Unravelling the Role of P300 and TMPRSS2 in Prostate Cancer: A Literature Review
Abstract
1. Introduction
2. Biological and Biochemical Features of TMPRSS2
2.1. Biological and Biochemical Features of TMPRSS2
2.2. The Biological Role of TMPRSS2 in the Normal Prostate Gland
2.3. The Role of TMPRSS2 in Prostate Carcinogenesis
3. Clinical Implications of TMPRSS2:ERG Fusion in Prostate Cancer
4. TMPRSS2:ERG Therapeutics
5. Biological and Biochemical Features of KATs
5.1. Lysine Acetyltransferases (KATs) of AR
5.2. Location of Gene
5.3. Role of P300/CBP Complex
5.4. p300 Protein Description
5.5. Role of p300 in the Cell Cycle and Proliferation
5.6. P300 Induces Oncogene Transcription, Promotes Cancer Cell Proliferation, Survival, Tumorigenesis, Metastasis, and Immune Evasion
5.7. The Transcriptional Acts of p300 in Prostate Cancer
5.8. p300 and Prostate Cancer
6. The Use of p300 as a Therapeutic Target in Prostate Cancer
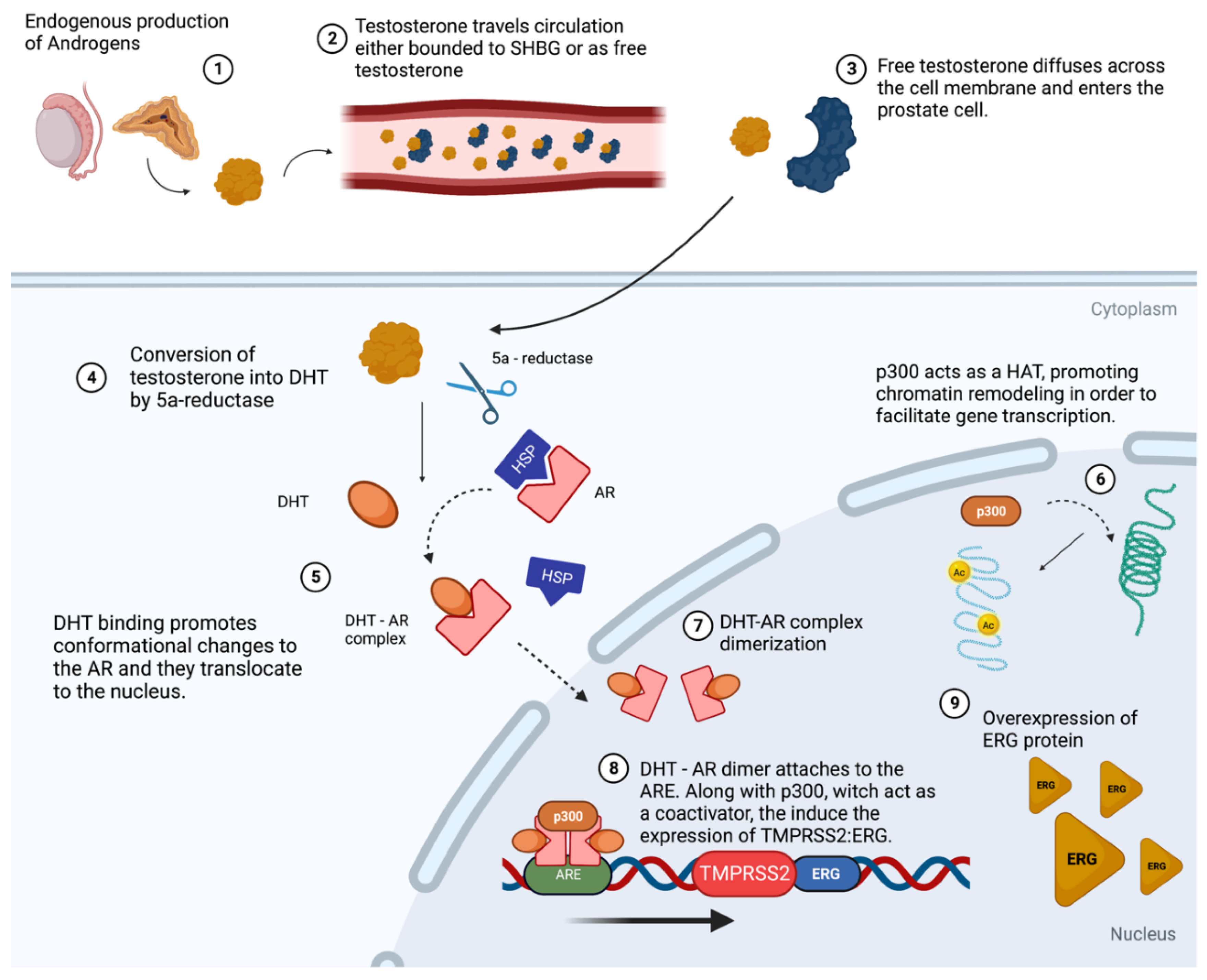
7. The Interplay between P300 and TMPRSS2 in Prostate Cancer
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Articles published in peer-reviewed journals.
- Articles written in English.
- Articles focusing on the role of p300 and TMPRSS2 in prostate cancer.
- Articles that provide insights into the molecular mechanisms, interactions, and functions of p300 and TMPRSS2 in prostate cancer development, progression, and prognosis.
- Articles published between January 2017 and February 2023, to ensure a contemporary review.
- Review articles and editorials without primary data.
- Articles that primarily focused on diseases other than prostate cancer.
- Articles lacking relevance to the role of p300 and TMPRSS2 in prostate cancer.
