Depression among Patients with an Implanted Left Ventricular Assist Device: Uncovering Pathophysiological Mechanisms and Implications for Patient Care
Abstract
1. Depression—Background and Treatment
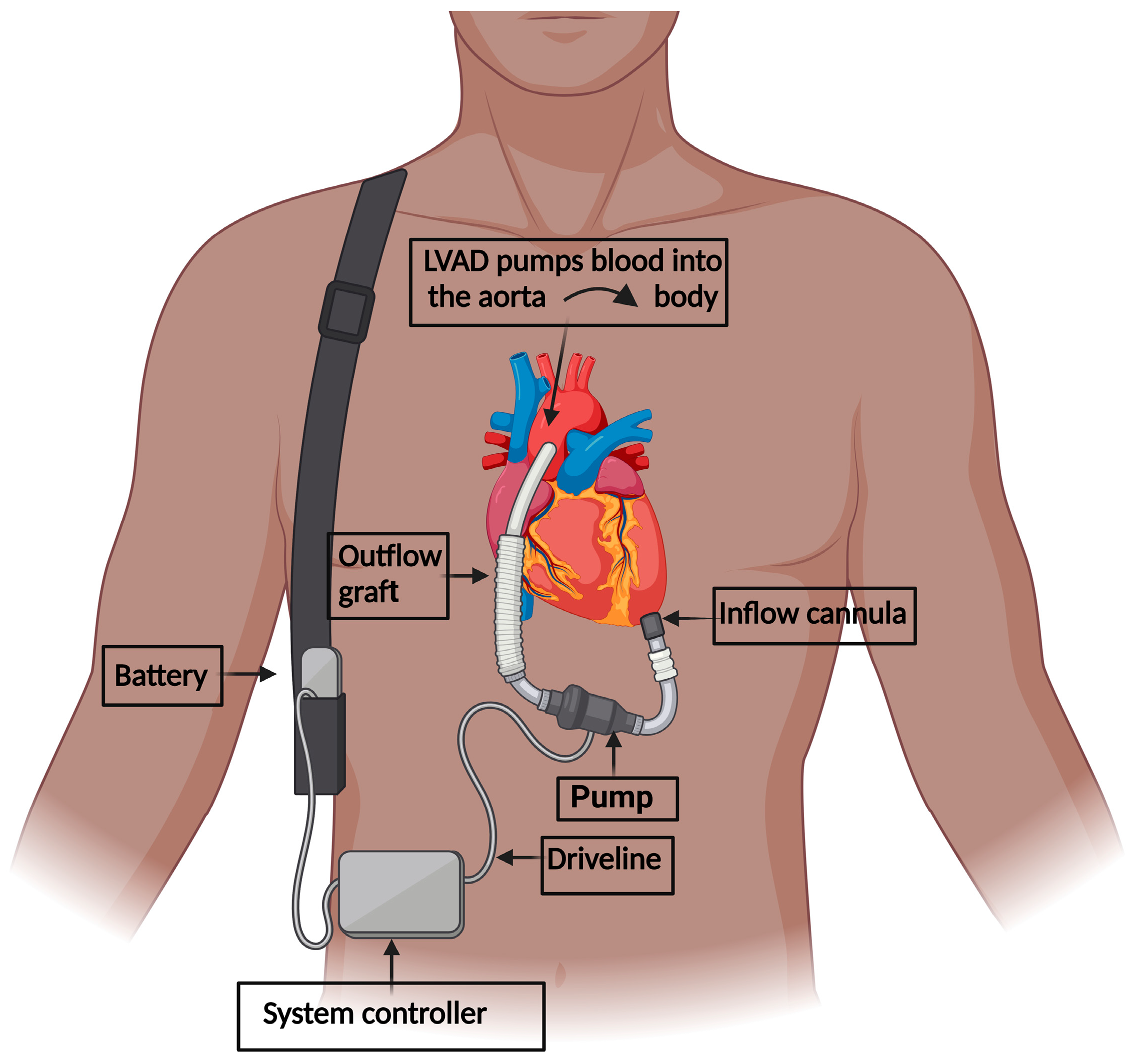
2. LVAD Implantation in Heart Failure
3. Depression among LVAD-Implanted Patients
3.1. Current Evidence of Depression among LVAD-Implanted Patients
3.2. Prognostic Implications
3.3. Suicidal Ideation
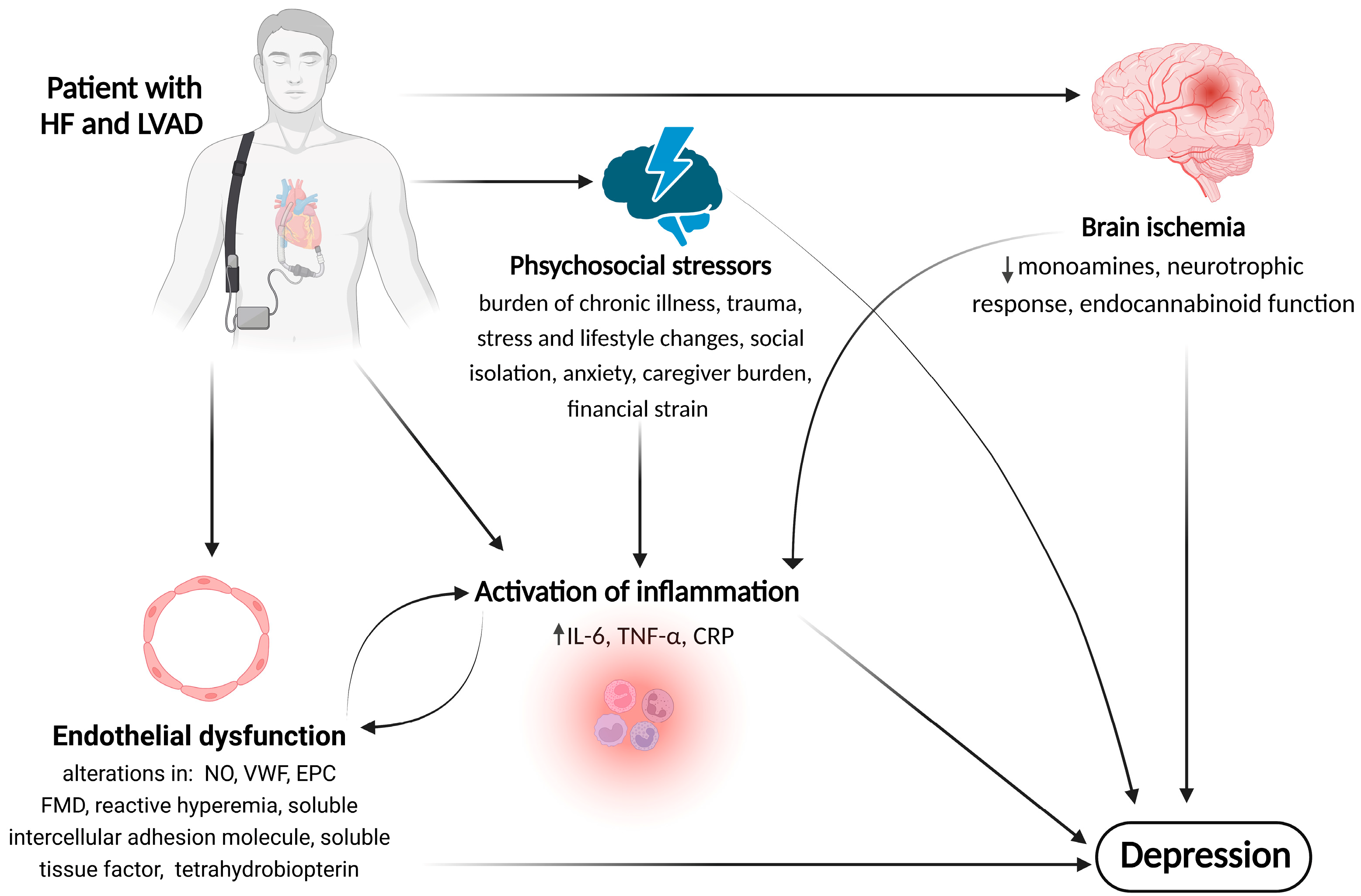
4. Possible Mechanisms and Pathophysiology of Depression in LVAD-Supported Patients
4.1. Psychosocial Factors
4.2. The Role of Inflammation
4.3. The Role of Endothelial Dysfunction (ED)
4.4. The Role of Brain Ischemia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, L.T.; Zarate, C.A. Clinical Practice Depression in the Primary Care Setting. N. Engl. J. Med. 2019, 380, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Jermann, F.; Perroud, N.; Favre, S.; Aubry, J.M.; Richard-Lepouriel, H. Quality of Life and Subjective Sleep-Related Measures in Bipolar Disorder and Major Depressive Disorder. Qual. Life Res. 2022, 31, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Atique-Ur-Rehman, H.; Neill, J.C. Cognitive Dysfunction in Major Depression: From Assessment to Novel Therapies. Pharmacol. Ther. 2019, 202, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijsen, K.; Verbeek, J.H.; Neumeyer-Gromen, A.; Verhoeven, A.C.; Bültmann, U.; Faber, B. Interventions to Improve Return to Work in Depressed People. Cochrane Database Syst. Rev. 2020, 10, CD006237. [Google Scholar] [CrossRef]
- König, H.; König, H.H.; Konnopka, A. The Excess Costs of Depression: A Systematic Review and Meta-Analysis. Epidemiol. Psychiatr. Sci. 2020, 29, E30. [Google Scholar] [CrossRef]
- Hughes, K.; Ford, K.; Bellis, M.A.; Glendinning, F.; Harrison, E.; Passmore, J. Health and Financial Costs of Adverse Childhood Experiences in 28 European Countries: A Systematic Review and Meta-Analysis. Lancet Public Health 2021, 6, e848–e857. [Google Scholar] [CrossRef]
- Kennis, M.; Gerritsen, L.; van Dalen, M.; Williams, A.; Cuijpers, P.; Bockting, C. Prospective Biomarkers of Major Depressive Disorder: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2020, 25, 321–338. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The Serotonin Theory of Depression: A Systematic Umbrella Review of the Evidence. Mol. Psychiatry, 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Köhler, C.A.; Evangelou, E.; Stubbs, B.; Solmi, M.; Veronese, N.; Belbasis, L.; Bortolato, B.; Melo, M.C.A.; Coelho, C.A.; Fernandes, B.S.; et al. Mapping Risk Factors for Depression across the Lifespan: An Umbrella Review of Evidence from Meta-Analyses and Mendelian Randomization Studies. J. Psychiatr. Res. 2018, 103, 189–207. [Google Scholar] [CrossRef]
- Chen, C.; Meier, S.T. Burnout and Depression in Nurses: A Systematic Review and Meta-Analysis. Int. J. Nurs. Stud. 2021, 124, 104099. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Clarke, T.K.; Hafferty, J.D.; Gibson, J.; Shirali, M.; Coleman, J.R.I.; Hagenaars, S.P.; Ward, J.; Wigmore, E.M.; et al. Genome-Wide Meta-Analysis of Depression Identifies 102 Independent Variants and Highlights the Importance of the Prefrontal Brain Regions. Nat. Neurosci. 2019, 22, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.R.I.; Gaspar, H.A.; Bryois, J.; Byrne, E.M.; Forstner, A.J.; Holmans, P.A.; de Leeuw, C.A.; Mattheisen, M.; McQuillin, A.; Whitehead Pavlides, J.M.; et al. The Genetics of the Mood Disorder Spectrum: Genome-Wide Association Analyses of over 185,000 Cases and 439,000 Controls. Biol. Psychiatry 2020, 88, 169. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Tian, C.; Hinds, D.; Agee, M.; Alipanahi, B.; Auton, A.; Bell, R.K.; Bryc, K.; Elson, S.L.; Fontanillas, P.; et al. Genome-Wide Association Studies of Antidepressant Class Response and Treatment-Resistant Depression. Transl. Psychiatry 2020, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Haeusler, D.; Kraus, C.; Höflich, A.S.; Kranz, G.S.; Baldinger, P.; Savli, M.; Mitterhauser, M.; Wadsak, W.; Karanikas, G.; et al. Attenuated Serotonin Transporter Association between Dorsal Raphe and Ventral Striatum in Major Depression. Hum. Brain Mapp. 2014, 35, 3857–3866. [Google Scholar] [CrossRef]
- Harmer, C.J.; Duman, R.S.; Cowen, P.J. How Do Antidepressants Work? New Perspectives for Refining Future Treatment Approaches. Lancet Psychiatry 2017, 4, 409–418. [Google Scholar] [CrossRef]
- Yohn, C.N.; Gergues, M.M.; Samuels, B.A. The Role of 5-HT Receptors in Depression. Mol. Brain 2017, 10, 28. [Google Scholar] [CrossRef]
- Yuan, N.; Chen, Y.; Xia, Y.; Dai, J.; Liu, C. Inflammation-Related Biomarkers in Major Psychiatric Disorders: A Cross-Disorder Assessment of Reproducibility and Specificity in 43 Meta-Analyses. Transl. Psychiatry 2019, 9, 233. [Google Scholar] [CrossRef]
- Kappelmann, N.; Lewis, G.; Dantzer, R.; Jones, P.B.; Khandaker, G.M. Antidepressant Activity of Anti-Cytokine Treatment: A Systematic Review and Meta-Analysis of Clinical Trials of Chronic Inflammatory Conditions. Mol. Psychiatry 2018, 23, 335–343. [Google Scholar] [CrossRef]
