Dectin-1-Independent Macrophage Phagocytosis of Mycobacterium abscessus
Abstract
1. Introduction
2. Results
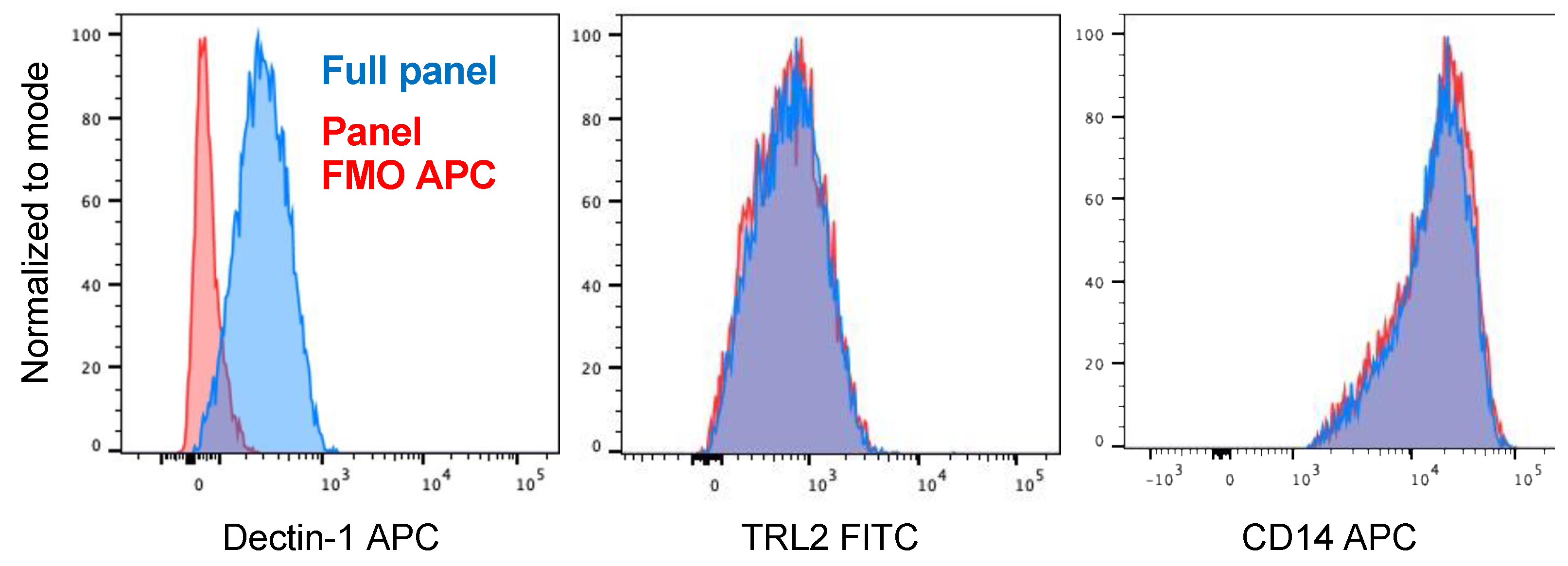
2.1. Blocking Antibody to Dectin-1 Reduces Phagocytosis of Zymosan by Human Monocyte Derived Macrophages but Does Not Decrease Uptake of M. abscessus
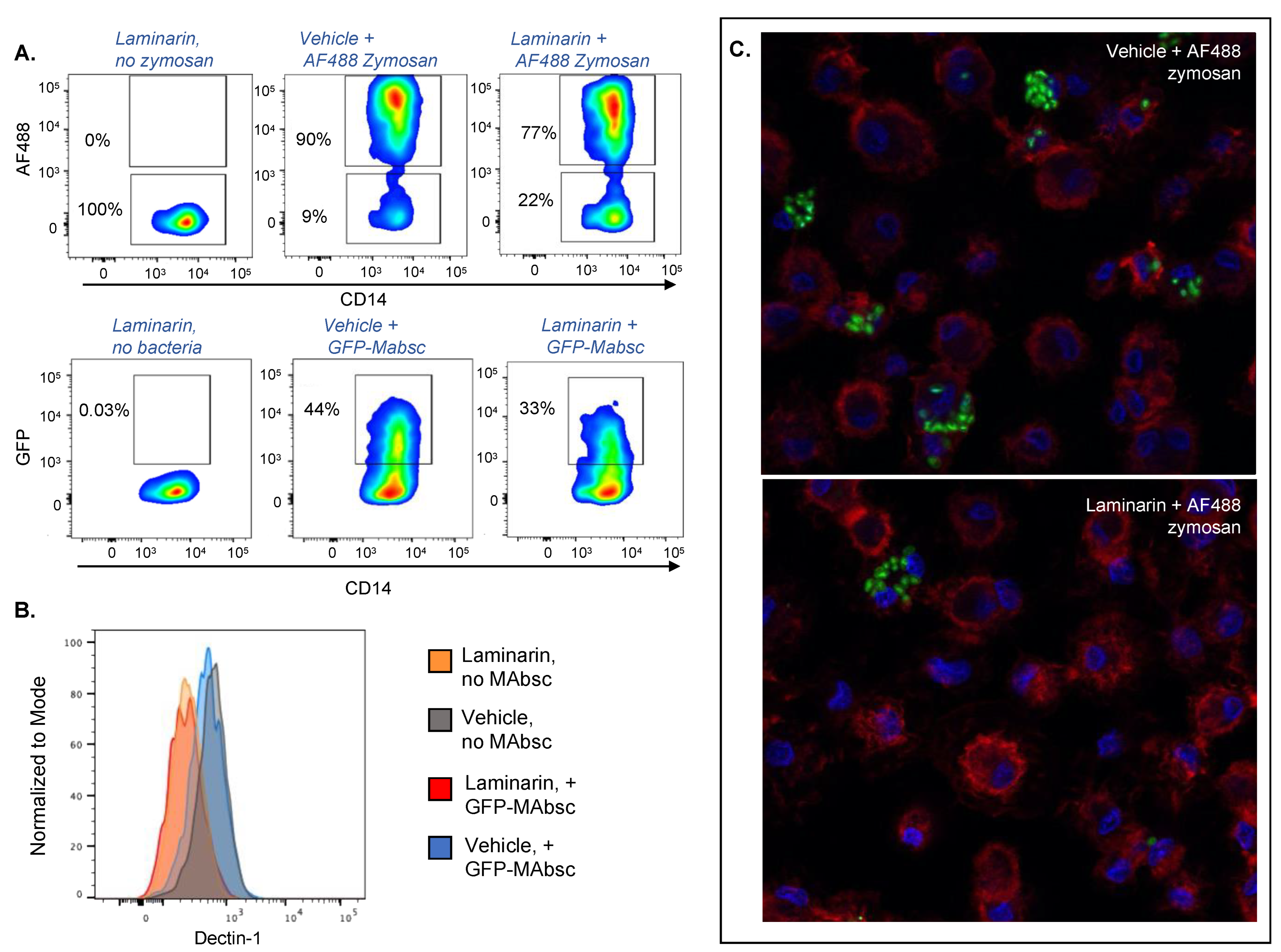
2.2. Laminarin Decreases Phagocytosis of Both M. abscessus and Zymosan by Human Monocyte Derived Macrophages
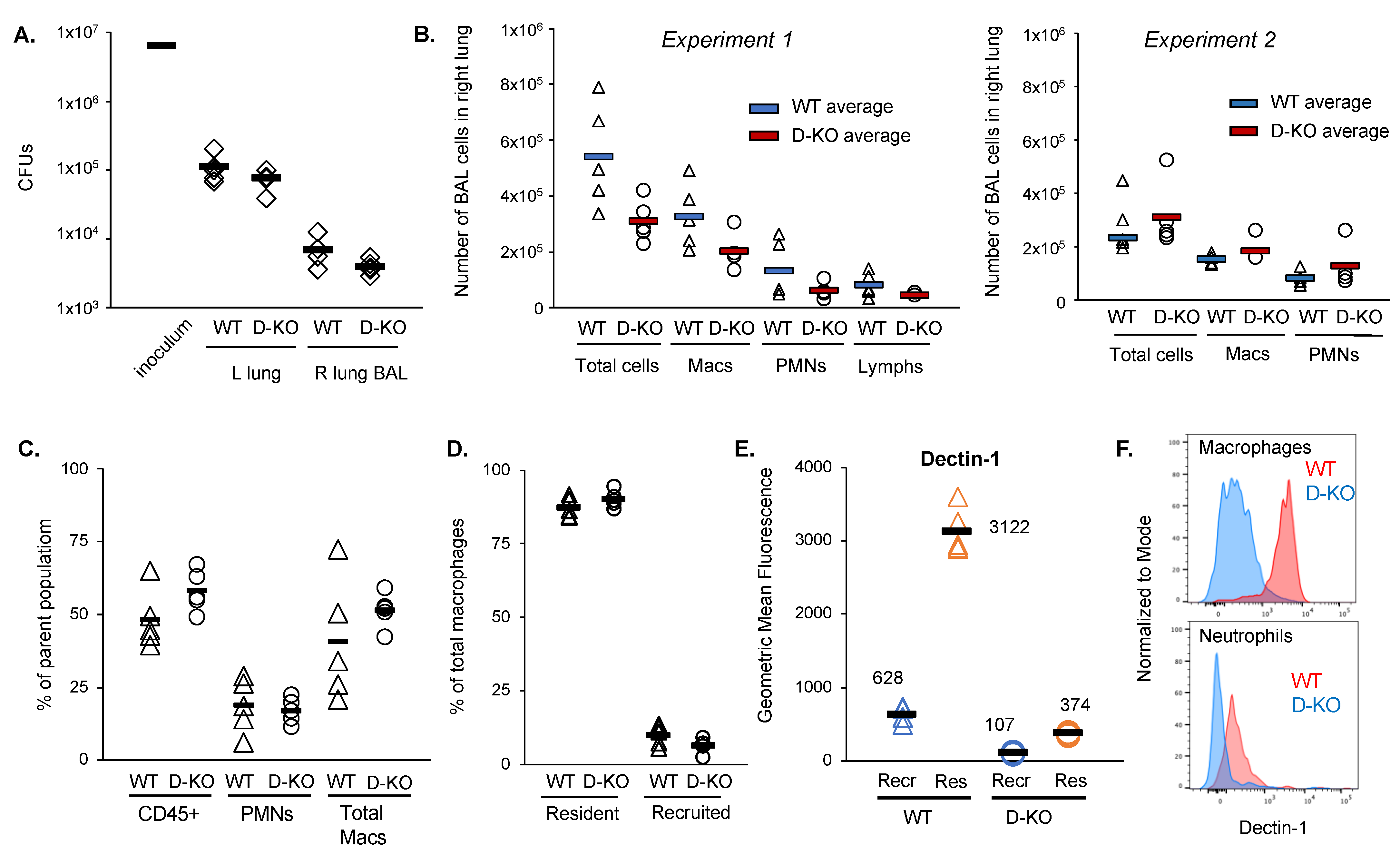
2.3. Dectin-1−/− Mice Do Not Exhibit Increased Susceptibility to M. abscessus Lung Infection
3. Discussion
4. Materials and Methods
4.1. Preparation of M. abscessus Inocula for Infections of Cells and Mice
4.2. Preparation of Zymosan Inoculum
4.3. Isolation of Human Monocyte Derived Macrophages (MDMs)
4.4. Monocyte-Derived Macrophage Pathogen Recognition Receptor Blocking Assay
4.5. Flow Cytometry of Monocyte-Derived Macrophages
4.6. Staining of Monocyte-Derived Macrophages for Confocal Microscopy
4.7. Murine Model of M. abscessus Pulmonary Infection
4.8. Lung Tissue Homogenization to Determine Bacterial Burden
4.9. Flow Cytometry on Murine Bronchoalveolar Lavage and Lung Tissue Homogenate Cells
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honda, J.R.; Virdi, R.; Chan, E.D. Global Environmental Nontuberculous Mycobacteria and Their Contemporaneous Man-Made and Natural Niches. Front. Microbiol. 2018, 9, 2029. [Google Scholar] [CrossRef] [PubMed]
- Honda, J.R.; Alper, S.; Bai, X.; Chan, E.D. Acquired and genetic host susceptibility factors and microbial pathogenic factors that predispose to nontuberculous mycobacterial infections. Curr. Opin. Immunol. 2018, 54, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Daniel-Wayman, S.; Ricotta, E.; Prevots, D.R. Epidemiology of Nontuberculous Mycobacteriosis. Semin. Respir. Crit. Care Med. 2018, 39, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Martiniano, S.L.; Nick, J.A.; Daley, C.L. Nontuberculous Mycobacterial Infections in Cystic Fibrosis. Thorac. Surg. Clin. 2019, 29, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Mirsaeidi, M.; Hadid, W.; Ericsoussi, B.; Rodgers, D.; Sadikot, R.T. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int. J. Infect. Dis. 2013, 17, e1000–e1004. [Google Scholar] [CrossRef]