- We excluded older articles in cases where subsequently updated data was discovered.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect Med. 2018, 8, a030361. [Google Scholar] [CrossRef] [PubMed]
- Vlajnic, T.; Bubendorf, L. Molecular pathology of prostate cancer: A practical approach. Pathology 2021, 53, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Assadi, M.; Jokar, N.; Ghasemi, M.; Nabipour, I.; Gholamrezanezhad, A.; Ahmadzadehfar, H. Precision Medicine Approach in Prostate Cancer. Curr. Pharm. Des. 2020, 26, 3783–3798. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Mens. Health 2019, 37, 288. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.E.; Huang, H.; Tindall, D.J. Androgen Receptor Signaling in Androgen-Refractory Prostate Cancer. JNCI J. Natl. Cancer Inst. 2001, 93, 1687–1697. [Google Scholar] [CrossRef]
- Xu, S.; Fan, L.; Jeon, H.-Y.; Zhang, F.; Cui, X.; Mickle, M.B.; Peng, G.; Hussain, A.; Fazli, L.; Gleave, M.E.; et al. p300-Mediated Acetylation of Histone Demethylase JMJD1A Prevents Its Degradation by Ubiquitin Ligase STUB1 and Enhances Its Activity in Prostate Cancer. Cancer Res. 2020, 80, 3074–3087. [Google Scholar] [CrossRef]
- Ligr, M.; Li, Y.; Zou, X.; Daniels, G.; Melamed, J.; Peng, Y.; Wang, W.; Wang, J.; Ostrer, H.; Pagano, M.; et al. Tumor Suppressor Function of Androgen Receptor Coactivator ARA70α in Prostate Cancer. Am. J. Pathol. 2010, 176, 1891–1900. [Google Scholar] [CrossRef]
- Thunders, M.; Delahunt, B. Gene of the month: TMPRSS2 (transmembrane serine protease 2). J. Clin. Pathol. 2020, 73, 773–776. [Google Scholar] [CrossRef]
- Epstein, R.J. The secret identities of TMPRSS2: Fertility factor, virus trafficker, inflammation moderator, prostate protector and tumor suppressor. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2021, 43, 159–176. [Google Scholar] [CrossRef]
- Lin, B.; Ferguson, C.; White, J.T.; Wang, S.; Vessella, R.; True, L.D.; Hood, L.; Nelson, P.S. Prostate-localized and Androgen-regulated Expression of the Membrane-bound Serine Protease TMPRSS2 1. 1999. Available online: http://aacrjournals.org/cancerres/article-pdf/59/17/4180/3243739/ch179904180p.pdf (accessed on 1 February 2023).
- Takeda, M. Proteolytic Activation of SARS-CoV-2 Spike Protein. Microbiol. Immunol. 2021, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Iwata-Yoshikawa, N.; Kakizaki, M.; Shiwa-Sudo, N.; Okura, T.; Tahara, M.; Fukushi, S.; Maeda, K.; Kawase, M.; Asanuma, H.; Tomita, Y.; et al. Essential role of TMPRSS2 in SARS-CoV-2 infection in murine airways. Nat. Commun. 2022, 13, 6100. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Fraser, B.J.; Beldar, S.; Seitova, A.; Hutchinson, A.; Mannar, D.; Li, Y.; Kwon, D.; Tan, R.; Wilson, R.P.; Leopold, K.; et al. Structure and activity of human TMPRSS2 protease implicated in SARS-CoV-2 activation. Nat. Chem. Biol. 2022, 18, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Zmora, P.; Moldenhauer, A.S.; Hofmann-Winkler, H.; Pöhlmann, S. TMPRSS2 isoform 1 activates respiratory viruses and is expressed in viral target cells. PLoS ONE 2015, 10, e0138380. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, S.H.; Hirsh, A.; Li, D.C.; Holloway, G.; Chao, J.; Boucher, R.C.; Gabriel, S.E. Regulation of the epithelial sodium channel by serine proteases in human airways. J. Biol. Chem. 2002, 277, 8338–8345. [Google Scholar] [CrossRef]
- Wilson, S.K.; Greer, B.; Hooper, J.; Zijlstra, A.; Walker, B.; Quigley, J.P.; Hawthorne, S.J. The Membrane-Anchored Serine Protease, TMPRSS2, Activates PAR-2 in Prostate Cancer Cells. Biochem. J. 2005, 388, 967–972. [Google Scholar] [CrossRef]
- Lucas, J.M.; Heinlein, C.; Kim, T.; Hernandez, S.A.; Malik, M.S.; True, L.D.; Morrissey, C.; Corey, E.; Montgomery, B.; Mostaghel, E.; et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014, 4, 1310–1325. [Google Scholar] [CrossRef]
- Kim, T.S.; Heinlein, C.; Hackman, R.C.; Nelson, P.S. Phenotypic Analysis of Mice Lacking the Tmprss2-Encoded Protease. Mol. Cell Biol. 2006, 26, 965–975. [Google Scholar] [CrossRef]
- Xiao, X.; Shan, H.; Niu, Y.; Wang, P.; Li, D.; Zhang, Y.; Wang, J.; Wu, Y.; Jiang, H. TMPRSS2 Serves as a Prognostic Biomarker and Correlated With Immune Infiltrates in Breast Invasive Cancer and Lung Adenocarcinoma. Front Mol. Biosci. 2022, 9, 647826. [Google Scholar] [CrossRef]