- Scaini, G.; Mason, B.L.; Diaz, A.P.; Jha, M.K.; Soares, J.C.; Trivedi, M.H.; Quevedo, J. Dysregulation of Mitochondrial Dynamics, Mitophagy and Apoptosis in Major Depressive Disorder: Does Inflammation Play a Role? Mol. Psychiatry 2022, 27, 1095–1102. [Google Scholar] [CrossRef]
- Bi, Y.; Ren, D.; Guo, Z.; Ma, G.; Xu, F.; Chen, Z.; An, L.; Zhang, N.; Ji, L.; Yuan, F.; et al. Influence and Interaction of Genetic, Cognitive, Neuroendocrine and Personalistic Markers to Antidepressant Response in Chinese Patients with Major Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 104, 110036. [Google Scholar] [CrossRef]
- Khoodoruth, M.A.S.; Estudillo-Guerra, M.A.; Pacheco-Barrios, K.; Nyundo, A.; Chapa-Koloffon, G.; Ouanes, S. Glutamatergic System in Depression and Its Role in Neuromodulatory Techniques Optimization. Front. Psychiatry 2022, 13, 886918. [Google Scholar] [CrossRef]
- Tartt, A.N.; Mariani, M.B.; Hen, R.; Mann, J.J.; Boldrini, M. Dysregulation of Adult Hippocampal Neuroplasticity in Major Depression: Pathogenesis and Therapeutic Implications. Mol. Psychiatry 2022, 27, 2689–2699. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, O. Investigational Drugs for the Treatment of Depression (Part 1): Monoaminergic, Orexinergic, GABA-Ergic, and Anti-Inflammatory Agents. Front. Pharmacol. 2022, 13, 884143. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, V.; Caterino, A.L.; Bianco, F.; Caputi, C.G.; Salerni, S.; Sciomer, S.; Maffei, S.; Gallina, S. Depression and Cardiovascular Disease: The Deep Blue Sea of Women’s Heart. Trends Cardiovasc. Med. 2020, 30, 170–176. [Google Scholar] [CrossRef]
- Liblik, K.; Mulvagh, S.L.; Hindmarch, C.C.T.; Alavi, N.; Johri, A.M. Depression and Anxiety Following Acute Myocardial Infarction in Women. Trends Cardiovasc. Med. 2022, 32, 341–347. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Y.; Luo, B.; Cui, J.; Liu, Y.; Liu, Y. Comorbidity of Type 2 Diabetes Mellitus and Depression: Clinical Evidence and Rationale for the Exacerbation of Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 9, 861110. [Google Scholar] [CrossRef]
- Rao, W.-W.; Zong, Q.-Q.; Zhang, J.-W.; An, F.-R.; Jackson, T.; Ungvari, G.S.; Xiang, Y.; Su, Y.-Y.; D’Arcy, C.; Xiang, Y.-T. Obesity Increases the Risk of Depression in Children and Adolescents: Results from a Systematic Review and Meta-Analysis. J. Affect. Disord. 2020, 267, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.J.; Hall, N.; Hudson, J.L.; Farrington, K.; Tucker, M.J.R.; Wellsted, D.; Jones, J.; Sharma, S.; Norton, S.; Ormandy, P.; et al. Approaches to the Identification and Management of Depression in People Living with Chronic Kidney Disease: A Scoping Review of 860 Papers. J. Ren. Care, 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Sun, W.; Liu, X. The Advances of Post-Stroke Depression: 2021 Update. J. Neurol. 2022, 269, 1236–1249. [Google Scholar] [CrossRef]
- Frank, D.; Gruenbaum, B.F.; Zlotnik, A.; Semyonov, M.; Frenkel, A.; Boyko, M. Pathophysiology and Current Drug Treatments for Post-Stroke Depression: A Review. Int. J. Mol. Sci. 2022, 23, 15114. [Google Scholar] [CrossRef]
- Botto, R.; Callai, N.; Cermelli, A.; Causarano, L.; Rainero, I. Anxiety and Depression in Alzheimer’s Disease: A Systematic Review of Pathogenetic Mechanisms and Relation to Cognitive Decline. Neurol. Sci. 2022, 43, 4107–4124. [Google Scholar] [CrossRef]
- Prange, S.; Klinger, H.; Laurencin, C.; Danaila, T.; Thobois, S. Depression in Patients with Parkinson’s Disease: Current Understanding of Its Neurobiology and Implications for Treatment. Drugs Aging 2022, 39, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Ikuta, T.; Sakuma, K.; Okuya, M.; Hatano, M.; Matsuda, Y.; Iwata, N. Antidepressants for the Treatment of Adults with Major Depressive Disorder in the Maintenance Phase: A Systematic Review and Network Meta-Analysis. Mol Psychiatry 2023, 28, 402–409. [Google Scholar] [CrossRef]
- Espinoza, R.T.; Kellner, C.H. Electroconvulsive Therapy. N. Engl. J. Med. 2022, 386, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. Available online: https://shop.lww.com/Kaplan-and-Sadock-s-Comprehensive-Textbook-of-Psychiatry/p/9781451100471 (accessed on 29 August 2022).
- Barth, J.; Munder, T.; Gerger, H.; Nüesch, E.; Trelle, S.; Znoj, H.; Jüni, P.; Cuijpers, P. Comparative Efficacy of Seven Psychotherapeutic Interventions for Patients with Depression: A Network Meta-Analysis. PLoS Med. 2013, 10, e1001454. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cui, L.; Li, J.; Liu, Y.; Chen, Y. Comparative Efficacy and Acceptability of Neuromodulation Procedures in the Treatment of Treatment-Resistant Depression: A Network Meta-Analysis of Randomized Controlled Trials. J. Affect. Disord. 2021, 287, 115–124. [Google Scholar] [CrossRef]
- Kato, M.; Hori, H.; Inoue, T.; Iga, J.; Iwata, M.; Inagaki, T.; Shinohara, K.; Imai, H.; Murata, A.; Mishima, K.; et al. Discontinuation of Antidepressants after Remission with Antidepressant Medication in Major Depressive Disorder: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2021, 26, 118–133. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults with Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Lewis, G.; Marston, L.; Duffy, L.; Freemantle, N.; Gilbody, S.; Hunter, R.; Kendrick, T.; Kessler, D.; Mangin, D.; King, M.; et al. Maintenance or Discontinuation of Antidepressants in Primary Care. N. Engl. J. Med. 2021, 385, 1257–1267. [Google Scholar] [CrossRef]