- Ratnatunga, C.N.; Lutzky, V.P.; Kupz, A.; Doolan, D.L.; Reid, D.W.; Field, M.; Bell, S.C.; Thomson, R.M.; Miles, J.J. The Rise of Non-Tuberculosis Mycobacterial Lung Disease. Front. Immunol. 2020, 11, 303. [Google Scholar] [CrossRef]
- Pyarali, F.F.; Schweitzer, M.; Bagley, V.; Salamo, O.; Guerrero, A.; Sharifi, A.; Campos, M.; Quartin, A.; Mirsaeidi, M. Increasing Non-tuberculous Mycobacteria Infections in Veterans With COPD and Association With Increased Risk of Mortality. Front. Med. 2018, 5, 311. [Google Scholar] [CrossRef]
- Esther, C.R., Jr.; Esserman, D.A.; Gilligan, P.; Kerr, A.; Noone, P.G. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J. Cyst. Fibros. 2010, 9, 117–123. [Google Scholar] [CrossRef]
- Henkle, E.; Novosad, S.A.; Shafer, S.; Hedberg, K.; Siegel, S.A.R.; Ku, J.; Varley, C.; Prevots, D.R.; Marras, T.K.; Winthrop, K.L. Long-Term Outcomes in a Population-based Cohort with Respiratory Nontuberculous Mycobacteria Isolation. Ann. Am. Thorac. Soc. 2017, 14, 1120–1128. [Google Scholar] [CrossRef]
- Park, I.K.; Olivier, K.N. Nontuberculous mycobacteria in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin. Respir. Crit. Care Med. 2015, 36, 217–224. [Google Scholar] [CrossRef]
- Bustamante, J.; Boisson-Dupuis, S.; Abel, L.; Casanova, J.L. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin. Immunol. 2014, 26, 454–470. [Google Scholar] [CrossRef]
- Fowler, C.J.; Olivier, K.N.; Leung, J.M.; Smith, C.C.; Huth, A.G.; Root, H.; Kuhns, D.B.; Logun, C.; Zelazny, A.; Frein, C.A.; et al. Abnormal nasal nitric oxide production, ciliary beat frequency, and Toll-like receptor response in pulmonary nontuberculous mycobacterial disease epithelium. Am. J. Respir. Crit. Care Med. 2013, 187, 1374–1381. [Google Scholar] [CrossRef]
- Johansen, M.D.; Herrmann, J.L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev. Microbiol. 2020, 18, 392–407. [Google Scholar] [CrossRef]
- Prasla, Z.; Sutliff, R.L.; Sadikot, R.T. Macrophage Signaling Pathways in Pulmonary Nontuberculous Mycobacteria Infections. Am. J. Respir. Cell Mol. Biol. 2020, 63, 144–151. [Google Scholar] [CrossRef]
