Abstract
In order to explore the effects of high temperature stress on the physiological characteristics of Paeonia ostii, the Paeonia ostii were subjected to 25 °C, 35 °C, 38 °C, and 40 °C for 7 days. Meanwhile, the physiological indicators of oxidative stress (hydrogen peroxide, H2O2; malondialdehyde, MDA; relative electrical conductivity, REC), antioxidant enzyme activity (superoxide dismutase, SOD; ascorbate peroxidase, APX; catalase, CAT; peroxidase, POD), photosynthetic pigment content (chlorophyll a, Chla; chlorophyll b, Chlb), photosynthetic characteristics (net photosynthetic rate, Pn; intercellular CO2 concentration, Ci; stomatal conductance, Gs; transpiration rate, Tr), and osmoregulatory substances content (soluble protein, SP; soluble sugar, SS) were determined. The results showed that, with the increase in temperature and stress time, the H2O2 content, MDA content, REC value, CAT activity, and APX activity increased, while Chla content, Chlb content, SS content, and SP content decreased. With the extension of stress time, the SOD activity, POD activity, and Tr value of each high temperature stress group first increased and then decreased; Ci first decreased, then increased, and then decreased; meanwhile, Pn and Gs showed an overall downward trend. PLS-DA (partial least squares discriminant analysis) was used to analyze the changes in physiological and biochemical indexes of peony leaves under 40 °C stress for different days. SOD was found to be the biggest factor affecting the changes in physiological and biochemical indexes of peony leaves treated with different days of stress.
1. Introduction
The peony (Paeonia suffruticosa Andr.) is a perennial deciduous shrub with beautiful and dignified flowers, known as the “national color and fragrance” and “king of flowers”. Its root has anti-hypertensive and anti-inflammatory properties [1]. The climate in the south of the Yangtze River is humid and hot, and the temperature in summer is high. The prolonged high temperature causes the peony leaves to curl and wilt, which is unfavorable for growth and affects large-scale production. P. ostii is one of the main varieties of the Jiangnan peony, which is more resistant to moisture and heat [2,3] and can adapt to the high temperature and high humidity environment in the Jiangnan area. Therefore, it is of great significance to explore its heat resistance mechanism under high temperature stress.
In the context of global warming and the increasing frequency of extreme temperatures, high temperature is considered to be an important environmental factor affecting plant growth. Heat stress in plants refers to the phenomenon whereby the ambient temperature rises above a critical threshold for a period of time, causing irreparable damage to plant growth and development [4]. Heat stress significantly affects plant developmental processes, such as seed germination, vegetative growth, and reproductive production [5]. In addition, heat stress can affect important plants’ physiological processes, namely photosynthesis and respiration rates, stomatal conductance, and leaf water potential homeostasis [6]. It is widely accepted that an increase in LMA is a common response to environmental stress [7]. Traditionally, high LMA has also been interpreted as a trait that increases a leaf’s structural resistance, as it protects leaves from phytosexual or mechanical damage [8]. Under normal circumstances, plants will produce ROS during normal metabolism, and there is a dynamic balance between ROS production and antioxidants [9]. However, when plants are under stress, ROS production is accelerated, and the balance is disturbed. Excessive ROS can lead to membrane lipid peroxidation, protein oxidation, enzyme inactivation, and DNA and RNA damage, resulting in plant injury. When high temperature causes a large accumulation of ROS in plants, the antioxidant defense system is activated to remove excessive ROS, thereby protecting plants from oxidative stress [10]. At the same time, osmoregulatory substances such as SS, SP, proline (Pro), etc., can work together with antioxidant enzymes to alleviate plant damage caused by high temperature stress. Some studies have shown that peonies reduce oxidative damage and osmotic stress by increasing the activity of antioxidant enzymes and accumulating Pro, thereby improving the plant’s heat tolerance [11].
At present, the research on peonies mainly focuses on pharmacological effects and germplasm resources, and there are relatively few studies on its physiological mechanism under high temperature and the breeding of high temperature-resistant cultivars [3]. Previous studies have found that light energy distribution in peony leaves is affected by high temperature, which can cause photoinhibition of PSⅡ, induce irreversible inactivation of the PSⅡ reaction center [12], and hinder electron transfer [13]. However, peony cultivars resistant to high temperature stress have a relatively low degree of time inhibition under high temperature stress and can maintain relatively high PSⅡ actual light energy conversion efficiency [14]. Ji et al. [15,16] investigated the changes in photosynthetic characteristics of P. ostii under high temperature stress and found that high temperature damaged the photosynthetic capacity and photosynthetic mechanism of peony leaves. At the same time, some studies have shown that exogenous additives can alleviate the photosynthetic characteristics of peonies under high temperature stress [17,18,19,20,21]. In terms of physiology and biochemistry, Qian et al. [22] treated peonies at different temperatures and found that the degree of damage of peonies was related to the intensity of high temperature stress. Li et al. [23] used principal component and membership function analysis to establish a comprehensive identification and evaluation system of peony heat tolerance, which is suitable for the screening and evaluating of peony cultivar resources with heat tolerance. Wang et al. [11] discussed the physiological response mechanism of peonies under high temperature. In recent years, researchers have started to study the mechanism of high temperature resistance of peonies from the molecular point of view [24,25,26,27].
According to previous studies, in response to high temperature stress, antioxidant enzymes, photosynthetic pigments, photosynthetic properties and osmoregulatory substances all change to mitigate the damage caused by high temperature. However, there are still few related reports in Peony, and the physiological and biochemical changes in peony treated for different days under different high temperature stress are still unclear. Therefore, in this study, the activities and contents of antioxidant enzymes (SOD, POD, CAT, APX), photosynthetic pigments (Chl a and Chl b), photosynthetic properties (Ci, Pn, Gs, Tr), and osmoregulatory substances (sp and ss) in peonies subjected to high temperature for different days were investigated and also analyzed using PLS-DA; meanwhile, scanning electron microscopy was used to observe the structure of peony leaves after high temperature treatment to study the effects of high temperature on the physiological and biochemical characteristics of peony leaves and to provide theoretical basis for the screening of peony germplasm resources and the cultivation of new varieties.
