Influence of Single-Wall Carbon Nanotube Suspension on the Mechanical Properties of Polymeric Films and Electrospun Scaffolds
Abstract
1. Introduction
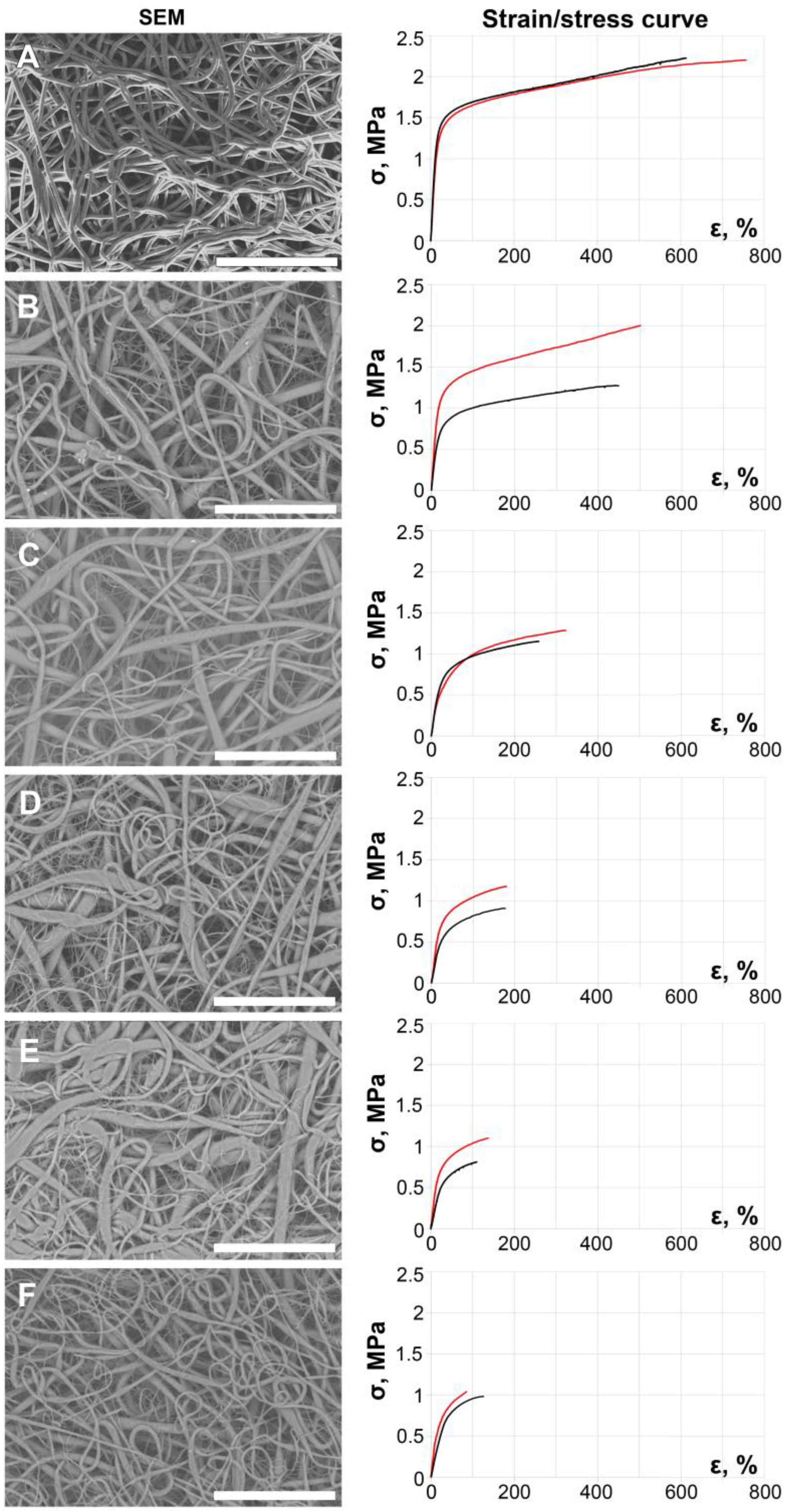
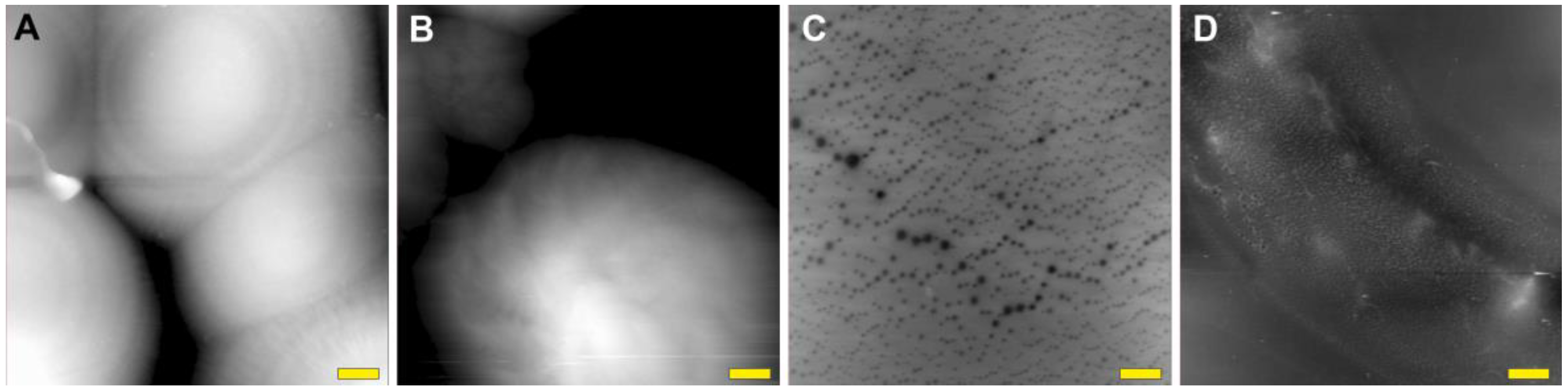
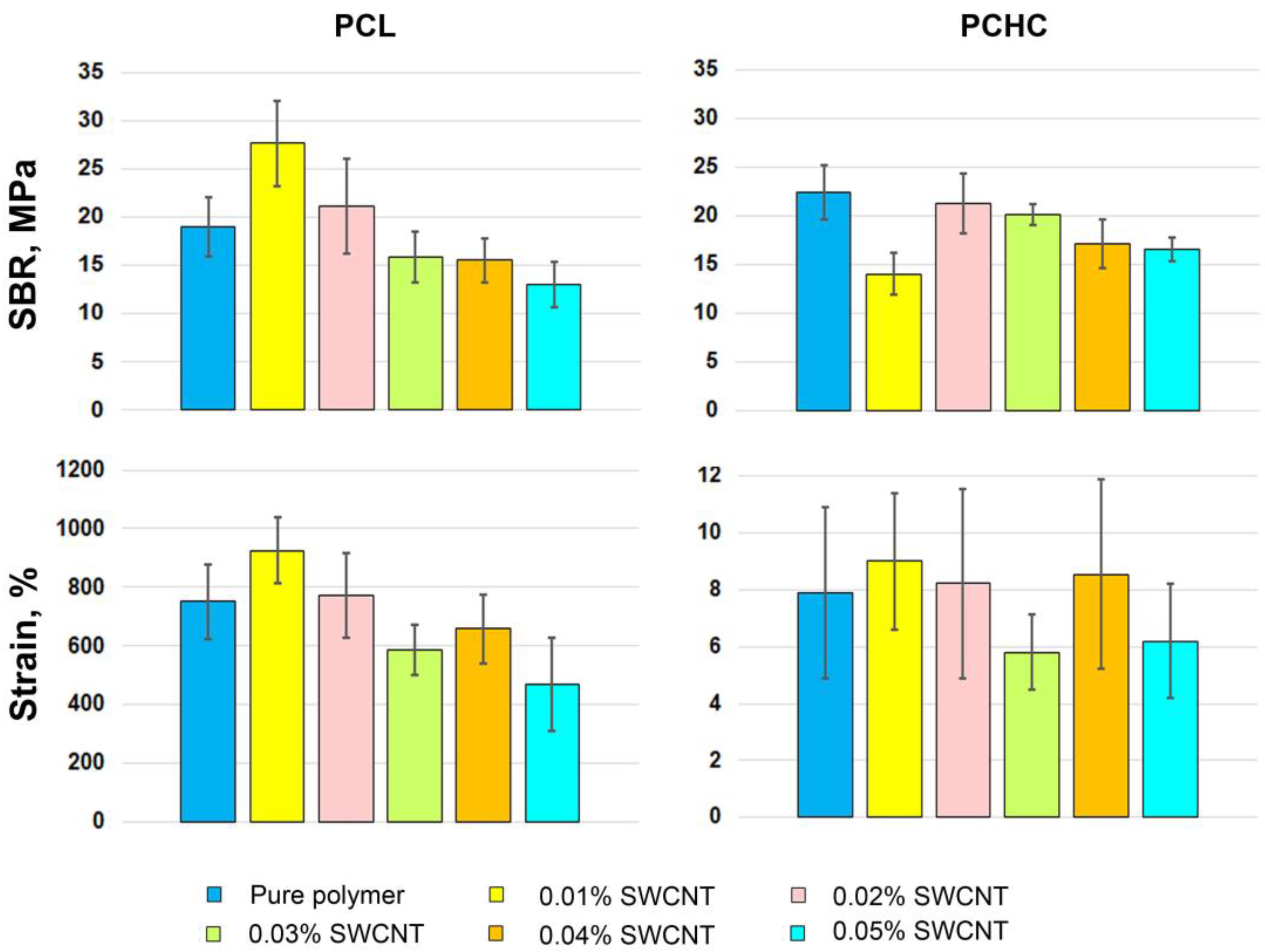
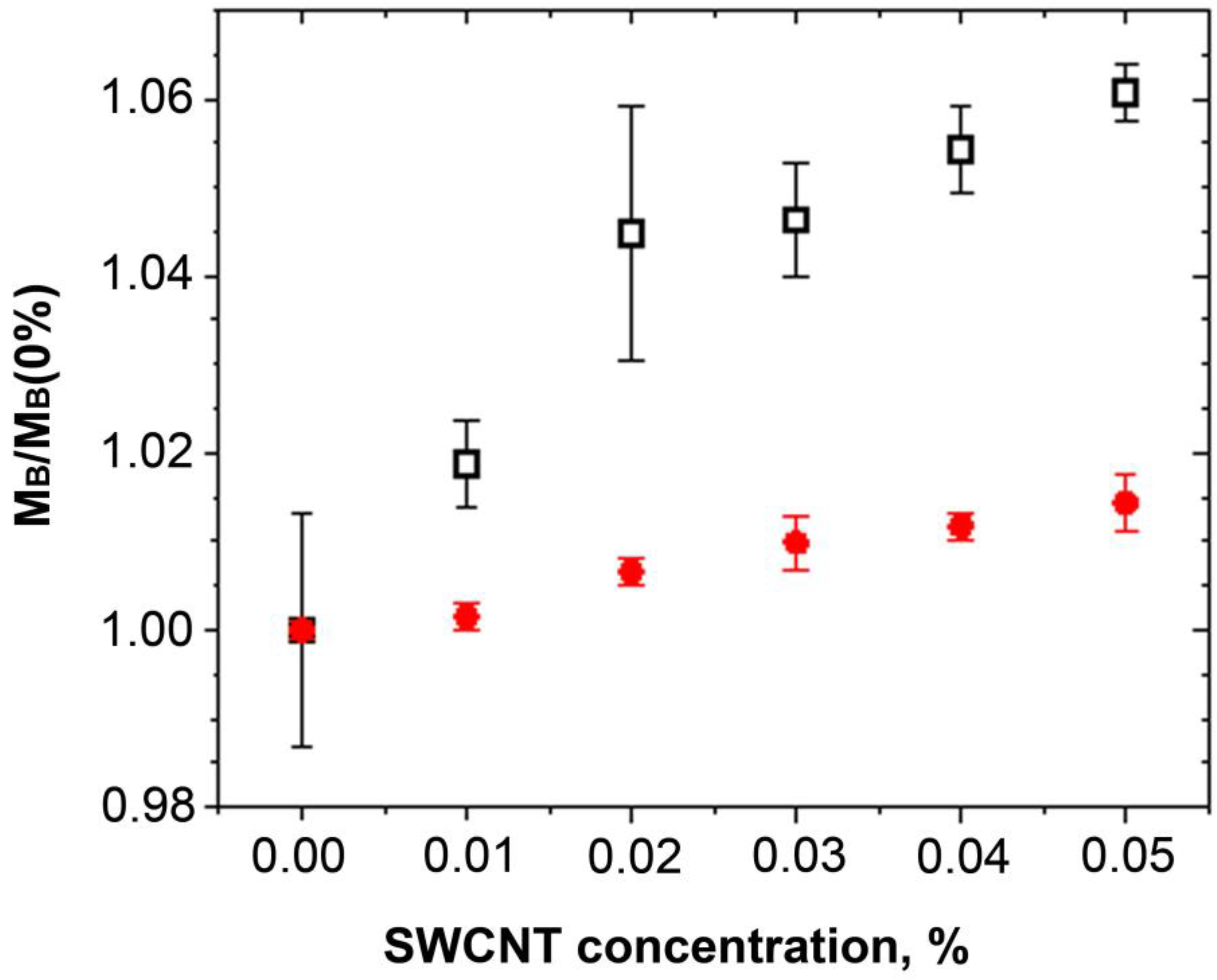
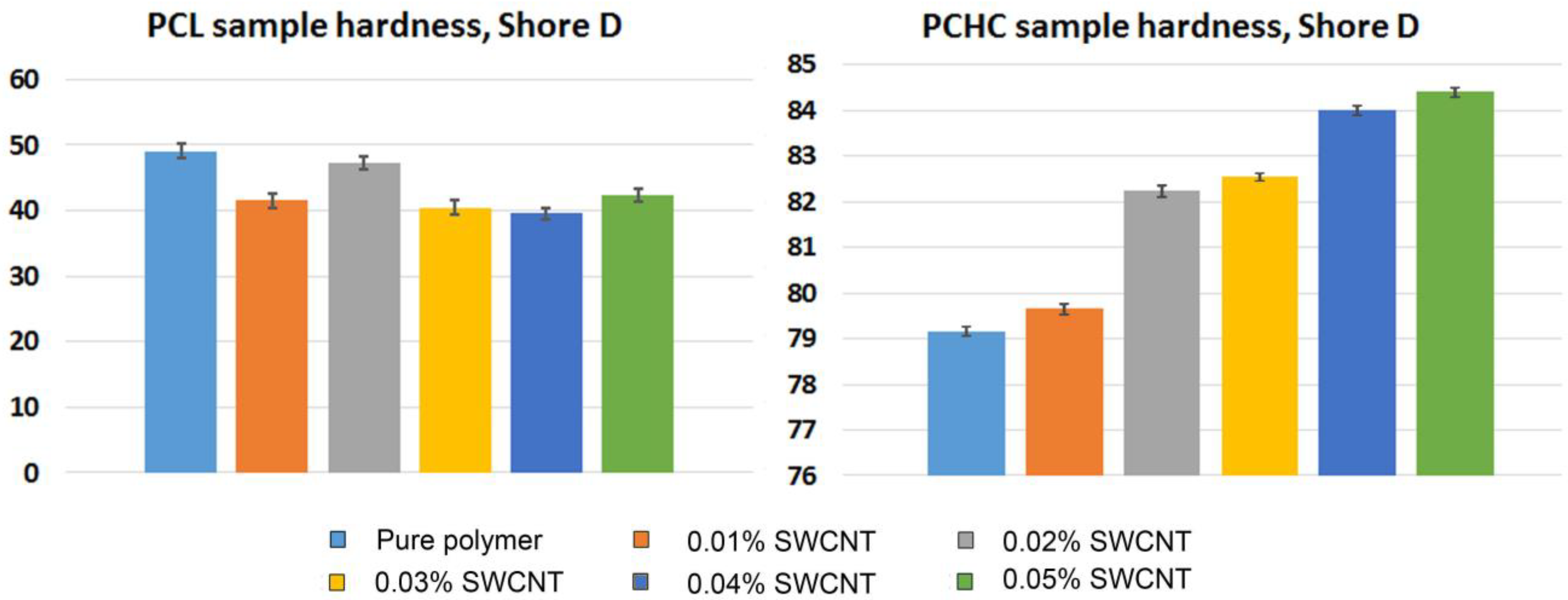
2. Results and Discussion
3. Materials and Methods
3.1. Nanotube Suspension Preparation
3.2. Polymer Composition
3.3. Scaffold Manufacturing
3.4. Film Fabrication
3.5. Mechanical Properties Evaluation
3.6. Sample Hardness Measurements
3.7. Brillouin Scattering Spectroscopy
3.8. Scanning Electron Microscopy (SEM)
3.9. Atomic Force Microscopy
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirillova, A.; Yeazel, T.R.; Asheghali, D.; Petersen, S.R.; Dort, S.; Gall, K.; Becker, M.L. Fabrication of biomedical scaffolds using biodegradable polymers. Chem. Rev. 2021, 121, 11238–11304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; King, M.W. Biodegradable polymers as the pivotal player in the design of tissue engineering scaffolds. Adv. Healthc. Mater. 2020, 9, 1901358. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Li, M.; Weng, H.; Gu, G.; Chen, Z. Design and comprehensive assessment of a biomimetic tri-layer tubular scaffold via biodegradable polymers for vascular tissue engineering applications. Mat. Sci. Eng. C 2020, 110, 110717. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.; Quint, C. The improvement of cell infiltration in an electrospun scaffold with multiple synthetic biodegradable polymers using sacrificial PEO microparticles. J. Biomed. Mat. Res. A 2019, 107, 1954–1964. [Google Scholar] [CrossRef] [PubMed]
