Opposite Auxin Dynamics Determine the Gametophytic and Embryogenic Fates of the Microspore
Abstract
1. Introduction
2. Results
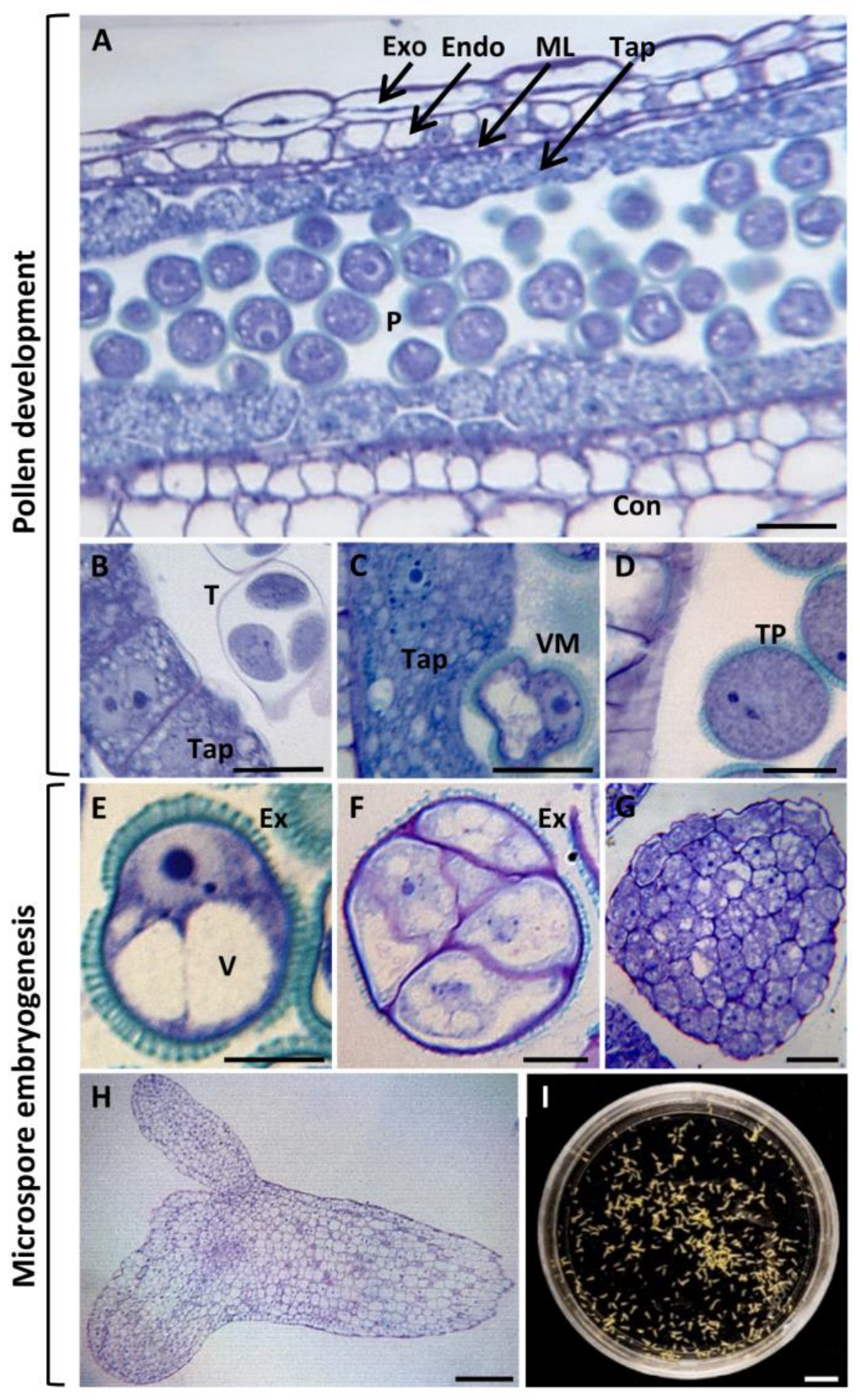
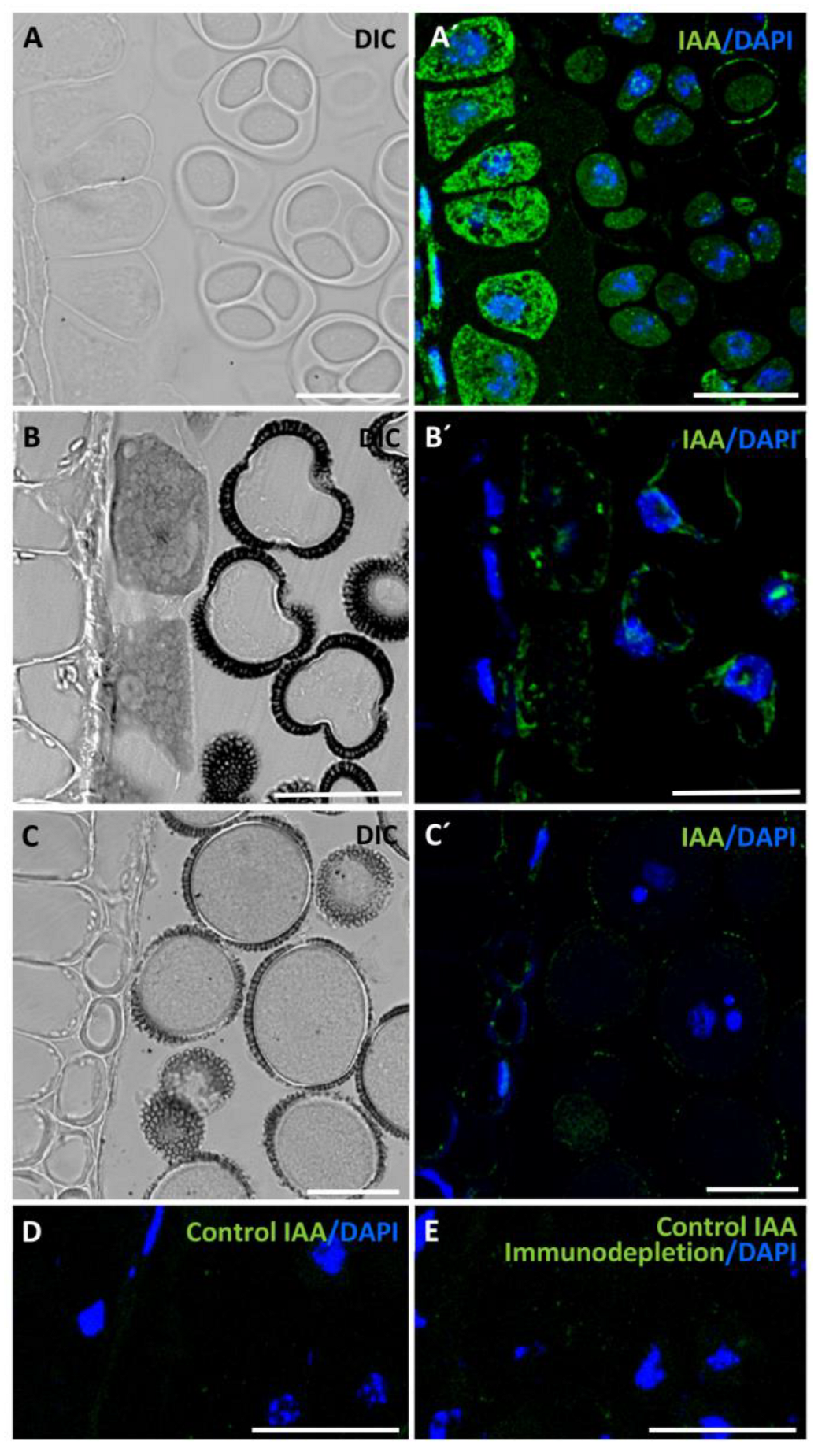
2.1. Endogenous Auxin Accumulation and Distribution Pattern during Pollen Development and Microspore Embryogenesis
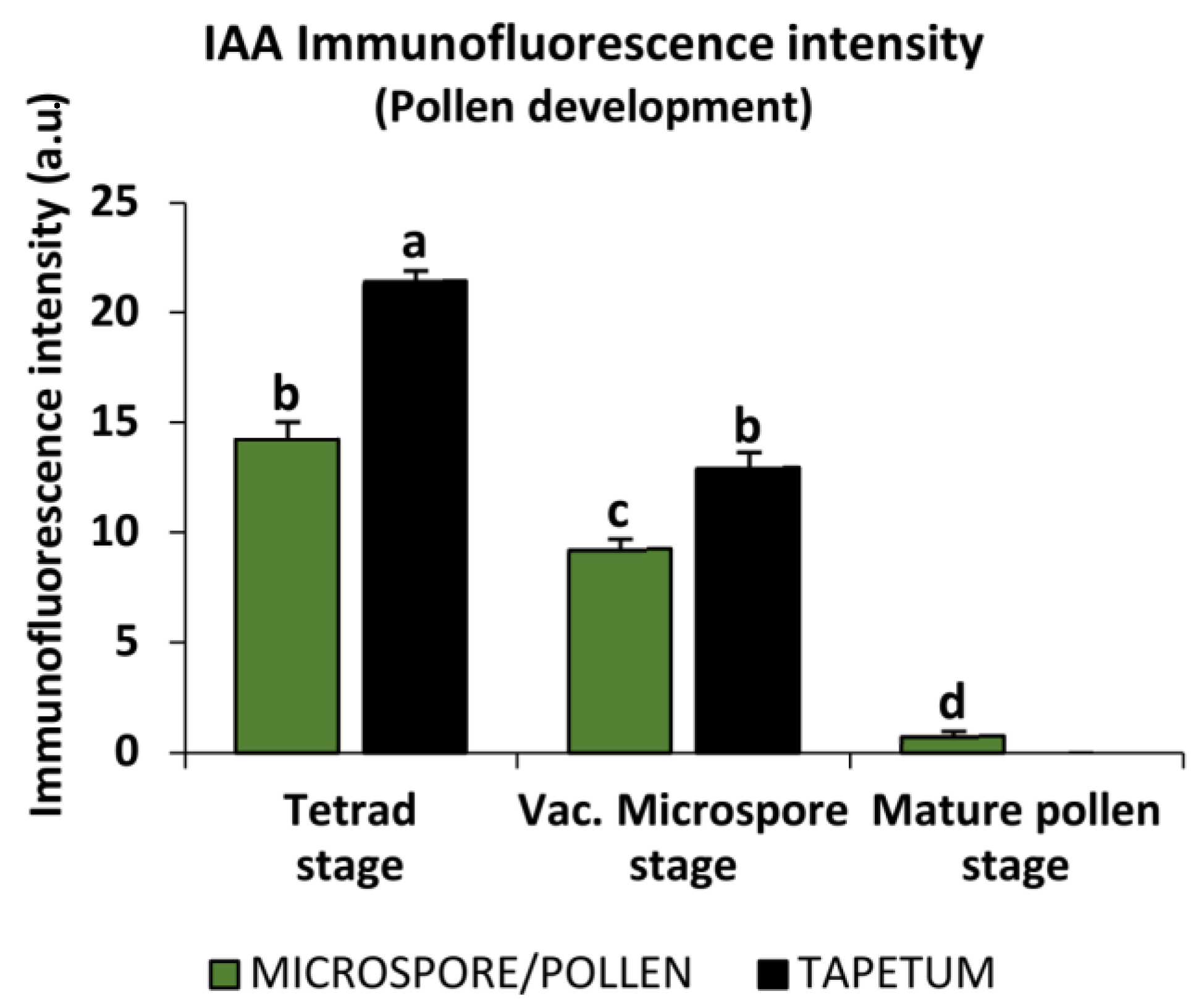
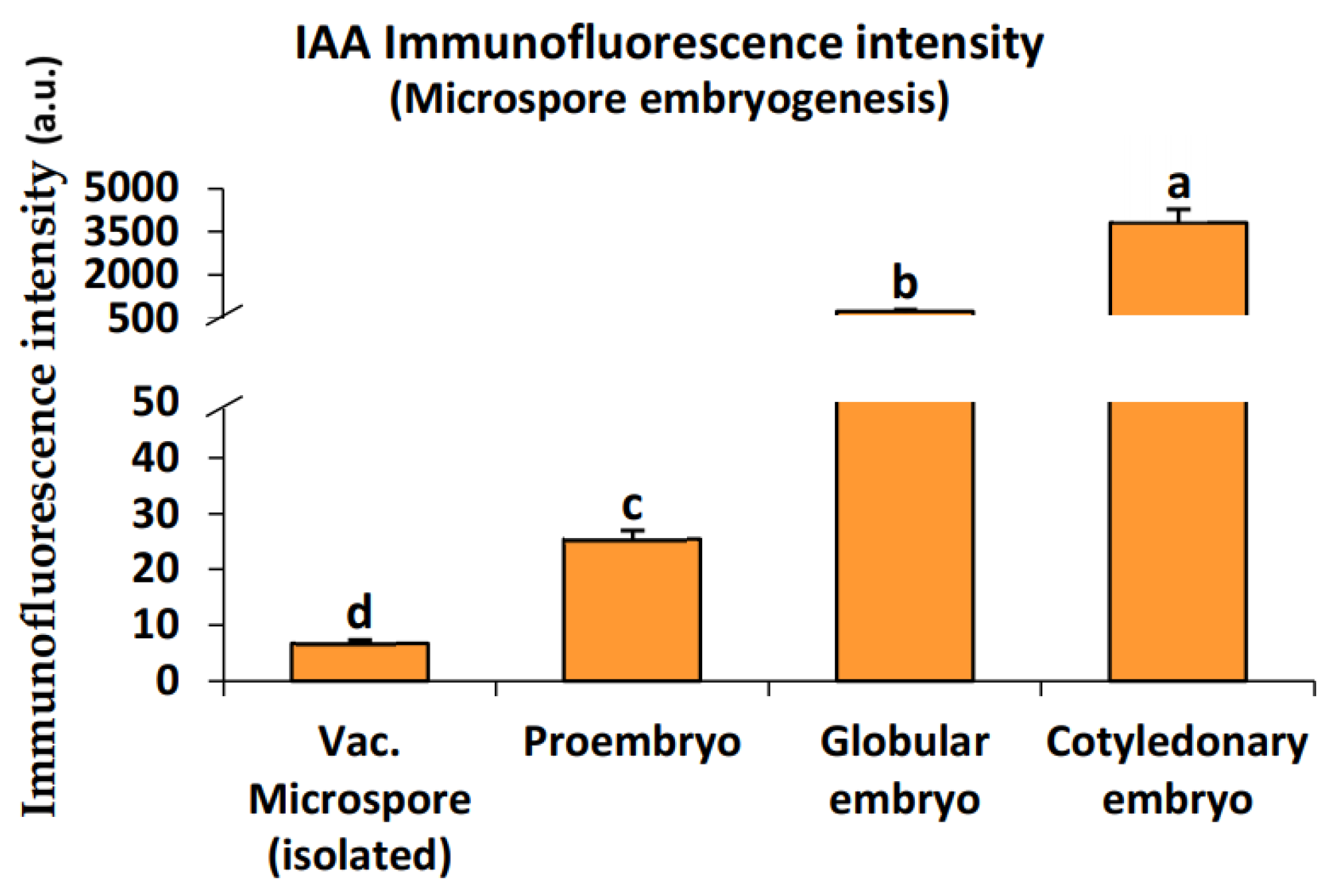
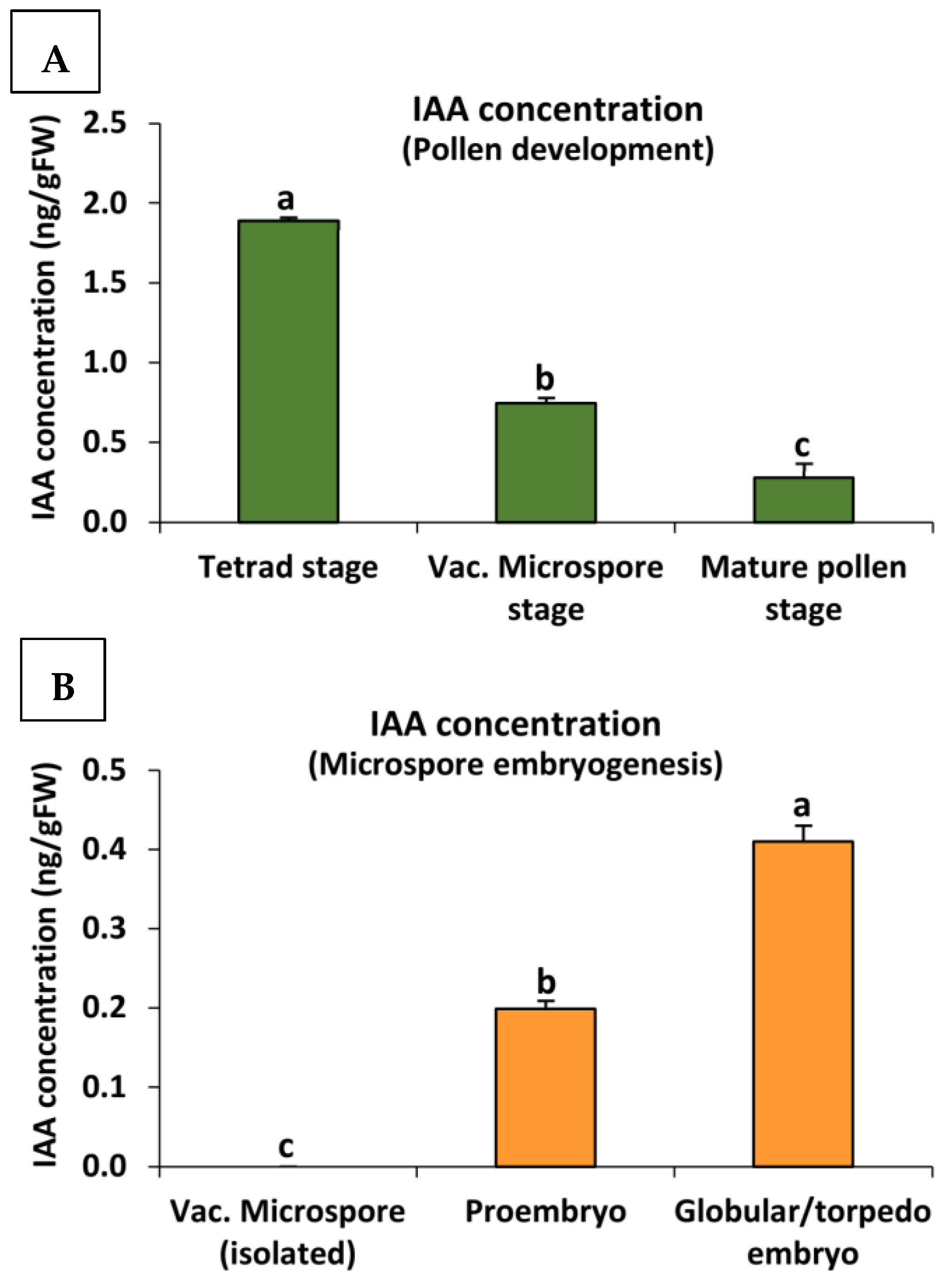
2.2. Endogenous Auxin Concentration during Pollen Development and Microspore Embryogenesis
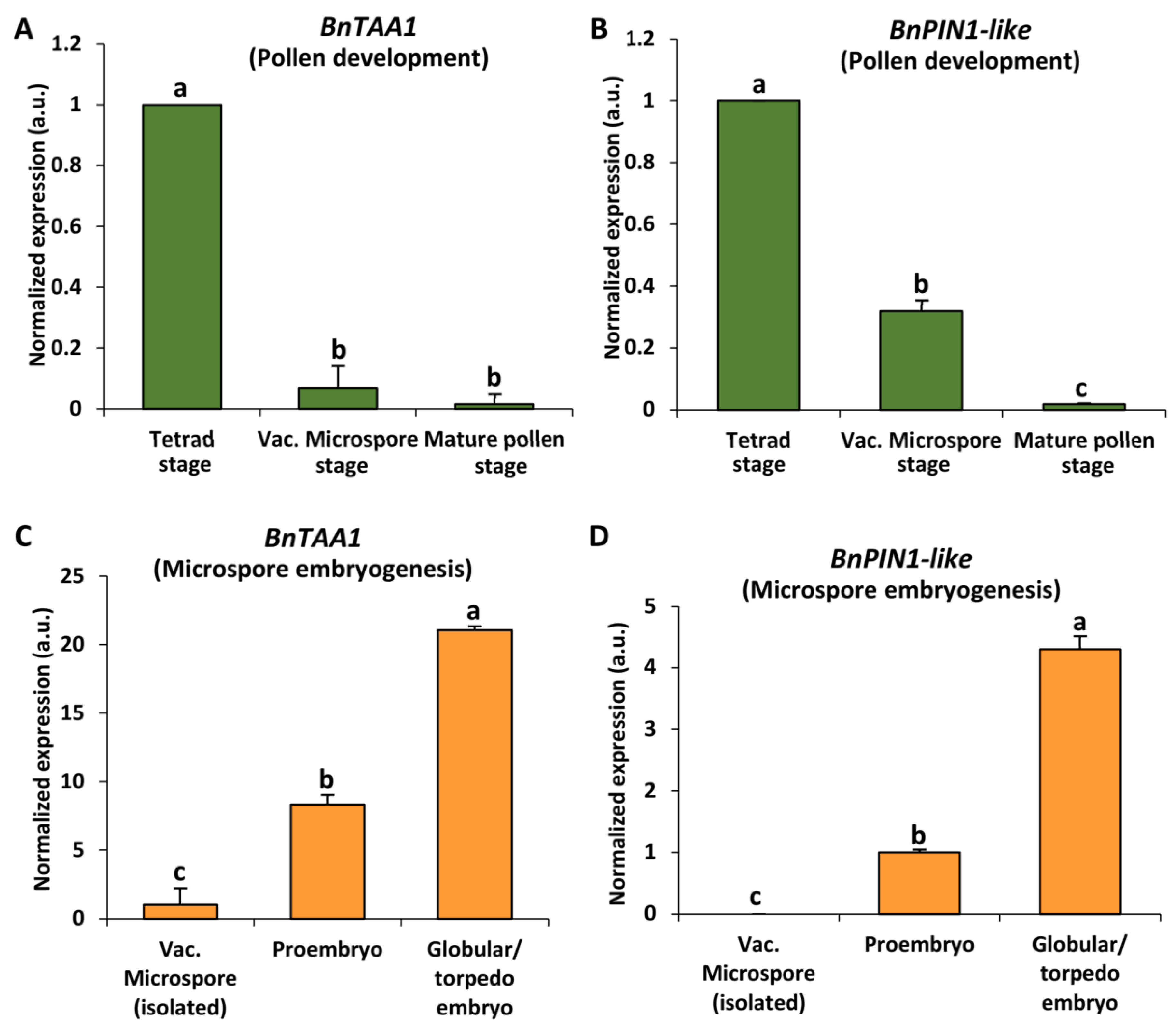
2.3. Expression Patterns of Auxin Biosynthesis and Transport Genes BnTAA1 and BnPIN1-like during Pollen Development and Microspore Embryogenesis
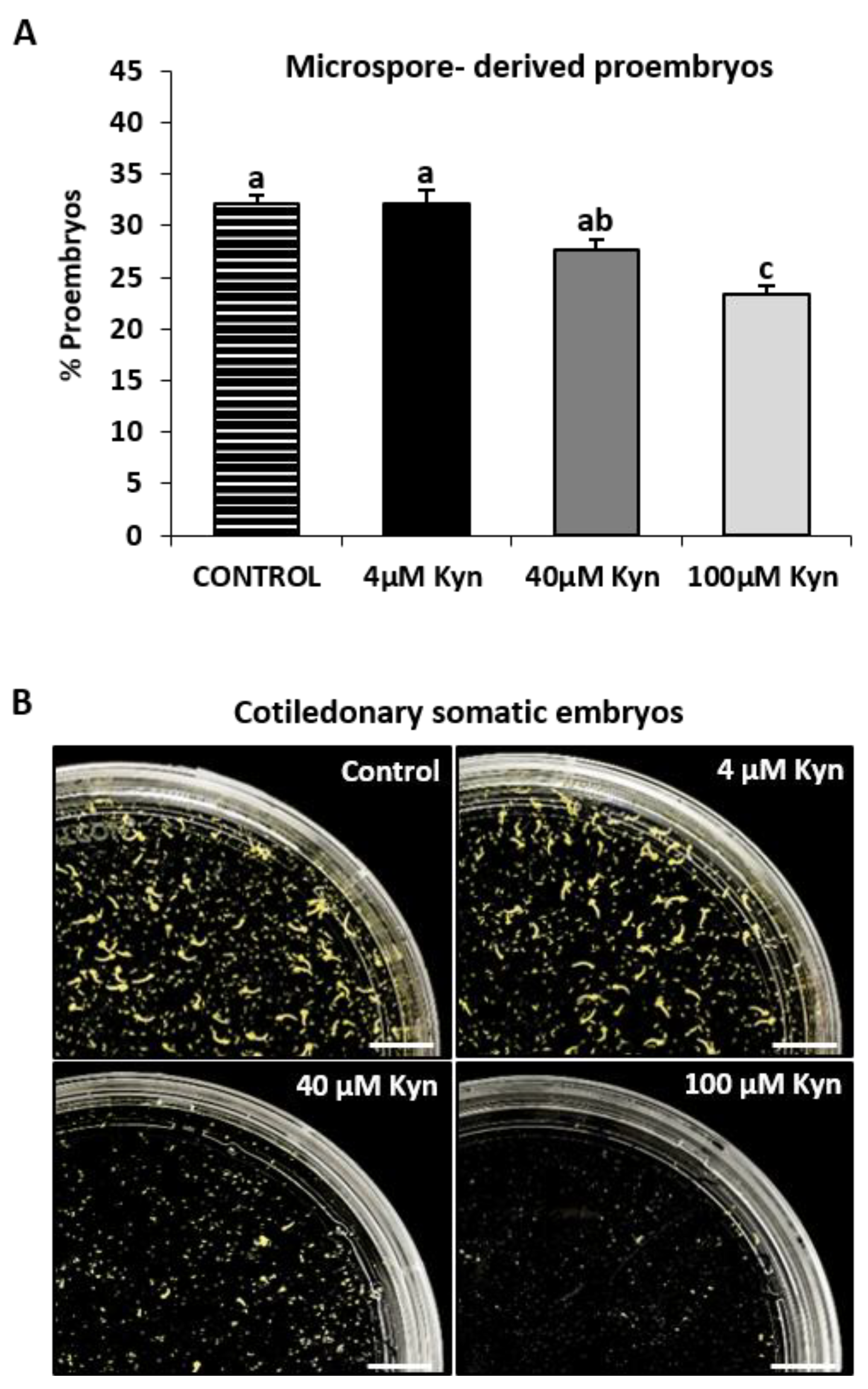
2.4. Effect of the Inhibition of Auxin Biosynthesis on Microspore Embryogenesis Initiation and Somatic Embryo Formation
3. Discussion
3.1. The Gametophytic Fate of the Microspore: Auxin Decreases during Pollen Development
3.2. The Change of Microspore Fate: Stress-Induced Cell Reprogramming Involve the Activation of Auxin Biosynthesis and Transport
3.3. Inhibition of Auxin Biosynthesis by Kynurenine Impaired Microspore Embryogenesis Initiation and Progression
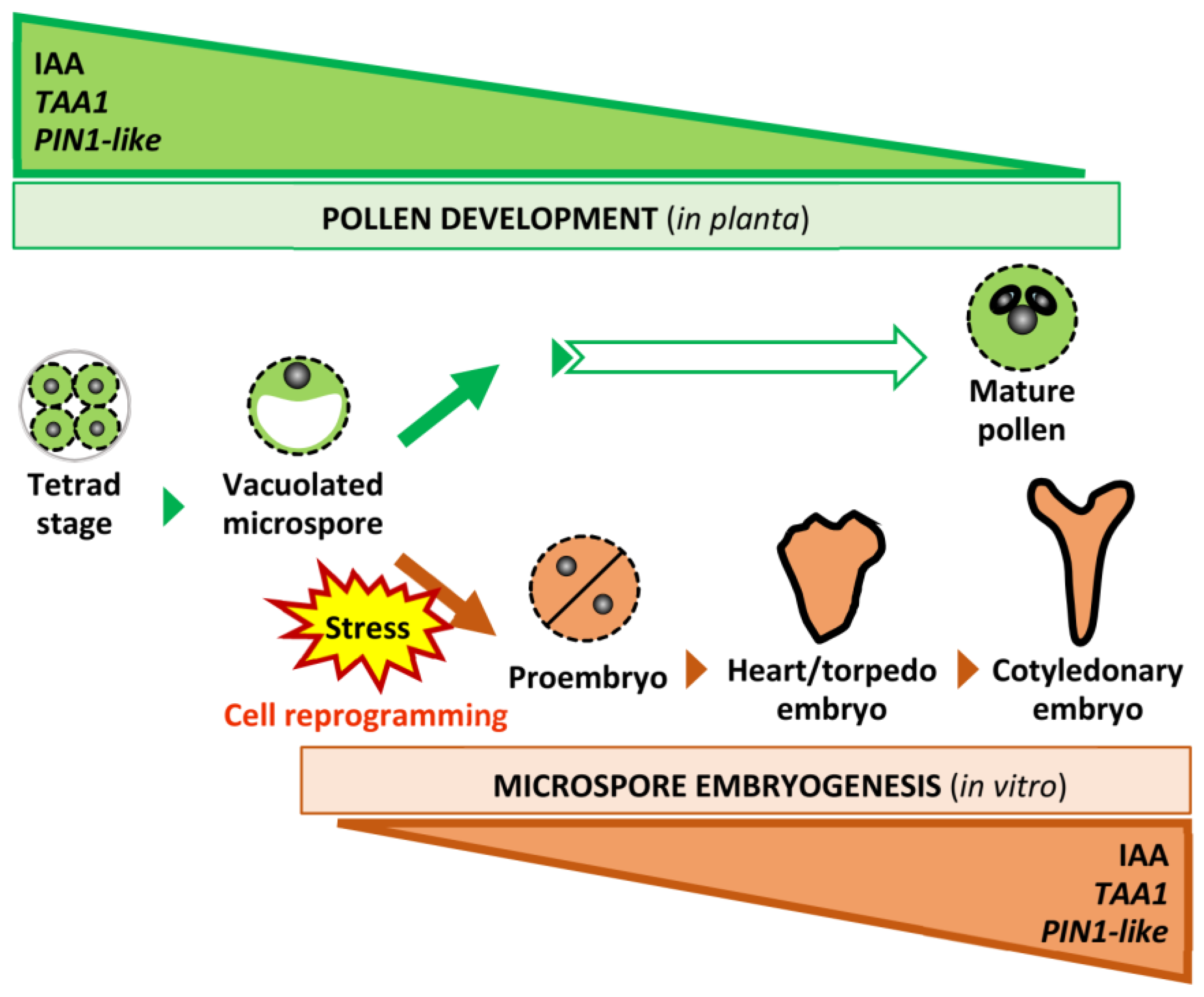
3.4. Opposite Auxin Dynamics Determine the Two Distinct Microspore Developmental Pathways and Cell Fate
4. Materials and Methods
4.1. Plant Material and Microspore Embryogenesis In Vitro Culture
4.2. Processing of Samples for Microscopy Analysis
4.3. Immunofluorescence Assays and Confocal Microscopy Analysis
4.4. Quantification of Auxin Immunofluorescence Signal
4.5. Quantification of Endogenous IAA Concentration
4.6. Expression Analysis by Reverse Transcriptase–Quantitative Polymerase Chain Reaction
4.7. Treatments with Kynurenine and Evaluation of Its Effect in Microspore Embryogenesis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Germaná, M.A.; Lambardi, M. In Vitro Embryogenesis in Higher Plants; Methods in Molecular Biology; Springer: New York, NY, USA, 2016; Volume 1359, ISBN 978-1-4939-3060-9. [Google Scholar]
- Testillano, P.S. Microspore Embryogenesis: Targeting the Determinant Factors of Stress-Induced Cell Reprogramming for Crop Improvement. J. Exp. Bot. 2019, 70, 2965–2978. [Google Scholar] [CrossRef]
- Stieglitz, H. Role of β-1,3-Glucanase in Postmeiotic Microspore Release. Dev. Biol. 1977, 57, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, H.G.; Lewis, D. The Formation of the Tryphine Coating the Pollen Grains of Raphanus, and Its Properties to the Relating. Proc. R. Soc. Lond. B Biol. Sci. 2014, 184, 149–165. [Google Scholar]
