Recombinant Human CD19 in CHO-K1 Cells: Glycosylation Patterns as a Quality Attribute of High Yield Processes
Abstract
1. Introduction
2. Results
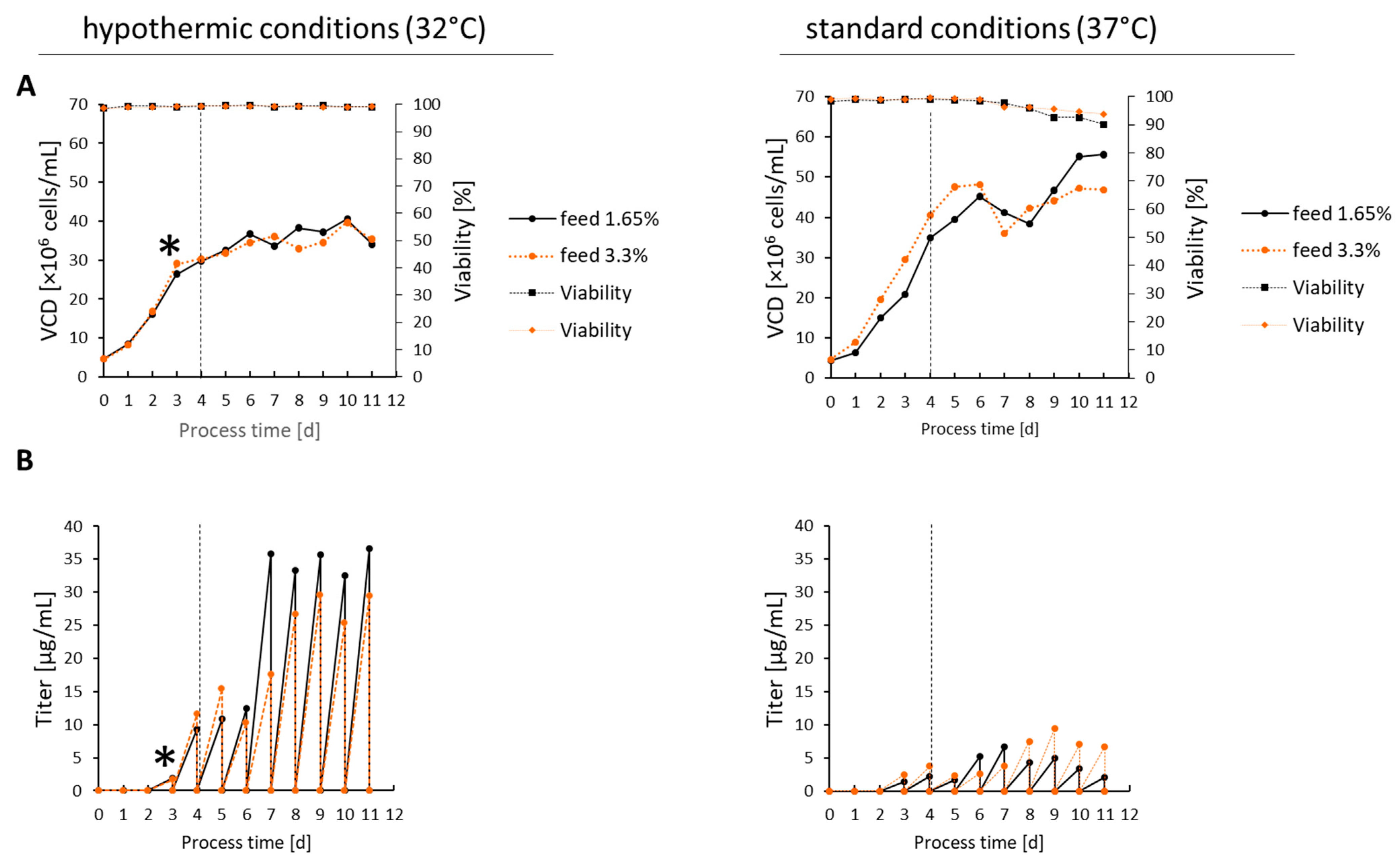
2.1. Bioprocess of the CD19-AD2 Fusion Protein at Hypothermic vs. Standard Culture Conditions
2.2. Purification of CD19-AD2
2.3. The Binding Kinetics of CD19-AD2 to Different Commercially Available mAbs
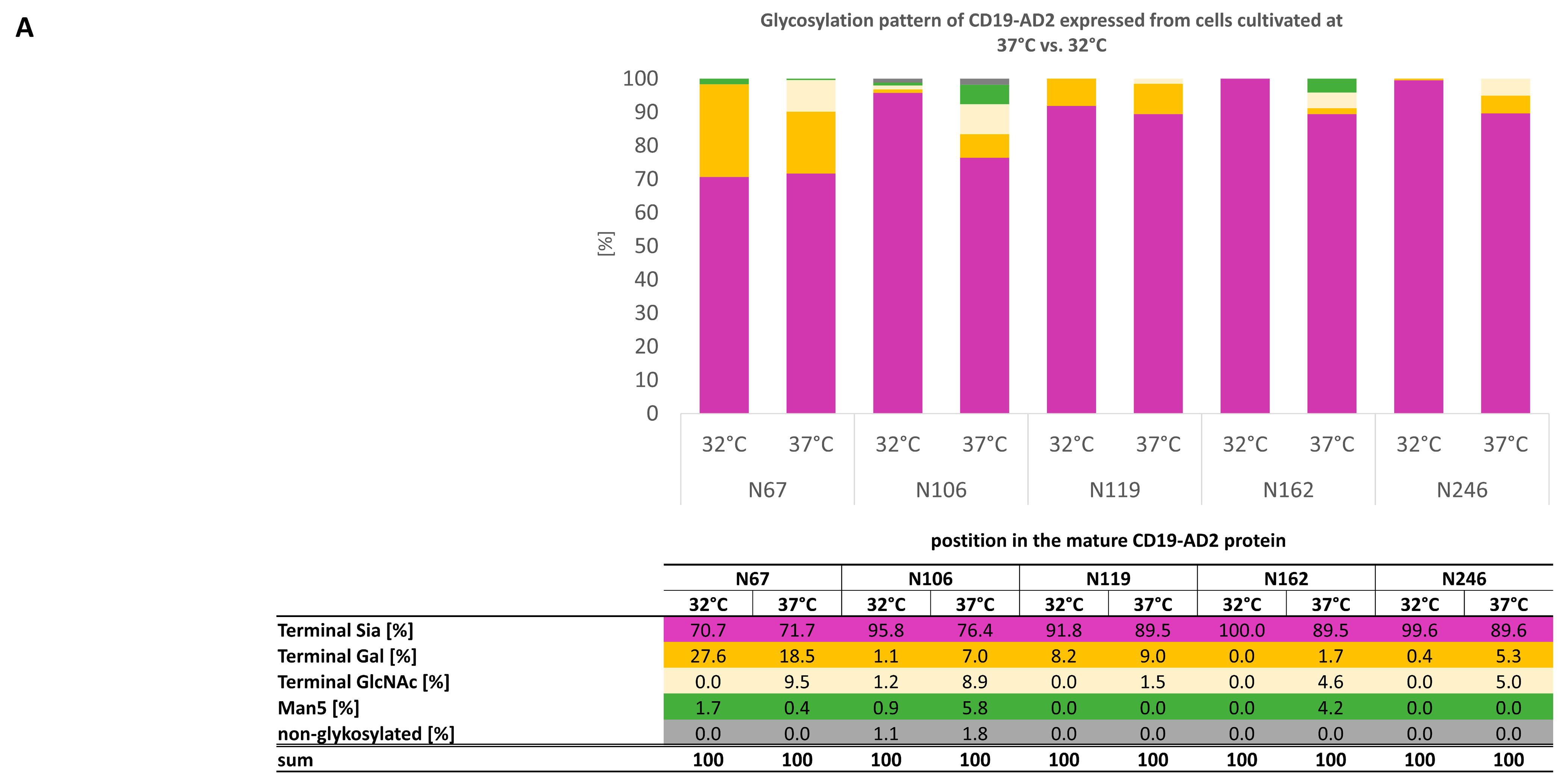
2.4. CD19-AD2 Glycoprofile Revealing Highly Complex Glycans
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Bioprocessing
4.3. Purification of CD19-AD2
4.4. SDS-PAGE Analysis
4.5. Binding Kinetics with Anti-CD19 Antibodies/Affinity Testing
4.6. Glycopeptide Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef]
- Laurent, E.; Sieber, A.; Salzer, B.; Wachernig, A.; Seigner, J.; Lehner, M.; Geyeregger, R.; Kratzer, B.; Jäger, U.; Kunert, R.; et al. Directed Evolution of Stabilized Monomeric CD19 for Monovalent CAR Interaction Studies and Monitoring of CAR-T Cell Patients. ACS Synth. Biol. 2021, 10, 1184–1198. [Google Scholar] [CrossRef]
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 626616. [Google Scholar] [CrossRef]
- Goebeler, M.-E.; Bargou, R. Blinatumomab: A CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leuk. Lymphoma 2016, 57, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Knoebl, P.; Thaler, J.; Jilma, P.; Quehenberger, P.; Gleixner, K.V.; Sperr, W.R. Emicizumab for the treatment of acquired hemophilia A. Blood 2021, 137, 410–419. [Google Scholar] [CrossRef]
- Kallehauge, T.B.; Kol, S.; Andersen, M.R.; Damgaard, C.K.; Lee, G.M.; Kildegaard, H.F. Endoplasmic reticulum-directed recombinant mRNA displays subcellular localization equal to endogenous mRNA during transient expression in CHO cells. Biotechnol. J. 2016, 11, 1362–1367. [Google Scholar] [CrossRef]
- Kawabe, Y.; Inao, T.; Komatsu, S.; Huang, G.; Ito, A.; Omasa, T.; Kamihira, M. Improved recombinant antibody production by CHO cells using a production enhancer DNA element with repeated transgene integration at a predetermined chromosomal site. J. Biosci. Bioeng. 2017, 123, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Yang, Y.; Ng, S.K. Advances in Mammalian Cell Line Development Technologies for Recombinant Protein Production. Pharmaceuticals 2013, 6, 579–603. [Google Scholar] [CrossRef] [PubMed]