- Henssler, J.; Alexander, D.; Schwarzer, G.; Bschor, T.; Baethge, C. Combining Antidepressants vs Antidepressant Monotherapy for Treatment of Patients with Acute Depression: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2022, 79, 300–312. [Google Scholar] [CrossRef]
- Murphy, S.E.; Capitão, L.P.; Giles, S.L.C.; Cowen, P.J.; Stringaris, A.; Harmer, C.J. The Knowns and Unknowns of SSRI Treatment in Young People with Depression and Anxiety: Efficacy, Predictors, and Mechanisms of Action. Lancet Psychiatry 2021, 8, 824–835. [Google Scholar] [CrossRef]
- Bahji, A.; Ermacora, D.; Stephenson, C.; Hawken, E.R.; Vazquez, G. Comparative Efficacy and Tolerability of Pharmacological Treatments for the Treatment of Acute Bipolar Depression: A Systematic Review and Network Meta-Analysis. J. Affect. Disord. 2020, 269, 154–184. [Google Scholar] [CrossRef]
- Johnson, D.A.W. Depression: Treatment Compliance in General Practice. Acta Psychiatr. Scand. 1981, 63, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Blease, C.R.; O’neill, S.; Walker, J.; Hägglund, M.; Torous, J. Treatment Outcomes for Depression: Challenges and Opportunities. Lancet Psychiatry 2020, 7, 925–927. [Google Scholar] [CrossRef]
- Maslej, M.M.; Furukawa, T.A.; Cipriani, A.; Andrews, P.W.; Sanches, M.; Tomlinson, A.; Volkmann, C.; McCutcheon, R.A.; Howes, O.; Guo, X.; et al. Individual Differences in Response to Antidepressants: A Meta-Analysis of Placebo-Controlled Randomized Clinical Trials. JAMA Psychiatry 2021, 78, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Canuso, C.M.; Singh, J.B.; Fedgchin, M.; Alphs, L.; Lane, R.; Lim, P.; Pinter, C.; Hough, D.; Sanacora, G.; Manji, H.; et al. Efficacy and Safety of Intranasal Esketamine for the Rapid Reduction of Symptoms of Depression and Suicidality in Patients at Imminent Risk for Suicide: Results of a Double-Blind, Randomized, Placebo-Controlled Study. Am. J. Psychiatry 2018, 175, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.L.; Norris, S.; Talbot, J.; Birmingham, M.; Hatchard, T.; Ortiz, A.; Owoeye, O.; Batten, L.A.; Blier, P. Single, Repeated, and Maintenance Ketamine Infusions for Treatment-Resistant Depression: A Randomized Controlled Trial. Am. J. Psychiatry 2019, 176, 401–409. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Gukasyan, N.; Davis, A.K.; Barrett, F.S.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Griffiths, R.R. Efficacy and Safety of Psilocybin-Assisted Treatment for Major Depressive Disorder: Prospective 12-Month Follow-Up. J. Psychopharmacol. 2022, 36, 151–158. [Google Scholar] [CrossRef]
- Wang, W.L.; Wang, S.Y.; Hung, H.Y.; Chen, M.H.; Juan, C.H.; Li, C.T. Safety of Transcranial Magnetic Stimulation in Unipolar Depression: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. J. Affect. Disord. 2022, 301, 400–425. [Google Scholar] [CrossRef]
- Wu, C.; Liu, P.; Fu, H.; Chen, W.; Cui, S.; Lu, L.; Tang, C. Transcutaneous Auricular Vagus Nerve Stimulation in Treating Major Depressive Disorder A Systematic Review and Meta-Analysis. Medicine 2018, 97, e13845. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report from the American Heart Association. Circulation 2023, 147, E93–E621. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the Impact of Heart Failure in the United States. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Colvin, M.; Smith, J.M.; Hadley, N.; Skeans, M.A.; Uccellini, K.; Goff, R.; Foutz, J.; Israni, A.K.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2018 Annual Data Report: Heart. Am. J. Transplant. 2020, 20, 340–426. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.J.; Naka, Y.; Horstmanshof, D.; Ravichandran, A.K.; Schroder, J.; Ransom, J.; Itoh, A.; Uriel, N.; Cleveland, J.C.; Raval, N.Y.; et al. Association of Clinical Outcomes with Left Ventricular Assist Device Use by Bridge to Transplant or Destination Therapy Intent. JAMA Cardiol. 2020, 5, 411. [Google Scholar] [CrossRef] [PubMed]
- Teuteberg, J.J.; Cleveland, J.C.; Cowger, J.; Higgins, R.S.; Goldstein, D.J.; Keebler, M.; Kirklin, J.K.; Myers, S.L.; Salerno, C.T.; Stehlik, J.; et al. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications. Ann. Thorac. Surg. 2020, 109, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.J.; Shah, P.; Kiernan, M.S.; Cornwell, W.K.; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann. Thorac. Surg. 2021, 111, 778–792. [Google Scholar] [CrossRef]
- Lundgren, S.; Poon, C.Y.M.; Selim, A.; Lowes, B.D.; Zolty, R.; Burdorf, A.; Potashnik-Peled, Y.; Moulton, M.J.; Um, J.Y.; Raichlin, E. Depression and Anxiety in Patients Undergoing Left Ventricular Assist Device Implantation. Int. J. Artif. Organs 2017, 41, 76–83. [Google Scholar] [CrossRef]
