Proteome Profiling of the Dura Mater in Patients with Moyamoya Angiopathy
Abstract
1. Introduction
2. Results
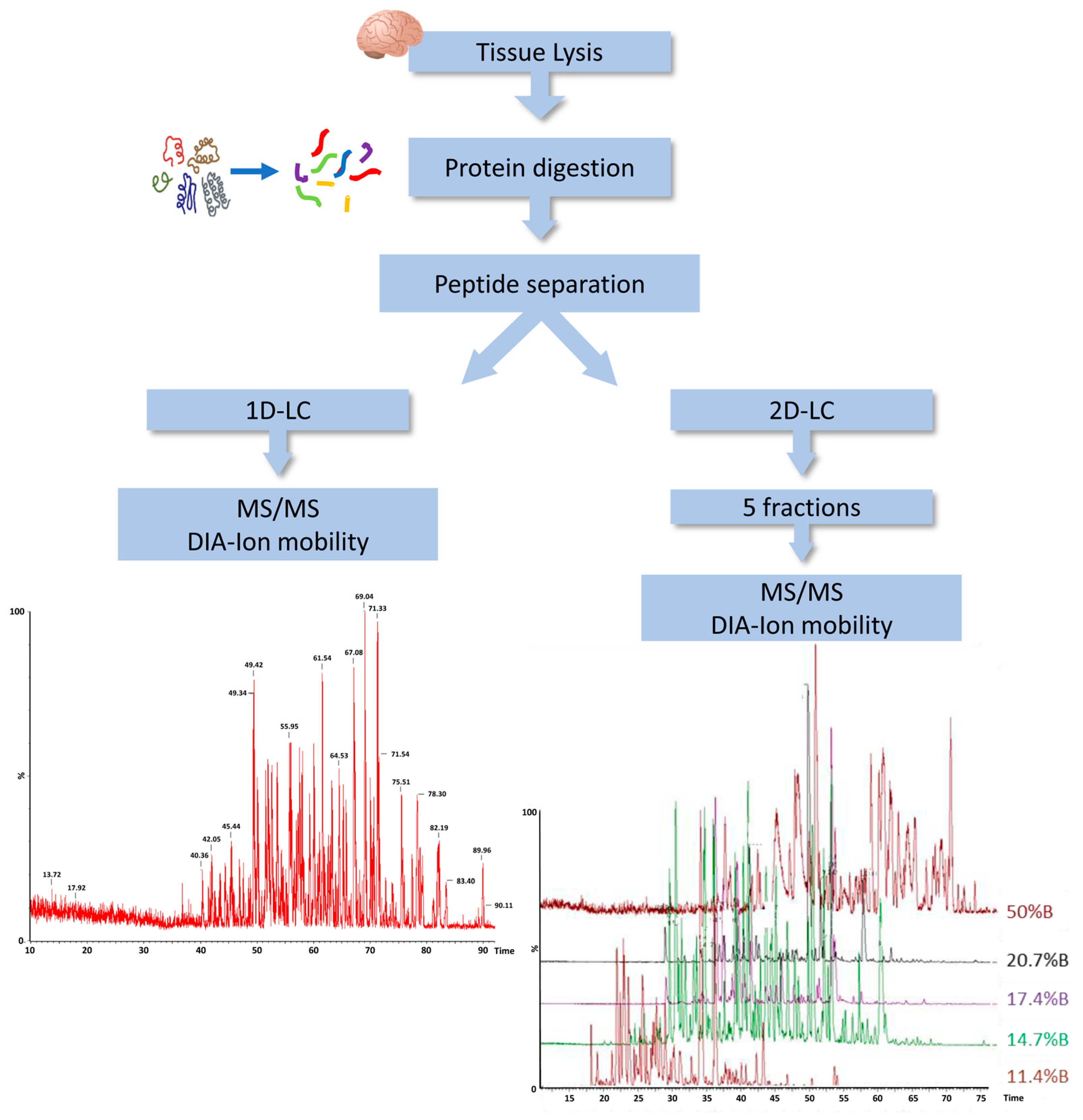
2.1. Protein Extraction from Dura Mater (DM) Samples Representing MMA Patient Cohort
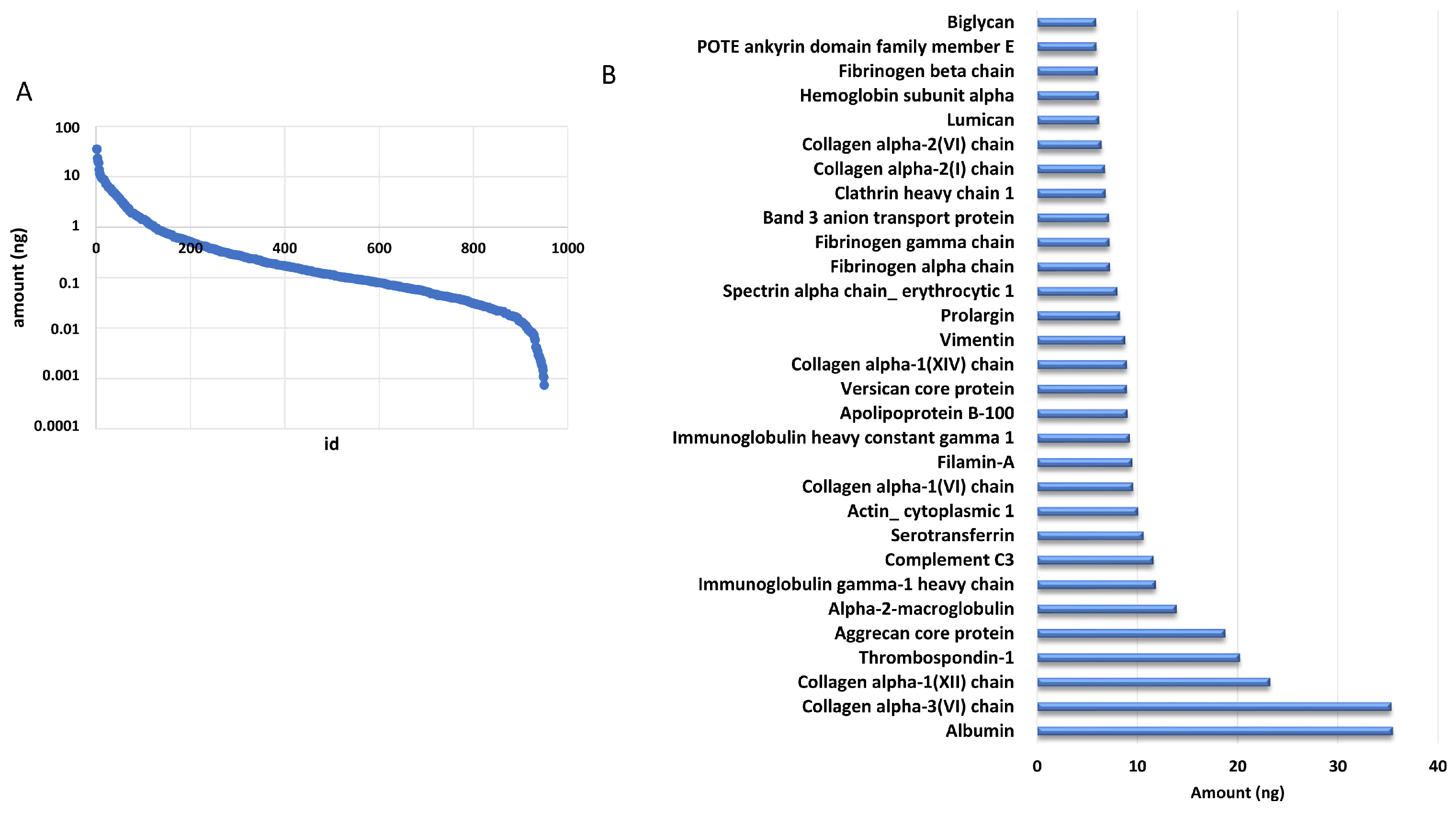
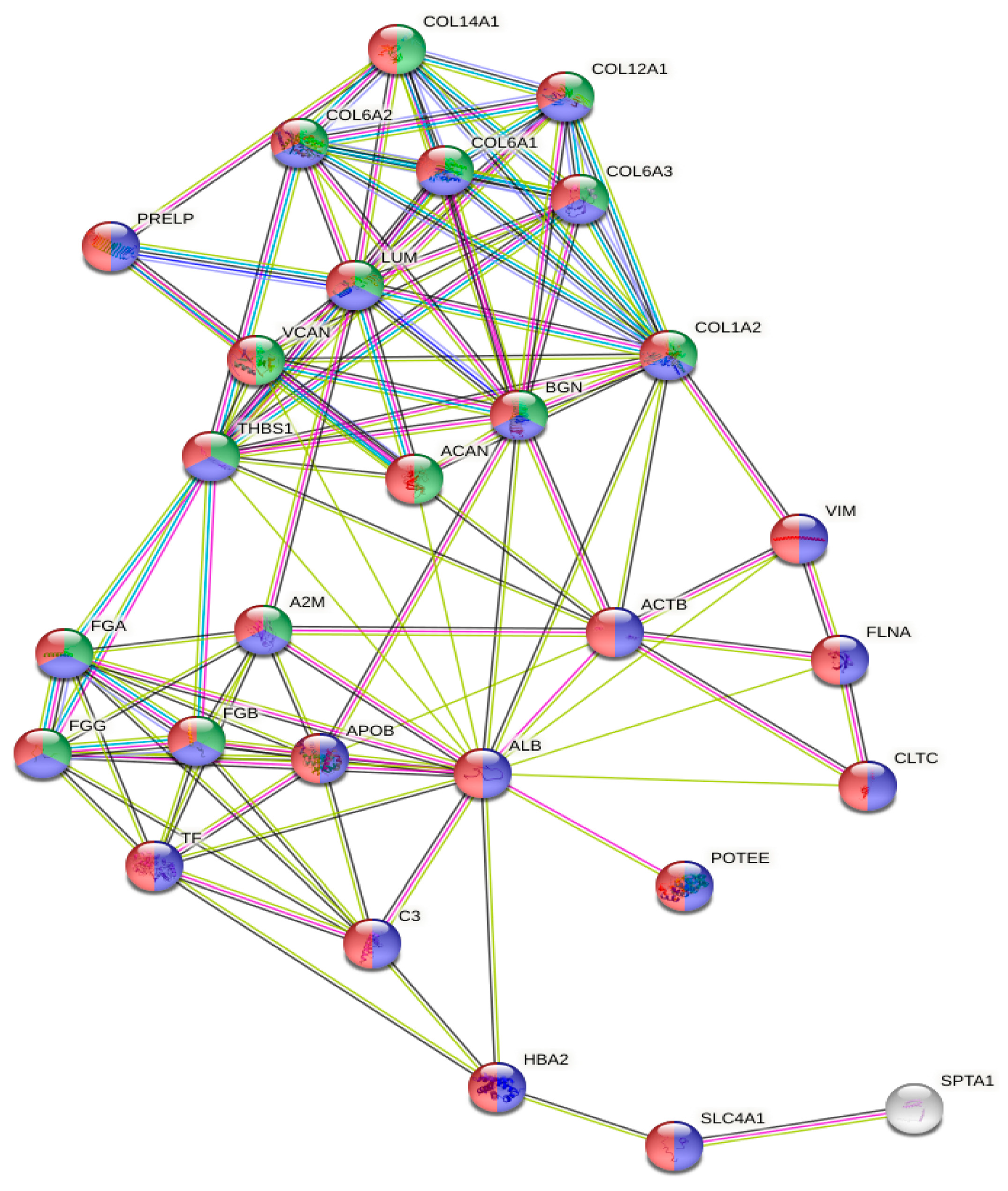
2.2. Mass Spectrometry Proteomic Approach
2.3. Gene Ontology Analyses
3. Discussion
4. Materials and Methods
4.1. Human Dura Mater (DM) Samples
4.2. Ethical Issues
4.3. Mass Spectrometry Analysis, Data Processing and Statistical Analysis
4.4. Gene Ontology Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1D | single-dimensional |
| 2D | two-dimensional |
| ACN | acetonitrile |
| ACVD | atherosclerotic cerebrovascular disease |
| ADH | alcohol dehydrogenase |
| CAPN1 | calpain1 |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| DIA | data independent acquisition |
| DM | dura mater |
| ECM | extracellular matrix |
| ECs | endothelial cells |
| FLNA | filamin A |
| GO | gene ontology |
| ICA | internal carotid artery |
| LC | liquid chromatography |
| MMA | moyamoya angiopathy |
| MS | mass spectrometry |
| PVNH | periventricular nodular heterotopia |
| VSMCs | vascular smooth muscle cells |
References
- Fukui, M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’ disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin. Neurol. Neurosurg. 1997, 99 (Suppl. S2), S238–S240. [Google Scholar] [CrossRef] [PubMed]
- Guey, S.; Tournier-Lasserve, E.; Hervé, D.; Kossorotoff, M. Moyamoya disease and syndromes: From genetics to clinical management. Appl. Clin. Genet. 2015, 8, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, I.G.; Bersano, A.; Canavero, I.; Restelli, F.; Raccuia, G.; Ciceri, E.F.; Faragò, G.; Gioppo, A.; Broggi, M.; Schiariti, M.; et al. Characteristics of Moyamoya Disease in the Older Population: Is It Possible to Define a Typical Presentation and Optimal Therapeutical Management? J. Clin. Med. 2021, 10, 2287. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, Y.; Fukai, N.; Tanaka, K.; Tsuruoka, S.; Inaba, Y.; Aoyagi, M.; Ohno, K. A new surgical treatment of moyamoya disease in children: A preliminary report. Surg. Neurol. 1981, 15, 313–320. [Google Scholar] [CrossRef]
- Kuroda, S.; Houkin, K.; Ishikawa, T.; Nakayama, N.; Iwasaki, Y. Novel bypass surgery for moyamoya disease using pericranial flap: Its impacts on cerebral hemodynamics and long-term outcome. Neurosurgery 2010, 66, 1093–1101. [Google Scholar] [CrossRef]
- Yamamoto, S.; Yamamoto, S.; Akai, T.; Sasahara, M.; Kuroda, S. Differentiation of Fibroblasts into Myofibroblasts in the Arachnoid Membrane of Moyamoya Disease. Stroke 2022, 53, 3465–3473. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, P.; Xia, X.; Wang, L.; Li, X. Living on the border of the CNS: Dural immune cells in health and disease. Cell Immunol. 2022, 377, 104545. [Google Scholar] [CrossRef]
- Argouarch, A.R.; Schultz, N.; Yang, A.C.; Jang, Y.; Garcia, K.; Cosme, C.G.; Corrales, C.I.; Nana, A.L.; Karydas, A.M.; Spina, S.; et al. Postmortem Human Dura Mater Cells Exhibit Phenotypic, Transcriptomic and Genetic Abnormalities that Impact their Use for Disease Modeling. Stem Cell Rev. Rep. 2022, 18, 3050–3065. [Google Scholar] [CrossRef]
- Malicek, D.; Wittig, I.; Luger, S.; Foerch, C. Proteomics-Based Approach to Identify Novel Blood Biomarker Candidates for Differentiating Intracerebral Hemorrhage from Ischemic Stroke-A Pilot Study. Front. Neurol. 2021, 12, 713124. [Google Scholar] [CrossRef]
- Dorschel, K.B.; Wanebo, J.E. Genetic and Proteomic Contributions to the Pathophysiology of Moyamoya Angiopathy and Related Vascular Diseases. Appl. Clin. Genet. 2021, 14, 145–171. [Google Scholar] [CrossRef]