- Singhania, A.; Wilkinson, R.J.; Rodrigue, M.; Haldar, P.; O’Garra, A. The value of transcriptomics in advancing knowledge of the immune response and diagnosis in tuberculosis. Nat. Immunol. 2018, 19, 1159–1168. [Google Scholar] [CrossRef]
- Tiberi, S.; du Plessis, N.; Walzl, G.; Vjecha, M.J.; Rao, M.; Ntoumi, F.; Mfinanga, S.; Kapata, N.; Mwaba, P.; McHugh, T.D.; et al. Tuberculosis: Progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect. Dis. 2018, 18, e183–e198. [Google Scholar] [CrossRef]
- Marakalala, M.J.; Graham, L.M.; Brown, G.D. The role of Syk/CARD9-coupled C-type lectin receptors in immunity to Mycobacterium tuberculosis infections. Clin. Dev. Immunol. 2010, 2010, 567571. [Google Scholar] [CrossRef]
- Wagener, M.; Hoving, J.C.; Ndlovu, H.; Marakalala, M.J. Dectin-1-Syk-CARD9 Signaling Pathway in TB Immunity. Front. Immunol. 2018, 9, 225. [Google Scholar] [CrossRef]
- Yonekawa, A.; Saijo, S.; Hoshino, Y.; Miyake, Y.; Ishikawa, E.; Suzukawa, M.; Inoue, H.; Tanaka, M.; Yoneyama, M.; Oh-Hora, M.; et al. Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity 2014, 41, 402–413. [Google Scholar] [CrossRef]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef]
- Taylor, P.R.; Tsoni, S.V.; Willment, J.A.; Dennehy, K.M.; Rosas, M.; Findon, H.; Haynes, K.; Steele, C.; Botto, M.; Gordon, S.; et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 2007, 8, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Rothfuchs, A.G.; Bafica, A.; Feng, C.G.; Egen, J.G.; Williams, D.L.; Brown, G.D.; Sher, A. Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J. Immunol. 2007, 179, 3463–3471. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.M.; Yang, C.S.; Yuk, J.M.; Lee, J.Y.; Kim, K.H.; Shin, S.J.; Takahara, K.; Lee, S.J.; Jo, E.K. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. 2008, 10, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Schorey, J.S. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood 2006, 108, 3168–3175. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, M.; Menzel, M.; Betz, R.; Kassner, G.; Weber, N.; Kohlhaufl, M.; Haussinger, K.; Ziegler-Heitbrock, L. Characterization of a population of small macrophages in induced sputum of patients with chronic obstructive pulmonary disease and healthy volunteers. Clin. Exp. Immunol. 2004, 138, 507–516. [Google Scholar] [CrossRef]