- Rutledge, T.; Reis, V.A.; Linke, S.E.; Greenberg, B.H.; Mills, P.J. Depression in Heart Failure. A Meta-Analytic Review of Prevalence, Intervention Effects, and Associations with Clinical Outcomes. J. Am. Coll. Cardiol. 2006, 48, 1527–1537. [Google Scholar] [CrossRef]
- Sladen, R.N.; Shulman, M.A.; Javaid, A.; Hodgson, C.; Myles, P.S.; Mcgiffin, D.; Nakagawa, S.; Amlani, A.M.; Hupf, J.; Takeda, K.; et al. Postdischarge Functional Capacity, Health-Related Quality of Life, Depression, Anxiety, and Post-Traumatic Stress Disorder in Patients Receiving a Long-Term Left Ventricular Assist Device. J. Card. Fail. 2022, 28, 83–92. [Google Scholar] [CrossRef]
- Kitagaki, K.; Ono, R.; Shimada, Y.; Yanagi, H.; Konishi, H.; Nakanishi, M. Depressive Symptoms Interfere with the Improvement in Exercise Capacity by Cardiac Rehabilitation after Left Ventricular Assist Device Implantation. Artif. Organs 2022, 46, 471–478. [Google Scholar] [CrossRef]
- Mullan, C.; Caraballo, C.; Ravindra, N.G.; Miller, P.E.; McCullough, M.; Brown, K.; Aw, T.W.; Gruen, J.; Clarke, J.-R.D.; Velazquez, E.J.; et al. Psychiatric Comorbidity and Outcomes after Left Ventricular Assist Device Implantation for End-Stage Heart Failure. JACC Heart Fail. 2020, 8, 569–577. [Google Scholar] [CrossRef]
- Mapelli, D.; Cavazzana, A.; Cavalli, C.; Bottio, T.; Tarzia, V.; Gerosa, G.; Volpe, B.R. Clinical Psychological and Neuropsychological Issues with Left Ventricular Assist Devices (LVADs). Ann. Cardiothorac. Surg. 2014, 3, 480–489. [Google Scholar] [CrossRef]
- Yost, G.; Bhat, G.; Mahoney, E.; Tatooles, A. Reduced Anxiety and Depression in Patients with Advanced Heart Failure after Left Ventricular Assist Device Implantation. Psychosomatics 2017, 58, 406–414. [Google Scholar] [CrossRef]
- Casida, J.M.; Abshire, M.; Ghosh, B.; Yang, J.J. The Relationship of Anxiety, Depression, and Quality of Life in Adults with Left Ventricular Assist Devices. ASAIO J. 2018, 64, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Weerahandi, H.; Goldstein, N.; Gelfman, L.P.; Jorde, U.; Kirkpatrick, J.N.; Meyerson, E.; Marble, J.; Naka, Y.; Pinney, S.; Slaughter, M.S.; et al. The Relationship between Psychological Symptoms and Ventricular Assist Device Implantation. J. Pain. Symptom Manag. 2017, 54, 870. [Google Scholar] [CrossRef] [PubMed]
- Stehlik, J.; Estep, J.D.; Selzman, C.H.; Rogers, J.G.; Spertus, J.A.; Shah, K.B.; Chuang, J.; Farrar, D.J.; Starling, R.C. Patient-Reported Health-Related Quality of Life Is a Predictor of Outcomes in Ambulatory Heart Failure Patients Treated with Left Ventricular Assist Device Compared with Medical Management: Results from the ROADMAP Study (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management). Circ. Heart Fail. 2017, 10, e003910. [Google Scholar] [CrossRef] [PubMed]
- Bidwell, J.T.; Lyons, K.S.; Mudd, J.O.; Gelow, J.M.; Chien, C.V.; Hiatt, S.O.; Grady, K.L.; Lee, C.S. Quality of Life, Depression, and Anxiety in Ventricular Assist Device Therapy: Longitudinal Outcomes for Patients and Family Caregivers. J. Cardiovasc. Nurs. 2017, 32, 455–463. [Google Scholar] [CrossRef] [PubMed]
- D’Aoust, R.F.; Antonsdottir, I.; Budhathoki, C.; Casida, J. Sleep Quality and Depression in Adults with Durable Left-Ventricular Assist Devices. Int. J. Artif. Organs 2021, 44, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Müller-Tasch, T.; Peters-Klimm, F.; Schellberg, D.; Holzapfel, N.; Barth, A.; Jünger, J.; Szecsenyi, J.; Herzog, W. Depression Is a Major Determinant of Quality of Life in Patients with Chronic Systolic Heart Failure in General Practice. J. Card. Fail. 2007, 13, 818–824. [Google Scholar] [CrossRef]
- Mbakwem, A.; Aina, F.; Amadi, C. Expert Opinion-Depression in Patients with Heart Failure: Is Enough Being Done? Card. Fail. Rev. 2016, 2, 110–112. [Google Scholar] [CrossRef]
- Warraich, H.J.; Kitzman, D.W.; Whellan, D.J.; Duncan, P.W.; Mentz, R.J.; Pastva, A.M.; Nelson, M.B.; Upadhya, B.; Reeves, G.R. Physical Function, Frailty, Cognition, Depression, and Quality of Life in Hospitalized Adults ≥60 Years with Acute Decompensated Heart Failure with Preserved versus Reduced Ejection Fraction. Circ. Heart Fail. 2018, 11, e005254. [Google Scholar] [CrossRef] [PubMed]
- Ishak, W.W.; Edwards, G.; Herrera, N.; Lin, T.; Hren, K.; Peterson, M.; Ngor, A.; Liu, A.; Kimchi, A.; Spiegel, B.; et al. Depression in Heart Failure: A Systematic Review. Innov. Clin. Neurosci. 2020, 17, 27–38. [Google Scholar] [PubMed]
- Gordon, R.J.; Weinberg, A.D.; Pagani, F.D.; Slaughter, M.S.; Pappas, P.S.; Naka, Y.; Goldstein, D.J.; Dembitsky, W.P.; Giacalone, J.C.; Ferrante, J.; et al. Prospective, Multicenter Study of Ventricular Assist Device Infections. Circulation 2013, 127, 691–702. [Google Scholar] [CrossRef]
- Köhler, A.-K.; Körperich, H.; Morshuis, M.; Freytag, C.C.; Gummert, J.; Burchert, W.; Preuss, R.; Körfer, J. Pre-Operative Risk Factors for Driveline Infection in Left Ventricular-Assist Device Patients. ESC Heart Fail. 2022, 9, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Snipelisky, D.; Stulak, J.M.; Schettle, S.D.; Sharma, S.; Kushwaha, S.S.; Dunlay, S.M. Psychosocial Characteristics and Outcomes in Patients with Left Ventricular Assist Device Implanted as Destination Therapy. Am. Heart J. 2015, 170, 887–894. [Google Scholar] [CrossRef]