- Rao, S.R.; Alham, N.K.; Upton, E.; McIntyre, S.; Bryant, R.J.; Cerundolo, L.; Bowes, E.; Jones, S.; Browne, M.; Mills, I.; et al. Detailed Molecular and Immune Marker Profiling of Archival Prostate Cancer Samples Reveals an Inverse Association between TMPRSS2:ERG Fusion Status and Immune Cell Infiltration. J. Mol. Diagn. 2020, 22, 652–669. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Wei, M.; Wang, S.; Dong, J.; Wei, J. Pan-Cancer Analysis of Immune Cell Infiltration Identifies a Prognostic Immune-Cell Characteristic Score (ICCS) in Lung Adenocarcinoma. Front. Immunol. 2020, 11, 1218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-C.; Hu, Z.-Q.; Long, J.-H.; Zhu, G.-M.; Wang, Y.; Jia, Y.; Zhou, J.; Ouyang, Y.; Zeng, Z. Clinical Implications of Tumor-Infiltrating Immune Cells in Breast Cancer. J. Cancer 2019, 10, 6175–6184. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-J.; Hsu, T.-W.; Wu, S.-R.; Lan, S.-W.; Hsiao, T.-F.; Lin, H.-Y.; Tu, H.-F.; Lee, C.-F.; Huang, C.-C.; Chen, M.-J.M.; et al. Inhibition of TMPRSS2 by HAI-2 reduces prostate cancer cell invasion and metastasis. Oncogene 2020, 39, 5950–5963. [Google Scholar] [CrossRef] [PubMed]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.-W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent Fusion of TMPRSS2 and ETS Transcription Factor Genes in Prostate Cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Tomlins, S.A.; Mehra, R.; Rhodes, D.R.; Smith, L.R.; Roulston, D.; Helgeson, B.E.; Cao, X.; Wei, J.T.; Rubin, M.A.; Shah, R.B.; et al. TMPRSS2:ETV4 Gene Fusions Define a Third Molecular Subtype of Prostate Cancer. Cancer Res. 2006, 66, 3396–3400. [Google Scholar] [CrossRef]
- Cernera, G.; Farooqi, A.A.; Latonen, L.; Scaravilli, M.; Koivukoski, S. Androgen-Driven Fusion Genes and Chimeric Transcripts in Prostate Cancer. Front. Cell Dev. Biol. 2021, 9, 623809. [Google Scholar] [CrossRef]
- Adamo, P.; Ladomery, M.R. The oncogene ERG: A key factor in prostate cancer. Oncogene 2016, 35, 403–414. [Google Scholar] [CrossRef]
- Rubin, M.A.; Chinnaiyan, A.M. Bioinformatics approach leads to the discovery of the TMPRSS2:ETS gene fusion in prostate cancer. Lab. Investig. 2006, 86, 1099–1102. [Google Scholar] [CrossRef]
- Perner, S.; Demichelis, F.; Beroukhim, R.; Schmidt, F.H.; Mosquera, J.-M.; Setlur, S.; Tchinda, J.; Tomlins, S.A.; Hofer, M.D.; Pienta, K.G.; et al. TMPRSS2:ERG Fusion-Associated Deletions Provide Insight into the Heterogeneity of Prostate Cancer. Cancer Res. 2006, 66, 8337–8378. [Google Scholar] [CrossRef]
- Zhou, F.; Gao, S.; Han, D.; Han, W.; Chen, S.; Patalano, S.; Macoska, J.A.; He, H.H.; Cai, C. TMPRSS2-ERG activates NO-cGMP signaling in prostate cancer cells. Oncogene 2019, 38, 4397–4411. [Google Scholar] [CrossRef] [PubMed]
- Deplus, R.; Delliaux, C.; Marchand, N.; Flourens, A.; Vanpouille, N.; Leroy, X.; de Launoit, Y.; Duterque-Coquillaud, M. TMPRSS2-ERG fusion promotes prostate cancer metastases in bone. Oncotarget 2017, 8, 11827–11840. [Google Scholar] [CrossRef]
- Taris, M.; Irani, J.; Blanchet, P.; Multigner, L.; Cathelineau, X.; Fromont, G. ERG Expression in Prostate Cancer:The Prognostic Paradox. Prostate 2014, 74, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, Z.R.; Burns, M.C.; Ebot, E.M.; Frampton, G.M.; Ross, J.S.; Hussain, M.H.A.; Abdulkadir, S.A. Early-onset metastatic and clinically advanced prostate cancer is a distinct clinical and molecular entity characterized by increased TMPRSS2–ERG fusions. Prostate Cancer Prostatic Dis. 2021, 24, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, F.; Cormio, A.; Brunelli, M.; D’Amuri, A.; Carrieri, G.; Bufo, P.; Cormio, L. Urine TMPRSS2: ERG Fusion Transcript as a Biomarker for Prostate Cancer: Literature Review. Clin. Genitourin. Cancer 2016, 14, 117–121. [Google Scholar] [CrossRef]
- Hessels, D.; Smit, F.P.; Verhaegh, G.W.; Witjes, J.A.; Cornel, E.B.; Schalken, J.A. Detection of TMPRSS2-ERG Fusion Transcripts and Prostate Cancer Antigen 3 in Urinary Sediments May Improve Diagnosis of Prostate Cancer. Clin. Cancer Res. 2007, 13, 5103–5108. [Google Scholar] [CrossRef]
- Song, C.; Chen, H. Predictive significance of TMRPSS2-ERG fusion in prostate cancer: A meta-analysis. Cancer Cell Int. 2018, 18, 177. [Google Scholar] [CrossRef]