2. Results
2.1. Effects of High Temperature Stress on ROS Accumulation and Lipid Peroxidation in Peony Leaves
As shown in Figure 1a, the content of H2O2 increased with the increase in stress temperature and treatment time. Beginning from the first day of high temperature stress, the H2O2 content in peony leaves under each high temperature treatment was significantly higher than that of CK (25 °C). After 7 days of stress, the content of H2O2 at 35 °C, 38 °C, and 40 °C was significantly higher than that in the CK group, increasing by 83.7%, 99.2%, and 246.1%, respectively.
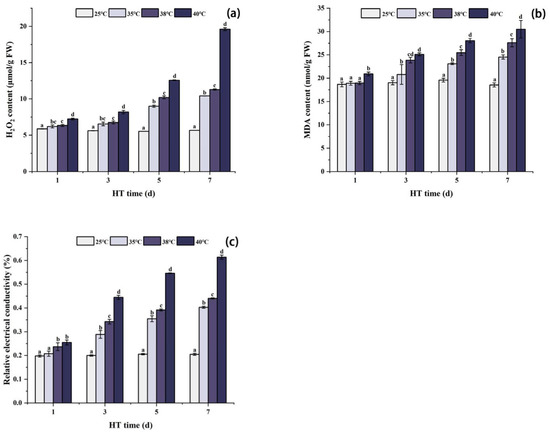
Figure 1.
Changes in H2O2 content, MDA content, and REC value in peony leaves under high temperature stress (a–c). FW, fresh weight; different letters in the same column indicate significant differences at the 0.05 level.
High temperature stress increased the MDA content and REC value, and the higher the temperature and the longer the stress time, the higher the MDA content and REC value. After 1 day of high temperature stress, the MDA content and REC value changed slightly under each high temperature treatment. The MDA content under 35 °C and 38 °C stress was not obviously different from CK, while the MDA content under 40 °C stress was significantly higher. The REC value under 35 °C stress was not significantly different from CK, but the REC value under 38 °C and 40 °C stress was significantly higher. After 3 and 5 days of stress, the MDA content and REC value of each high temperature treatment group were significantly higher than those of the CK group. After 7 days of stress, the MDA content of each high temperature treatment group reached the maximum, which was significantly higher than that in the CK group, increasing by 32.4%, 48.8%, and 64.5%, respectively. At the same time, the REC value of each group also reached the maximum, which was significantly higher than that in the CK group, increasing by 96.8%, 115.1%, and 199.9%, respectively (Figure 1b,c).
2.2. Effects of High Temperature Stress on the Activities of Antioxidant Enzymes in Peony Leaves
Figure 2 shows that SOD, POD, CAT, and APX of each high temperature treatment group were significantly higher than those of CK at 1d of high temperature stress. The SOD activity of the 35 °C, 38 °C, and 40 °C treatment groups reached the maximum after 3 days of stress, and the POD activity of the 38 °C and 40 °C treatment groups also reached the maximum, while the POD activity of the 35 °C treatment group reached the maximum after 5 days of stress. After 7 days of stress, the CAT and APX activities of each high temperature stress group reached the maximum, and the SOD and POD activities reached the minimum. The POD activity of each high temperature stress group was significantly lower than that in the CK group. Although the SOD activity under 40 °C stress was still significantly higher than that in the CK group, the SOD activity under 35 °C and 38 °C stress was lower than that in the CK group.
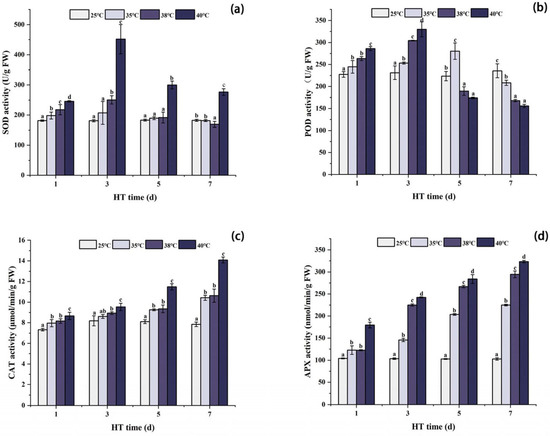
Figure 2.
Changes in antioxidant enzyme activities in peony leaves under high temperature stress. (a) Superoxide dismutase (SOD); (b) ascorbic acid peroxidase (APX); (c) catalase (CAT); (d) peroxidase (POD). FW, fresh weight; different letters in the same column indicate significant differences at the 0.05 level.
2.3. Effects of High Temperature Stress on the Photosynthetic Pigment Content of Peony Leaves
Figure 3 shows that under the same high temperature stress, the Chla content decreases with the stress time. Compared with CK in the same period, Chla content was the lowest at 7 days of stress, and decreased by 42.2%, 53.6%, and 67.3% at 35 °C, 38 °C, and 40 °C, respectively. The trend of Chlb content was similar to that of Chla; under the same high temperature stress, Chlb content showed a decreasing trend with the extension of stress days. Compared with CK in the same period, Chlb content at 40 °C decreased significantly by 19.7% on the 5th day of stress. On the 7th day of stress, Chlb content at 38 °C and 40 °C decreased by 20.8% and 48.3%, respectively, compared with CK.
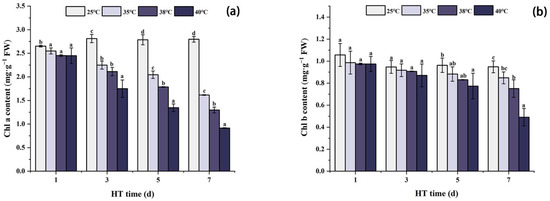
Figure 3.
Changes in chlorophyll content of peony leaves under high temperature stress. (a) Chla content; (b) Chlb content. FW, fresh weight; different letters in the same column indicate significant differences at the 0.05 level.