- Lundgren, S.; Lowes, B.D.; Zolty, R.; Burdorf, A.; Raichlin, E.; Um, J.Y.; Poon, C. Do Psychosocial Factors Have Any Impact on Outcomes after Left Ventricular Assist Device Implantation? ASAIO J. 2018, 64, e43–e47. [Google Scholar] [CrossRef]
- Tigges-Limmer, K.; Schönbrodt, M.; Roefe, D.; Arusoglu, L.; Morshuis, M.; Gummert, J.F. Suicide after Ventricular Assist Device Implantation. J. Heart Lung Transplant. 2010, 29, 692–694. [Google Scholar] [CrossRef]
- Charton, M.; Flécher, E.; Leclercq, C.; Delmas, C.; Dambrin, C.; Goeminne, C.; Vincentelli, A.; Michel, M.; Lehelias, L.; Verdonk, C.; et al. Suicide Attempts among LVAD Recipients: Real-Life Data from the ASSIST-ICD Study. Circulation 2020, 141, 934–936. [Google Scholar] [CrossRef]
- Waldenburger, N.; Steinecke, M.; Peters, L.; Jünemann, F.; Bara, C.; Zimmermann, T. Depression, Anxiety, Fear of Progression, and Emotional Arousal in Couples after Left Ventricular Assist Device Implantation. ESC Heart Fail. 2020, 7, 3022–3028. [Google Scholar] [CrossRef]
- Okam, N.A.; Ahmad, W.; Rana, D.; Torrilus, C.; Jahan, N.; Sedrakyan, S. Psychological Spectrum Experienced by Heart Failure Patients after Left Ventricular Assist Device Implantation. Cureus 2020, 12, e9671. [Google Scholar] [CrossRef]
- Casida, J.M.; Marcuccilli, L.; Peters, R.M.; Wright, S. Lifestyle Adjustments of Adults with Long-Term Implantable Left Ventricular Assist Devices: A Phenomenologic Inquiry. Heart Lung 2011, 40, 511–520. [Google Scholar] [CrossRef]
- Melnikov, S.; Abuhazira, M.; Golobov, D.; Yaari, V.; Jaarsma, T.; Ben Gal, T. Depression and Anxiety Moderate the Relationship between Body Image and Personal Well-Being among Patients with an Implanted Left Ventricular Assist Device. J. Cardiovasc. Nurs. 2020, 35, 149–155. [Google Scholar] [CrossRef]
- Kugler, C.; Meng, M.; Rehn, E.; Morshuis, M.; Gummert, J.F.; Tigges-Limmer, K. Sexual Activity in Patients with Left Ventricular Assist Devices and Their Partners: Impact of the Device on Quality of Life, Anxiety and Depression. Eur. J. Cardiothorac. Surg. 2018, 53, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Grosman-Rimon, L.; McDonald, M.A.; Jacobs, I.; Tumiati, L.C.; Bar-Ziv, S.P.; Shogilev, D.J.; Mociornita, A.G.; Ghashghai, A.; Chruscinski, A.; Cherney, D.Z.I.; et al. Markers of Inflammation in Recipients of Continuous-Flow Left Ventricular Assist Devices. ASAIO J. 2014, 60, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Westaby, S.; Piggot, D.; Dudnikov, S.; Robson, D.; Catarino, P.A.; Clelland, C.; Nojiri, C. End-Organ Function during Chronic Nonpulsatile Circulation. Ann. Thorac. Surg. 2002, 74, 1080–1085. [Google Scholar] [CrossRef]
- Ozawa, Y.; Kobori, H.; Suzaki, Y.; Navar, L.G. Sustained Renal Interstitial Macrophage Infiltration Following Chronic Angiotensin II Infusions. Am. J. Physiol. Ren. Physiol. 2007, 292, F330. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Ruperez, M.; Lorenzo, O.; Esteban, V.; Blanco, J.; Mezzano, S.; Egido, J. Angiotensin II Regulates the Synthesis of Proinflammatory Cytokines and Chemokines in the Kidney. Kidney Int. Suppl. 2002, 62, S12–S22. [Google Scholar] [CrossRef]
- Sorescu, D. Smad3 Mediates Angiotensin II- and TGF-Β1-Induced Vascular Fibrosis: Smad3 Thickens the Plot. Circ. Res. 2006, 98, 988–989. [Google Scholar] [CrossRef]
- Wang, A.K.; Miller, B.J. Meta-Analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons between Schizophrenia, Bipolar Disorder, and Depression. Schizophr. Bull. 2018, 44, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Shelton, R.C.; Claiborne, J.; Sidoryk-Wegrzynowicz, M.; Reddy, R.; Aschner, M.; Lewis, D.A.; Mirnics, K. Altered Expression of Genes Involved in Inflammation and Apoptosis in Frontal Cortex in Major Depression. Mol. Psychiatry 2011, 16, 751–762. [Google Scholar] [CrossRef]
- Köhler-Forsberg, O.; Lydholm, C.N.; Hjorthøj, C.; Nordentoft, M.; Mors, O.; Benros, M.E. Efficacy of Anti-inflammatory Treatment on Major Depressive Disorder or Depressive Symptoms: Meta-Analysis of Clinical Trials. Acta Psychiatr. Scand. 2019, 139, 404–419. [Google Scholar] [CrossRef]
- Eller, T.; Vasar, V.; Shlik, J.; Maron, E. Pro-Inflammatory Cytokines and Treatment Response to Escitaloprsam in Major Depressive Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 445–450. [Google Scholar] [CrossRef]
- Mansur, R.B.; Delgado-Peraza, F.; Subramaniapillai, M.; Lee, Y.; Iacobucci, M.; Rodrigues, N.; Rosenblat, J.D.; Brietzke, E.; Cosgrove, V.E.; Kramer, N.E.; et al. Extracellular Vesicle Biomarkers Reveal Inhibition of Neuroinflammation by Infliximab in Association with Antidepressant Response in Adults with Bipolar Depression. Cells 2020, 9, 895. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A.H. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression: The Role of Baseline Inflammatory Biomarkers. JAMA Psychiatry 2013, 70, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Majd, M.; Saunders, E.F.H.; Engeland, C.G. Inflammation and the Dimensions of Depression: A Review. Front. Neuroendocrinol. 2020, 56, 100800. [Google Scholar] [CrossRef] [PubMed]