- Le Fourn, V.; Girod, P.-A.; Buceta, M.; Regamey, A.; Mermod, N. CHO cell engineering to prevent polypeptide aggregation and improve therapeutic protein secretion. Metab. Eng. 2014, 21, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, A.; Hust, M.; Schirrmann, T. Expression of Recombinant Antibodies. Front. Immunol. 2013, 4, 217. [Google Scholar] [CrossRef]
- Cobb, B.A. The history of IgG glycosylation and where we are now. Glycobiology 2020, 30, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Ecker, D.M.; Jones, S.D.; Levine, H.L. The therapeutic monoclonal antibody market. Mabs 2015, 7, 9–14. [Google Scholar] [CrossRef]
- Lobner, E.; Wachernig, A.; Gudipati, V.; Mayrhofer, P.; Salzer, B.; Lehner, M.; Huppa, J.B.; Kunert, R. Getting CD19 Into Shape: Expression of Natively Folded “Difficult-to- Express” CD19 for Staining and Stimulation of CAR-T Cells. Front. Bioeng. Biotechnol. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Kunert, R.; Reinhart, D. Advances in recombinant antibody manufacturing. Appl. Microbiol. Biotechnol. 2016, 100, 3451–3461. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, P.; Castan, A.; Kunert, R. Shake tube perfusion cell cultures are suitable tools for the prediction of limiting substrate, CSPR, bleeding strategy, growth and productivity behavior. J. Chem. Technol. Biotechnol. 2021, 96, 2930–2939. [Google Scholar] [CrossRef]
- Mayrhofer, P.; Kunert, R. Screening of Media Supplements for High-Performance Perfusion Cultures by Design of Experiment. In Animal Cell Biotechnology: Methods and Protocols; Pörtner, R., Ed.; Springer: New York, NY, USA, 2020; pp. 27–39. [Google Scholar]
- Reinhart, D.; Damjanovic, L.; Kaisermayer, C.; Sommeregger, W.; Gili, A.; Gasselhuber, B.; Castan, A.; Mayrhofer, P.; Grünwald-Gruber, C.; Kunert, R. Bioprocessing of Recombinant CHO-K1, CHO-DG44, and CHO-S: CHO Expression Hosts Favor Either mAb Production or Biomass Synthesis. Biotechnol. J. 2019, 14, e1700686. [Google Scholar] [CrossRef]
- Kumar, N.; Gammell, P.; Clynes, M. Proliferation control strategies to improve productivity and survival during CHO based production culture: A summary of recent methods employed and the effects of proliferation control in product secreting CHO cell lines. Cytotechnology 2007, 53, 33–46. [Google Scholar] [CrossRef]