- Garratt, L.W.; Wright, A.K.; Ranganathan, S.C.; Grigg, J.; Sly, P.D.; behalf of AREST CF. Small macrophages are present in early childhood respiratory disease. J. Cyst. Fibros. 2012, 11, 201–208. [Google Scholar] [CrossRef]
- Schupp, J.C.; Khanal, S.; Gomez, J.L.; Sauler, M.; Adams, T.S.; Chupp, G.L.; Yan, X.; Poli, S.; Zhao, Y.; Montgomery, R.R.; et al. Single-Cell Transcriptional Archetypes of Airway Inflammation in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 202, 1419–1429. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Elder, M.J.; Webster, S.J.; Williams, D.L.; Gaston, J.S.; Goodall, J.C. TSLP production by dendritic cells is modulated by IL-1beta and components of the endoplasmic reticulum stress response. Eur. J. Immunol. 2016, 46, 455–463. [Google Scholar] [CrossRef]
- Li, D.; Dong, B.; Tong, Z.; Wang, Q.; Liu, W.; Wang, Y.; Liu, W.; Chen, J.; Xu, L.; Chen, L.; et al. MBL-mediated opsonophagocytosis of Candida albicans by human neutrophils is coupled with intracellular Dectin-1-triggered ROS production. PLoS ONE 2012, 7, e50589. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Lee, H.M.; Shin, D.M.; Choi, D.K.; Lee, Z.W.; Kim, K.H.; Yuk, J.M.; Kim, C.D.; Lee, J.H.; Jo, E.K. Innate immune responses to Mycobacterium ulcerans via toll-like receptors and dectin-1 in human keratinocytes. Cell Microbiol. 2009, 11, 678–692. [Google Scholar] [CrossRef]
- Karuppusamy, S.; Rajauria, G.; Fitzpatrick, S.; Lyons, H.; McMahon, H.; Curtin, J.; Tiwari, B.K.; O’Donnell, C. Biological Properties and Health-Promoting Functions of Laminarin: A Comprehensive Review of Preclinical and Clinical Studies. Mar. Drugs. 2022, 20, 772. [Google Scholar] [CrossRef]
- Bouchery, T.; Volpe, B.; Doolan, R.; Coakley, G.; Moyat, M.; Esser-von Bieren, J.; Wickramasinghe, L.C.; Hibbs, M.L.; Sotillo, J.; Camberis, M.; et al. beta-Glucan receptors on IL-4 activated macrophages are required for hookworm larvae recognition and trapping. Immunol. Cell Biol. 2022, 100, 223–234. [Google Scholar] [CrossRef]