- Touraev, A.; Pfosser, M.; Vicente, O.; Heberle-Bors, E. Stress as the Major Signal Controlling the Developmental Fate of Tobacco Microspores: Towards a Unified Model of Induction of Microspore/Pollen Embryogenesis. Planta 1996, 200, 144–152. [Google Scholar] [CrossRef]
- Shariatpanahi, M.E.; Belogradova, K.; Hessamvaziri, L.; Heberle-Bors, E.; Touraev, A. Efficient Embryogenesis and Regeneration in Freshly Isolated and Cultured Wheat (Triticum aestivum L.) Microspores without Stress Pretreatment. Plant Cell Rep. 2006, 25, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Juzoń, K.; Krzewska, M.; Dziurka, M.; Dubas, E.; Kopeć, P.; Zieliński, K.; Żur, I. Chemically-Induced DNA de-Methylation Alters the Effectiveness of Microspore Embryogenesis in Triticale. Plant Sci. 2019, 287, 110189. [Google Scholar] [CrossRef]
- Maluszynski, M.; Kasha, K.J.; Forster, B.P.; Szarejko, I. Doubled Haploid Production in Crop Plants: A Manual; Springer: Dordrecht, The Netherlands, 2003; ISBN 978-90-481-6393-9. [Google Scholar]
- Germanà, M.A. Anther Culture for Haploid and Doubled Haploid Production. Plant Cell Tissue Organ. Cult. 2011, 104, 283–300. [Google Scholar] [CrossRef]
- Germanà, M.A.; Chiancone, B.; Guarda, N.L.; Testillano, P.S.; Risueño, M.C. Development of Multicellular Pollen of Eriobotrya japonica Lindl. through Anther Culture. Plant Sci. 2006, 171, 718–725. [Google Scholar] [CrossRef]
- Forster, B.P.; Heberle-Bors, E.; Kasha, K.J.; Touraev, A. The Resurgence of Haploids in Higher Plants. Trends Plant Sci. 2007, 12, 368–375. [Google Scholar] [CrossRef]
- Dunwell, J.M. Haploids in Flowering Plants: Origins and Exploitation. Plant Biotechnol. J. 2010, 8, 377–424. [Google Scholar] [CrossRef]
- Smýkal, P. Pollen Embryogenesis—The Stress Mediated Switch from Gametophytic to Sporophytic Development. Current Status and Future Prospects. Biol. Plant 2000, 43, 481–489. [Google Scholar] [CrossRef]
- Ibáñez, S.; Carneros, E.; Testillano, P.S.; Pérez-Pérez, J.M. Advances in Plant Regeneration: Shake, Rattle and Roll. Plants 2020, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.; Bemer, M.; Boutilier, K. A Transcriptional View on Somatic Embryogenesis. Regeneration 2017, 4, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A. Somatic Embryogenesis—Stress-Induced Remodeling of Plant Cell Fate. Biochim. Biophys. Acta Gene Regul. Mech. BBA-Gene Regul. Mech. 2015, 1849, 385–402. [Google Scholar] [CrossRef]
- Díaz-Sala, C. Molecular Dissection of the Regenerative Capacity of Forest Tree Species: Special Focus on Conifers. Front. Plant Sci. 2019, 9, 1943. [Google Scholar] [CrossRef]
- Möller, B.; Weijers, D. Auxin Control of Embryo Patterning. Cold Spring Harb. Perspect. Biol. 2009, 1, 1545. [Google Scholar] [CrossRef]