- Bollati-Fogolín, M.; Forno, G.; Nimtz, M.; Conradt, H.S.; Etcheverrigaray, M.; Kratje, R. Temperature Reduction in Cultures of hGM-CSF-expressing CHO Cells: Effect on Productivity and Product Quality. Biotechnol. Prog. 2005, 21, 17–21. [Google Scholar] [CrossRef]
- Torres, M.; Zúñiga, R.; Gutierrez, M.; Vergara, M.; Collazo, N.; Reyes, J.; Berrios, J.; Aguillón, J.C.; Molina, M.C.; Altamirano, C. Mild hypothermia upregulates myc and xbp1s expression and improves anti-TNFα production in CHO cells. PLoS ONE 2018, 13, e0194510. [Google Scholar] [CrossRef]
- Borys, M.C.; Dalal, N.G.; Abu-Absi, N.R.; Khattak, S.F.; Jing, Y.; Xing, Z.; Li, Z.J. Effects of culture conditions on N-glycolylneuraminic acid (Neu5Gc) content of a recombinant fusion protein produced in CHO cells. Biotechnol. Bioeng. 2010, 105, 1048–1057. [Google Scholar] [CrossRef]
- Xu, J.; Tang, P.; Yongky, A.; Drew, B.; Borys, M.C.; Liu, S.; Li, Z.J. Systematic development of temperature shift strategies for Chinese hamster ovary cells based on short duration cultures and kinetic modeling. mAbs 2019, 11, 191–204. [Google Scholar] [CrossRef]
- Reinhart, D.; Damjanovic, L.; Kaisermayer, C.; Kunert, R. Benchmarking of commercially available CHO cell culture media for antibody production. Appl. Microbiol. Biotechnol. 2015, 99, 4645–4657. [Google Scholar] [CrossRef]
- Teplyakov, A.; Obmolova, G.; Luo, J.; Gilliland, G.L. Crystal structure of B-cell co-receptor CD19 in complex with antibody B43 reveals an unexpected fold. Proteins Struct. Funct. Bioinform. 2018, 86, 495–500. [Google Scholar] [CrossRef]
- Lau, K.S.; Partridge, E.A.; Grigorian, A.; Silvescu, C.I.; Reinhold, V.N.; Demetriou, M.; Dennis, J.W. Complex N-Glycan Number and Degree of Branching Cooperate to Regulate Cell Proliferation and Differentiation. Cell 2007, 129, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Phang, R.; Lin, C.-H. Synthesis of Type-I and Type-II LacNAc-Repeating Oligosaccharides as the Backbones of Tumor-Associated Lewis Antigens. Front. Immunol. 2022, 13, 858894. [Google Scholar] [CrossRef]
- Fischöder, T.; Laaf, D.; Dey, C.; Elling, L. Enzymatic Synthesis of N-Acetyllactosamine (LacNAc) Type 1 Oligomers and Characterization as Multivalent Galectin Ligands. Molecules 2017, 22, 1320. [Google Scholar] [CrossRef]