- Jozefowski, S.; Yang, Z.; Marcinkiewicz, J.; Kobzik, L. Scavenger receptors and beta-glucan receptors participate in the recognition of yeasts by murine macrophages. Inflamm. Res. 2012, 61, 113–126. [Google Scholar] [CrossRef]
- van Bruggen, R.; Drewniak, A.; Jansen, M.; van Houdt, M.; Roos, D.; Chapel, H.; Verhoeven, A.J.; Kuijpers, T.W. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for beta-glucan-bearing particles. Mol. Immunol. 2009, 47, 575–581. [Google Scholar] [CrossRef]
- He, X.; Howard, B.A.; Liu, Y.; Neumann, A.K.; Li, L.; Menon, N.; Roach, T.; Kale, S.D.; Samuels, D.C.; Li, H.; et al. LYSMD3: A mammalian pattern recognition receptor for chitin. Cell Rep. 2021, 36, 109392. [Google Scholar] [CrossRef]
- Yokoyama, C.C.; Baldridge, M.T.; Leung, D.W.; Zhao, G.; Desai, C.; Liu, T.C.; Diaz-Ochoa, V.E.; Huynh, J.P.; Kimmey, J.M.; Sennott, E.L.; et al. LysMD3 is a type II membrane protein without an in vivo role in the response to a range of pathogens. J. Biol. Chem. 2018, 293, 6022–6038. [Google Scholar] [CrossRef]
- Marakalala, M.J.; Guler, R.; Matika, L.; Murray, G.; Jacobs, M.; Brombacher, F.; Rothfuchs, A.G.; Sher, A.; Brown, G.D. The Syk/CARD9-coupled receptor Dectin-1 is not required for host resistance to Mycobacterium tuberculosis in mice. Microbes Infect. 2011, 13, 198–201. [Google Scholar] [CrossRef]
- Wilson, G.J.; Marakalala, M.J.; Hoving, J.C.; van Laarhoven, A.; Drummond, R.A.; Kerscher, B.; Keeton, R.; van de Vosse, E.; Ottenhoff, T.H.; Plantinga, T.S.; et al. The C-type lectin receptor CLECSF8/CLEC4D is a key component of anti-mycobacterial immunity. Cell Host. Microbe 2015, 17, 252–259. [Google Scholar] [CrossRef]
- Carvalho, N.B.; Oliveira, F.S.; Duraes, F.V.; de Almeida, L.A.; Florido, M.; Prata, L.O.; Caliari, M.V.; Appelberg, R.; Oliveira, S.C. Toll-like receptor 9 is required for full host resistance to Mycobacterium avium infection but plays no role in induction of Th1 responses. Infect. Immun. 2011, 79, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Killick, K.E.; Ni Cheallaigh, C.; O’Farrelly, C.; Hokamp, K.; MacHugh, D.E.; Harris, J. Receptor-mediated recognition of mycobacterial pathogens. Cell Microbiol. 2013, 15, 1484–1495. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Yoon, J.H.; Shin, M.K.; Shin, S.J. Infection of Dendritic Cells with Mycobacterium avium subspecies hominissuis Exhibits a Functionally Tolerogenic Phenotype in Response to Toll-Like Receptor Agonists via IL-10/Cox2/PGE2/EP2 Axis. Front. Microbiol. 2019, 10, 1795. [Google Scholar] [CrossRef] [PubMed]