- Van Bochove, B.; Grijpma, D.W. Photo-crosslinked synthetic biodegradable polymer networks for biomedical applications. J. Biomater. Sci. 2019, 30, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.W.; Toong, D.W.Y.; Ng, J.C.K.; Ow, V.; Lu, S.; Tan, L.P.; Wong, P.E.H.; Venkatraman, S.; Huang, Y.; Ang, H.Y. Polymer blends and polymer composites for cardiovascular implants. Eur. Polym. J. 2021, 146, 110249. [Google Scholar] [CrossRef]
- Leal, B.B.; Wakabayashi, N.; Oyama, K.; Kamiya, H.; Braghirolli, D.I.; Pranke, P. Vascular tissue engineering: Polymers and methodologies for small caliber vascular grafts. Front. Cardiovasc. Med. 2021, 7, 592361. [Google Scholar] [CrossRef]
- Barrows, T.H. Degradable implant materials: A review of synthetic absorbable polymers and their applications. Clin. Mater. 1986, 1, 233–257. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 10, 930–968. [Google Scholar] [CrossRef]
- Xing, Y.; Gu, Y.; Guo, L.; Guo, J.; Xu, Z.; Xiao, Y.; Fang, Z.; Wang, C.; Feng, Z.; Wang, Z. Gelatin coating promotes in situ endothelialization of electrospun polycaprolactone vascular grafts. J. Biomater. Sci. 2021, 32, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, B.; Yin, A.; Zhang, B.; Luo, R.; Pan, J.; Wang, Y. Polycaprolactone vascular graft with epigallocatechin gallate embedded sandwiched layer-by-layer functionalization for enhanced antithrombogenicity and anti-inflammation. J. Control. Release 2020, 320, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Rickel, A.P.; Deng, X.; Engebretson, D.; Hong, Z. Electrospun nanofiber scaffold for vascular tissue engineering. Mater. Sci. Eng. C 2021, 129, 112373. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, J.P.; Kang, H.J.; Fahad, M.A.A.; Park, M.; Choi, M.; Lee, H.Y.; Lee, B.T. Physico-mechanical and biological evaluation of heparin/VEGF-loaded electrospun polycaprolactone/decellularized rat aorta extracellular matrix for small-diameter vascular grafts. J. Biomater. Sci. 2022, 33, 1664–1684. [Google Scholar] [CrossRef]
- Agarwal, R.; Blum, K.M.; Musgrave, A.; Onwuka, E.A.; Yi, T.; Reinhardt, J.W.; Best, C.A.; Breuer, C.K. Degradation and in vivo evaluation of polycaprolactone, poly (ε-caprolactone-co-L-lactide), and poly-L-lactic acid as scaffold sealant polymers for murine tissue-engineered vascular grafts. Reg. Med. 2019, 14, 627–637. [Google Scholar] [CrossRef]
- Furdella, K.J.; Higuchi, S.; Behrangzade, A.; Kim, K.; Wagner, W.R.; Geest, J.P.V. In-vivo assessment of a tissue engineered vascular graft computationally optimized for target vessel compliance. Acta Biomater. 2021, 123, 298–311. [Google Scholar] [CrossRef]
- Oztemur, J.; Yalcin-Enis, I. Development of biodegradable webs of PLA/PCL blends prepared via electrospinning: Morphological, chemical, and thermal characterization. JBMA B 2021, 109, 1844–1856. [Google Scholar] [CrossRef]
- Tiyek, I.; Gunduz, A.; Yalcinkaya, F.; Chaloupek, J. Influence of electrospinning parameters on the hydrophilicity of electrospun polycaprolactone nanofibres. J. Nanosci. Nanotechnol. 2019, 19, 7251–7260. [Google Scholar] [CrossRef]
- Sharma, D.; Satapathy, B.K. Optimization and physical performance evaluation of electrospun nanofibrous mats of PLA, PCL and their blends. J. Ind. Text. 2022, 51, 6640S–6665S. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.; Park, K.; Kim, B.K. Non-Toxic Natural Additives to Improve the Electrical Conductivity and Viscosity of Polycaprolactone for Melt Electrospinning. App. Sci. 2023, 13, 1844. [Google Scholar] [CrossRef]