- Weijers, D.; Nemhauser, J.; Yang, Z. Auxin: Small Molecule, Big Impact. J. Exp. Bot. 2018, 69, 133–136. [Google Scholar] [CrossRef]
- Müller, D.; Leyser, O. Auxin, Cytokinin and the Control of Shoot Branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef]
- Prasad, K.; Dhonukshe, P. Polar Auxin Transport: Cell Polarity to Patterning. In Polar Auxin Transport. Signaling and Communication in Plants; Chen, R., Baluška, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 17, pp. 25–44. [Google Scholar]
- Mattsson, J.; Ckurshumova, W.; Berleth, T. Auxin Signaling in Arabidopsis Leaf Vascular Development. Plant Physiol. 2003, 131, 1327–1339. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Frimi, J.; Heldstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN Auxin Efflux Facilitator Network Controls Growth and Patterning in Arabidopsis Roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef]
- Moreno-Risueno, M.A.; Van Norman, J.M.; Moreno, A.; Zhang, J.; Ahnert, S.E.; Benfey, P.N. Oscillating Gene Expression Determines Competence for Periodic Arabidopsis Root Branching. Science 2010, 329, 1306–1311. [Google Scholar] [CrossRef]
- Chen, D.; Ren, Y.; Deng, Y.; Zhao, J. Auxin Polar Transport Is Essential for the Development of Zygote and Embryo in Nicotiana Tabacum L. and Correlated with ABP1 and PM H+-ATPase Activities. J. Exp. Bot. 2010, 61, 1853–1867. [Google Scholar] [CrossRef]
- Rademacher, E.H.; Möller, B.; Lokerse, A.S.; Llavata-Peris, C.I.; Van Den Berg, W.; Weijers, D. A Cellular Expression Map of the Arabidopsis Auxin Response Factor Gene Family. Plant J. 2011, 68, 597–606. [Google Scholar] [CrossRef]
- Petrášek, J.; Friml, J. Auxin Transport Routes in Plant Development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef] [PubMed]
- Mironova, V.; Teale, W.; Shahriari, M.; Dawson, J.; Palme, K. The Systems Biology of Auxin in Developing Embryos. Trends Plant Sci. 2017, 22, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Leyser, O. Auxin Signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [PubMed]
- McSteen, P. Auxin and Monocot Development. Cold Spring Harb. Perspect. Biol. 2010, 2, 1479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin Biosynthesis. In The Arabidopsis Book; The American Society of Plant Biologists: Washington, DC, USA, 2014; Volume 12, pp. 1–14. [Google Scholar]
- Brumos, J.; Alonso, J.M.; Stepanova, A.N. Genetic Aspects of Auxin Biosynthesis and Its Regulation. Physiol. Plant 2014, 151, 3–12. [Google Scholar] [CrossRef]