- Fine, S.W.; Gopalan, A.; Leversha, M.A.; Al-Ahmadie, H.A.; Tickoo, S.K.; Zhou, Q.; Satagopan, J.M.; Scardino, P.T.; Gerald, W.L.; Reuter, V.E. TMPRSS2-ERG gene fusion is associated with low Gleason scores and not with high-grade morphological features. Mod. Pathol. 2010, 23, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Graff, R.E.; Bauer, S.R.; Pitt, M.J.; Lis, R.T.; Stack, E.C.; Martin, N.E.; Kunz, L.; Penney, K.L.; Ligon, A.H.; et al. The TMPRSS2:ERG Rearrangement, ERG Expression, and Prostate Cancer Outcomes: A Cohort Study and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1497–1509. [Google Scholar] [CrossRef]
- Ankerst, D.P.; Goros, M.; Tomlins, S.A.; Patil, D.; Feng, Z.; Wei, J.T.; Sanda, M.G.; Gelfond, J.; Thompson, I.M.; Leach, R.J.; et al. Incorporation of Urinary Prostate Cancer Antigen 3 and TMPRSS2:ERG into Prostate Cancer Prevention Trial Risk Calculator. Eur. Urol. Focus 2019, 5, 54–61. [Google Scholar] [CrossRef]
- Reig, Ò.; Marín-Aguilera, M.; Carrera, G.; Jiménez, N.; Paré, L.; García-Recio, S.; Gaba, L.; Pereira, M.V.; Fernández, P.; Prat, A.; et al. TMPRSS2-ERG in Blood and Docetaxel Resistance in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2016, 70, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Perez, M.P.; Perez-Navarro, E.; Alonso-Gordoa, T.; Conteduca, V.; Font, A.; Vázquez-Estévez, S.; González-Del-Alba, A.; Wetterskog, D.; Antonarakis, E.S.; Mellado, B.; et al. A correlative biomarker study and integrative prognostic model in chemotherapy-naïve metastatic castration-resistant prostate cancer treated with enzalutamide. Prostate 2023, 83, 376–384. [Google Scholar] [CrossRef]
- Lorenzin, F.; Demichelis, F. Past, Current, and Future Strategies to Target ERG Fusion-Positive Prostate Cancer. Cancers 2022, 14, 1118. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-X.; Wei, B.-F.; Zhang, X.; Gong, Y.-P.; Ma, L.-Y.; Zhao, W. Current development of CBP/p300 inhibitors in the last decade. Eur. J. Med. Chem. 2021, 209, 112861. [Google Scholar] [CrossRef] [PubMed]
- Ogryzko, V.V.; Schiltz, R.; Russanova, V.; Howard, B.H.; Nakatani, Y. The Transcriptional Coactivators p300 and CBP Are Histone Acetyltransferases. Cell 1996, 87, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Shiama, N. The p300/CBP family: Integrating signals with transcription factors and chromatin. Trends Cell Biol. 1997, 7, 230–236. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Wu, Y.-S.; Hung, C.-Y.; Wang, S.-A.; Young, M.-J.; Hsu, T.-I.; Hung, J.-J. USP24 induces IL-6 in tumor-associated microenvironment by stabilizing p300 and β-TrCP and promotes cancer malignancy. Nat. Commun. 2018, 9, 3996. [Google Scholar] [CrossRef]
- Weinert, B.T.; Narita, T.; Satpathy, S.; Srinivasan, B.; Hansen, B.K.; Schölz, C.; Hamilton, W.B.; Zucconi, B.E.; Wang, W.W.; Liu, W.R.; et al. Time-Resolved Analysis Reveals Rapid Dynamics and Broad Scope of the CBP/p300 Acetylome. Cell 2018, 174, 231–244.e12. [Google Scholar] [CrossRef]
- Raisner, R.; Kharbanda, S.; Jin, L.; Jeng, E.; Chan, E.; Merchant, M.; Haverty, P.M.; Bainer, R.; Cheung, T.; Arnott, D.; et al. Enhancer Activity Requires CBP/P300 Bromodomain-Dependent Histone H3K27 Acetylation. Cell Rep. 2018, 24, 1722–1729. [Google Scholar] [CrossRef]
- Narita, T.; Ito, S.; Higashijima, Y.; Chu, W.K.; Neumann, K.; Walter, J.; Satpathy, S.; Liebner, T.; Hamilton, W.B.; Maskey, E.; et al. Enhancers are activated by p300/CBP activity-dependent PIC assembly, RNAPII recruitment, and pause release. Mol. Cell 2021, 81, 2166–2182.e6. [Google Scholar] [CrossRef]
- Wu, T.; Kamikawa, Y.F.; Donohoe, M.E. Brd4’s Bromodomains Mediate Histone H3 Acetylation and Chromatin Remodeling in Pluripotent Cells through P300 and Brg1. Cell Rep. 2018, 25, 1756–1771. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Lan, Y.; Li, C.Y.; Sidoli, S.; Donahue, G.; Dou, Z.; Frederick, B.; Chen, Q.; Luense, L.J.; Garcia, B.A.; et al. Histone Acetyltransferase p300 Induces De Novo Super-Enhancers to Drive Cellular Senescence. Mol. Cell 2019, 73, 684–698.e8. [Google Scholar] [CrossRef] [PubMed]
- Ferreon, J.C.; Lee, C.W.; Arai, M.; Martinez-Yamout, M.A.; Dyson, H.J.; Wright, P.E. Cooperative regulation of p53 by modulation of ternary complex formation with CBP/p300 and HDM2. Proc. Natl. Acad. Sci. USA 2009, 106, 6591–6596. [Google Scholar] [CrossRef] [PubMed]
- Ruas, J.L.; Poellinger, L.; Pereira, T. Role of CBP in regulating HIF-1-mediated activation of transcription. J. Cell Sci. 2005, 118, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Zor, T.; De Guzman, R.N.; Dyson, H.; Wright, P.E. Solution Structure of the KIX Domain of CBP Bound to the Transactivation Domain of c-Myb. J. Mol. Biol. 2004, 337, 521–534. [Google Scholar] [CrossRef]