2.4. Effects of High Temperature Stress on the Photosynthetic Characteristics of Peony Leaves
As shown in Figure 4, the photosynthetic characteristics of peony leaves in each temperature treatment group changed with the high temperature stress treatment. With the extension of high temperature treatment time, Ci first decreased, then increased, and then decreased. After 1 day of stress, the Ci of each high temperature treatment group was higher than that in the CK group, and the Ci of 40 °C was significantly higher than that of CK. After 3 days of stress, the Ci of each high temperature treatment group decreased. Although the Ci at 40 °C was slightly lower than that of CK, the Ci at 35 °C and 38 °C was still significantly higher than that of CK. After 5 days of stress, Ci of each temperature treatment group increased, which was higher than that of CK. After 7 days of stress, the Ci of each high temperature treatment group decreased.
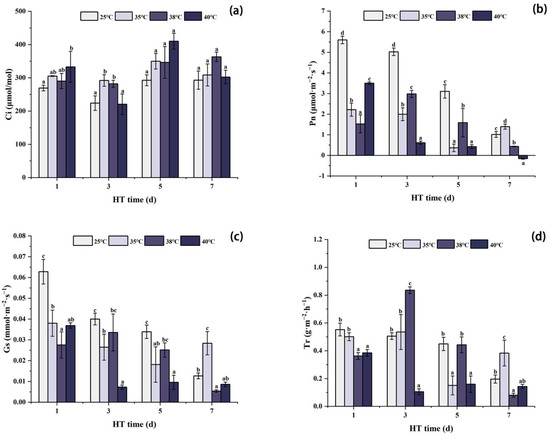
Figure 4.
Changes in photosynthetic characteristics of peony leaves under high temperature stress. (a) Intercellular CO2 content (Ci); (b) net photosynthetic rate (Pn); (c) stomatal conductance (Gs); (d) transpiration rate (Tr). Different letters in the same column indicate significant differences at the 0.05 level.
The changes in Pn and Gs were similar and showed an overall decreasing trend with the increase in high temperature treatment time. After 1 day of stress, Pn and Gs of each high temperature treatment group were significantly lower than those of CK, and the values of Pn and Gs at 38 °C were the lowest. After 3 days of stress, Pn and Gs continued to decrease at 35 °C and 40 °C, while they were increased at 38 °C compared with 1 day, but still significantly lower than those of CK. After 5 days of stress, Pn and Gs of each high temperature treatment group decreased, which were significantly lower than those of CK. After 7 days of stress, Pn and Gs at 38 °C and 40 °C continued to decrease, while Pn and Gs at 35 °C were higher than those at 5 days, and significantly higher than those of CK.
As shown in Figure 4d, the results of Tr showed an overall trend of first increasing and then decreasing with the extension of high temperature treatment time. After 1 day of stress, Tr of each high temperature treatment group was significantly lower than that of CK. After 3 days of stress, compared with 1d, Tr at 40 °C decreased, which was significantly lower than that of CK at the same period. Under the stress conditions of 35 °C and 38 °C, Tr was increased, and Tr at 38 °C was significantly higher than that of CK. After 5 days of stress, Tr at 40 °C increased slightly compared with that at 3 days, while Tr at 35 °C and 38 °C decreased, and Tr at 35 °C was significantly lower than that of CK. After 7 days of stress, compared with 5 days of stress, the Tr of 35 °C stress increased, which was significantly higher than that of CK, while the Tr of 38 °C and 40 °C stress decreased, and the Tr of 38 °C stress was significantly lower than that of CK.
2.5. Effects of High Temperature Stress on Changes in Osmotic Regulators in Peony Leaves
As shown in Figure 5, the changes in SP and SS contents in peony leaves under high temperature stress were similar, and both showed a decreasing trend with the extension of high temperature stress time. Compared with CK, the contents of SP and SS were the lowest in each high temperature treatment group after 7 days of stress. Compared with CK, SP at 35 °C, 38 °C, and 40 °C decreased by 17.7%, 25.9%, and 36.2%, respectively, and SS at 35 °C, 38 °C, and 40 °C decreased by 41.5%, 57.7%, and 68.1%, respectively.
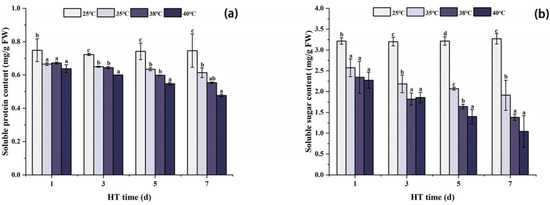
Figure 5.
Changes in osmotic regulator content in peony leaves under high temperature stress. (a) Soluble protein content. (b) Soluble sugar content. FW, fresh weight; different letters in the same column indicate significant differences at the 0.05 level.
2.6. Correlation Analysis of Factors under Different Stress Temperatures
Correlation analysis of the indicators of P. ostii at 40 °C was performed, and the results are shown in Table 1.

Table 1.
Correlation analysis of 13 indicators at 40 °C.
At 40 °C, there was a very significant positive correlation between any two of the five indicators of H2O2, MDA, REC, CAT, and APX; between any two of the four indicators of ss, sp, Gs and Tr; between Chla, Chlb, SS, and sp; and between any two of the three indicators of Gs, Tr and Pn. Meanwhile, Chla was very positively correlated with Gs and Tr, and Pn was very positively correlated with Chla, SS, and sp. Then, Chlb was positively correlated with Pn, Gs, and Tr.
H2O2 was very significantly negatively correlated with Chla, Chlb, ss, and sp. In addition, REC, MDA, CAT, and APX were very significantly negatively correlated with Pn, Chla, Chlb, ss, and sp, and CAT, APX, REC, and SOD were very significantly negatively correlated with Gs and Tr. There was a significant negative correlation between H2O2 with POD, Pn, and Gs; MDA with Gs and Tr; and POD with Ci.
The indicators in Table 1 represent different meanings, and the complex correlation between them indicates that the change in heat tolerance of peonies cannot be evaluated by a single indicator, and multiple indicators should be integrated for analysis.