- He, W.; Brumos, J.; Li, H.; Ji, Y.; Ke, M.; Gong, X.; Zeng, Q.; Li, W.; Zhang, X.; An, F.; et al. A Small-Molecule Screen Identifies L-Kynurenine as a Competitive Inhibitor of TAA1/TAR Activity in Ethylene-Directed Auxin Biosynthesis and Root Growth in Arabidopsis. Plant Cell 2011, 23, 3944–3960. [Google Scholar] [CrossRef]
- de Wit, M.; Ljung, K.; Fankhauser, C. Contrasting Growth Responses in Lamina and Petiole during Neighbor Detection Depend on Differential Auxin Responsiveness Rather than Different Auxin Levels. New Phytol. 2015, 208, 198–209. [Google Scholar] [CrossRef]
- Nomura, T.; Itouga, M.; Kojima, M.; Kato, Y.; Sakakibara, H.; Hasezawa, S. Copper Mediates Auxin Signalling to Control Cell Differentiation in the Copper Moss Scopelophila cataractae. J. Exp. Bot. 2015, 66, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Ljung, K. Auxin Metabolism and Homeostasis during Plant Development. Development 2013, 140, 943–950. [Google Scholar] [CrossRef]
- Adamowski, M.; Friml, J. PIN-Dependent Auxin Transport: Action, Regulation, and Evolution. Plant Cell 2015, 27, 20–32. [Google Scholar] [CrossRef]
- Bennett, T. PIN Proteins and the Evolution of Plant Development. Trends Plant Sci. 2015, 20, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Zazimalova, E.; Murphy, A.S.; Yang, H.; Hoyerova, K.; Hosek, P. Auxin Transporters-Why So Many? Cold Spring Harb. Perspect. Biol. 2010, 2, a001552. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.; Sitbon, F.; Ljung, K.; Von Arnold, S. Inhibited Polar Auxin Transport Results in Aberrant Embryo Development in Norway spruce. New Phytol. 2008, 177, 356–366. [Google Scholar] [CrossRef]
- Hakman, I.; Hallberg, H.; Palovaara, J. The Polar Auxin Transport Inhibitor NPA Impairs Embryo Morphology and Increases the Expression of an Auxin Efflux Facilitator Protein PIN during Picea abies Somatic Embryo Development. Tree Physiol. 2009, 29, 483–496. [Google Scholar] [CrossRef]
- Abrahamsson, M.; Valledares, S.; Larsson, E.; Clapham, D.; Von Arnold, S. Patterning during Somatic Embryogenesis in Scots ine in Relation to Polar Auxin Transport and Programmed Cell Death. Plant Cell Tiss. Organ. Cult. 2012, 109, 391–400. [Google Scholar] [CrossRef]
- Prem, D.; Solís, M.T.; Bárány, I.; Rodríguez-Sanz, H.; Risueño, M.C.; Testillano, P.S. A New Microspore Embryogenesis System under Low Temperature Which Mimics Zygotic Embryogenesis Initials, Expresses Auxin and Efficiently Regenerates Doubled-Haploid Plants in Brassica napus. BMC Plant Biol. 2012, 12, 127. [Google Scholar] [CrossRef]