- Bersano, A.; Bedini, G.; Nava, S.; Acerbi, F.; Sebastiano, D.R.; Binelli, S.; Franceschetti, S.; Faragò, G.; Grisoli, M.; Gioppo, A.; et al. GEN-O-MA project: An Italian network studying clinical course and pathogenic pathways of moyamoya disease-study protocol and preliminary results. Neurol. Sci. 2019, 40, 561–570. [Google Scholar] [CrossRef]
- Bersano, A.; Khan, N.; Fuentes, B.; Acerbi, F.; Canavero, I.; Tournier-Lasserve, E.; Vajcoczy, P.; Zedde, M.L.; Hussain, S.; Lémeret, S.; et al. European Stroke Organisation (ESO) Guidelines on Moyamoya angiopathy Endorsed by Vascular European Reference Network (VASCERN). Eur. Stroke J. 2023, 8, 55–84. [Google Scholar] [CrossRef]
- Tinelli, F.; Nava, S.; Arioli, F.; Bedini, G.; Scelzo, E.; Lisini, D.; Faragò, G.; Gioppo, A.; Ciceri, E.F.; Acerbi, F.; et al. Vascular Remodeling in Moyamoya Angiopathy: From Peripheral Blood Mononuclear Cells to Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 5763. [Google Scholar] [CrossRef]
- Dei Cas, M.; Carrozzini, T.; Pollaci, G.; Potenza, A.; Nava, S.; Canavero, I.; Tinelli, F.; Gorla, G.; Vetrano, I.G.; Acerbi, F.; et al. Plasma Lipid Profiling Contributes to Untangle the Complexity of Moyamoya Arteriopathy. Int. J. Mol. Sci. 2021, 22, 13410. [Google Scholar] [CrossRef]
- Canavero, I.; Vetrano, I.G.; Zedde, M.; Pascarella, R.; Gatti, L.; Acerbi, F.; Nava, S.; Ferroli, P.; Parati, E.A.; Bersano, A. Clinical Management of Moyamoya Patients. J. Clin. Med. 2021, 10, 3628. [Google Scholar] [CrossRef]
- Araki, Y.; Yoshikawa, K.; Okamoto, S.; Sumitomo, M.; Maruwaka, M.; Wakabayashi, T. Identification of novel biomarker candidates by proteomic analysis of cerebrospinal fluid from patients with moyamoya disease using SELDI-TOF-MS. BMC Neurol. 2010, 10, 112. [Google Scholar] [CrossRef]
- Kashiwazaki, D.; Uchino, H.; Kuroda, S. Downregulation of Apolipoprotein-E and Apolipoprotein-J in Moyamoya Disease-A Proteome Analysis of Cerebrospinal Fluid. J. Stroke Cerebrovasc. Dis. 2017, 26, 2981–2987. [Google Scholar] [CrossRef]
