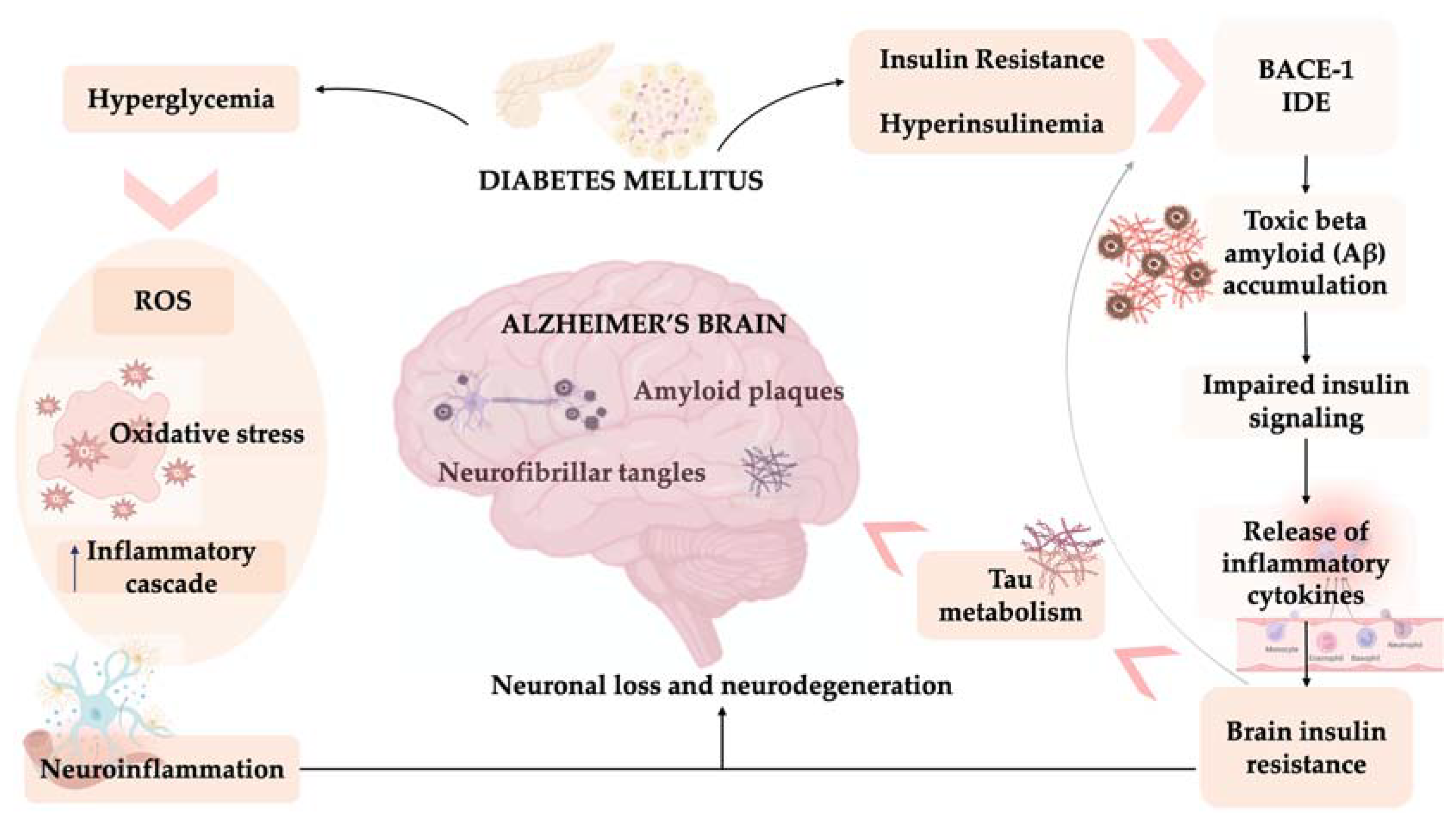
2. Diabetes, Cognitive Dysfunction and Dementia
3. Diabetes of the Brain: AD
4. GLP-1 Reduces Neuroinflammation
5. In Vitro and Animal Evidence
6. Clinical Evidence
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AGE | Advanced glycation end product |
| AP-1 | Activator protein-1 |
| APOE | Apolipoprotein E |
| APP | Amyloid precursor protein |
| Aβ | Beta amyloid |
| BACE-1 | β-site amyloid precursor protein cleaving enzyme 1 |
| Bcl2 | B-cell lymphoma 2 |
| BDNF | Brain derived neurotropic factor |
| cAMP | Cyclic adenosine monophosphate |
| CDC | Centres for Disease Control and Prevention |
| CDR | Clinical Dementia Rating |
| CI | Cognitive impairment |
| CNS | Central nervous system |
| CRP | C-reactive protein |
| CSF | Cerebrospinal fluid |
| DM | Diabetes Mellitus |
| DSST | Digit Symbol Substitution Test |
| Ex-4 | Exenatide acetate |
| FDA | Food and Drug Administration |
| GcgR | Glucagon Receptor |
| GIPR | GIP receptor |
| GLP-1 | Glucagon-like peptide-1 |
| GLP1-RA | Glucagon-like peptide 1- receptor agonist |
| GLP-1Rs | GLP-1 receptors |
| H2O2 | Hydrogen peroxide |
| HRs | Hazard ratios |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IDE | Insulin degrading enzyme |
| IGF-1 | insulin-like growth factor-1 |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IRS-1 | Insulin Receptor Substrates-1 |
| JNK | c-Jun N-terminal kinase |
| MAPK | Mitogen activated protein kinase |
| MCI | Mild cognitive impairement |
| MoCA | Montreal Cognitive Assessment |
| MRI | Magnetic Resonance imaging |
| mTOR | Mammalian target of rapamycin |
| NC | patients without Cognitive impairment |
| NFTs | Neurofibrillary tangles |
| NF-κB | nuclear factor kappa B |
| NTS | Nucleus of the solitary tract |
| OA | Okadaic acid |
| PET | Positron Emission Tomography |
| PHFs | Paired helical fibers |
| PIB | Pittsburgh compound B |
| PPG | Preproglucagon |
| PS1 | Presenilin 1 |
| PSD95 | Postsynaptic density protein 95 |
| ROS | Reactive forms of oxygen |
| SB | Sum-of-boxes |
| STAT | Signal transducers and activator of transcription |
| STZ | Streptozotocin |
| sVCAM-1 | Soluble vascular cell adhesion molecule-1 |
| T1DM | Type 1 Diabetes Mellitus |
| T2DM | Type 2 Diabetes Mellitus |
| TNF-α | tumour necrosis factor α |
| WHO | World Health Organization |
| VaD | Vascular Dementia |
References
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed. 2017. Available online: https://www.diabetesatlas.org (accessed on 6 June 2023).