- Custers, J.B.M.; Cordewener, J.H.G.; Niillen, Y.; Dons, H.J.M.; Van, M.M.; Campagne, L. Plant Cell Reports Temperature Controls Both Gametophytic and Sporophytic Development in Microspore Cultures of Brassica napus. Plant Cell Rep. 1994, 13, 267–271. [Google Scholar] [CrossRef]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C.I. Role of Auxin in Regulating Arabidopsis Flower Development. Planta 2006, 223, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin Biosynthesis by the YUCCA Flavin Monooxygenases Controls the Formation of Floral Organs and Vascular Tissues in Arabidopsis. Genes. Dev. 2006, 20, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, E.; Østergaard, L. Distinct and Dynamic Auxin Activities during Reproductive Development. Cold Spring Harb. Perspect. Biol. 2009, 1, a001628. [Google Scholar] [CrossRef] [PubMed]
- Nic-Can, G.I.; Loyola-Vargas, V.M. The Role of the Auxins During Somatic Embryogenesis. In Somatic Embryogenesis: Fundamental Aspects and Applications; Springer International Publishing: Cham, Switzerland, 2016; pp. 171–182. ISBN 9783319337050. [Google Scholar]
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-La-Peña, C.; Loyola-Vargas, V.M. Signaling overview of plant somatic embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef]
- Awada, R.; Campa, C.; Gibault, E.; Déchamp, E.; Georget, F.; Lepelley, M.; Abdallah, C.; Erban, A.; Martinez-Seidel, F.; Kopka, J.; et al. Unravelling the Metabolic and Hormonal Machinery during Key Steps of Somatic Embryogenesis: A Case Study in Coffee. Int. J. Mol. Sci. 2019, 20, 4665. [Google Scholar] [CrossRef]
- Fan, Y.; Tang, Z.; Wei, J.; Yu, X.; Guo, H.; Li, T.; Guo, H.; Zhang, L.; Fan, Y.; Zhang, C.; et al. Dynamic Transcriptome Analysis Reveals Complex Regulatory Pathway Underlying Induction and Dose Effect by Different Exogenous Auxin IAA and 2,4-D During In vitro Embryogenic Redifferentiation in Cotton. Front. Plant Sci. 2022, 13, 931105. [Google Scholar] [CrossRef]
- Hale, B.; Phipps, C.; Rao, N.; Wijeratne, A.; Phillips, G.C. Differential Expression Profiling Reveals Stress-Induced Cell Fate Divergence in Soybean Microspores. Plants 2020, 9, 1510. [Google Scholar] [CrossRef]
- Smertenko, A.; Bozhkov, P.V. Somatic Embryogenesis: Life and Death Processes during Apical-Basal Patterning. J. Exp. Bot. 2014, 65, 1343–1360. [Google Scholar] [CrossRef]
- Solís, M.T.; Chakrabarti, N.; Corredor, E.; Cortés-Eslava, J.; Rodríguez-Serrano, M.; Biggiogera, M.; Risueño, M.C.; Testillano, P.S. Epigenetic Changes Accompany Developmental Programmed Cell Death in Tapetum Cells. Plant Cell Physiol. 2014, 55, 16–29. [Google Scholar] [CrossRef]
- Salinas-Grenet, H.; Herrera-Vásquez, A.; Parra, S.; Cortez, A.; Gutiérrez, L.; Pollmann, S.; León, G.; Blanco-Herrera, F. Modulation of Auxin Levels in Pollen Grains Affects Stamen Development and Anther Dehiscence in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 2480. [Google Scholar] [CrossRef]