- Ortega, E.; Rengachari, S.; Ibrahim, Z.; Hoghoughi, N.; Gaucher, J.; Holehouse, A.S.; Khochbin, S.; Panne, D. Transcription factor dimerization activates the p300 acetyltransferase. Nature 2018, 562, 538–544. [Google Scholar] [CrossRef]
- Wojciak, J.M.; A Martinez-Yamout, M.; Dyson, H.J.; E Wright, P. Structural basis for recruitment of CBP/p300 coactivators by STAT1 and STAT2 transactivation domains. EMBO J. 2009, 28, 948–958. [Google Scholar] [CrossRef]
- Vervoorts, J.; Lüscher-Firzlaff, J.M.; Rottmann, S.; Lilischkis, R.; Walsemann, G.; Dohmann, K.; Austen, M.; Lüscher, B. Stimulation of c-MYC transcriptional activity and acetylation by recruitment of the cofactor CBP. EMBO Rep. 2003, 4, 484–490. [Google Scholar] [CrossRef]
- Boyes, J.; Byfield, P.; Nakatani, Y.; Ogryzko, V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature 1998, 396, 594–598. [Google Scholar] [CrossRef]
- Kalkhoven, E. CBP and p300: HATs for different occasions. Biochem. Pharmacol. 2004, 68, 1145–1155. [Google Scholar] [CrossRef]
- Medrano-Fernández, A.; Delgado-Garcia, J.M.; del Blanco, B.; Llinares, M.; Sánchez-Campusano, R.; Olivares, R.; Gruart, A.; Barco, A. The Epigenetic Factor CBP Is Required for the Differentiation and Function of Medial Ganglionic Eminence-Derived Interneurons. Mol. Neurobiol. 2019, 56, 4440–4454. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.G.; Özdag, H.; Caldas, C. p300/CBP and cancer. Oncogene 2004, 23, 4225–4231. [Google Scholar] [CrossRef] [PubMed]
- Yee, S.-P.; Branton, P.E. Detection of cellular proteins associated with human adenovirus type 5 early region 1A polypeptides. Virology 1985, 147, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Eckner, R.; E Ewen, M.; Newsome, D.; Gerdes, M.; A DeCaprio, J.; Lawrence, J.B.; Livingston, D.M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994, 8, 869–884. [Google Scholar] [CrossRef]
- Chrivia, J.C.; Kwok, R.P.S.; Lamb, N.; Hagiwara, M.; Montminy, M.R.; Goodman, R.H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 1993, 365, 855–859. [Google Scholar] [CrossRef]
- Stein, R.W.; Corrigan, M.; Yaciuk, P.; Whelan, J.; Moran, E. Analysis of E1A-mediated growth regulation functions: Binding of the 300-kilodalton cellular product correlates with E1A enhancer repression function and DNA synthesis-inducing activity. J. Virol. 1990, 64, 4421–4427. [Google Scholar] [CrossRef]
- Kwok, R.P.S.; Lundblad, J.R.; Chrivia, J.C.; Richards, J.P.; Bächinger, H.P.; Brennan, R.G.; Roberts, S.G.E.; Green, M.R.; Goodman, R.H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature 1994, 370, 223–226. [Google Scholar] [CrossRef]
- Das, C.; Lucia, M.S.; Hansen, K.C.; Tyler, J.K. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature 2009, 459, 113–117. [Google Scholar] [CrossRef]
- Black, J.C.; Choi, J.E.; Lombardo, S.R.; Carey, M. A Mechanism for Coordinating Chromatin Modification and Preinitiation Complex Assembly. Mol Cell 2006, 23, 809–818. [Google Scholar] [CrossRef]
- Sheikh, B.N.; Akhtar, A. The many lives of KATs—Detectors, integrators and modulators of the cellular environment. Nat. Rev. Genet. 2019, 20, 7–23. [Google Scholar] [CrossRef]
- Goodman, R.H.; Smolik, S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000, 14, 1553–1577. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wang, K.; Zhao, Y.; Ma, Q.; Chen, Z.; Huang, W. Effects of the Acetyltransferase p300 on Tumour Regulation from the Novel Perspective of Posttranslational Protein Modification. Biomolecules 2023, 13, 417. [Google Scholar] [CrossRef] [PubMed]
- Kemper, J.K.; Xiao, Z.; Ponugoti, B.; Miao, J.; Fang, S.; Kanamaluru, D.; Tsang, S.; Wu, S.-Y.; Chiang, C.-M.; Veenstra, T.D. FXR Acetylation Is Normally Dynamically Regulated by p300 and SIRT1 but Constitutively Elevated in Metabolic Disease States. Cell Metab. 2009, 10, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.H.; Sze, S.K.; Tay, A.S.L.; Lin, V.C.-L. Acetylation at Lysine 183 of Progesterone Receptor by p300 Accelerates DNA Binding Kinetics and Transactivation of Direct Target Genes. J. Biol. Chem. 2014, 289, 2180–2194. [Google Scholar] [CrossRef]
- Anyetei-Anum, C.; Evans, R.M.; Back, A.M.; Roggero, V.R.; Allison, L.A. Acetylation modulates thyroid hormone receptor intracellular localization and intranuclear mobility. Mol. Cell Endocrinol. 2019, 495, 110509. [Google Scholar] [CrossRef]