- Maccaferri, E.; Mazzocchetti, L.; Benelli, T.; Brugo, T.M.; Zucchelli, A.; Giorgini, L. Rubbery nanofibers by co-electrospinning of almost immiscible NBR and PCL blends. Mater. Des. 2020, 186, 108210. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, K.; Liu, Y.; Zhang, C.; Wang, B. Using wet electrospun PCL/gelatin/CNT yarns to fabricate textile-based scaffolds for vascular tissue engineering. ACS Biomat. Sci. Eng. 2021, 7, 2627–2637. [Google Scholar] [CrossRef]
- Lin, H.C.; Ko, B.T.; Wu, T.M. Thermal and Mechanical Properties of CO 2-Based Biodegradable Poly (cyclohexene carbonate)/Organically Modified Layered Zinc Phenylphosphonate Nanocomposites. J. Polym. Environ. 2019, 27, 1065–1070. [Google Scholar] [CrossRef]
- Lin, L.; Xu, Y.; Qin, J.; Wang, S.; Xiao, M.; Meng, Y. Correlation between crystallization behavior and mechanical properties of biodegradable poly (caprolactone-co-cyclohexene carbonate). Polym. Plast. Technol. Eng. 2018, 57, 1530–1541. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Z.I.; Yang, J.; Liu, G.L.; Hu, Z.; Huang, H.; Li, X.; Liu, Q.; Ma, M.; Xu, Z.; et al. Carbon dioxide-derived biodegradable and cationic polycarbonates as a new siRNA carrier for gene therapy in pancreatic cancer. Nanomaterials 2021, 11, 2312. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.L.; Wu, H.W.; Lin, Z.I.; Liao, M.G.; Su, Y.C.; Chen, C.K.; Ko, B.T. Synthesis of functional CO 2-based polycarbonates via dinuclear nickel nitrophenolate-based catalysis for degradable surfactant and drug-loaded nanoparticle applications. Polym. Chem. 2021, 12, 1244–1259. [Google Scholar] [CrossRef]