2.7. PLS-DA Analysis of Different Days of High Temperature Stress at 40 °C
This experiment analyzed the PLS-DA of various physiological and biochemical indexes of peonies leaves under high temperature stress of 40 °C, which is a discriminant analysis method in multivariate data analysis technology and is widely used in genomics, proteomics, and metabolomics [28]. As shown in the PLS-DA model (Figure 6), various indices of peony leaves under high temperature stress at 40 °C were different in different stress days. The VIP score map showed that SOD was the most important contribution model, followed by APX and Ci.
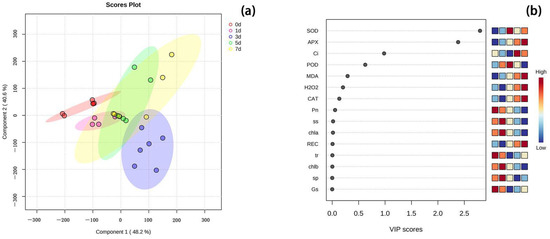
Figure 6.
PLS-DA analysis and related VIP scores of various indices of peony leaves under high temperature stress at 40 °C for different stress days. (a) Score plot of PLS-DA analysis based on each value for different days of 40 °C stress. The different color spaces represent a separate group. (b) The score map of each physiological and biochemical index identified by VIP score; the physiological and biochemical index with a score greater than 1 has reference value. The indicator with the highest VIP score was the variable with the largest differential contribution in the model.
2.8. Leaf Anatomy during Different Days of High Temperature Stress at 40 °C
As shown in Figure 7, the SEM observation shows that the structure of leaf midvein will extend, along with the time of high temperature stress, to be long and loose. The longitudinal section of the leaf surface showed that the mesophyll tissue of P. Ostii was composed of fence tissue and sponge tissue. With the extension of high temperature stress time, the arrangement of fence tissue changed, and the structure of sponge tissue was loose. As shown in Table 2, leaf LMA and LD at a high temperature of 40 °C had similar trends with MT/LT, rising first and then falling back to healthy levels. PT/LT decreased, and ST/LT increased on day 0 and day 5 of the heat stress, and PT/LT increased on day 1 and day 5 of the heat stress. From day 5 to day 7 of the heat stress, PT/LT significantly decreased, and ST/LT significantly increased.

Figure 7.
Scanning structure of leaves treated at 40 °C for different days. (a) Treatment at 40 °C for 0d; (b) treatment at 40 °C for 1d; (c) treatment at 40 °C for 3d; (d) treatment at 40 °C for 5d; (e) treatment at 40 °C for 7d; pp: palisade parenchyma; sp: spongy parenchyma.

Table 2.
Effects of heat treatment at 40 °C for different days on the structure of peony leaves.
3. Discussion
3.1. Changes in ROS Accumulation and Lipid Peroxidation in Peony Leaves under High Temperature
High temperature stress will affect the PSII of plants, resulting in a decrease in photochemical efficiency, the inhibition of electron transport, and excess of light energy, thus forming a large number of reactive oxygen species, which will change the permeability of cell membrane and cause membrane lipid peroxidation, causing damage to plants [29,30]. In this experiment, the H2O2 content increased with the increase in high temperature stress temperature and treatment time, which was consistent with the results of previous studies on tomatoes (Solanum lycopersicum L) [31] and sorghum (Sorghum bicolor L. Moench) [32]. Membrane lipid peroxidation can cause the accumulation of MDA, the final product of lipid peroxidation in plants, so the level of MDA can reflect the degree of cell membrane damage [33]. At the same time, the structure of the cell membrane is destroyed, which significantly increases the membrane’s permeability, resulting in a large amount of extravasation of intracellular electrolyte solution and some small molecular ions, which results in an increase in the conductivity of the tissue leachate [34]. Therefore, REC can be used to determine the extravasation of electrolytes in plant cells and the degree of cell membrane damage [35]. The results of this experiment showed that H2O2, MDA, and REC were significantly positively correlated under high temperature stress, and the levels of MDA and REC increased with the stress, confirming that high temperature stress damaged the membrane structure of peony cells. These results were consistent with the research results of Wang et al. [11].
3.2. Changes in Antioxidant Enzyme Activities in Peony Leaves under High Temperature
If there is an imbalance between ROS production and scavenging in plants, plant cells will produce oxidative stress, which will eventually lead to cell death, and then inhibit plant growth and development [36]. ROS accumulation in plants is controlled by a complex antioxidant defense system [29]. SOD, POD, APX, and CAT are the key enzymes that constitute the antioxidant defense system. In the enzymatic system, SOD, as the first line of defense of a plant’s enzymatic antioxidant system, can catalyze the dismutation reaction of 2 molecules’ O2− to generate H2O2 and O2, and H2O2 is then catalyzed by CAT, POD, APX, and other antioxidant enzymes to generate H2O to achieve the purpose of scavenging ROS [37,38]. Therefore, in this experiment, the increase rates of MDA and REC in each temperature group were higher than those of H2O2, indicating that H2O2 was partially removed by antioxidant enzymes. Previous studies have shown that the antioxidant defense system is initiated by various external environmental pressures [9]; during abiotic stress, especially environmental stress (e.g., UV radiation), a plant produces ROS when the plant is exposed to stress, and plant-produced antioxidants, flavonoids, and secondary metabolites play the role of protecting the plant for detoxifying ROS and protecting the plant from abnormal conditions (i.e., stress) and to aid in protein and amino acid stabilization [39,40,41]. Consistent with this conclusion, the activities of antioxidant enzymes were increased under heat stress for a certain period of time (3d) in this experiment. This indicates that peonies can reduce the damage of reactive oxygen species by enhancing the activities of antioxidant enzymes under 3d heat stress, but the activities of APX and CAT still show an increasing trend, while the activities of SOD and POD decreased under long-term (5d–7d) high temperature stress, which is different from the results of previous studies [17,42], indicating that different antioxidant enzymes were sensitive to different temperatures and activated at different temperature ranges, and that activation occurs at different temperature ranges [43]. At the same time, under high temperature stress conditions, H2O2, MDA, REC, CAT, and APX are positively correlated with each other, indicating that a large amount of H2O2 induces a significant increase in the activity of antioxidant enzymes involved in ROS scavenging [44,45].