- Wang, C.; Fu, M.; Angeletti, R.H.; Siconolfi-Baez, L.; Reutens, A.T.; Albanese, C.; Lisanti, M.P.; Katzenellenbogen, B.S.; Kato, S.; Hopp, T.; et al. Direct Acetylation of the Estrogen Receptor α Hinge Region by p300 Regulates Transactivation and Hormone Sensitivity. J. Biol. Chem. 2001, 276, 18375–18383. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Y.; Juan, L.-J.; Chung, B. SF-1 (Nuclear Receptor 5A1) Activity Is Activated by Cyclic AMP via p300-Mediated Recruitment to Active Foci, Acetylation, and Increased DNA Binding. Mol. Cell Biol. 2005, 25, 10442–10453. [Google Scholar] [CrossRef]
- Zhao, W.-X.; Tian, M.; Zhao, B.-X.; Li, G.-D.; Liu, B.; Zhan, Y.-Y.; Chen, H.-Z.; Wu, Q. Orphan Receptor TR3 Attenuates the p300-Induced Acetylation of Retinoid X Receptor-α. Mol. Endocrinol. 2007, 21, 2877–2889. [Google Scholar] [CrossRef]
- Waddell, A.R.; Huang, H.; Liao, D. CBP/p300: Critical Co-Activators for Nuclear Steroid Hormone Receptors and Emerging Therapeutic Targets in Prostate and Breast Cancers. Cancers 2021, 13, 2872. [Google Scholar] [CrossRef]
- Schneider, A.; Chatterjee, S.; Bousiges, O.; Selvi, B.R.; Swaminathan, A.; Cassel, R.; Blanc, F.; Kundu, T.K.; Boutillier, A.-L. Acetyltransferases (HATs) as Targets for Neurological Therapeutics. Neurotherapeutics 2013, 10, 568–588. [Google Scholar] [CrossRef]
- Li, P.; Ge, J.; Li, H. Lysine acetyltransferases and lysine deacetylases as targets for cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Hogg, S.J.; Motorna, O.; Cluse, L.A.; Johanson, T.M.; Coughlan, H.D.; Raviram, R.; Myers, R.M.; Costacurta, M.; Todorovski, I.; Pijpers, L.; et al. Targeting histone acetylation dynamics and oncogenic transcription by catalytic P300/CBP inhibition. Mol. Cell 2021, 81, 2183–2200.e13. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, R.; Li, Z.; Mei, L.; Wan, S.; Ding, H.; Chen, Z.; Xing, J.; Feng, H.; Han, J.; et al. Discovery of Highly Potent, Selective, and Orally Efficacious p300/CBP Histone Acetyltransferases Inhibitors. J. Med. Chem. 2020, 63, 1337–1360. [Google Scholar] [CrossRef]
- Jaiswal, B.; Agarwal, A.; Gupta, A. Lysine Acetyltransferases and Their Role in AR Signaling and Prostate Cancer. Front. Endocrinol. 2022, 13, 886594. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, M.; Gaucher, J.; Aguilar-Gurrieri, C.; Ortega, E.; Panne, D. Structure of the p300 catalytic core and implications for chromatin targeting and HAT regulation. Nat. Struct. Mol. Biol. 2013, 20, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.L.; Manning, E.T.; Kadonaga, J.T. Biochemical Analysis of Distinct Activation Functions in p300 That Enhance Transcription Initiation with Chromatin Templates. Mol. Cell Biol. 1999, 19, 8123–8135. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, Y.; Shi, J.; Ahn, J.; Mi, W.; Ali, M.; Wang, X.; Klein, B.J.; Wen, H.; Li, W.; et al. The ZZ domain of p300 mediates specificity of the adjacent HAT domain for histone H3. Nat. Struct. Mol. Biol. 2018, 25, 841–849. [Google Scholar] [CrossRef]
- Dyson, H.J.; Wright, P.E. Role of Intrinsic Protein Disorder in the Function and Interactions of the Transcriptional Coactivators CREB-binding Protein (CBP) and p300. J. Biol. Chem. 2016, 291, 6714–6722. [Google Scholar] [CrossRef]
- Chan, H.M.; La Thangue, N.B. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 2001, 114, 2363–2373. [Google Scholar] [CrossRef]
- Dancy, B.M.; Cole, P.A. Protein Lysine Acetylation by p300/CBP. Chem. Rev. 2015, 115, 2419–2452. [Google Scholar] [CrossRef]
- Narita, T.; Weinert, B.T.; Choudhary, C. Functions and mechanisms of non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 156–174. [Google Scholar] [CrossRef] [PubMed]
- Merika, M.; Williams, A.J.; Chen, G.; Collins, T.; Thanos, D. Recruitment of CBP/p300 by the IFNβ Enhanceosome Is Required for Synergistic Activation of Transcription. Mol. Cell 1998, 1, 277–287. [Google Scholar] [CrossRef]
- Kaypee, S.; Sahadevan, S.A.; Patil, S.; Ghosh, P.; Roy, N.S.; Roy, S.; Kundu, T.K. Mutant and Wild-Type Tumor Suppressor p53 Induces p300 Autoacetylation. iScience 2018, 4, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Sevinç, K.; Sevinç, G.G.; Cribbs, A.P.; Philpott, M.; Uyulur, F.; Morova, T.; Dunford, J.E.; Göklemez, S.; Arı, Ş.; et al. Bromodomain inhibition of the coactivators CBP/EP300 facilitate cellular reprogramming. Nat. Chem. Biol. 2019, 15, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Santoso, B.; Kadonaga, J.T. Reconstitution of chromatin transcription with purified components reveals a chromatin-specific repressive activity of p300. Nat. Struct. Mol. Biol. 2006, 13, 131–139. [Google Scholar] [CrossRef]