- Welle, A.; Kröger, M.; Döring, M.; Niederer, K.; Pindel, E.; Chronakis, I.S. Electrospun aliphatic polycarbonates as tailored tissue scaffold materials. Biomaterials 2007, 28, 2211–2219. [Google Scholar] [CrossRef]
- Kamphuis, A.J.; Picchioni, F.; Pescarmona, P.P. CO2-fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, L.; Xiao, M.; Wang, S.; Smith, A.T.; Sun, L.; Meng, Y. Synthesis and properties of CO2-based plastics: Environmentally-friendly, energy-saving and biomedical polymeric materials. Prog. Polym. Sci. 2018, 80, 163–182. [Google Scholar] [CrossRef]
- Koning, C.; Wildeson, J.; Parton, R.; Plum, B.; Steeman, P.; Darensbourg, D.J. Synthesis and physical characterization of poly (cyclohexane carbonate), synthesized from CO2 and cyclohexene oxide. Polymer 2001, 42, 3995–4004. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, T.; Zhou, Y.; Zhou, D.; Shen, Z.; Lin, L. Mechanism investigation of thermal degradation of CO2-based poly (cyclohexene carbonate caprolactone). Polym. Degrad. Stab. 2019, 168, 108957. [Google Scholar] [CrossRef]
- Dror, Y.; Salalha, W.; Khalfin, R.L.; Cohen, Y.; Yarin, A.L.; Zussman, E. Carbon nanotubes embedded in oriented polymer nanofibers by electrospinning. Langmuir 2003, 19, 7012–7020. [Google Scholar] [CrossRef]
- Zadehnajar, P.; Akbari, B.; Karbasi, S.; Mirmusavi, M.H. Preparation and characterization of poly ε-caprolactone-gelatin/multi-walled carbon nanotubes electrospun scaffolds for cartilage tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 326–337. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Su, Y.; Toftdal, M.S.; Le Friec, A.; Dong, M.; Han, X.; Chen, M. 3D electrospun synthetic extracellular matrix for tissue regeneration. Small Sci. 2021, 1, 2100003. [Google Scholar] [CrossRef]