- Chawla, A.; Chawla, R.; Jaggi, S. Microvascular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Chornenkyy, Y.; Wang, W.X.; Wei, A.; Nelson, P.T. Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol. 2019, 29, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Akter, K.; Lanza, E.A.; Martin, S.A.; Myronyuk, N.; Rua, M.; Raffa, R.B. Diabetes mellitus and Alzheimer’s disease: Shared pathology and treatment? Br. J. Clin. Pharmacol. 2011, 71, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Baglietto-Vargas, D.; Shi, J.; Yaeger, D.M.; Ager, R.; LaFerla, F.M. Diabetes and Alzheimer’s disease crosstalk. Neurosci. Biobehav. Rev. 2016, 64, 272–287. [Google Scholar] [CrossRef]
- Mamelak, M. Energy and the Alzheimer brain. Neurosci. Biobehav. Rev. 2017, 75, 297–313. [Google Scholar] [CrossRef]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol 2018, 14, 591–604. [Google Scholar] [CrossRef]
- Li, X.; Song, D.; Leng, S.X. Link between type 2 diabetes and Alzheimer’s disease: From epidemiology to mechanism and treatment. Clin. Interv. Aging 2015, 10, 549–560. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Huerta, M.; González-Usigli, H.A.; Torres-Sánchez, E.D.; Delgado-Lara, D.L.; Pacheco-Moisés, F.P.; Mireles-Ramírez, M.A.; Torres-Mendoza, B.M.; Moreno-Cih, R.I.; Velázquez-Brizuela, I.E. Cognitive disorder and dementia in type 2 diabetes mellitus. World J. Diabetes 2022, 13, 319–337. [Google Scholar] [CrossRef]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Gispen, W.H.; Biessels, G.J. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000, 23, 542–549. [Google Scholar] [CrossRef]
- Biessels, G.J.; van der Heide, L.P.; Kamal, A.; Bleys, R.L.; Gispen, W.H. Ageing and diabetes: Implications for brain function. Eur. J. Pharmacol. 2002, 441, 1–14. [Google Scholar] [CrossRef]
- Ott, A.; Stolk, R.P.; van Harskamp, F.; Pols, H.A.; Hofman, A.; Breteler, M.M. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999, 53, 1937–1942. [Google Scholar] [CrossRef]
- McCall, A.L. The impact of diabetes on the CNS. Diabetes 1992, 41, 557–570. [Google Scholar] [CrossRef]
- Koekkoek, P.S.; Kappelle, L.J.; van den Berg, E.; Rutten, G.E.; Biessels, G.J. Cognitive function in patients with diabetes mellitus: Guidance for daily care. Lancet Neurol. 2015, 14, 329–340. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Hua, S.; Liao, H.; Wang, M.; Xiong, Y.; Cao, F. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract. 2017, 124, 41–47. [Google Scholar] [CrossRef]
- Xue, M.; Xu, W.; Ou, Y.N.; Cao, X.P.; Tan, M.S.; Tan, L.; Yu, J.T. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res. Rev. 2019, 55, 100944. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group; Jacobson, A.M.; Musen, G.; Ryan, C.M.; Silvers, N.; Cleary, P.; Waberski, B.; Burwood, A.; Weinger, K.; Bayless, M.; et al. Long-term effect of diabetes and its treatment on cognitive function. N. Engl. J. Med. 2007, 356, 1842–1852. [Google Scholar] [CrossRef]
- Brands, A.M.; Van den Berg, E.; Manschot, S.M.; Biessels, G.J.; Kappelle, L.J.; De Haan, E.H.; Kessels, R.P. A detailed profile of cognitive dysfunction and its relation to psychological distress in patients with type 2 diabetes mellitus. J. Int. Neuropsychol. Soc. 2007, 13, 288–297. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Zhang, W.; Zhang, Z.; Yang, X.; Wang, P.; Yuan, H. Association Between Inflammatory Biomarkers and Cognitive Dysfunction Analyzed by MRI in Diabetes Patients. Diabetes Metab. Syndr. Obes. 2020, 13, 4059–4065. [Google Scholar] [CrossRef] [PubMed]
- Moheet, A.; Mangia, S.; Seaquist, E.R. Impact of diabetes on cognitive function and brain structure. Ann. N. Y. Acad. Sci. 2015, 1353, 60–71. [Google Scholar] [CrossRef]
- Arbelaez, A.M.; Semenkovich, K.; Hershey, T. Glycemic extremes in youth with T1DM: The structural and functional integrity of the developing brain. Pediatr. Diabetes 2013, 14, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Reitz, C.; Patel, B.; Tang, M.X.; Manly, J.J.; Mayeux, R. Relation of diabetes to mild cognitive impairment. Arch. Neurol. 2007, 64, 570–575. [Google Scholar] [CrossRef]
- Petersen, R.C. Normal aging, mild cognitive impairment, and early Alzheimer’s disease. Neurologia 1995, 1, 326–344. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Espeland, M.A.; Bryan, R.N.; Goveas, J.S.; Robinson, J.G.; Siddiqui, M.S.; Liu, S.; Hogan, P.E.; Casanova, R.; Coker, L.H.; Yaffe, K.; et al. WHIMS-MRI Study Group. Influence of type 2 diabetes on brain volumes and changes in brain volumes: Results from the Women’s Health Initiative Magnetic Resonance Imaging studies. Diabetes Care 2013, 36, 90–97. [Google Scholar] [CrossRef]
- Moran, C.; Phan, T.G.; Chen, J.; Blizzard, L.; Beare, R.; Venn, A.; Münch, G.; Wood, A.G.; Forbes, J.; Greenaway, T.M.; et al. Brain atrophy in type 2 diabetes: Regional distribution and influence on cognition. Diabetes Care 2013, 36, 4036–4042. [Google Scholar] [CrossRef]
- Leibson, C.L.; Rocca, W.A.; Hanson, V.A.; Cha, R.; Kokmen, E.; O’Brien, P.C.; Palumbo, P.J. Risk of dementia among persons with diabetes mellitus: A population-based cohort study. Am. J. Epidemiol. 1997, 145, 301–308. [Google Scholar] [CrossRef]
- Marszałek, M. Cukrzyca typu 2 a choroba Alzheimera-jedna czy dwie choroby? Mechanizmy asocjacji [Diabetes type 2 and Alzheimer disease—One or two diseases? Mechanisms of association]. Adv. Hyg. Exp. Med. 2013, 67, 653–671. [Google Scholar] [CrossRef]