- Sankar, N.; Baluchamy, S.; Kadeppagari, R.-K.; Singhal, G.; Weitzman, S.; Thimmapaya, B. p300 provides a corepressor function by cooperating with YY1 and HDAC3 to repress c-Myc. Oncogene 2008, 27, 5717–5728. [Google Scholar] [CrossRef]
- Martire, S.; Nguyen, J.; Sundaresan, A.; Banaszynski, L.A. Differential contribution of p300 and CBP to regulatory element acetylation in mESCs. BMC Mol. Cell Biol. 2020, 21, 55. [Google Scholar] [CrossRef]
- Tsang, F.H.; Law, C.; Tang, T.C.; Cheng, C.L.; Chin, D.W.; Tam, W.V.; Wei, L.; Wong, C.C.; Ng, I.O.; Wong, C. Aberrant Super-Enhancer Landscape in Human Hepatocellular Carcinoma. Hepatology 2019, 69, 2502–2517. [Google Scholar] [CrossRef]
- Kim, E.; Zucconi, B.E.; Wu, M.; Nocco, S.E.; Meyers, D.J.; McGee, J.S.; Venkatesh, S.; Cohen, D.L.; Gonzalez, E.C.; Ryu, B.; et al. MITF Expression Predicts Therapeutic Vulnerability to p300 Inhibition in Human Melanoma. Cancer Res. 2019, 79, 2649–2661. [Google Scholar] [CrossRef]
- Bi, Y.; Kong, P.; Zhang, L.; Cui, H.; Xu, X.; Chang, F.; Yan, T.; Li, J.; Cheng, C.; Song, B.; et al. EP300 as an oncogene correlates with poor prognosis in esophageal squamous carcinoma. J. Cancer 2019, 10, 5413–5426. [Google Scholar] [CrossRef]
- Xiao, X.-S.; Cai, M.-Y.; Chen, J.-W.; Guan, X.-Y.; Kung, H.-F.; Zeng, Y.-X.; Xie, D. High expression of p300 in human breast cancer correlates with tumor recurrence and predicts adverse prognosis. Chin. J. Cancer Res. 2011, 23, 201–207. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Liu, C.; Li, X.; Zhang, B.; Wang, B.; Zhang, Y.; Song, C.; Zhang, T.; Liu, M.; et al. Acetylation of PHF5A Modulates Stress Responses and Colorectal Carcinogenesis through Alternative Splicing-Mediated Upregulation of KDM3A. Mol. Cell 2019, 74, 1250–1263.e6. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Tan, J.; Lim, K.J.; Koh, J.; Ooi, W.F.; Li, Z.; Huang, D.; Xing, M.; Chan, Y.S.; Qu, J.Z.; et al. VHL Deficiency Drives Enhancer Activation of Oncogenes in Clear Cell Renal Cell Carcinoma. Cancer Discov. 2017, 7, 1284–1305. [Google Scholar] [CrossRef] [PubMed]
- Diesch, J.; Le Pannérer, M.-M.; Winkler, R.; Casquero, R.; Muhar, M.; van der Garde, M.; Maher, M.; Herráez, C.M.; Bech-Serra, J.J.; Fellner, M.; et al. Inhibition of CBP synergizes with the RNA-dependent mechanisms of Azacitidine by limiting protein synthesis. Nat. Commun. 2021, 12, 6060. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Nagai, Y.; Deng, G.; Ohtani, T.; Zhu, Z.; Zhou, Z.; Zhang, H.; Ji, M.Q.; Lough, J.W.; Samanta, A.; et al. Dynamic Interactions between TIP60 and p300 Regulate FOXP3 Function through a Structural Switch Defined by a Single Lysine on TIP60. Cell Rep. 2014, 7, 1471–1480. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Predina, J.; Han, R.; Beier, U.H.; Wang, L.C.; Kapoor, V.; Bhatti, T.R.; Akimova, T.; Singhal, S.; et al. Inhibition of p300 impairs Foxp3+ T regulatory cell function and promotes antitumor immunity. Nat. Med. 2013, 19, 1173–1177. [Google Scholar] [CrossRef]
- de Almeida Nagata, D.E.; Chiang, E.Y.; Jhunjhunwala, S.; Caplazi, P.; Arumugam, V.; Modrusan, Z.; Chan, E.; Merchant, M.; Jin, L.; Arnott, D.; et al. Regulation of Tumor-Associated Myeloid Cell Activity by CBP/EP300 Bromodomain Modulation of H3K27 Acetylation. Cell Rep. 2019, 27, 269–281.e4. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Han, R.; Beier, U.H.; Akimova, T.; Bhatti, T.; Xiao, H.; Cole, P.A.; Brindle, P.K.; Hancock, W.W. Two Histone/Protein Acetyltransferases, CBP and p300, Are Indispensable for Foxp3 + T-Regulatory Cell Development and Function. Mol. Cell Biol. 2014, 34, 3993–4007. [Google Scholar] [CrossRef]
- Ianculescu, I.; Wu, D.-Y.; Siegmund, K.D.; Stallcup, M.R. Selective Roles for cAMP Response Element-binding Protein Binding Protein and p300 Protein as Coregulators for Androgen-regulated Gene Expression in Advanced Prostate Cancer Cells. J. Biol. Chem. 2012, 287, 4000–4013. [Google Scholar] [CrossRef]
- Abraham, S.E.; Lobo, S.; Yaciuk, P.; Wang, H.G.; Moran, E. p300, and p300-associated proteins, are components of TATA-binding protein (TBP) complexes. Oncogene 1993, 8, 1639–1647. [Google Scholar]
- Sawant, M.; Mahajan, K.; Renganathan, A.; Weimholt, C.; Luo, J.; Kukshal, V.; Jez, J.M.; Jeon, M.S.; Zhang, B.; Li, T.; et al. Chronologically modified androgen receptor in recurrent castration-resistant prostate cancer and its therapeutic targeting. Sci. Transl. Med. 2022, 14, eabg4132. [Google Scholar] [CrossRef]