3.3. Changes in the Photosynthetic Capacity of Peony Leaves under High Temperature
Chloroplasts are extremely sensitive to high temperature stress in photosynthesis [46,47]. Studies have shown that high temperature can inhibit photosynthetic pigment synthesis in peony seedling leaves [17]. The chlorophyll content measured in this experiment showed that the chlorophyll content decreased with increasing stress, which was consistent with the results of previous studies [11]. Meanwhile, correlation analysis results showed that Chla and Chlb of peony leaves subjected to high temperature stress were very significantly negatively correlated with H2O2, MDA, and REC, indicating that chlorophyll may be affected by H2O2 under high temperature stress, resulting in reduced content [48]. At the same time, studies have shown that high temperature stress promotes the activity of chlorophyll-degrading enzymes, thereby accelerating chlorophyll degradation [49].
Pn can directly represent the photosynthetic capacity of an individual leaf [50]. Reduced photosynthetic capacity is associated with lower chlorophyll content [51], which allows leaves to capture less light and thus reduces Pn. In addition, stomatal limitation and non-stomatal limitation are two of the important factors leading to the decrease in the photosynthetic capacity of plants [52]. The gas exchange parameters of P. ostii in this experiment showed that the Pn index of P. ostii in the first 3 days was limited by stomatal factors. Under high temperature conditions, the decrease in Gs led to the decrease in Tr and Ci, indicating a decrease in plant stomatal conductance, the closure of stomata, a reduction in plant water dissipation, a reduction in the mesophyll cells’ absorption of carbon dioxide, and a further reduction in Pn. At 3d–7d, Pn and Gs decreased significantly, and Ci increased significantly under the three high temperature stresses, indicating that the decrease in Pn in P. ostii was not caused by insufficient CO2, but by non-stomatal factors. These results were consistent with the results of maize [53] and paeoniflora [54]. Studies have shown that Rubisco activase plays a key role in photosynthesis under heat stress conditions (non-stomatal limitation) [55]. High temperature inactivates the electron acceptor and donor sides of PSII, inactivates enzymes in the Calvin cycle, reduces Rubisco activity, and leads to heat inactivation of Rubisco [56,57]. Rubisco activase can be reversibly inhibited when exposed to moderate high temperature stress, but when exposed to prolonged high temperature, Rubisco activase activity can be irreversibly inhibited due to the insolubility of the Rubisco activase protein and its own degradation [55], thereby affecting photosynthesis. Therefore, the decrease in Pn at 3D–7D may be related to Rubisco activase activity.
3.4. Changes in Osmotic Regulatory Substances in Peony Leaves under High Temperature
In addition to antioxidants, osmoregulatory substances (OA) can resist the damage caused by external stress to plants by regulating cell osmotic balance [58,59]. As an OA, SS content can reduce the thermal sensitivity of photosynthetic electron transport [60], protect photosynthetic organs such as chloroplasts from high temperature stress [61], maintain cell osmotic potential, and reduce cytoplasmic membrane damage [61]. Studies have shown that high temperature stress can change sugar metabolism in peonies and promote the accumulation of soluble sugar [22,42]. In this experiment, the content of SS showed a decreasing trend with the increase in stress time, which was different from the previous conclusion [22,42]. High concentrations of H2O2 generated by high temperatures oxidize proteins in the Calvin cycle, such as cysteine (-SH) or methionine (-SCH3) residues, further inactivating enzymes in the Calvin cycle [62]. Calvin cycle is a carbon-fixation pathway in photosynthesis that provides essential monosaccharides for sucrose synthesis [63]. Therefore, H2O2 can affect the content of SS and other carbohydrates in plants by affecting the Calvin cycle. Under high temperature stress, SS is negatively correlated with H2O2, and positively correlated with Pn, Chla, and Chlb, which also confirms the effect of H2O2 and photosynthesis on SS. Therefore, SS content in this experiment was reduced under high temperature stress.
As an osmoregulatory substance, soluble proteins mostly exist in the form of enzymes in plants and can participate in many physiological activities of plants, such as improving cell osmotic potential and preventing cytoplasmic dehydration. Soluble proteins can be used as a basis for evaluating a plant’s metabolic capacity. Studies have shown that peonies can increase soluble protein content in leaves under short-term high temperature stress, thereby reducing water loss of cells and maintaining cell morphology. However, with the increase in stress time, the regulation of this pathway is impaired, and soluble protein content decreases [42]. Consistent with this conclusion, in the present experiment, the soluble protein content showed a decreasing trend with the stress time.
4. Materials and Methods
4.1. Materials and Treatment
In this study, strong and consistent 4-year old peonies (P. ostii) were planted in a plastic basin with an upper diameter of 28 cm, a lower diameter of 19 cm, and a height of 23 cm. The substrate was composed of garden soil, sand, and perlite (mass ratio: 5:3:2), and water and fertilizer management was normal. Four-year-old peonies with basically the same growth and size were selected. The control group was treated at 25 °C, and the experimental group was treated at 35 °C, 38 °C, and 40 °C, repeated three times. During the experiment, the air humidity in the incubator was set at 70%, the light intensity was set at 8000 lx, and the light and night were 14 h/10 h each day. The vapor pressure deficit was constant at all temperatures. Samples (the first pair of leaves under the top bud) were collected at 0, 1, 3, 5, and 7d after treatment, and three plants were randomly selected from each treatment as replicates. During sampling, photosynthetic indices were measured first, and then leaves were taken for chlorophyll content determination and antioxidant enzyme activity determination. Each measurement was replicated three times.
4.2. Determination of H2O2 Content, MDA Content, and REC Value
H2O2 content and MDA content were determined using a kit (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China), and the procedure was repeated three times. The specific steps were as follows:
H2O2 content: 0.1 g (W) of the leaves of different treatment groups were added with reagents, and the supernatant was extracted for the test. At the same time, the same volume of reagents was used as A control, and the microplate reader was used for determination: H2O2 content (μmol/g fresh weight) = 2.67 × (ΔA − 0.0006) ÷ W, (ΔA = A determination − A control).