- Ott, A.; Stolk, R.P.; Hofman, A.; van Harskamp, F.; Grobbee, D.E.; Breteler, M.M. Association of diabetes mellitus and dementia: The Rotterdam Study. Diabetologia 1996, 39, 1392–1397. [Google Scholar] [CrossRef]
- Cooper, C.; Sommerlad, A.; Lyketsos, C.G.; Livingston, G. Modifiable predictors of dementia in mild cognitive impairment: A systematic review and meta-analysis. Am. J. Psychiatry 2015, 172, 323–334. [Google Scholar] [CrossRef]
- Gudala, K.; Bansal, D.; Schifano, F.; Bhansali, A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J. Diabetes Investig. 2013, 4, 640–650. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Bir, S.C.; Khan, M.W.; Javalkar, V.; Toledo, E.G.; Kelley, R.E. Emerging Concepts in Vascular Dementia: A Review. J. Stroke Cerebrovasc. Dis. 2021, 30, 105864. [Google Scholar] [CrossRef] [PubMed]
- Chang Wong, E.; Chang Chui, H. Vascular Cognitive Impairment and Dementia. Contin. Lifelong Learn. Neurol. 2022, 28, 750–780. [Google Scholar] [CrossRef]
- Luchsinger, J.A. Type 2 diabetes, related conditions, in relation and dementia: An opportunity for prevention? J. Alzheimers Dis. 2010, 20, 723–736. [Google Scholar] [CrossRef]
- Chatterjee, S.; Peters, S.A.; Woodward, M.; Mejia Arango, S.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.; et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared with Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef]
- Cole, A.R.; Astell, A.; Green, C.; Sutherland, C. Molecular connexions between dementia and diabetes. Neurosci. Biobehav. Rev. 2007, 31, 1046–1063. [Google Scholar] [CrossRef]
- Sonnen, J.A.; Larson, E.B.; Brickell, K.; Crane, P.K.; Woltjer, R.; Montine, T.J.; Craft, S. Different patterns of cerebral injury in dementia with or without diabetes. Arch. Neurol. 2009, 66, 315–322. [Google Scholar] [CrossRef]
- Biessels, G.J.; Strachan, M.W.; Visseren, F.L.; Kappelle, L.J.; Whitmer, R.A. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: Towards targeted interventions. Lancet Diabetes Endocrinol. 2014, 2, 246–255. [Google Scholar] [CrossRef]
- Candasamy, M.; Mohamed Elhassan, S.A.; Kumar Bhattamisra, S.; Hua, W.Y.; Sern, L.M.; Binti Busthamin, N.A.; Mohamad Ilni, N.B.; Shun, N.S.; Baohong, L.; Ya, N.S.; et al. Type 3 diabetes (Alzheimer’s disease): New insight for promising therapeutic avenues. Panminerva Med. 2020, 62, 155–163. [Google Scholar] [CrossRef]
- Wittenauer, B.R.; Smith, L. Priority Medicines for Europe and the World “A Public Health Approach to Innovation”. Update on 2004 Background Paper. Who 2012, 61–79. Available online: https://apps.who.int/medicinedocs/documents/s20245en/s20245en.pdf (accessed on 10 April 2020).
- Weuve, J.; Hebert, L.E.; Scherr, P.A.; Evans, D.A. Deaths in the United States among persons with Alzheimer’s disease (2010–2050). Alzheimer’s Dement. 2014, 10, e40–e46. [Google Scholar] [CrossRef]
- Alzheimer Europe. Dementia in Europe Yearbook 2019: Estimating the Prevalence of Dementia in Europe. 2020. Available online: https://www.alzheimereurope.org/content/download/195515/1457520/file/FINAL%2005707%20Alzheimer%20Europe%20yearbook%202019.pdf (accessed on 24 January 2021).
- Murphy, S.L.; Xu, J.; Kochanek, K.D. Deaths: Final data for 2010. National vital statistics reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics. Natl. Vital Stat. Rep. 2013, 61, 1–117. [Google Scholar]
- Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Su, D.; Diao, W.; Li, J.; Pan, L.; Zhang, X.; Wu, X.; Mao, W. Strategic Design of Amyloid-β Species Fluorescent Probes for Alzheimer’s Disease. CS Chem. Neurosci. 2022, 13, 540–551. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Corrada, M.M.; Curriero, F.C.; Kawas, C. Survival following a diagnosis of Alzheimer disease. ArchNeurol 2002, 59, 1764–1767. [Google Scholar] [CrossRef]
- Piaceri, I.; Nacmias, B.; Sorbi, S. Genetics of familial and sporadic Alzheimer’s disease. Front. Biosci. 2013, 5, 167–177. [Google Scholar] [CrossRef]
- Yang, Y.; Song, W. Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience 2013, 250, 140–150. [Google Scholar] [CrossRef]
- Michailidis, M.; Tata, D.A.; Moraitou, D.; Kavvadas, D.; Karachrysafi, S.; Papamitsou, T.; Vareltzis, P.; Papaliagkas, V. Antidiabetic Drugs in the Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 4641. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Yao, Z.G.; Jing, H.Y.; Wang, D.M.; Lv, B.B.; Li, J.M.; Liu, F.F.; Fan, H.; Sun, X.C.; Qin, Y.J.; Zhao, M.Q. Valproic acid ameliorates olfactory dysfunction in APP/PS1 transgenic mice of Alzheimer’s disease: Ameliorations from the olfactory epithelium to the olfactory bulb. Pharmacol. Biochem. Behav. 2016, 144, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, N.Q.; Yan, F.; Jin, H.; Zhou, S.Y.; Shi, J.S.; Jin, F. Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav. Brain Res. 2018, 339, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Wilson, R.S.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch. Neurol. 2004, 61, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Woung, L.C.; Tsai, M.T.; Liu, C.C.; Su, Y.H.; Li, C.Y. Risk of Alzheimer’s disease in relation to diabetes: A population-based cohort study. Neuroepidemiology 2012, 38, 237–244. [Google Scholar] [CrossRef]
- Ohyagi, Y.; Miyoshi, K.; Nakamura, N. Therapeutic Strategies for Alzheimer’s Disease in the View of Diabetes Mellitus. Adv. Exp. Med. Biol. 2019, 1128, 227–248. [Google Scholar] [CrossRef]
- Mushtaq, G.; Khan, J.A.; Kumosani, T.A.; Kamal, M.A. Alzheimer’s disease and type 2 diabetes via chronic inflammatory mechanisms. Saudi J. Biol. Sci. 2015, 22, 4–13. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016, 12, 459–509. [Google Scholar] [CrossRef]