- Liu, X.; Jin, F.; Wang, C.; Zhao, S.; Han, S.; Jiang, P.; Cui, C. Targeted metabolomics analysis of serum amino acid profiles in patients with Moyamoya disease. Amino Acids. 2022, 54, 137–146. [Google Scholar] [CrossRef]
- Wang, X.; Han, C.; Jia, Y.; Wang, J.; Ge, W.; Duan, L. Proteomic Profiling of Exosomes from Hemorrhagic Moyamoya Disease and Dysfunction of Mitochondria in Endothelial Cells. Stroke 2021, 52, 3351–3361. [Google Scholar] [CrossRef]
- Xu, S.; Wei, W.; Zhang, F.; Chen, T.; Dong, L.; Shi, J.; Wu, X.; Zhang, T.; Li, Z.; Zhang, J.; et al. Transcriptomic Profiling of Intracranial Arteries in Adult Patients with Moyamoya Disease Reveals Novel Insights into Its Pathogenesis. Front. Mol. Neurosci. 2022, 15, 881954. [Google Scholar] [CrossRef]
- Goldschmidt, E.; Cacicedo, M.; Kornfeld, S.; Valinoti, M.; Ielpi, M.; Ajler, P.M.; Yampolsky, C.; Rasmussen, J.; Castro, G.R.; Argibay, P. Construction and in vitro testing of a cellulose dura mater graft. Neurol. Res. 2016, 38, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, I.; Abberton, T.; Fuller, M.; Tipper, J.L.; Fisher, J.; Ingham, E. Biological Effects of Clinically Relevant CoCr Nanoparticles in the Dura Mater: An Organ Culture Study. Nanomaterials 2014, 4, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.; Kwon, W.K.; Kim, J.H.; Park, Y.K.; Yoon, W.; Kim, J.H.; Kwon, T.H.; Moon, H.J. Inflammation by activated macrophage-like THP-1 cells increases human dura mater cell adhesion with alteration of integrin α2 β1 and matrix metalloproteinase. J. Orthop. Res. 2019, 37, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Imai, H.; Konno, K.; Kubota, C.; Seki, K.; Puentes, S.; Faried, A.; Yokoo, H.; Hata, H.; Yoshimoto, Y.; et al. Experimental investigation of encephalomyosynangiosis using gyrencephalic brain of the miniature pig: Histopathological evaluation of dynamic reconstruction of vessels for functional anastomosis. Laboratory investigation. J. Neurosurg. Pediatr. 2009, 3, 488–495. [Google Scholar] [CrossRef]