MDA content: 0.1 g (W) leaves of different treatment groups were extracted, and the absorbance values at 532 nm and 600 nm were measured and calculated: MDA content (nmol/g fresh weight) = 51.6 × ΔA ÷ W, (ΔA = A532 − A600).
REC was determined using the conductivity meter method. First, 0.1 g leaves were ground into fine powder in liquid nitrogen, and the zero value of conductivity (E0) was measured after adding distilled water. The initial value of conductivity (E1) was measured after the sample was static in the dark for 3h, and the final value of conductivity (E2) was measured after 10 min of boiling water bath. REC% = (E1 − E0)/(E2 − E0) ∗ 100%. The procedure was repeated three times.
4.3. Determination of Antioxidant Enzyme Activity
SOD activity, POD activity, CAT activity, and APX activity were determined using kits (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China), and the procedure was repeated three times. The specific steps were as follows:
SOD activity: 0.1 g (W) of leaf supernatant from different treatment groups was selected as the experimental group, and the same volume of distilled water was selected as the control group. The absorbance values at 560 nm of the two groups were measured and calculated: percentage of inhibition = (A control tube−A assay tube) ÷ A control tube 100%; SOD (U/g fresh weight) = 11.11 × percentage inhibition ÷ (1 − percentage inhibition) ÷ W.
POD activity: The supernatant of 0.1 g (W) leaves from different treatment groups was extracted and measured at a wavelength of 470 nm A1 (absorbance at 1 min) and A2 (absorbance at 2 min), and POD activity was calculated: POD (U/g fresh weight) = 4000 × ΔA ÷ W, ΔA = A2 − A1.
CAT activity: 0.1 g (W) of the leaves of different treatment groups were added with reagents, and the supernatant was extracted for the test. At the same time, the same volume of reagents was used as A control, and the microplate reader was used for determination and calculation: CAT (μmol/min/g fresh weight) = 8.9 × (ΔA − 0.0013) ÷ W, ΔA = A control − A determination.
APX activity: The supernatant of 0.1 g (W) leaves from different treatment groups was extracted and measured at a wavelength of 290 nm A1 (absorbance at 10 s) and A2 (absorbance at 130 s), and APX activity was calculated: APX (nmol/min/g fresh weight) = 1786 × ΔA ÷ W, ΔA = A1 − A2.
4.4. Determination of Photosynthetic Pigment Content
The chlorophyll was extracted using the absolute ethanol extraction method, and absolute ethanol was used as blank control. The absorbance of chlorophyll extract in the experimental group and absolute ethanol in the control group at the wavelength of 663 nm and 645 nm was measured, respectively. Each replicate was measured three times at different wavelengths, and the average value was taken.
The chlorophyll content was calculated using the following formula:
where D663 nm and D645 nm are the absorbance of the solution to be measured at 663 nm and 645 nm, respectively. V is the volume of liquid to be measured (mL); m is leaf fresh mass (g) or leaf area (m2).
Chla content = (12.7D663 nm − 2.69D645 nm) × V/(1000 × m);
Chlb content = (22.9D645 nm − 4.68D663 nm) × V/(1000 × m).
4.5. Measurement of Photosynthetic Parameters
Ci, Pn, Gs, and Tr were measured using LI-6400 a portable Photosynthesis Instrument 400 (LI-Cor6400XTPSC-4817, US). The first determination of leaves were to be numbered and labeled for the next determination.
4.6. Determination of Osmotic Regulator Content
Soluble sugar and soluble protein contents were determined using kits (Suzhou Keming Biotechnology Co., Ltd, Suzhou, China) according to the instructions.
Soluble sugar content determination: the absorbance value at A wavelength of 620 nm was measured after adding reagents to 0.1 g (W) leaves. The same volume of reagents was set as the control group for determination: soluble sugar (mg/g fresh weight) = 1.17 × (ΔA + 0.07) ÷ W, ΔA = A determination − A control.
Soluble protein content determination: the absorbance value at A wavelength of 620 nm was measured after adding reagent to 0.05 g (W) leaves. The same volume of reagent was set as the control group for determination: Cpr (mg/g fresh weight) = 0.1403 × (ΔA + 0.0007) ÷ W, ΔA = A determination − A control.
4.7. Data Analysis
SPSS 25 software was used for one-way analysis of variance and correlation analysis. Excel and Origin2021 software was used for data processing and mapping. PLS-DA analysis used https://www.metaboanalyst.ca/ (accessed on 12 March 2023.)