- Exalto, L.G.; Biessels, G.J.; Karter, A.J.; Huang, E.S.; Katon, W.J.; Minkoff, J.R.; Whitmer, R.A. Risk score for prediction of 10 year dementia risk in individuals with type 2 diabetes: A cohort study. Lancet Diabetes Endocrinol. 2013, 1, 183–190. [Google Scholar] [CrossRef]
- Cukierman-Yaffe, T.; Gerstein, H.C.; Williamson, J.D.; Lazar, R.M.; Lovato, L.; Miller, M.E.; Coker, L.H.; Murray, A.; Sullivan, M.D.; Marcovina, S.M.; et al. Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) Investigators. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: The action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care 2009, 32, 221–226. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef]
- Vieira, M.N.N.; Lima-Filho, R.A.S.; De Felice, F.G. Connecting Alzheimer’s disease to diabetes: Underlying mechanisms and potential therapeutic targets. Neuropharmacology 2018, 136 Pt B, 160–171. [Google Scholar] [CrossRef]
- De la Monte, S.M.; Wands, J.R. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef]
- Kar, S.; Chabot, J.G.; Quirion, R. Quantitative autoradiographic localization of [125I]insulin-like growth factor I, [125I]insulin-like growth factor II, and [125I]insulin receptor binding sites in developing and adult rat brain. J. Comp. Neurol. 1993, 333, 375–397. [Google Scholar] [CrossRef]
- O’Kusky, J.; Ye, P. Neurodevelopmental effects of insulin-like growth factor signaling. Front. Neuroendocrinol. 2012, 33, 230–251. [Google Scholar] [CrossRef]
- Grillo, C.A.; Piroli, G.G.; Lawrence, R.C.; Wrighten, S.A.; Green, A.J.; Wilson, S.P.; Sakai, R.R.; Kelly, S.J.; Wilson, M.A.; Mott, D.D.; et al. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes 2015, 64, 3927–3936. [Google Scholar] [CrossRef]
- van der Heide, L.P.; Kamal, A.; Artola, A.; Gispen, W.H.; Ramakers, G.M. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J. Neurochem. 2005, 94, 1158–1166. [Google Scholar] [CrossRef]
- Ronaghi, A.; Zibaii, M.I.; Pandamooz, S.; Nourzei, N.; Motamedi, F.; Ahmadiani, A.; Dargahi, L. Entorhinal cortex stimulation induces dentate gyrus neurogenesis through insulin receptor signaling. Brain Res. Bull. 2019, 144, 75–84. [Google Scholar] [CrossRef]
- Kuwabara, T.; Kagalwala, M.N.; Onuma, Y.; Ito, Y.; Warashina, M.; Terashima, K.; Sanosaka, T.; Nakashima, K.; Gage, F.H.; Asashima, M. Insulin biosynthesis in neuronal progenitors derived from adult hippocampus and the olfactory bulb. EMBO Mol. Med. 2011, 3, 742–754. [Google Scholar] [CrossRef]
- Watson, G.S.; Peskind, E.R.; Asthana, S.; Purganan, K.; Wait, C.; Chapman, D.; Schwartz, M.W.; Plymate, S.; Craft, S. Insulin increases CSF Abeta42 levels in normal older adults. Neurology 2003, 60, 1899–1903. [Google Scholar] [CrossRef]
- Rawlings, A.M.; Sharrett, A.R.; Mosley, T.H.; Ballew, S.H.; Deal, J.A.; Selvin, E. Glucose Peaks and the Risk of Dementia and 20-Year Cognitive Decline. Diabetes Care 2017, 40, 879–886. [Google Scholar] [CrossRef]
- Leclerc, M.; Bourassa, P.; Tremblay, C.; Caron, V.; Sugère, C.; Emond, V.; Bennett, D.A.; Calon, F. Cerebrovascular insulin receptors are defective in Alzheimer’s disease. Brain 2023, 146, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Yamaguchi, K.; Matsui, K.; Sano, T.; Kubota, T.; Hashimoto, T.; Mano, A.; Yamada, K.; Matsuo, Y.; Kubota, N.; et al. Differential effects of diet and genetically-induced brain insulin resistance on amyloid pathology in a mouse model of Alzheimer’s disease. Mol. Neurodegener 2019, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Jantrapirom, S.; Nimlamool, W.; Chattipakorn, N.; Chattipakorn, S.; Temviriyanukul, P.; Inthachat, W.; Govitrapong, P.; Potikanond, S. Liraglutide Suppresses Tau Hyperphosphorylation, Amyloid Beta Accumulation through Regulating Neuronal Insulin Signaling and BACE-1 Activity. Int. J. Mol. Sci. 2020, 21, 1725. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, J.R.M.; Marques, D.F.F.; Baptista, S.J.; Pereira, C.M.F.; Moreira, P.I.; Dinis, T.C.P.; Santos, A.E.; Salvador, J.A.R. Highlights in BACE1 Inhibitors for Alzheimer’s Disease Treatment. Front. Chem. 2018, 6, 178. [Google Scholar] [CrossRef] [PubMed]
- Kurochkin, I.V.; Guarnera, E.; Berezovsky, I.N. Insulin-Degrading Enzyme in the Fight against Alzheimer’s Disease. Trends Pharmacol. Sci. 2018, 39, 49–58. [Google Scholar] [CrossRef]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.A.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Guenette, S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef]
- Edland, S.D. Insulin-degrading enzyme, apolipoprotein E, and Alzheimer’s disease. J. Mol. Neurosci. 2004, 23, 213–217. [Google Scholar] [CrossRef]
- Michailidis, M.; Moraitou, D.; Tata, D.A.; Kalinderi, K.; Papamitsou, T.; Papaliagkas, V. Alzheimer’s Disease as Type 3 Diabetes: Common Pathophysiological Mechanisms between Alzheimer’s Disease and Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 2687. [Google Scholar] [CrossRef]
- Mohandas, E.; Rajmohan, V.; Raghunath, B. Neurobiology of Alzheimer’s disease. Indian J. Psychiatry 2009, 51, 55–61. [Google Scholar] [CrossRef]
- Daval, M.; Bedrood, S.; Gurlo, T.; Huang, C.J.; Costes, S.; Butler, P.C.; Langen, R. The effect of curcumin on human islet amyloid polypeptide misfolding and toxicity. Amyloid 2010, 17, 118–128. [Google Scholar] [CrossRef]