- Hayley, S.; Brebner, K.; Lacosta, S.; Merali, Z.; Anisman, H. Sensitization to the Effects of Tumor Necrosis Factor-Alpha: Neuroendocrine, Central Monoamine, and Behavioral Variations. J. Neurosci. 1999, 19, 5654–5665. [Google Scholar] [CrossRef]
- Zhao, Q.; Peng, C.; Wu, X.; Chen, Y.; Wang, C.; You, Z. Maternal Sleep Deprivation Inhibits Hippocampal Neurogenesis Associated with Inflammatory Response in Young Offspring Rats. Neurobiol. Dis. 2014, 68, 57–65. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Lee, R.; Breen, E.C.; Irwin, M.R. Plasma and Cerebrospinal Fluid Inflammatory Markers and Human Aggression. Neuropsychopharmacology 2023, 48, 1060–1066. [Google Scholar] [CrossRef]
- McCloskey, M.S.; Ammerman, B.A. Suicidal Behavior and Aggression-Related Disorders. Curr. Opin. Psychol. 2018, 22, 54–58. [Google Scholar] [CrossRef]
- Lee, P.H.; Doyle, A.E.; Silberstein, M.; Jung, J.-Y.; Liu, R.T.; Perlis, R.H.; Roffman, J.; Smoller, J.W.; Fava, M.; Kessler, R.C. Associations between Genetic Risk for Adult Suicide Attempt and Suicidal Behaviors in Young Children in the US. JAMA Psychiatry 2022, 79, 971–980. [Google Scholar] [CrossRef]
- Rabbany, J.M.; Ellis, S.; Metts, A.; Burke, A.; Brent, D.A.; Melhem, N.; Marcott, S.; Mann, J.J. Mood Disorders and Aggressive Traits Mediate Effects of Reported Childhood Adversity on Suicide Attempt Risk. Arch. Suicide Res. 2022, 1–24. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Vitkovic, L.; Konsman, J.P.; Bockaert, J.; Dantzer, R.; Homburger, V.; Jacque, C. Cytokine Signals Propagate through the Brain. Mol. Psychiatry 2000, 5, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. The Blood–Brain Barrier in Psychoneuroimmunology. Immunol. Allergy Clin. N. Am. 2009, 29, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Desse, S.; Martinez, A.; Worthen, R.J.; Jope, R.S.; Beurel, E. TNFα Disrupts Blood Brain Barrier Integrity to Maintain Prolonged Depressive-like Behavior in Mice. Brain Behav. Immun. 2018, 69, 556–567. [Google Scholar] [CrossRef]
- Takeuchi, H.; Jin, S.; Wang, J.; Zhang, G.; Kawanokuchi, J.; Kuno, R.; Sonobe, Y.; Mizuno, T.; Suzumura, A. Tumor Necrosis Factor-α Induces Neurotoxicity via Glutamate Release from Hemichannels of Activated Microglia in an Autocrine Manner. J. Biol. Chem. 2006, 281, 21362–21368. [Google Scholar] [CrossRef]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Choudhury, S.; Musaelyan, K.; Myint, A.M.; Thuret, S.; Price, J.; Pariante, C.M. Interleukin-1β: A New Regulator of the Kynurenine Pathway Affecting Human Hippocampal Neurogenesis. Neuropsychopharmacology 2012, 37, 939–949. [Google Scholar] [CrossRef]
- Myint, A.M.; Kim, Y.K. Cytokine–Serotonin Interaction through IDO: A Neurodegeneration Hypothesis of Depression. Med. Hypotheses 2003, 61, 519–525. [Google Scholar] [CrossRef]
- Bremner, J.D.; Narayan, M.; Anderson, E.R.; Staib, L.H.; Miller, H.L.; Charney, D.S. Hippocampal Volume Reduction in Major Depression. Am. J. Psychiatry 2000, 157, 115–117. [Google Scholar] [CrossRef]
- Leonard, B.E. Inflammation and Depression: A Causal or Coincidental Link to the Pathophysiology? Acta Neuropsychiatr. 2018, 30, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Poredos, P.; Jezovnik, M.K.; Radovancevic, R.; Gregoric, I.D. Endothelial Function in Patients with Continuous-Flow Left Ventricular Assist Devices. Angiology 2021, 72, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.N.; Cornwell, W.K.; Pal, J.D.; Lindenfeld, J.; Ambardekar, A. V Living Without a Pulse: The Vascular Implications of Continuous-Flow Left Ventricular Assist Devices. Circ. Heart Fail. 2018, 11, e004670. [Google Scholar] [CrossRef]
- Lappegård, K.T.; Bergseth, G.; Riesenfeld, J.; Pharo, A.; Magotti, P.; Lambris, J.D.; Mollnes, T.E. The Artificial Surface-Induced Whole Blood Inflammatory Reaction Revealed by Increases in a Series of Chemokines and Growth Factors Is Largely Complement Dependent. J. Biomed. Mater. Res. A 2008, 87, 129–135. [Google Scholar] [CrossRef]
- Chia, P.Y.; Teo, A.; Yeo, T.W. Overview of the Assessment of Endothelial Function in Humans. Front. Med. 2020, 7, 542567. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, H.; Tsuchimine, S.; O’Hashi, K.; Sakai, K.; Hattori, K.; Hidese, S.; Nakajima, S.; Chiba, S.; Yoshimura, A.; Fukuzato, N.; et al. Association between Vascular Endothelial Growth Factor-Mediated Blood-Brain Barrier Dysfunction and Stress-Induced Depression. Mol. Psychiatry 2022, 27, 3822–3832. [Google Scholar] [CrossRef]
- Waclawovsky, A.J.; de Brito, E.; Smith, L.; Vancampfort, D.; da Silva, A.M.V.; Schuch, F.B. Endothelial Dysfunction in People with Depressive Disorders: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2021, 141, 152–159. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Kollia, N.; Tousoulis, D. The Link between Depression and Atherosclerosis through the Pathways of Inflammation and Endothelium Dysfunction. Maturitas 2018, 109, 1–5. [Google Scholar] [CrossRef]
- Cooper, D.C.; Tomfohr, L.M.; Milic, M.S.; Natarajan, L.; Bardwell, W.A.; Ziegler, M.G.; Dimsdale, J.E. Depressed Mood and Flow-Mediated Dilation: A Systematic Review and Meta-Analysis. Psychosom. Med. 2011, 73, 360. [Google Scholar] [CrossRef]