- Marinho, F.A.; de Paula, R.R.; Mendes, A.C.; de Almeida, L.A.; Gomes, M.T.; Carvalho, N.B.; Oliveira, F.S.; Caliari, M.V.; Oliveira, S.C. Toll-like receptor 6 senses Mycobacterium avium and is required for efficient control of mycobacterial infection. Eur. J. Immunol. 2013, 43, 2373–2385. [Google Scholar] [CrossRef]
- Middleton, A.M.; Chadwick, M.V.; Nicholson, A.G.; Dewar, A.; Groger, R.K.; Brown, E.J.; Wilson, R. The role of Mycobacterium avium complex fibronectin attachment protein in adherence to the human respiratory mucosa. Mol. Microbiol. 2000, 38, 381–391. [Google Scholar] [CrossRef]
- Secott, T.E.; Lin, T.L.; Wu, C.C. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein facilitates M-cell targeting and invasion through a fibronectin bridge with host integrins. Infect. Immun. 2004, 72, 3724–3732. [Google Scholar] [CrossRef]
- Noh, K.T.; Shin, S.J.; Son, K.H.; Jung, I.D.; Kang, H.K.; Lee, S.J.; Lee, E.K.; Shin, Y.K.; You, J.C.; Park, Y.M. The Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein, a toll-like receptor 4 agonist, enhances dendritic cell-based cancer vaccine potency. Exp. Mol. Med. 2012, 44, 340–349. [Google Scholar] [CrossRef]
- Altemeier, W.A.; Hung, C.F.; Matute-Bello, G. Mouse Models of Acute Lung Injury. In Acute Lung Injury and Repair; Schnapp, L.M., Feghali-Bostwick, C., Eds.; Respiratory Medicine; Springer International Publishing: Cham, Switzerland, 2017; pp. 5–23. [Google Scholar]
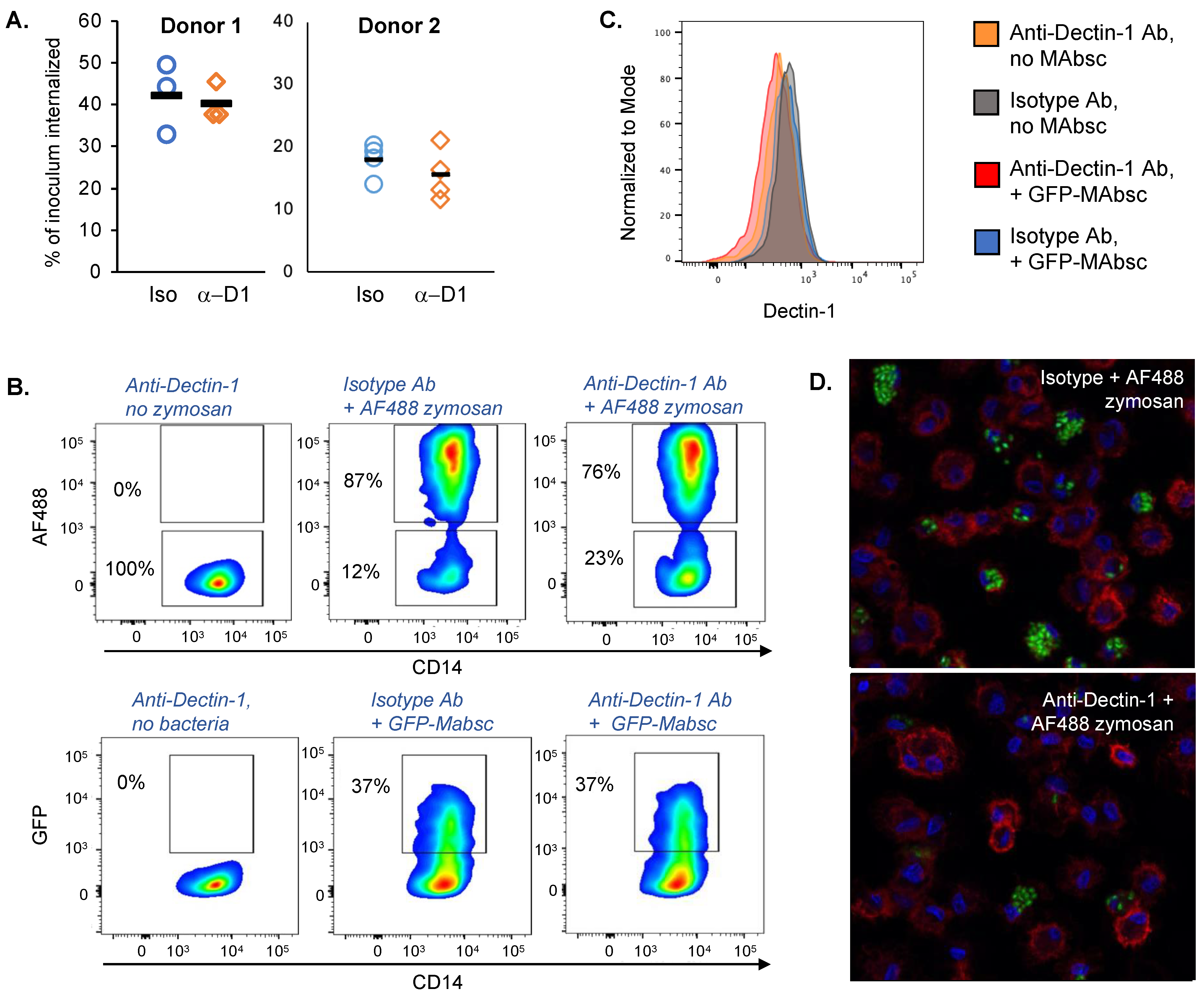
| AF488 Zymosan | |||
|---|---|---|---|
| Cell Type (% Parent Population) | Anti-Dectin-1 Antibody | Isotype Control Antibody | p Value |
| CD14+ cells | 85.47 (1.6) | 86.63 (0.9) | 0.33 |
| AF488+ CD14+ cells | 76.17 (0.6) | 86.43 (0.7) | 4.3 × 10−5 |
| AF488− CD14+ cells | 23.03 (0.8) | 12.63 (0.6) | 5.9 × 10−5 |
| Geometric Mean Fluorescence Dectin-1 | |||
| AF488+ CD14+ cells | 203.33 (13.1) | 244.67 (11.2) | 0.014 |