Author Contributions
Conceptualization, E.H. and X.X. (Xuanze Xia); methodology, E.H.; software, X.X.; validation, W.J., T.L. and X.X. (Xianyi Xu); data curation, J.C.; measurement, X.X. (Xuanze Xia); writing—review and editing, E.H.; project administration, X.C.; supervision, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China (2019YFD1001500), Public Welfare Fund of Zhejiang Province (LGN22C160006) and Talent Program of Zhejiang A&F University Jiyang College (RQ2020B04/RQ1911B05).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, S.R.; Zhao, H.S.; Dong, M.R.; Xiong, W.J.; Wang, X.; Wu, M.Y.; Zhai, H.Y.; Wu, D.L.; Zhang, C. Chemical constituents, pharmacological effects of Moutan Cortex and predictive analysis on its quality marker (Q-Marker). Chin. Tradit. Herb. Drugs 2020, 53, 5215–5224. [Google Scholar]
- Wang, J.; Hu, Y.H.; Zhang, Q.X. Studies on Cuitivar Resources of Tree Peony from South Yangtse River of China. North. Hortic. 2007, 4, 160–162. [Google Scholar]
- Zhao, B.; Huang, Q. Research Progress on Peony under High Temperature Stress Caused by Climate Warming. E3S Web Conf. 2021, 252, 03056. [Google Scholar] [CrossRef]
- Xalxo, R.; Yadu, B.; Chandra, J.; Chandrakar, V.; Keshavkant, S. Alteration in Carbohydrate Metabolism Modulates Thermotolerance of Plant under Heat Stress. In Heat Stress Tolerance in Plants; Wani, S.H., Kumar, V., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Goraya, G.K.; Kaur, B.; Asthir, B.; Bala, S.; Kaur, G.; Farooq, M. Rapid injuries of high temperature in plants. J. Plant Biol. 2017, 60, 298–305. [Google Scholar] [CrossRef]
- Sharma, S.; Manjeet, M. Heat stress effects in fruit crops. Agric. Rev. 2020, 41, 73–78. [Google Scholar] [CrossRef]
- Alonso-Forn, D.; Sancho-Knapik, D.; Ferrio, J.P.; Peguero-Pina, J.J.; Bueno, A.; Onoda, Y.; Cavender-Bares, J.; Niinemets, Ü.; Jansen, S.; Riederer, M.; et al. Revisiting the Functional Basis of Sclerophylly Within the Leaf Economics Spectrum of Oaks: Different Roads to Rome. Curr. For. Rep. 2020, 6, 260–281. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K.; Kushwaha, S.R.; Singh, T.P. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006, 171, 382–388. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Z.; Zhao, D.; Tao, J. Effects of High-Temperature Stress on Photosynthetic Characteristics and Antioxidant Enzyme System of Paeonia ostii. Phyton 2022, 91, 599–615. [Google Scholar] [CrossRef]
- Liu, C.Y.; Chen, D.Y.; Gai, S.P.; Zhan, Y.X.; Zheng, G.S. Effects of high-and low temperature stress on the leaf PS II functions and physiological characteristics of tree peony (Paeonia suffruticosa cV. ‘Roufurong’). Chin. J. Appl. Ecol. 2012, 23, 133–139. [Google Scholar]
- Liu, C.; Yuan, Y.; Gai, S.P.; Zhan, Y.X.; Liu, C.Y.; Zheng, G.S. Effects of Strong Light Coupled with High Temperature Treatment on Energy Transfer Between PS II and PS I in Tree Peony Leaves. Acta Hortic. Sin. 2014, 41, 311–318. [Google Scholar]
- Liu, J.J. Effects of High Temperature and Drought Stress on PS II Function and Light Distribution in Peony Leaves with Different Resistance. North. Hortic. 2019, 11, 72–79. [Google Scholar]
- Ji, W.; Luo, H.; Song, Y.; Hong, E.; Li, Z.; Lin, B.; Fan, C.; Wang, H.; Song, X.; Jin, S.; et al. Changes in Photosynthetic Characteristics of Paeonia suffruticosa under High Temperature Stress. Agronomy 2022, 12, 1203. [Google Scholar] [CrossRef]
- Li, Z.; Ji, W.; Hong, E.; Fan, Z.; Lin, B.; Xia, X.; Chen, X.; Zhu, X. Study on Heat Resistance of Peony Using Photosynthetic Indexes and Rapid Fluorescence Kinetics. Horticulturae 2023, 9, 100. [Google Scholar] [CrossRef]
- Wu, S.; Jin, X.L.; Zhang, M.H.; Zhang, F.J.; Luo, F. Effects of Exogenous Salicylic Acid on Heat Tolerance of Tree Peony Seedlings under High Temperature Stress. J. Henan Agric. Sci. 2018, 47, 98–103. [Google Scholar]
- Wu, S. Heat Tolerance of Tree Peony Seedlings Induced by Exogenous Chemical Substances. Master’s Thesis, Central South University of Forestry and Technolog, Changsha, China, May 2018. [Google Scholar]
- Wu, S.; Jin, X.L.; Zhang, M.H.; Shun, L.X.; Chen, R. Effects of Exogenous Abscisic Acid on Heat Tolerance in Tree Peony Seedlings under High Temperature Stress. Adv. Ornam. Hortic. China 2018, 2018, 346–352. [Google Scholar]
- Ren, Z.B.; Chen, F.Z.; Shu, C.Q.; Li, X.L.; Liu, K.H.; Ji, X.M. Effects of Exogenous 2,4-epibrassinolide on Heat Resistance of Peony. J. Jianghan Univ. (Nat. Sci. Ed.) 2018, 46, 446–453. [Google Scholar]
- Zhao, D.; Wang, X.; Cheng, Z.; Tang, Y.; Tao, J. Multi-walled carbon nanotubes prevent high temperature-induced damage by activating the ascorbate-glutathione cycle in Paeonia ostii T. Hong et J. X. Zhang. Ecotoxicol. Environ. Saf. 2021, 227, 112948. [Google Scholar] [CrossRef]
- Qian, G.Y.; Kong, X.S.; Zhang, S.L. Physiological responses of three peony cultivars to high temperature stress. Jiangsu Agric. Sci. 2017, 45, 103–105. [Google Scholar]
- Li, M.; Cheng, Z.T.; Jun, M.J. Comparative Study on Heat Tolerance of 38 Peony Varieties. Mol. Plant Breed. 2021. [Google Scholar]
- Ma, J.; Wang, Q.; Wei, L.L.; Zhao, Y.; Zhang, G.Z.; Wang, J.; Gu, C.H. Responses of the tree peony (Paeonia suffruticosa, Paeoniaceae) cultivar ‘Yu Hong’ to heat stress revealed by iTRAQ-based quantitative proteomics. Proteome Sci. 2022, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Cheng, Y.W.; Ya, H.Y.; Han, J.M.; Zheng, L. Identification of heat shock proteins via transcriptome profiling of tree peony leaf exposed to high temperature. Genet. Mol. Res. 2015, 14, 8431–8442. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.H. Functional Analysis of High Temperature Stress Transcriptome and Small Molecular Heat Shock Protein Gene in Paeonia suffruticosa. Master’s Thesis, Zhejiang A&F University, Hanzhou, China, June 2021. [Google Scholar]