- de la Monte, S.M. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 35–66. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Yu, C.J.; Ma, D.; Song, L.L.; Zhai, Z.N.; Tao, Y.; Zhang, Y.; Cai, L.Y.; Hou, Y.H.; Chen, H.Y.; Wang, L. The role of GLP-1/GIP receptor agonists in Alzheimer’s disease. Adv. Clin. Exp. Med. 2020, 29, 661–668. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Wang, Z.; Zhang, X.; Yao, L.; Wang, F.; Liu, S.; Yin, J.; Ling, E.A.; Wang, L.; et al. High glucose-induced expression of inflammatory cytokines and reactive oxygen species in cultured astrocytes. Neuroscience 2012, 202, 58–68. [Google Scholar] [CrossRef]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef]
- Craft, S. Insulin resistance and Alzheimer’s disease pathogenesis: Potential mechanisms and implications for treatment. Curr. Alzheimer Res. 2007, 4, 147–152. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Giau, V.V. Type 3 Diabetes and Its Role Implications in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef]
- Akhtar, A.; Sah, S.P. Insulin signaling pathway and related molecules: Role in neurodegeneration and Alzheimer’s disease. Neurochem. Int. 2020, 135, 104707. [Google Scholar] [CrossRef]
- Blasko, I.; Stampfer-Kountchev, M.; Robatscher, P.; Veerhuis, R.; Eikelenboom, P.; Grubeck-Loebenstein, B. How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: The role of microglia and astrocytes. Aging Cell 2004, 3, 169–176. [Google Scholar] [CrossRef]
- Kshirsagar, V.; Thingore, C.; Juvekar, A. Insulin resistance: A connecting link between Alzheimer’s disease and metabolic disorder. Metab. Brain Dis. 2021, 36, 67–83. [Google Scholar] [CrossRef]
- Femminella, G.D.; Bencivenga, L.; Petraglia, L.; Visaggi, L.; Gioia, L.; Grieco, F.V.; de Lucia, C.; Komici, K.; Corbi, G.; Edison, P.; et al. Antidiabetic Drugs in Alzheimer’s Disease: Mechanisms of Action and Future Perspectives. J. Diabetes Res. 2017, 2017, 7420796. [Google Scholar] [CrossRef]
- Larsen, P.J.; Tang-Christensen, M.; Holst, J.J.; Orskov, C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997, 77, 257–270. [Google Scholar] [CrossRef]
- Llewellyn-Smith, I.J.; Reimann, F.; Gribble, F.M.; Trapp, S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience 2011, 180, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.X.; Gao, H.; Guo, Y.X.; Wang, B.Y.; Hua, R.X.; Gao, L.; Shang, H.W.; Lu, X.; Xu, J.D. GLP-1 and Underlying Beneficial Actions in Alzheimer’s Disease, Hypertension, and NASH. Front. Endocrinol. 2021, 12, 721198. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Jeon, S.J.; Cho, K.S.; Moon, E.; Sapkota, A.; Jun, H.S.; Ryu, J.H.; Choi, J.W. Activation of Glucagon-Like Peptide-1 Receptor Promotes Neuroprotection in Experimental Autoimmune Encephalomyelitis by Reducing Neuroinflammatory Responses. Mol. Neurobiol. 2018, 55, 3007–3020. [Google Scholar] [CrossRef]
- Du, H.; Meng, X.; Yao, Y.; Xu, J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front. Endocrinol. 2022, 13, 1033479. [Google Scholar] [CrossRef]
- Cork, S.C.; Richards, J.E.; Holt, M.K.; Gribble, F.M.; Reimann, F.; Trapp, S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol. Metab. 2015, 4, 718–731. [Google Scholar] [CrossRef]
- McClean, P.L.; Gault, V.A.; Harriott, P.; Hölscher, C. Glucagon-like peptide-1 analogues enhance synaptic plasticity in the brain: A link between diabetes and Alzheimer’s disease. Eur. J. Pharmacol. 2010, 630, 158–162. [Google Scholar] [CrossRef]
- McClean, P.L.; Hölscher, C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology 2014, 76 Pt A, 57–67. [Google Scholar] [CrossRef]
- McClean, P.L.; Parthsarathy, V.; Faivre, E.; Hölscher, C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 6587–6594. [Google Scholar] [CrossRef]
- Long-Smith, C.M.; Manning, S.; McClean, P.L.; Coakley, M.F.; O’Halloran, D.J.; Holscher, C.; O’Neill, C. The diabetes drug liraglutide ameliorates aberrant insulin receptor localisation and signalling in parallel with decreasing both amyloid-β plaque and glial pathology in a mouse model of Alzheimer’s disease. Neuromolecular Med. 2013, 15, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Parthsarathy, V.; Hölscher, C. Chronic treatment with the GLP1 analogue liraglutide increases cell proliferation and differentiation into neurons in an AD mouse model. PLoS ONE 2013, 8, e58784. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Ma, D.; Zhang, M.; Hu, S.; Shao, S.; Gong, C.X. Subcutaneous administration of liraglutide ameliorates Alzheimer-associated tau hyperphosphorylation in rats with type 2 diabetes. J. Alzheimers Dis. 2013, 37, 637–648. [Google Scholar] [CrossRef]
- Palleria, C.; Leo, A.; Andreozzi, F.; Citraro, R.; Iannone, M.; Spiga, R.; Sesti, G.; Constanti, A.; De Sarro, G.; Arturi, F.; et al. Liraglutide prevents cognitive decline in a rat model of streptozotocin-induced diabetes independently from its peripheral metabolic effects. Behav. Brain Res. 2017, 321, 157–169. [Google Scholar] [CrossRef]
- Li, Y.; Glotfelty, E.J.; Karlsson, T.; Fortuno, L.V.; Harvey, B.K.; Greig, N.H. The metabolite GLP-1 (9-36) is neuroprotective and anti-inflammatory in cellular models of neurodegeneration. J. Neurochem. 2021, 159, 867–886. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kam, T.I.; Lee, S.; Park, H.; Oh, Y.; Kwon, S.H.; Song, J.J.; Kim, D.; Kim, H.; Jhaldiyal, A.; et al. Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer’s disease. Acta Neuropathol. Commun. 2021, 9, 78. [Google Scholar] [CrossRef]
- Iwai, T.; Sawabe, T.; Tanimitsu, K.; Suzuki, M.; Sasaki-Hamada, S.; Oka, J. Glucagon-like peptide-1 protects synaptic and learning functions from neuroinflammation in rodents. J. Neurosci. Res. 2014, 92, 446–454. [Google Scholar] [CrossRef]