- Mukawa, M.; Nariai, T.; Inaji, M.; Tamada, N.; Maehara, T.; Matsushima, Y.; Ohno, K.; Negi, M.; Kobayashi, D. First autopsy analysis of a neovascularized arterial network induced by indirect bypass surgery for moyamoya disease: Case report. J. Neurosurg. 2016, 124, 1211–1214. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, L.; Jia, X.; Zhang, Y.; Liu, T.; Zhang, L. iTRAQ-based Quantitative Proteomic Analysis of Dural Tissues Reveals Upregulated Haptoglobin to be a Potential Biomarker of Moyamoya Disease. Curr. Proteom. 2021, 18, 27–37. [Google Scholar] [CrossRef]
- Pollaci, G.; Gorla, G.; Potenza, A.; Carrozzini, T.; Canavero, I.; Bersano, A.; Gatti, L. Novel Multifaceted Roles for RNF213 Protein. Int. J. Mol. Sci. 2022, 23, 4492. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Zhang, X.; Ma, X.; He, X.; Gan, C.; Zou, X.; Wang, S.; Shu, K.; Lei, T.; et al. CircZXDC Promotes Vascular Smooth Muscle Cell Transdifferentiation via Regulating miRNA-125a-3p/ABCC6 in Moyamoya Disease. Cells 2022, 11, 3792. [Google Scholar] [CrossRef]
- Stossel, T.P.; Condeelis, J.; Cooley, L.; Hartwig, J.H.; Noegel, A.; Schleicher, M.; Shapiro, S.S. Filamins as integrators of cell mechanics and signalling. Nat. Rev. Mol. Cell Biol. 2001, 2, 138–145. [Google Scholar] [CrossRef]
- Zhou, A.X.; Hartwig, J.H.; Akyürek, L.M. Filamins in cell signaling, transcription and organ development. Trends Cell Biol. 2010, 20, 113–123. [Google Scholar] [CrossRef]
- Scott, R.M.; Smith, E.R. Moyamoya disease and moyamoya syndrome. N. Engl. J. Med. 2009, 360, 1226–1237. [Google Scholar] [CrossRef]