- Xu, B.; Li, Y.; Zhu, C.; Cook, W.D.; Forsythe, J.; Chen, Q. Fabrication, mechanical properties and cytocompatibility of elastomeric nanofibrous mats of poly (glycerol sebacate). Eur. Polym. J. 2015, 64, 79–92. [Google Scholar] [CrossRef]
- Vogt, L.; Ruther, F.; Salehi, S.; Boccaccini, A.R. Poly (glycerol sebacate) in biomedical applications—A review of the recent literature. Adv. Healthc. Mater. 2021, 10, 2002026. [Google Scholar] [CrossRef]
- Castro, K.C.; Campos, M.G.N.; Mei, L.H.I. Hyaluronic acid electrospinning: Challenges, applications in wound dressings and new perspectives. Int. J. Biol. Macromol. 2021, 173, 251–266. [Google Scholar] [CrossRef]
- Steel, E.M.; Azar, J.Y.; Sundararaghavan, H.G. Electrospun hyaluronic acid-carbon nanotube nanofibers for neural engineering. Materialia 2020, 9, 100581. [Google Scholar] [CrossRef]
- Weisman, J.A.; Jammalamadaka, U.; Tappa, K.; Mills, D.K. Doped halloysite nanotubes for use in the 3D printing of medical devices. Bioengineering 2017, 4, 96. [Google Scholar] [CrossRef]
- Huang, B. Carbon nanotubes and their polymeric composites: The applications in tissue engineering. Biomanufacturing Rev. 2020, 5, 3. [Google Scholar] [CrossRef]
- Kiran, A.R.; Kumari, G.K.; Krishnamurthy, P.T. Carbon nanotubes in drug delivery: Focus on anticancer therapies. J. Drug Deliv. Sci. Technol. 2020, 59, 101892. [Google Scholar] [CrossRef]