- Cai, H.Y.; Yang, J.T.; Wang, Z.J.; Zhang, J.; Yang, W.; Wu, M.N.; Qi, J.S. Lixisenatide reduces amyloid plaques, neurofibrillary tangles and neuroinflammation in an APP/PS1/tau mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2018, 495, 1034–1040. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Gao, R.; Chen, X.; Chen, R.; Chen, Z. Glucagon-like peptide-1 analogs mitigate neuroinflammation in Alzheimer’s disease by suppressing NLRP2 activation in astrocytes. Mol. Cell Endocrinol. 2022, 542, 111529. [Google Scholar] [CrossRef]
- Qian, Z.; Chen, H.; Xia, M.; Chang, J.; Li, X.; Ye, S.; Wu, S.; Jiang, S.; Bao, J.; Wang, B.; et al. Activation of glucagon-like peptide-1 receptor in microglia attenuates neuroinflammation-induced glial scarring via rescuing Arf and Rho GAP adapter protein 3 expressions after nerve injury. Int. J. Biol. Sci. 2022, 18, 1328–1346. [Google Scholar] [CrossRef] [PubMed]
- Candeias, E.; Sebastião, I.; Cardoso, S.; Carvalho, C.; Santos, M.S.; Oliveira, C.R.; Moreira, P.I.; Duarte, A.I. Brain GLP-1/IGF-1 Signaling and Autophagy Mediate Exendin-4 Protection Against Apoptosis in Type 2 Diabetic Rats. Mol. Neurobiol. 2018, 55, 4030–4050. [Google Scholar] [CrossRef]
- Emsley, J.G.; Mitchell, B.D.; Kempermann, G.; Macklis, J.D. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog. Neurobiol. 2005, 75, 321–341. [Google Scholar] [CrossRef]
- Hamilton, A.; Holscher, C. The effect of ageing on neurogenesis and oxidative stress in the APP(swe)/PS1(deltaE9) mouse model of Alzheimer’s disease. Brain Res. 2012, 1449, 83–93. [Google Scholar] [CrossRef]
- During, M.J.; Cao, L.; Zuzga, D.S.; Francis, J.S.; Fitzsimons, H.L.; Jiao, X.; Bland, R.J.; Klugmann, M.; Banks, W.A.; Drucker, D.J.; et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med. 2003, 9, 1173–1179. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Zhang, W.; Li, T.; Yang, T.; Yuan, X.; Zhou, Y.; Zou, Q.; Yang, H.; Gao, F.; Tian, Y.; et al. GLP-1R activation ameliorated novel-object recognition memory dysfunction via regulating hippocampal AMPK/NF-κB pathway in neuropathic pain mice. Neurobiol. Learn Mem. 2021, 182, 107463. [Google Scholar] [CrossRef]
- Meyer, D.; Bonhoeffer, T.; Scheuss, V. Balance and stability of synaptic structures during synaptic plasticity. Neuron 2014, 82, 430–443. [Google Scholar] [CrossRef]
- Mullins, R.J.; Mustapic, M.; Chia, C.W.; Carlson, O.; Gulyani, S.; Tran, J.; Li, Y.; Mattson, M.P.; Resnick, S.; Egan, J.M.; et al. A Pilot Study of Exenatide Actions in Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 741–752. [Google Scholar] [CrossRef]
- Egefjord, L.; Gejl, M.; Møller, A.; Brændgaard, H.; Gottrup, H.; Antropova, O.; Møller, N.; Poulsen, H.E.; Gjedde, A.; Brock, B.; et al. Effects of liraglutide on neurodegeneration, blood flow and cognition in Alzheimer’s disease—Protocol for a controlled, randomized double-blinded trial. Dan. Med. J. 2012, 59, A4519. [Google Scholar]
- Gejl, M.; Gjedde, A.; Egefjord, L.; Møller, A.; Hansen, S.B.; Vang, K.; Rodell, A.; Brændgaard, H.; Gottrup, H.; Schacht, A.; et al. In Alzheimer’s Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Front. Aging Neurosci. 2016, 8, 108. [Google Scholar] [CrossRef]
- Femminella, G.D.; Frangou, E.; Love, S.B.; Busza, G.; Holmes, C.; Ritchie, C.; Lawrence, R.; McFarlane, B.; Tadros, G.; Ridha, B.H.; et al. Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: Study protocol for a randomised controlled trial (ELAD study). Trials 2019, 20, 191. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, Y.; Zhuang, X.; Sun, A.; Zhang, Y.; Ni, Y. Low glucagon-like peptide-1 (GLP-1) concentration in serum is indicative of mild cognitive impairment in type 2 diabetes patients. Clin. Neurol. Neurosurg. 2018, 174, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Vadini, F.; Simeone, P.G.; Boccatonda, A.; Guagnano, M.T.; Liani, R.; Tripaldi, R.; Di Castelnuovo, A.; Cipollone, F.; Consoli, A.; Santilli, F. Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: A randomized, controlled study. Int. J. Obes. 2020, 44, 1254–1263. [Google Scholar] [CrossRef]
- Ishøy, P.L.; Fagerlund, B.; Broberg, B.V.; Bak, N.; Knop, F.K.; Glenthøj, B.Y.; Ebdrup, B.H. No cognitive-enhancing effect of GLP-1 receptor agonism in antipsychotic-treated, obese patients with schizophrenia. Acta Psychiatr. Scand. 2017, 136, 52–62. [Google Scholar] [CrossRef]
- Li, Q.; Jia, M.; Yan, Z.; Li, Q.; Sun, F.; He, C.; Li, Y.; Zhou, X.; Zhang, H.; Liu, X.; et al. Activation of Glucagon-Like Peptide-1 Receptor Ameliorates Cognitive Decline in Type 2 Diabetes Mellitus Through a Metabolism-Independent Pathway. J. Am. Heart Assoc. 2021, 10, e020734. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, Z.; Zhang, B.; Zhang, W.; Wang, J.; Ni, W.; Miao, Y.; Liu, J.; Bi, Y. Enhancement of Impaired Olfactory Neural Activation and Cognitive Capacity by Liraglutide, but Not Dapagliflozin or Acarbose, in Patients with Type 2 Diabetes: A 16-Week Randomized Parallel Comparative Study. Diabetes Care 2022, 45, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Cukierman-Yaffe, T.; Gerstein, H.C.; Colhoun, H.M.; Diaz, R.; García-Pérez, L.E.; Lakshmanan, M.; Bethel, A.; Xavier, D.; Probstfield, J.; Riddle, M.C.; et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: An exploratory analysis of the REWIND trial. Lancet Neurol. 2020, 19, 582–590. [Google Scholar] [CrossRef]