- Osika, W.; Montgomery, S.M.; Dangardt, F.; Währborg, P.; Gan, L.M.; Tideman, E.; Friberg, P. Anger, Depression and Anxiety Associated with Endothelial Function in Childhood and Adolescence. Arch. Dis. Child. 2011, 96, 38–43. [Google Scholar] [CrossRef]
- Lopez-Vilchez, I.; Diaz-Ricart, M.; Navarro, V.; Torramade, S.; Zamorano-Leon, J.; Lopez-Farre, A.; Galan, A.M.; Gasto, C.; Escolar, G. Endothelial Damage in Major Depression Patients Is Modulated by SSRI Treatment, as Demonstrated by Circulating Biomarkers and an In Vitro Cell Model. Transl. Psychiatry 2016, 6, e886. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M.; Long, J.W.; et al. Advanced Heart Failure Treated with Continuous-Flow Left Ventricular Assist Device. N. Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef]
- Slaughter, M.S.; Pagani, F.D.; McGee, E.C.; Birks, E.J.; Cotts, W.G.; Gregoric, I.; Howard Frazier, O.; Icenogle, T.; Najjar, S.S.; Boyce, S.W.; et al. HeartWare Ventricular Assist System for Bridge to Transplant: Combined Results of the Bridge to Transplant and Continued Access Protocol Trial. J. Heart Lung Transplant. 2013, 32, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Starling, R.C.; Estep, J.D.; Horstmanshof, D.A.; Milano, C.A.; Stehlik, J.; Shah, K.B.; Bruckner, B.A.; Lee, S.; Long, J.W.; Selzman, C.H.; et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: The ROADMAP Study 2-Year Results. JACC Heart Fail. 2017, 5, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Teuteberg, J.J.; Slaughter, M.S.; Rogers, J.G.; McGee, E.C.; Pagani, F.D.; Gordon, R.; Rame, E.; Acker, M.; Kormos, R.L.; Salerno, C.; et al. The HVAD Left Ventricular Assist Device: Risk Factors for Neurological Events and Risk Mitigation Strategies. JACC Heart Fail. 2015, 3, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.J.; Jorde, U.P.; Sun, B.; Park, S.J.; Milano, C.A.; Frazier, O.H.; Sundareswaran, K.S.; Farrar, D.J.; Russell, S.D. Pre-Operative Risk Factors of Bleeding and Stroke During Left Ventricular Assist Device Support. J. Am. Coll. Cardiol. 2014, 63, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.H.; Cho, S.M.; Prayson, R.A.; Hassett, C.E.; Starling, R.C.; Uchino, K. Cerebral Microvascular Injury in Patients with Left Ventricular Assist Device: A Neuropathological Study. Transl. Stroke Res. 2022, 13, 257–264. [Google Scholar] [CrossRef]
- Kannapadi, N.V.; White, B.; Woo Choi, C.; Chen, L.L.; Cho, S.M. Clinically Silent Brain Injury and Perioperative Neurological Events in Patients with Left Ventricular Assist Device: A Brain Autopsy Study. ASAIO J. 2021, 67, 917–922. [Google Scholar] [CrossRef]
- Cheng, A.; Williamitis, C.A.; Slaughter, M.S. Comparison of Continuous-Flow and Pulsatile-Flow Left Ventricular Assist Devices: Is There an Advantage to Pulsatility? Ann. Cardiothorac. Surg. 2014, 3, 573. [Google Scholar] [CrossRef]
- Li, Y.S.J.; Haga, J.H.; Chien, S. Molecular Basis of the Effects of Shear Stress on Vascular Endothelial Cells. J. Biomech. 2005, 38, 1949–1971. [Google Scholar] [CrossRef]
- Ono, M.; Joshi, B.; Brady, K.; Easley, R.B.; Kibler, K.; Conte, J.; Shah, A.; Russell, S.D.; Hogue, C.W. Cerebral Blood Flow Autoregulation Is Preserved after Continuous-Flow Left Ventricular Assist Device Implantation. J. Cardiothorac. Vasc. Anesth. 2012, 26, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Van Mook, W.N.K.A.; Rennenberg, R.J.M.W.; Schurink, G.W.; Van Oostenbrugge, R.J.; Mess, W.H.; Hofman, P.A.M.; De Leeuw, P.W. Cerebral Hyperperfusion Syndrome. Lancet Neurol. 2005, 4, 877–888. [Google Scholar] [CrossRef]
- Goodwin, K.; Kluis, A.; Alexy, T.; John, R.; Voeller, R. Neurological Complications Associated with Left Ventricular Assist Device Therapy. Expert. Rev. Cardiovasc. Ther. 2018, 16, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Starling, R.; Cho, S.M.; Nowacki, A.S.; Uchino, K.; Hussain, M.S.; Mountis, M.; Moazami, N. Risk Factors, Mortality, and Timing of Ischemic and Hemorrhagic Stroke with Left Ventricular Assist Devices. J. Heart Lung Transplant. 2017, 36, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.; Brewer, R.J.; Nemeh, H.W.; Gerlach, B.; Lanfear, D.E.; Williams, C.T.; Paone, G. Stroke While on Long-Term Left Ventricular Assist Device Support: Incidence, Outcome, and Predictors. ASAIO J. 2014, 60, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Stewart, G.C.; Uber, P.A. The Vexing Problem of Thrombosis in Long-Term Mechanical Circulatory Support. J. Heart Lung Transplant. 2014, 33, 1–11. [Google Scholar] [CrossRef]
- Lee, E.-J.; Kwon, O.D.; Kim, S.J. Prevalence, Awareness, and Treatment of Depression among Community-Dwelling Stroke Survivors in Korea. Sci. Rep. 2022, 12, 4050. [Google Scholar] [CrossRef]
- Almhdawi, K.A.; Alazrai, A.; Kanaan, S.; Shyyab, A.A.; Oteir, A.O.; Mansour, Z.M.; Jaber, H. Post-Stroke Depression, Anxiety, and Stress Symptoms and Their Associated Factors: A Cross-Sectional Study. Neuropsychol. Rehabil. 2021, 31, 1091–1104. [Google Scholar] [CrossRef]