| AF488− CD14+ cells | 319.33 (20.7) | 444.67 (4.5) | 0.00051 |
| GFP− M. abscessus | |||
| Cell Type (% parent population) | Anti-Dectin-1 Antibody | Isotype Control Antibody | p value |
| CD14+ cells | 88.00 (0.4) | 87.87 (1.3) | 0.87 |
| AF488+ CD14+ cells | 36.27 (2.8) | 37.90 (2.6) | 0.49 |
| AF488− CD14+ cells | 63.03 (2.9) | 61.30 (2.2) | 0.46 |
| Geometric Mean Fluorescence Dectin-1 | |||
| AF488+ CD14+ cells | 352.87 (16.0) | 523.33 (8.0) | 7.9 × 10−5 |
| AF488− CD14+ cells | 359.67 (9.3) | 513.33 (16.8) | 0.00016 |
| AF488 Zymosan | |||
|---|---|---|---|
| Cell Type (% Parent Population) | Anti-Dectin-1 Antibody | Isotype Control Antibody | p Value |
| CD14+ cells | 84.50 (2.2) | 87.87 (2.6) | 0.15 |
| AF488+ CD14+ cells | 78.30 (1.9) | 90.33 (0.3) | 0.00040 |
| AF488− CD14+ cells | 21.13 (1.9) | 9.12 (0.3) | 0.00044 |
| Geometric Mean Fluorescence Dectin-1 | |||
| AF488+ CD14+ cells | 133.67 (12.0) | 248.67 (4.9) | 0.00011 |
| AF488− CD14+ cells | 231.67 (4.5) | 443.33 (21.2) | 7.1 × 10−5 |
| GFP− M. abscessus | |||
| Cell Type (% parent population) | Anti-Dectin-1 Antibody | Isotype Control Antibody | p value |
| CD14+ cells | 85.67 (1.0) | 88.80 (0.90) | 0.015 |
| AF488+ CD14+ cells | 34.30 (1.8) | 42.70 (2.7) | 0.011 |
| AF488− CD14+ cells | 64.57 (1.6) | 56.37 (2.6) | 0.0099 |
| Geometric Mean Fluorescence Dectin-1 | |||
| AF488+ CD14+ cells | 255.00 (3.0) | 528.00 (19.5) | 1.8 × 10−5 |
| AF488− CD14+ cells | 259.00 (2.7) | 521.00 (9.6) | 1.4 × 10−6 |
| Cell Type (% Parent Population) | Wild-Type Mice | Dectin-1−/− Mice | p Value |
|---|---|---|---|
| BAL | |||
| CD45+ | 48.5 (1.0) | 58.2 (7.1) | 0.11 |
| PMN | 19.0 (9.3) | 17.2 (4.3) | 0.71 |
| Total macrophages | 40.9 (20.9) | 51.5 (5.97) | 0.29 |
| % Dectin-1+ total macrophages | 90.0 (3.5) | 7.1 (1.4) | 3 × 10−11 |
| Resident macrophages | 88.2 (3.8) | 91.9 (2.5) | 0.11 |
| Recruited macrophages | 10.5 (4.0) | 7.0 (2.4) | 0.12 |
| Lung Homogenate | |||
| CD45+ | 73.74 (6.8) | 72.46 (4.8) | 0.79 |
| PMN | 5.61 (1.9) | 6.18 (0.8) | 0.50 |
| Total macrophages | 10.53 (5.2) | 11.64 (4.7) | 0.75 |
| Airspace macrophages | 62.80 (12.4) | 63.44 (9.8) | 0.82 |