- Pérez-Pérez, Y.; El-Tantawy, A.A.; Solís, M.T.; Risueño, M.C.; Testillano, P.S. Stress-Induced Microspore Embryogenesis Requires Endogenous Auxin Synthesis and Polar Transport in Barley. Front. Plant Sci. 2019, 10, 1200. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sanz, H.; Solís, M.T.; Lopez, M.F.; Gómez-Cadenas, A.; Risueño, M.C.; Testillano, P.S. Auxin Biosynthesis, Accumulation, Action and Transport Are Involved in Stress-Induced Microspore Embryogenesis Initiation and Progression in Brassica napus. Plant Cell Physiol. 2015, 56, 1401–1417. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.L.; Ni, W.M.; Elge, S.; Mueller-Roeber, B.; Xu, Z.H.; Xue, H.W. Auxin Flow in Anther Filaments Is Critical for Pollen Grain Development through Regulating Pollen Mitosis. Plant Mol. Biol. 2006, 61, 215–226. [Google Scholar] [CrossRef]
- Cecchetti, V.; Celebrin, D.; Napoli, N.; Ghelli, R.; Brunetti, P.; Costantino, P.; Cardarelli, M. An Auxin Maximum in the Middle Layer Controls Stamen Development and Pollen Maturation in Arabidopsis. New Phytol. 2017, 213, 1194–1207. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, M.; Cecchetti, V. Auxin Polar Transport in Stamen Formation and Development: How Many Actors? Front. Plant Sci. 2014, 5, 333. [Google Scholar] [CrossRef] [PubMed]
- Carneros, E.; Sánchez-Muñoz, J.; Pérez-Pérez, Y.; Pintos, B.; Gómez-Garay, A.; Testillano, P.S. Dynamics of Endogenous Auxin and Its Role in Somatic Embryogenesis Induction and Progression in Cork Oak. Plants 2023, 12, 1542. [Google Scholar] [CrossRef]
- Corredoira, E.; Cano, V.; Bárány, I.; Solís, M.T.; Rodríguez, H.; Vieitez, A.M.; Risueño, M.C.; Testillano, P.S. Initiation of Leaf Somatic Embryogenesis Involves High Pectin Esterification, Auxin Accumulation and DNA Demethylation in Quercus alba. J. Plant Physiol. 2017, 213, 42–54. [Google Scholar] [CrossRef]
- Vondrakova, Z.; Dobrev, P.I.; Pesek, B.; Fischerova, L.; Vagner, M.; Motyka, V. Profiles of Endogenous Phytohormones over the Course of Norway Spruce Somatic Embryogenesis. Front. Plant Sci. 2018, 9, 1283. [Google Scholar] [CrossRef]
- Caeiro, A.; Caeiro, S.; Correia, S.; Canhoto, J. Induction of Somatic Embryogenesis in Tamarillo (Solanum betaceum Cav.) Involves Increases in the Endogenous Auxin Indole-3Acetic Acid. Plants 2022, 11, 1347. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Qi, L.; Zhang, S. Integrated Transcriptomic and Metabolic Analyses Provide Insights into the Maintenance of Embryogenic Potential and the Biosynthesis of Phenolic Acids and Flavonoids Involving Transcription Factors in Larix kaempferi (Lamb.) Carr. Front. Plant Sci. 2022, 13, 1056930. [Google Scholar] [CrossRef]