- Anita, N.Z.; Zebarth, J.; Chan, B.; Wu, C.Y.; Syed, T.; Shahrul, D.; Nguyen, M.M.; Pakosh, M.; Herrmann, N.; Lanctôt, K.L.; et al. Inflammatory markers in type 2 diabetes with vs. without cognitive impairment; a systematic review and meta-analysis. Brain Behav. Immun. 2022, 100, 55–69. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Li, Y.; Li, L.; Melchiorsen, J.U.; Rosenkilde, M.; Hölscher, C. The Novel Dual GLP-1/GIP Receptor Agonist DA-CH5 Is Superior to Single GLP-1 Receptor Agonists in the MPTP Model of Parkinson’s Disease. J. Park. Dis. 2020, 10, 523–542. [Google Scholar] [CrossRef]
- Salles, G.N.; Calió, M.L.; Hölscher, C.; Pacheco-Soares, C.; Porcionatto, M.; Lobo, A.O. Neuroprotective and restorative properties of the GLP-1/GIP dual agonist DA-JC1 compared with a GLP-1 single agonist in Alzheimer’s disease. Neuropharmacology 2020, 162, 107813. [Google Scholar] [CrossRef]
- Maskery, M.; Goulding, E.M.; Gengler, S.; Melchiorsen, J.U.; Rosenkilde, M.M.; Hölscher, C. The Dual GLP-1/GIP Receptor Agonist DA4-JC Shows Superior Protective Properties Compared to the GLP-1 Analogue Liraglutide in the APP/PS1 Mouse Model of Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Demen. 2020, 35, 1533317520953041. [Google Scholar] [CrossRef]
- Cai, H.Y.; Yang, D.; Qiao, J.; Yang, J.T.; Wang, Z.J.; Wu, M.N.; Qi, J.S.; Hölscher, C. A GLP-1/GIP Dual Receptor Agonist DA4-JC Effectively Attenuates Cognitive Impairment and Pathology in the APP/PS1/Tau Model of Alzheimer’s Disease. J. Alzheimers Dis. 2021, 83, 799–818. [Google Scholar] [CrossRef]
- Tamargo, I.A.; Bader, M.; Li, Y.; Yu, S.J.; Wang, Y.; Talbot, K.; DiMarchi, R.D.; Pick, C.G.; Greig, N.H. Novel GLP-1R/GIPR co-agonist “twincretin” is neuroprotective in cell and rodent models of mild traumatic brain injury. Exp. Neurol. 2017, 288, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.M.; Pathak, V.; Gault, V.A.; McClean, S.; Irwin, N.; Flatt, P.R. Novel dual incretin agonist peptide with antidiabetic and neuroprotective potential. Biochem. Pharmacol. 2018, 155, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.J.; Hölscher, C.; Li, T.; Dong, X.F.; Qu, X.S.; Cao, Y.; Wu, M.N.; Wang, Z.J.; Qi, J.S. Sheng li xue bao. Acta Physiol. Sin. 2017, 69, 135–145. [Google Scholar]
- Tai, J.; Liu, W.; Li, Y.; Li, L.; Hölscher, C. Neuroprotective effects of a triple GLP-1/GIP/glucagon receptor agonist in the APP/PS1 transgenic mouse model of Alzheimer’s disease. Brain Res. 2018, 1678, 64–74. [Google Scholar] [CrossRef]
- Li, Y.; Glotfelty, E.J.; Namdar, I.; Tweedie, D.; Olson, L.; Hoffer, B.J.; DiMarchi, R.D.; Pick, C.G.; Greig, N.H. Neurotrophic and neuroprotective effects of a monomeric GLP-1/GIP/Gcg receptor triagonist in cellular and rodent models of mild traumatic brain injury. Exp. Neurol. 2020, 324, 113113. [Google Scholar] [CrossRef]
- Li, T.; Jiao, J.J.; Su, Q.; Hölscher, C.; Zhang, J.; Yan, X.D.; Zhao, H.M.; Cai, H.Y.; Qi, J.S. A GLP-1/GIP/Gcg receptor triagonist improves memory behavior, as well as synaptic transmission, neuronal excitability and Ca2+ homeostasis in 3xTg-AD mice. Neuropharmacology 2020, 170, 108042. [Google Scholar] [CrossRef] [PubMed]
- Palleria, C.; Leporini, C.; Maida, F.; Succurro, E.; De Sarro, G.; Arturi, F.; Russo, E. Potential effects of current drug therapies on cognitive impairment in patients with type 2 diabetes. Front. Neuroendocrinol. 2016, 42, 76–92. [Google Scholar] [CrossRef]
| Study | Aim of the Study | Results |
|---|---|---|
| Michailidis M. et al. (2022) [53] | Evaluate the efficacy of antidiabetic drugs in AD treatment | Intranasal insulin improves: cognitive, verbal, working and audiovisual memory and functional ability. Metformin: inconclusive results because some studies demonstrated that it reduces risk of CI and Dementia and improves verbal memory, attention and executive function; other studies demonstrated that its long-term use increases risk of CI and Dementia. Liraglutide: improves cerebral glucose metabolism. So it has moderate neuroprotective effects. Rosiglitazone: improves attention and delayed recall and protects against cognitive impairment. Pioglitazone: improves cognition, metabolism and cerebral blood flow to the parietal lobe. |
| Livingston G. et al. (2020) [54] | Evaluate prevention strategy and treatment strategy | Modifying risk factors (such as hypertension, tobacco and alcohol use, obesity, noise exposure etc.) might prevent or delay up to 40% of dementias. For patients with dementia: it’s necessary farmacological help (to reduce neuropsychiatric symptoms) and social support for patient and family carers. |
| Yao Z. G. et al. (2016) [55] | Analyse the effects of Valproic Acid on olfactory dysfunction of APP/PS1 double transgenic mouse models of AD | Valproic Acid improves olfactory performances, reduces β-amyloid deposition in olfactory epithelium, decreases cell apoptosis of olfactory epithelium, reduces senile plaques and levels of soluble and insoluble Aβ42 peptides in olfactory bulb so it improves olfactory performances and prevented degenerative changes. |
| Ohyagi Y. et al. (2019) [59] | Analyse of apomorphine that promoted intracellular Aβ degradation and improved memory function | Apomorphine treatment reduces neuronal insulin resistance and activates insulin-degrading enzyme, an Aβ-degrading enzyme. |
| Study | Population | Sample Size | Intervention | Outcome | Results |
|---|---|---|---|---|---|
| Mullins R.J. et al. (2019) [121] | Patients (more than 60 years old) with evidence of early-stage Alzheimer’s disease or mild cognitive impairment in screening testing. | 27 Exenatide (n = 13) vs. placebo (n = 14) | Exenatide 5 mcg or 10 mcg SC twice daily vs. Placebo | Safety and tolerability of exenatide and responses for clinical, cognitive, and biomarker outcomes in early Alzheimer Disease. | Exenatide treatment produced no differences on Alzheimer Disease outcome. |