| Non-airspace macrophages (recruited and interstitial) | 35.30 (12.7) | 34.72 (9.7) | 0.84 |
| Fluorophore | Antibody Target | Clone | Company | Catalog Number |
|---|---|---|---|---|
| APC | CD14 | 61D3 | Invitrogen (Waltham, MA, USA) | 17-0149-42 |
| PerCP Cy5.5 | CD369 (Dectin-1) | 15e2 | Biolegend (San Diego, CA, USA) | 355408 |
| FITC | TLR2 | TL2.2 | Invitrogen | 11.99.22.42 |
| Calcein Blue-AM | Intact, viable cells | n/a | Invitrogen | 65-0855 39 |
| Fluorophore | Antibody Target | Clone | Company | Catalog Number |
|---|---|---|---|---|
| AlexaFluor 405 | CD45 | 30.F11 | Invitrogen | 48045182 |
| FITC | Ly-6G | 1A8 | BD Biosciences (Franklin Lakes, NJ, USA) | 551460 |
| PE | Siglec-F | E50-2440 | BD Biosciences | 552126 |
| PE | CD88 | 20/70 | Biolegend | 135806 |
| APC | CD88 | 20/70 | Biolegend | 135808 |
| PerCP-eFluor 710 | Clec7a/Dectin-1 | bg1fpJ | Invitrogen | 46-5859-82 |
| PE-Cy7 | CD64 | X54-5/7.1 | Biolegend | 139314 |
| APC-Cy7 | CD11c | N418 | Biolegend | 117324 |
| BUV395 | Ly-6G | 1A8 | BD Biosciences | 563978 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochoa, A.E.; Congel, J.H.; Corley, J.M.; Janssen, W.J.; Nick, J.A.; Malcolm, K.C.; Hisert, K.B. Dectin-1-Independent Macrophage Phagocytosis of Mycobacterium abscessus. Int. J. Mol. Sci. 2023, 24, 11062. https://doi.org/10.3390/ijms241311062
Ochoa AE, Congel JH, Corley JM, Janssen WJ, Nick JA, Malcolm KC, Hisert KB. Dectin-1-Independent Macrophage Phagocytosis of Mycobacterium abscessus. International Journal of Molecular Sciences. 2023; 24(13):11062. https://doi.org/10.3390/ijms241311062
Chicago/Turabian StyleOchoa, Alma E., Jack H. Congel, Jodi M. Corley, William J. Janssen, Jerry A. Nick, Kenneth C. Malcolm, and Katherine B. Hisert. 2023. "Dectin-1-Independent Macrophage Phagocytosis of Mycobacterium abscessus" International Journal of Molecular Sciences 24, no. 13: 11062. https://doi.org/10.3390/ijms241311062
APA StyleOchoa, A. E., Congel, J. H., Corley, J. M., Janssen, W. J., Nick, J. A., Malcolm, K. C., & Hisert, K. B. (2023). Dectin-1-Independent Macrophage Phagocytosis of Mycobacterium abscessus. International Journal of Molecular Sciences, 24(13), 11062. https://doi.org/10.3390/ijms241311062