- Loubinoux, I.; Kronenberg, G.; Endres, M.; Schumann-Bard, P.; Freret, T.; Filipkowski, R.K.; Kaczmarek, L.; Popa-Wagner, A. Post-Stroke Depression: Mechanisms, Translation and Therapy. J. Cell. Mol. Med. 2012, 16, 1961–1969. [Google Scholar] [CrossRef]
- Medeiros, G.C.; Roy, D.; Kontos, N.; Beach, S.R. Post-Stroke Depression: A 2020 Updated Review. Gen Hosp Psychiatry 2020, 66, 70–80. [Google Scholar] [CrossRef]
- Robinson, R.G.; Jorge, R.E. Post-Stroke Depression: A Review. Am. J. Psychiatry 2016, 173, 221–231. [Google Scholar] [CrossRef]
- Levy, M.J.F.; Boulle, F.; Steinbusch, H.W.; van den Hove, D.L.A.; Kenis, G.; Lanfumey, L. Neurotrophic Factors and Neuroplasticity Pathways in the Pathophysiology and Treatment of Depression. Psychopharmacology 2018, 235, 2195–2220. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Z.; Sun, D.; Xu, Z.; Yuan, Y.; Zhang, X.; Li, N. Low Serum BDNF May Indicate the Development of PSD in Patients with Acute Ischemic Stroke. Int. J. Geriatr. Psychiatry 2011, 26, 495–502. [Google Scholar] [CrossRef]
- Kolb, B.; Saber, H.; Fadel, H.; Rajah, G. The Endocannabinoid System and Stroke: A Focused Review. Brain Circ. 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Backes, D.; Van den Bergh, W.M.; Van Duijn, A.L.; Lahpor, J.R.; van Dijk, D.; Slooter, A.J.C. Cerebrovascular Complications of Left Ventricular Assist Devices. Eur. J. Cardiothorac. Surg. 2012, 42, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Gupta, A.; Kumar, S.; Baumblatt, J.A.; Pauwaa, S.; Gallagher, C.; Treitman, A.; Pappas, P.; Tatooles, A.; Bhat, G. Are Blood Stream Infections Associated with an Increased Risk of Hemorrhagic Stroke in Patients with a Left Ventricular Assist Device? ASAIO J. 2012, 58, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Eshelman, A.K.; Mason, S.; Nemeh, H.; Williams, C. LVAD Destination Therapy: Applying What We Know about Psychiatric Evaluation and Management from Cardiac Failure and Transplant. Heart Fail. Rev. 2009, 14, 21–28. [Google Scholar] [CrossRef]
- Rossi Ferrario, S.; Bacich, D.; Beltrame, L.; Balestroni, G.; Pistono, M. Does a Comprehensive Inpatient Rehabilitation Program Improve Patients’ and Caregivers’ Emotional State in LVAD Patients? Artif. Organs 2019, 43, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Tigges-Limmer, K.; Brocks, Y.; Winkler, Y.; Stock Gissendanner, S.; Morshuis, M.; Gummert, J.F. Mental Health Interventions during Ventricular Assist Device Therapy: A Scoping Review. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.; Peters, C.J.; Nickels, M.W. Arranging Inpatient Psychiatric Treatment for a Patient with a Left Ventricular Assist Device. Prog. Transplant. 2022, 32, 248–251. [Google Scholar] [CrossRef]
- Rossi Ferrario, S.; Panzeri, A.; Pistono, M. Psychological Difficulties of LVAD Patients and Caregivers: A Follow up over One Year from Discharge. Artif. Organs 2022, 46, 479–490. [Google Scholar] [CrossRef]
- Chai, M.; Su, G.; Gao, J.; Chen, W.; Wu, Q.; Dong, Y.; Wang, H.; Chen, D.; Li, Y.; Gao, X.; et al. Molecular Mechanism of the Protective Effects of M2 Microglia on Neurons: A Review Focused on Exosomes and Secretory Proteins. Neurochem. Res. 2022, 47, 3556–3564. [Google Scholar] [CrossRef]
- Schwartz, M.; Deczkowska, A. Neurological Disease as a Failure of Brain-Immune Crosstalk: The Multiple Faces of Neuroinflammation. Trends Immunol. 2016, 37, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Perez-Caballero, L.; Pérez-Egea, R.; Romero-Grimaldi, C.; Puigdemont, D.; Molet, J.; Caso, J.-R.; Mico, J.-A.; Pérez, V.; Leza, J.-C.; Berrocoso, E. Early Responses to Deep Brain Stimulation in Depression Are Modulated by Anti-Inflammatory Drugs. Mol. Psychiatry 2014, 19, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.; Camprodon, J.A. Understanding the Mechanisms of Action of Electroconvulsive Therapy: Revisiting Neuroinflammatory and Neuroplasticity Hypotheses. JAMA Psychiatry 2023, 80, 643–644. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnsasra, H.; Khalil, F.; Kanneganti Perue, R.; Azab, A.N. Depression among Patients with an Implanted Left Ventricular Assist Device: Uncovering Pathophysiological Mechanisms and Implications for Patient Care. Int. J. Mol. Sci. 2023, 24, 11270. https://doi.org/10.3390/ijms241411270
Alnsasra H, Khalil F, Kanneganti Perue R, Azab AN. Depression among Patients with an Implanted Left Ventricular Assist Device: Uncovering Pathophysiological Mechanisms and Implications for Patient Care. International Journal of Molecular Sciences. 2023; 24(14):11270. https://doi.org/10.3390/ijms241411270
Chicago/Turabian StyleAlnsasra, Hilmi, Fouad Khalil, Radha Kanneganti Perue, and Abed N. Azab. 2023. "Depression among Patients with an Implanted Left Ventricular Assist Device: Uncovering Pathophysiological Mechanisms and Implications for Patient Care" International Journal of Molecular Sciences 24, no. 14: 11270. https://doi.org/10.3390/ijms241411270
APA StyleAlnsasra, H., Khalil, F., Kanneganti Perue, R., & Azab, A. N. (2023). Depression among Patients with an Implanted Left Ventricular Assist Device: Uncovering Pathophysiological Mechanisms and Implications for Patient Care. International Journal of Molecular Sciences, 24(14), 11270. https://doi.org/10.3390/ijms241411270