- Welti, J.; Sharp, A.; Brooks, N.; Yuan, W.; McNair, C.; Chand, S.N.; Pal, A.; Figueiredo, I.; Riisnaes, R.; Gurel, B.; et al. Targeting the p300/CBP Axis in Lethal Prostate Cancer. Cancer Discov. 2021, 11, 1118–1137. [Google Scholar] [CrossRef]
- Heemers, H.V.; Debes, J.D.; Tindall, D.J. The Role of the Transcriptional Coactivator p300 in Prostate Cancer Progression. Adv. Exp. Med. Biol. 2008, 617, 535–540. [Google Scholar] [CrossRef]
- Gruber, M.; Ferrone, L.; Puhr, M.; Santer, F.R.; Furlan, T.; E Eder, I.; Sampson, N.; Schäfer, G.; Handle, F.; Culig, Z. p300 is upregulated by docetaxel and is a target in chemoresistant prostate cancer. Endocr. Relat. Cancer 2020, 27, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sawyers, C.L.; I Scher, H. Targeting the androgen receptor pathway in prostate cancer. Curr. Opin. Pharmacol. 2008, 8, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Aurilio, G.; Cimadamore, A.; Mazzucchelli, R.; Lopez-Beltran, A.; Verri, E.; Scarpelli, M.; Massari, F.; Cheng, L.; Santoni, M.; Montironi, R. Androgen Receptor Signaling Pathway in Prostate Cancer: From Genetics to Clinical Applications. Cells 2020, 9, 2653. [Google Scholar] [CrossRef] [PubMed]
- Michmerhuizen, A.R.; Spratt, D.E.; Pierce, L.J.; Speers, C.W. ARe we there yet? Understanding androgen receptor signaling in breast cancer. NPJ Breast Cancer 2020, 6, 47. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, B.; Liu, X.; Zhang, X.D.; Zhang, L.; Liu, T. Histone acetyltransferases CBP/p300 in tumorigenesis and CBP/p300 inhibitors as promising novel anticancer agents. Theranostics 2022, 12, 4935–4948. [Google Scholar] [CrossRef]
- Eickhoff, N.; Bergman, A.M.; Zwart, W. Homing in on a Moving Target: Androgen Receptor Cistromic Plasticity in Prostate Cancer. Endocrinology 2022, 163, bqac153. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Gordon, M.S.; Reimers, M.A.; Sedkov, A.; Lipford, K.; Snavely-Merhaut, J.; Kumar, S.; Guichard, S.M.; Shore, N. The Courage study: A first-in-human phase 1 study of the CBP/p300 inhibitor FT-7051 in men with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2021, 39, TPS5085. [Google Scholar] [CrossRef]
- Chen, Z.; Song, X.; Li, Q.; Xie, L.; Guo, T.; Su, T.; Tang, C.; Chang, X.; Liang, B.; Huang, D. Androgen Receptor-Activated Enhancers Simultaneously Regulate Oncogene TMPRSS2 and lncRNA PRCAT38 in Prostate Cancer. Cells 2019, 8, 864. [Google Scholar] [CrossRef] [PubMed]
| Clinical trial | Phase (Participants) | Disease | Drug | Primary Outcome Measures | Secondary Outcome Measures |
|---|---|---|---|---|---|
| NCT04575766 | I (25) | Metastatic Castration-Resistant Prostate Cancer | FT-7051 | DLTs AEs Clinical laboratory abnormalities | PSA response rTTP ORR CRR AUC Cmax Tmax T1/2 |
| NCT03568656 | I/IIa (350) | Metastatic Castration-Resistant Prostate Cancer | CCS1477 ± abiraterone acetate OR ± enzalutamide | Treatment-related AEs Laboratory assessments | PSA response ORR rPFS Cmax AUC |
| NCT05488548 | I (50) | Castration-Resistant Prostate Cancer | EP31670 | MTD DLTs RP2D | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gioukaki, C.; Georgiou, A.; Gkaralea, L.E.; Kroupis, C.; Lazaris, A.C.; Alamanis, C.; Thomopoulou, G.E. Unravelling the Role of P300 and TMPRSS2 in Prostate Cancer: A Literature Review. Int. J. Mol. Sci. 2023, 24, 11299. https://doi.org/10.3390/ijms241411299
Gioukaki C, Georgiou A, Gkaralea LE, Kroupis C, Lazaris AC, Alamanis C, Thomopoulou GE. Unravelling the Role of P300 and TMPRSS2 in Prostate Cancer: A Literature Review. International Journal of Molecular Sciences. 2023; 24(14):11299. https://doi.org/10.3390/ijms241411299
Chicago/Turabian StyleGioukaki, Charitomeni, Alexandros Georgiou, Lydia Evangelia Gkaralea, Christos Kroupis, Andreas C. Lazaris, Christos Alamanis, and Georgia Eleni Thomopoulou. 2023. "Unravelling the Role of P300 and TMPRSS2 in Prostate Cancer: A Literature Review" International Journal of Molecular Sciences 24, no. 14: 11299. https://doi.org/10.3390/ijms241411299
APA StyleGioukaki, C., Georgiou, A., Gkaralea, L. E., Kroupis, C., Lazaris, A. C., Alamanis, C., & Thomopoulou, G. E. (2023). Unravelling the Role of P300 and TMPRSS2 in Prostate Cancer: A Literature Review. International Journal of Molecular Sciences, 24(14), 11299. https://doi.org/10.3390/ijms241411299