| Egefjord L. et al. (2012) [122] | Patients from 50 years to 80 years with Alzheimer Disease (AD) diagnosis. | 34 Liraglutide vs. Placebo | Liraglutide 1.8 mg vs. Placebo | Liraglutide will change the intra-cerebral amyloid deposit in the CNS in patients with Alzheimer’s disease assessed by PIB PET scan. | Closed, but no results posted. |
| Gejl M. et al. (2016) [123] | Patients from 50 years to 80 years with Alzheimer Disease (AD) diagnosis. | 38 Liraglutide (n = 18) vs. Placebo (n = 20) | Liraglutide 1.8 mg daily vs. Placebo | Change in deposition ofamyloid deposit (Aβ) in the Central Nervous System assessed by PIB PET scan followed by improvement of cognition. | In Liraglutide group, the Glucose metabolism (CMRglc)increased numerically but non-significantly (all p ≥ 0.49) compared to Placebo group in which Glucose metabolism (CMRglc) declined significantly (p = 0.04). |
| Femminella G.D. et al. 2019 [124] | Patients (more than 50 years old) with mild Alzheimer’s dementia. | 206 Liraglutide (n = 103) vs. Placebo (n = 103) | Liraglutide 1.8 mg vs. Placebo | Change in cerebral glucose metabolic rate in the cortical regions from baseline to follow up (12 months). | Closed, but no results posted. |
| Vadini F. et al. (2020) [126] | Patients with IFG or T2DM, BMI > 30 in treatment with diet therapy plus metformin at the highest tolerated dose. | 32 Liraglutide (n = 16) vs. Placebo (n = 16) | Liraglutide 1.8 mg vs. Placebo | Liraglutide effects on cognitive functions in type 2 diabetic subjects independently on the weight loss it might induce. | Liraglutide significantly increased short term memory (p = 0.024) and memory composite z-score (p = 0.0065). |
| Li et al (2021) [128] | Patients aged 18 to 65 years with a glycated hemoglobin (HbA1c) value of >7.0%, whowere treated with oral antidiabetic drugs or insulin for at least 3 months. | 47 Liraglutide (n = 24) Vs Placebo (n = 23) | Liraglutide from 0.6 mg to 1.8 mg daily vs. placebo | Change in scores of neuropsychological cognition tests and brain activation monitoring by functional near-infrared spectroscopy | At 12 weeks, the liraglutide group demonstrated better scores in all cognitive tests, above all as regard memory and attention (p = 0.04) when compared to control group. Liraglutide increased activation of the dorsolateral prefrontal cortex and orbitofrontal cortex brain regions (p = 0.0038) and the improvement of cognitive decline in this group was correlated with morphological changes in brain regions (p < 0.05), compared to metabolic changes. |
| Cheng et al. (2022) [129] | All patients were aged 40 to 75 years, had hemoglobin A1c (HbA1c) of 7.0–10.0% and a BMI of $25 kg/m2, and had been on a stable dose of metformin monotherapy (1500 mg daily) for at least 90 days. | 36 Liraglutide (n = 12); dapagliflozin (n = 12); acarbose (n = 12) | Liraglutide from 0.6 mg to 1.8 mg once daily; dapagliflozin 10 mg once daily; acarbose from 50 mg to 100 mg threetimes daily | The primary end point was the change in odor-induced brain activation from baseline to week 16 evaluated with fMRI and cognitive assessments. | In the group treated with liraglutide, a significant improvement in impaired odor-induced left hippocampal activation and an improvement of cognitive subdomains of delayed memory, attention and executive function were demonstrated (all p < 0.05), unlike the groups treated with dapagliflozin or acarbose. |
| Cukierman-Yaffe T. et al. (2020) [130] | Patients more than 50 years old with T2DM and additional cardiovascular risk factors, glycated haemoglobin of up to 9 5% on a maximum of two oral glucose-lowering drugs with or without basal insulin and a body-mass index of at least 23 kg/m2. | 8828 Dulaglutide (n = 4456) vs. Placebo (n = 4372) | Dulaglutide 1.5 mg once weekly vs. Placebo | Exploratory analysis within REWIND trial to evaluate association between dulaglutide and cognitive impairment. | Long-term treatment with dulaglutide might reduce cognitive impairment that occours in 4 05 per 100 patient-years in participants assigned dulaglutide and 4 35 per 100 patient-years in people assigned placebo. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelle, M.C.; Zaffina, I.; Giofrè, F.; Pujia, R.; Arturi, F. Potential Role of Glucagon-like Peptide-1 Receptor Agonists in the Treatment of Cognitive Decline and Dementia in Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 11301. https://doi.org/10.3390/ijms241411301
Pelle MC, Zaffina I, Giofrè F, Pujia R, Arturi F. Potential Role of Glucagon-like Peptide-1 Receptor Agonists in the Treatment of Cognitive Decline and Dementia in Diabetes Mellitus. International Journal of Molecular Sciences. 2023; 24(14):11301. https://doi.org/10.3390/ijms241411301
Chicago/Turabian StylePelle, Maria Chiara, Isabella Zaffina, Federica Giofrè, Roberta Pujia, and Franco Arturi. 2023. "Potential Role of Glucagon-like Peptide-1 Receptor Agonists in the Treatment of Cognitive Decline and Dementia in Diabetes Mellitus" International Journal of Molecular Sciences 24, no. 14: 11301. https://doi.org/10.3390/ijms241411301
APA StylePelle, M. C., Zaffina, I., Giofrè, F., Pujia, R., & Arturi, F. (2023). Potential Role of Glucagon-like Peptide-1 Receptor Agonists in the Treatment of Cognitive Decline and Dementia in Diabetes Mellitus. International Journal of Molecular Sciences, 24(14), 11301. https://doi.org/10.3390/ijms241411301