- Rodríguez-Sanz, H.; Manzanera, J.A.; Solís, M.T.; Gómez-Garay, A.; Pintos, B.; Risueño, M.C.; Testillano, P.S. Early Markers Are Present in Both Embryogenesis Pathways from Microspores and Immature Zygotic Embryos in Cork Oak, Quercus suber L. BMC Plant Biol. 2014, 14, 224. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Yuan, D.; Jin, F.; Zhang, Y.; Xu, J. Transcript Profiling Reveals Complex Auxin Signalling Pathway and Transcription Regulation Involved in Dedifferentiation and Redifferentiation during Somatic Embryogenesis in Cotton. BMC Plant Biol. 2012, 12, 110. [Google Scholar] [CrossRef]
- Wójcikowska, B.; Gaj, M.D. Expression Profiling of AUXIN RESPONSE FACTOR Genes during Somatic Embryogenesis Induction in Arabidopsis. Plant Cell Rep. 2017, 36, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, A.M.; Wójcikowska, B.; Gaj, M.D. Current Perspectives on the Auxin-Mediated Genetic Network That Controls the Induction of Somatic Embryogenesis in Plants. Int. J. Mol. Sci. 2020, 21, 1333. [Google Scholar] [CrossRef] [PubMed]
- Uc-Chuc, M.A.; Pérez-Hernández, C.; Galaz-ávalos, R.M.; Brito-Argaez, L.; Aguilar-Hernández, V.; Loyola-Vargas, V.M. YUCCA-Mediated Biosynthesis of the Auxin IAA Is Required during the Somatic Embryogenic Induction Process in Coffea canephora. Int. J. Mol. Sci. 2020, 21, 4751. [Google Scholar] [CrossRef]
- Li, M.; Wrobel-Marek, J.; Heidmann, I.; Horstman, A.; Chen, B.; Reis, R.; Angenent, G.C.; Boutilier, K. Auxin Biosynthesis Maintains Embryo Identity and Growth during BABY BOOM-Induced Somatic Embryogenesis. Plant Physiol. 2022, 188, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.S.; Park, C.; Gutièrrez, C.L.; Wójcikowska, B.; Pěnčík, A.; Novák, O.; Chen, J.; Grunewald, W.; Dresselhaus, T.; Friml, J.; et al. Maternal Auxin Supply Contributes to Early Embryo Patterning in Arabidopsis. Nat. Plants 2018, 4, 548–553. [Google Scholar] [CrossRef]
- Robert, H.S.; Grones, P.; Stepanova, A.N.; Robles, L.M.; Lokerse, A.S.; Alonso, J.M.; Weijers, D.; Friml, J. Local Auxin Sources Orient the Apical-Basal Axis in Arabidopsis Embryos. Curr. Biol. 2013, 23, 2506–2512. [Google Scholar] [CrossRef]
- Supena, E.D.J.; Winarto, B.; Riksen, T.; Dubas, E.; Van Lammeren, A.; Offringa, R.; Boutilier, K.; Custers, J. Regeneration of Zygotic-like Microspore-Derived Embryos Suggests an Important Role for the Suspensor in Early Embryo Patterning. J. Exp. Bot. 2008, 59, 803–8014. [Google Scholar] [CrossRef]