- De la Cuesta, F.; Zubiri, I.; Maroto, A.S.; Posada, M.; Padial, L.R.; Vivanco, F.; Alvarez-Llamas, G.; Barderas, M.G. Deregulation of smooth muscle cell cytoskeleton within the human atherosclerotic coronary media layer. J. Proteom. 2013, 82, 155–165. [Google Scholar] [CrossRef]
- Nallapalli, R.K.; Ibrahim, M.X.; Zhou, A.X.; Bandaru, S.; Sunkara, S.N.; Redfors, B.; Pazooki, D.; Zhang, Y.; Borén, J.; Cao, Y.; et al. Targeting filamin A reduces K-RAS-induced lung adenocarcinomas and endothelial response to tumor growth in mice. Mol. Cancer 2012, 11, 50. [Google Scholar] [CrossRef]
- Fox, J.W.; Lamperti, E.D.; Ekşioğlu, Y.Z.; Hong, S.E.; Feng, Y.; Graham, D.A.; Scheffer, I.E.; Dobyns, W.B.; Hirsch, B.A.; Radtke, R.A.; et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron 1998, 21, 1315–1325. [Google Scholar] [CrossRef]
- Ikeuchi, Y.; Kitayama, J.; Sahara, N.; Okata, T.; Miyake, N.; Matsumoto, N.; Kitazono, T.; Ago, T. Filamin A Variant as a Possible Second-Hit Gene Promoting Moyamoya Disease-like Vascular Formation Associated with RNF213 p.R4810K Variant. Neurol. Genet. 2022, 8, e200017. [Google Scholar] [CrossRef]
- Scholz, B.; Korn, C.; Wojtarowicz, J.; Mogler, C.; Augustin, I.; Boutros, M.; Niehrs, C.; Augustin, H.G. Endothelial RSPO3 Controls Vascular Stability and Pruning through Non-canonical WNT/Ca(2+)/NFAT Signaling. Dev. Cell. 2016, 36, 79–93. [Google Scholar] [CrossRef]
- Banfi, C.; Brioschi, M.; Vicentini, L.M.; Cattaneo, M.G. The Effects of Silencing PTX3 on the Proteome of Human Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 13487. [Google Scholar] [CrossRef]
- Brioschi, M.; Eligini, S.; Crisci, M.; Fiorelli, S.; Tremoli, E.; Colli, S.; Banfi, C. A mass spectrometry-based workflow for the proteomic analysis of in vitro cultured cell subsets isolated by means of laser capture microdissection. Anal. Bioanal. Chem. 2014, 406, 2817–2825. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; Von Mering, C.; et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013, 41, D808–D815. [Google Scholar] [CrossRef]