- Ma, J.; Wang, J.; Wang, Q.; Shang, L.; Zhao, Y.; Zhang, G.; Ma, Q.; Hong, S.; Gu, C. Physiological and transcriptional responses to heat stress and functional analyses of PsHSPs in tree peony (Paeonia suffruticosa). Front. Plant Sci. 2022, 13, 926900. [Google Scholar] [CrossRef] [PubMed]
- Zontov, Y.; Rodionova, O.Y.; Kucheryavskiy, S.; Pomerantsev, A. PLS-DA–A MATLAB GUI tool for hard and soft approaches to partial least squares discriminant analysis. Chemom. Intell. Lab. Syst. 2020, 203, 104064. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding Oxidative Stress and Antioxidant Functions to Enhance Photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Jahan, M.S.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L.) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef]
- Kandziora-Ciupa, M.; Gospodarek, J.; Nadgórska-Socha, A. Pollution and ecological risk assessment of heavy metals in forest soils with changes in the leaf traits and membrane integrity of Vaccinium myrtillus L. Eur. J. For. Res. 2022, 141, 409–419. [Google Scholar] [CrossRef]
- Parida, A.K.; Jha, B. Antioxidative Defense Potential to Salinity in the Euhalophyte Salicornia brachiata. J. Plant Growth Regul. 2010, 29, 137–148. [Google Scholar] [CrossRef]
- Gulen, H.; Eris, A. Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci. 2004, 166, 739–744. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef]
- Narayan, B.; Minsu, L.; Hojin, L.; Arjun, A.; Ah, R.H.; Areum, H.; Hyun, S.K. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 2020, 10, 208. [Google Scholar] [CrossRef]
- Bendou, O.; Gutiérrez-Fernández, I.; Marcos-Barbero, E.L.; Bueno-Ramos, N.; Miranda-Apodaca, J.; González-Hernández, A.I.; Morcuende, R.; Arellano, J.B. Physiological and Antioxidant Response to Different Water Deficit Regimes of Flag Leaves and Ears of Wheat Grown under Combined Elevated CO2 and High Temperature. Plants 2022, 11, 2384. [Google Scholar] [CrossRef]
- Zhang, M.H. The Research on Genetic Diversity and Heat to lerance of Peony Resources in Hunan Province. Ph.D. Thesis, Central South University of Forestry and Technolog, Changsha, China, May 2019. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Silva, P.C.C.; Neto, A.D.D.A.; Gheyi, H.R.; Ribas, R.F.; Silva, C.R.D.R.; Cova, A.M.W. Salt tolerance induced by hydrogen peroxide priming on seed is related to improvement of ion homeostasis and antioxidative defense in sunflower plants. J. Plant Nutr. 2020, 44, 1207–1221. [Google Scholar] [CrossRef]
- Khosrowshahi, Z.T.; Ghassemi-Golezani, K.; Salehi-Lisar, S.Y.; Motafakkerazad, R. Changes in antioxidants and leaf pigments of safflower (Carthamus tinctorius L.) affected by exogenous spermine under water deficit. Biol. Futur. 2020, 71, 313–321. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Rai, K.K.; Rai, N.; Rai, S.P. Investigating the impact of high temperature on growth and yield of Lablab purpureus L. inbred lines using integrated phenotypical, physiological, biochemical and molecular approaches. Indian J. Plant Physiol. 2018, 23, 209–226. [Google Scholar] [CrossRef]
- Jahan, M.S.; Hasan, M.; Alotaibi, F.S.; Alabdallah, N.M.; Alharbi, B.M.; Ramadan, K.M.A.; Bendary, E.S.A.; Alshehri, D.; Jabborova, D.; Al-Balawi, D.A.; et al. Exogenous Putrescine Increases Heat Tolerance in Tomato Seedlings by Regulating Chlorophyll Metabolism and Enhancing Antioxidant Defense Efficiency. Plants 2022, 11, 1038. [Google Scholar] [CrossRef]
- Jiang, D.; Dai, T.; Jing, Q.; Cao, W.; Zhou, Q.; Zhao, H.; Fan, X. Effects of Long-Term Fertilization on Leaf Photosynthetic Characteristics and Grain Yield in Winter Wheat. Photosynthetica 2004, 42, 439–446. [Google Scholar] [CrossRef]
- Narayan, B.; Su, G.H.; Tae, M.Y. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar]
- Salmon, Y.; Lintunen, A.; Dayet, A.; Chan, T.; Dewar, R.; Vesala, T.; Hölttä, T. Leaf carbon and water status control stomatal and nonstomatal limitations of photosynthesis in trees. New Phytol. 2020, 226, 690–703. [Google Scholar] [CrossRef]
- Li, Y.; Xu, W.; Ren, B.; Zhao, B.; Zhang, J.; Liu, P.; Zhang, Z. High temperature reduces photosynthesis in maize leaves by damaging chloroplast ultrastructure and photosystem II. J. Agron. Crop. Sci. 2020, 206, 548–564. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, Q.; Hou, X.; Wang, J.; Chen, S.; Zhang, Q.; Wang, Z.; Yin, Y.; Liu, J. The Effect of High-Temperature Stress on the Physiological Indexes, Chloroplast Ultrastructure, and Photosystems of two Herbaceous Peony Cultivars. J. Plant Growth Regul. 2022, 42, 1631–1646. [Google Scholar] [CrossRef]
- Feller, U. Drought stress and carbon assimilation in a warming climate: Reversible and irreversible impacts. J. Plant Physiol. 2016, 203, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Balfagón, D.; Zandalinas, S.I.; Mittler, R.; Gómez-Cadenas, A. High temperatures modify plant responses to abiotic stress conditions. Physiol. Plant. 2020, 170, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, J.A.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and Rubisco Activase Play an Important Role in the Biochemical Limitations of Photosynthesis in Rice, Wheat, and Maize under High Temperature and Water Deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Huve, K.; Bichele, I.; Tobias, M.; Niinemets, U. Heat sensitivity of photosynthetic electron transport varies during the day due to changes in sugars and osmotic potential. Plant Cell Environ. 2006, 29, 212–228. [Google Scholar] [CrossRef]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of Drought on Soluble Sugars and Free Proline Content in Selected Arabidopsis Mutants. Biology 2020, 9, 367. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Sinha, A.K. ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front. Plant Sci. 2015, 6, 769. [Google Scholar] [CrossRef]
- Cséke, C.; Buchanan, B.B. Regulation of the formation and utilization of photosynthate in leaves. Biochim. Biophys. Acta (BBA) Rev. Bioenerg. 1986, 853, 43–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).