- Pérez-Pastrana, J.; Testillano, P.S.; Bárány, I.; Canto-Flick, A.; Álvarez-López, D.; Pijeira-Fernández, G.; Avilés-Viñas, S.A.; Peña-Yam, L.; Muñoz-Ramírez, L.; Nahuat-Dzib, S.; et al. Endogenous Auxin Accumulation/Localization during Zygotic and Somatic Embryogenesis of Capsicum chinense Jacq. J. Plant Physiol. 2021, 258–259, 153333. [Google Scholar] [CrossRef]
- Forestan, C.; Meda, S.; Varotto, S. ZmPIN1-Mediated Auxin Transport Is Related to Cellular Differentiation during Maize Embryogenesis and Endosperm Development. Plant Physiol. 2010, 152, 1373–1390. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, Y. Auxin and Above-Ground Meristems. J. Exp. Bot. 2018, 69, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Teale, W.; Palme, K. Naphthylphthalamic Acid and the Mechanism of Polar Auxin Transport. J. Exp. Bot. 2018, 69, 303–312. [Google Scholar] [CrossRef]
- Xie, Q.; Frugis, G.; Colgan, D.; Chua, N. Arabidopsis NAC1 Transduces Auxin Signal Downstream of TIR1 to Promote Lateral Root Development. Genes. Dev. 2000, 14, 3024–3036. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; McSteen, P. The Role of Auxin Transport during Inflorescence Development in Maize (Zea Mays, Poaceae). Am. J. Bot. 2007, 94, 1745–1755. [Google Scholar] [CrossRef]
- Durgbanshi, A.; Arbona, V.; Pozo, O.; Miersch, O.; Sancho, J.V.; Gómez-Cadenas, A. Simultaneous Determination of Multiple Phytohormones in Plant Extracts by Liquid Chromatography-Electrospray Tandem Mass Spectrometry. J. Agric. Food Chem. 2005, 53, 8437–8442. [Google Scholar] [CrossRef]
- Arbona, C.V.; Go, A. Hormonal Modulation of Citrus Responses to Flooding. J. Plant Growth Regul. 2008, 27, 241–250. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Pérez, Y.; Solís, M.T.; Albacete, A.; Testillano, P.S. Opposite Auxin Dynamics Determine the Gametophytic and Embryogenic Fates of the Microspore. Int. J. Mol. Sci. 2023, 24, 11177. https://doi.org/10.3390/ijms241311177
Pérez-Pérez Y, Solís MT, Albacete A, Testillano PS. Opposite Auxin Dynamics Determine the Gametophytic and Embryogenic Fates of the Microspore. International Journal of Molecular Sciences. 2023; 24(13):11177. https://doi.org/10.3390/ijms241311177
Chicago/Turabian StylePérez-Pérez, Yolanda, María Teresa Solís, Alfonso Albacete, and Pilar S. Testillano. 2023. "Opposite Auxin Dynamics Determine the Gametophytic and Embryogenic Fates of the Microspore" International Journal of Molecular Sciences 24, no. 13: 11177. https://doi.org/10.3390/ijms241311177
APA StylePérez-Pérez, Y., Solís, M. T., Albacete, A., & Testillano, P. S. (2023). Opposite Auxin Dynamics Determine the Gametophytic and Embryogenic Fates of the Microspore. International Journal of Molecular Sciences, 24(13), 11177. https://doi.org/10.3390/ijms241311177