- Kaur, J.; Gill, G.S.; Jeet, K. Applications of carbon nanotubes in drug delivery: A comprehensive review. In Micro and Nano Technologies, Characterization and Biology of Nanomaterials for Drug Delivery. Nanoscience and Nanotechnology in Drug Delivery, 1st ed.; Mohapatra, S., Ranjan, S., Dasgupta, N., Kumar, R., Thomas, S., Eds.; Elsvier: Amsterdam, The Netherlands, 2019; pp. 113–135. [Google Scholar] [CrossRef]
- Kassem, A.; Ayoub, G.M.; Malaeb, L. Antibacterial activity of chitosan nano-composites and carbon nanotubes: A review. Sci. Total Environ. 2019, 668, 566–576. [Google Scholar] [CrossRef]
- Bartlet, K.; Movafaghi, S.; Kota, A.; Popat, K.C. Superhemophobic titania nanotube array surfaces for blood contacting medical devices. RSC Adv. 2017, 7, 35466–35476. [Google Scholar] [CrossRef]
- Feng, Y.; Luo, X.; Wu, F.; Liu, H.; Liang, E.; He, R.R.; Liu, M. Systematic studies on blood coagulation mechanisms of halloysite nanotubes-coated PET dressing as superior topical hemostatic agent. Chem. Eng. J. 2022, 428, 132049. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y.; Park, S.J. Modeling the roles of carbon nanotubes and interphase dimensions in the conductivity of nanocomposites. Results Phys. 2019, 15, 102562. [Google Scholar] [CrossRef]
- Arrigo, R.; Malucelli, G. Rheological behavior of polymer/carbon nanotube composites: An overview. Materials 2020, 13, 2771. [Google Scholar] [CrossRef]
- Omastová, M.; Číková, E.; Mičušík, M. Electrospinning of ethylene vinyl acetate/carbon nanotube nanocomposite fibers. Polymers 2019, 11, 550. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocompositesu. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef]
- Method for Obtaining Carbon Nanostructures and Apparatus. Available online: https://patents.google.com/patent/RU2573035C2/en (accessed on 2 May 2023).