- Pontremoli, M.; Brioschi, M.; Baetta, R.; Ghilardi, S.; Banfi, C. Identification of DKK-1 as a novel mediator of statin effects in human endothelial cells. Sci. Rep. 2018, 8, 16671. [Google Scholar] [CrossRef] [PubMed]
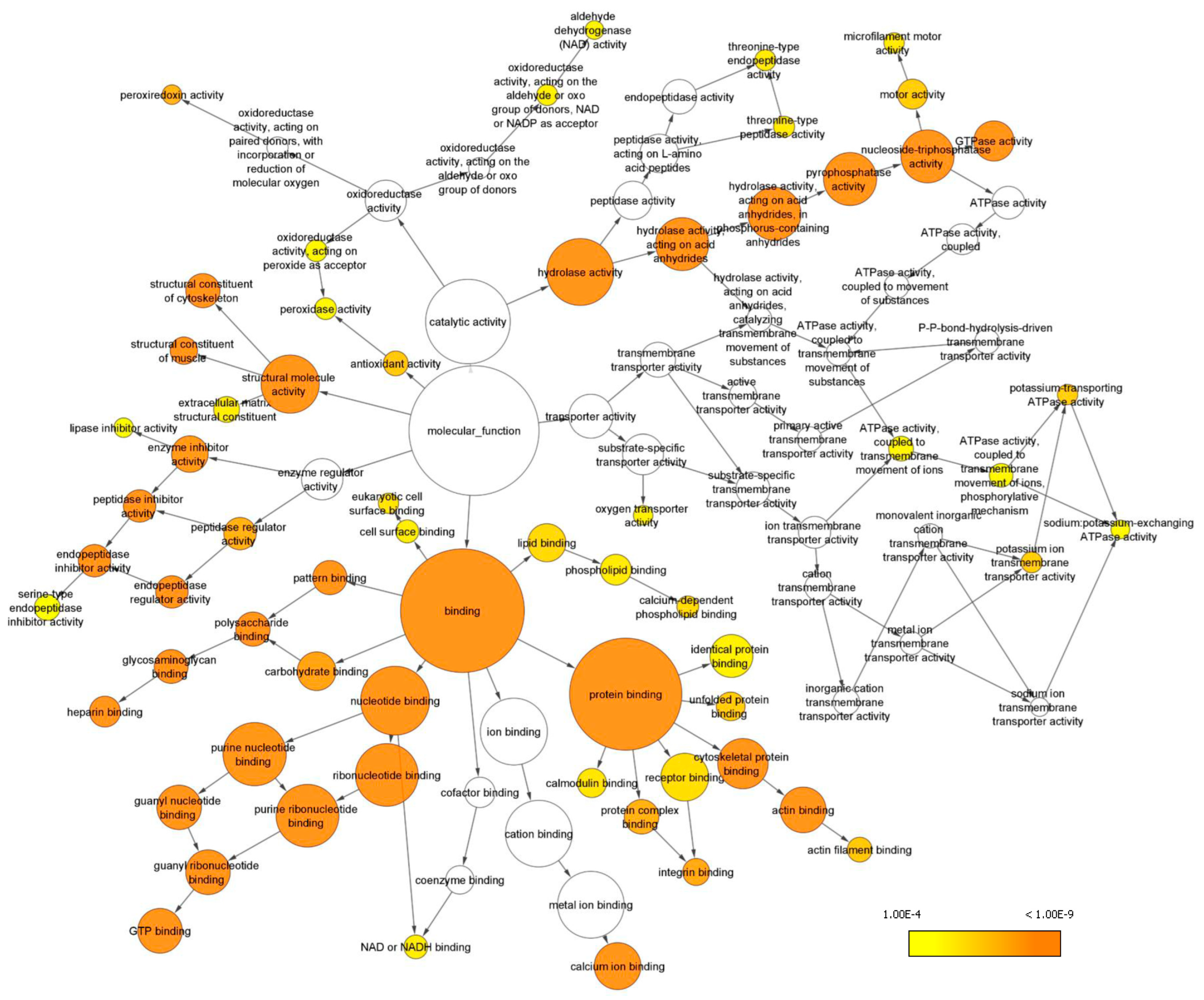
| #Term ID | Term Description | Observed Gene Count | Strength | False Discovery Rate |
|---|---|---|---|---|
| GO:0030901 | Midbrain development | 17 | 0.62 | 7.81 × 10−5 |
| GO:0061564 | Axon development | 40 | 0.31 | 0.00074 |
| GO:0014012 | Peripheral nervous system axon regeneration | 5 | 1.26 | 0.0011 |
| GO:0048678 | Response to axon injury | 11 | 0.67 | 0.0013 |
| GO:0031175 | Neuron projection development | 55 | 0.24 | 0.0017 |
| GO:0048812 | Neuron projection morphogenesis | 43 | 0.27 | 0.0022 |
| GO:0008088 | Axo-dendritic transport | 12 | 0.59 | 0.0024 |
| GO:0031102 | Neuron projection regeneration | 8 | 0.78 | 0.0027 |
| GO:0048666 | Neuron development | 62 | 0.21 | 0.0039 |
| GO:0031103 | Axon regeneration | 7 | 0.82 | 0.0040 |
| GO:0022008 | Neurogenesis | 108 | 0.15 | 0.0046 |
| GO:0098930 | Axonal transport | 10 | 0.59 | 0.0071 |
| GO:0008090 | Retrograde axonal transport | 6 | 0.83 | 0.0083 |
| GO:0042063 | Gliogenesis | 23 | 0.34 | 0.0102 |
| GO:0099640 | Axo-dendritic protein transport | 5 | 0.92 | 0.0111 |
| GO:0007409 | Axonogenesis | 33 | 0.27 | 0.0117 |
| GO:0060052 | Neurofilament cytoskeleton organization | 4 | 1.09 | 0.0121 |
| GO:0098974 | Postsynaptic actin cytoskeleton organization | 5 | 0.86 | 0.0175 |
| GO:0099173 | Postsynapse organization | 12 | 0.47 | 0.0178 |
| GO:1901215 | Negative regulation of neuron death | 21 | 0.33 | 0.0180 |
| GO:0033693 | Neurofilament bundle assembly | 3 | 1.33 | 0.0181 |
| GO:0010976 | Positive regulation of neuron projection development | 26 | 0.29 | 0.0191 |