- Rubel, R.I.; Ali, M.H.; Jafor, M.A.; Alam, M.M. Carbon nanotubes agglomeration in reinforced composites: A review. AIMS Mater. Sci. 2019, 6, 756–780. [Google Scholar] [CrossRef]
- Dokuchaeva, A.A.; Timchenko, T.P.; Karpova, E.V.; Vladimirov, S.V.; Soynov, I.A.; Zhuravleva, I.Y. Effects of Electrospinning Parameter Adjustment on the Mechanical Behavior of Poly-ε-caprolactone Vascular Scaffolds. Polymers 2022, 14, 349. [Google Scholar] [CrossRef]
- Hua, C.; Chen, Z.; Xu, Q.; He, L. Ring-banded spherulites in PCL and PCL/MWCNT solution-casting films and effect of compressed CO2 on them. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 784–792. [Google Scholar] [CrossRef]
- Wu, G.P.; Jiang, S.D.; Lu, X.B.; Ren, W.M.; Yan, S.K. Stereoregular poly (cyclohexene carbonate) s: Unique crystallization behavior. Chin. J. Polym. Sci. 2012, 30, 487–492. [Google Scholar] [CrossRef]
- Maghsoudlou, M.A.; Isfahani, R.B.; Saber-Samandari, S.; Sadighi, M. Effect of interphase, curvature and agglomeration of SWCNTs on mechanical properties of polymer-based nanocomposites: Experimental and numerical investigations. Compos. Part B Eng. 2019, 175, 107119. [Google Scholar] [CrossRef]
- ISO Standard No. 10868:2017; Nanotechnologies—Characterization of Single-Wall Carbon Nanotubes Using Ultraviolet-Visible-Near Infrared (UV-Vis-NIR) Absorption Spectroscopy. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/69547.html (accessed on 1 May 2023).




| Polymer | Parameter | SWCNT Concentration (%) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 | ||
| PCHC | νB, GHz | 12.26 ± 0.08 | 12.38 ± 0.03 | 12.53 ± 0.09 | 12.54 ± 0.04 | 12.59 ± 0.03 | 12.63 ± 0.02 |
| PCL | νB, GHz | 12.99 ± 0.006 | 13.00 ± 0.01 | 13.033 ± 0.01 | 13.054 ± 0.02 | 13.066 ± 0.01 | 13.083 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dokuchaeva, A.A.; Vladimirov, S.V.; Borodin, V.P.; Karpova, E.V.; Vaver, A.A.; Shiliaev, G.E.; Chebochakov, D.S.; Kuznetsov, V.A.; Surovtsev, N.V.; Adichtchev, S.V.; et al. Influence of Single-Wall Carbon Nanotube Suspension on the Mechanical Properties of Polymeric Films and Electrospun Scaffolds. Int. J. Mol. Sci. 2023, 24, 11092. https://doi.org/10.3390/ijms241311092
Dokuchaeva AA, Vladimirov SV, Borodin VP, Karpova EV, Vaver AA, Shiliaev GE, Chebochakov DS, Kuznetsov VA, Surovtsev NV, Adichtchev SV, et al. Influence of Single-Wall Carbon Nanotube Suspension on the Mechanical Properties of Polymeric Films and Electrospun Scaffolds. International Journal of Molecular Sciences. 2023; 24(13):11092. https://doi.org/10.3390/ijms241311092
Chicago/Turabian StyleDokuchaeva, Anna A., Sergey V. Vladimirov, Vsevolod P. Borodin, Elena V. Karpova, Andrey A. Vaver, Gleb E. Shiliaev, Dmitry S. Chebochakov, Vasily A. Kuznetsov, Nikolay V. Surovtsev, Sergey V. Adichtchev, and et al. 2023. "Influence of Single-Wall Carbon Nanotube Suspension on the Mechanical Properties of Polymeric Films and Electrospun Scaffolds" International Journal of Molecular Sciences 24, no. 13: 11092. https://doi.org/10.3390/ijms241311092
APA StyleDokuchaeva, A. A., Vladimirov, S. V., Borodin, V. P., Karpova, E. V., Vaver, A. A., Shiliaev, G. E., Chebochakov, D. S., Kuznetsov, V. A., Surovtsev, N. V., Adichtchev, S. V., Malikov, A. G., Gulov, M. A., & Zhuravleva, I. Y. (2023). Influence of Single-Wall Carbon Nanotube Suspension on the Mechanical Properties of Polymeric Films and Electrospun Scaffolds. International Journal of Molecular Sciences, 24(13), 11092. https://doi.org/10.3390/ijms241311092