| GO:0099641 | Anterograde axonal protein transport | 4 | 0.98 | 0.0220 |
| GO:0098935 | Dendritic transport | 4 | 0.94 | 0.0280 |
| GO:0098971 | Anterograde dendritic transport of neurotransmitter receptor complex | 3 | 1.21 | 0.0280 |
| GO:0048699 | Generation of neurons | 96 | 0.13 | 0.0317 |
| GO:1990138 | Neuron projection extension | 9 | 0.48 | 0.0449 |
| GO:0061900 | Glial cell activation | 7 | 0.57 | 0.0459 |
| GO:0050808 | Synapse organization | 24 | 0.26 | 0.0479 |
| GO:0007420 | Brain development | 51 | 0.17 | 0.0483 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrozzini, T.; Pollaci, G.; Gorla, G.; Potenza, A.; Rifino, N.; Acerbi, F.; Vetrano, I.G.; Ferroli, P.; Bersano, A.; Gianazza, E.; et al. Proteome Profiling of the Dura Mater in Patients with Moyamoya Angiopathy. Int. J. Mol. Sci. 2023, 24, 11194. https://doi.org/10.3390/ijms241311194
Carrozzini T, Pollaci G, Gorla G, Potenza A, Rifino N, Acerbi F, Vetrano IG, Ferroli P, Bersano A, Gianazza E, et al. Proteome Profiling of the Dura Mater in Patients with Moyamoya Angiopathy. International Journal of Molecular Sciences. 2023; 24(13):11194. https://doi.org/10.3390/ijms241311194
Chicago/Turabian StyleCarrozzini, Tatiana, Giuliana Pollaci, Gemma Gorla, Antonella Potenza, Nicola Rifino, Francesco Acerbi, Ignazio G. Vetrano, Paolo Ferroli, Anna Bersano, Erica Gianazza, and et al. 2023. "Proteome Profiling of the Dura Mater in Patients with Moyamoya Angiopathy" International Journal of Molecular Sciences 24, no. 13: 11194. https://doi.org/10.3390/ijms241311194
APA StyleCarrozzini, T., Pollaci, G., Gorla, G., Potenza, A., Rifino, N., Acerbi, F., Vetrano, I. G., Ferroli, P., Bersano, A., Gianazza, E., Banfi, C., & Gatti, L. (2023). Proteome Profiling of the Dura Mater in Patients with Moyamoya Angiopathy. International Journal of Molecular Sciences, 24(13), 11194. https://doi.org/10.3390/ijms241311194