- Kotidis, P.; Donini, R.; Arnsdorf, J.; Hansen, A.H.; Voldborg, B.G.R.; Chiang, A.W.; Haslam, S.M.; Betenbaugh, M.; del Val, I.J.; Lewis, N.E.; et al. CHOGlycoNET: Comprehensive glycosylation reaction network for CHO cells. Metab. Eng. 2023, 76, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G.; Jefferis, R. Post-translational modifications in the context of therapeutic proteins. Nat. Biotechnol. 2006, 24, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Heard, A.; Landmann, J.H.; Hansen, A.R.; Papadopolou, A.; Hsu, Y.-S.; Selli, M.E.; Warrington, J.M.; Lattin, J.; Chang, J.; Ha, H.; et al. Antigen glycosylation regulates efficacy of CAR T cells targeting CD19. Nat. Commun. 2022, 13, 3367. [Google Scholar] [CrossRef]
- Lee, C.-G.; Oh, M.J.; Park, S.-Y.; An, H.J.; Kim, J.H. Inhibition of poly-LacNAc biosynthesis with release of CMP-Neu5Ac feedback inhibition increases the sialylation of recombinant EPO produced in CHO cells. Sci. Rep. 2018, 8, 7273. [Google Scholar] [CrossRef]
- Delorme, E.; Lorenzini, T.; Giffin, J.; Martin, F.; Jacobsen, F.; Boone, T.; Elliott, S. Role of glycosylation on the secretion and biological activity of erythropoietin. Biochemistry 1992, 31, 9871–9876. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Lee, S.M.; Gil, J.Y.; Kwon, O.; Kim, J.Y.; Park, S.J.; Chung, H.-S.; Oh, D.-B. N-glycan analysis of human α1-antitrypsin produced in Chinese hamster ovary cells. Glycoconj. J. 2012, 30, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Kadirvelraj, R.; Yang, J.-Y.; Kim, H.W.; Sanders, J.H.; Moremen, K.W.; Wood, Z.A. Comparison of human poly-N-acetyl-lactosamine synthase structure with GT-A fold glycosyltransferases supports a modular assembly of catalytic subsites. J. Biol. Chem. 2021, 296, 100110. [Google Scholar] [CrossRef]
- Zhou, D. Why are Glycoproteins Modified by Poly-N-Acetyllactosamine Glycoconjugates? Curr. Protein Pept. Sci. 2003, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gudipati, V.; Rydzek, J.; Doel-Perez, I.; Gonçalves, V.D.R.; Scharf, L.; Königsberger, S.; Lobner, E.; Kunert, R.; Einsele, H.; Stockinger, H.; et al. Inefficient CAR-proximal signaling blunts antigen sensitivity. Nat. Immunol. 2020, 21, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Fry, T.J.; Shah, N.N.; Orentas, R.J.; Stetler-Stevenson, M.; Yuan, C.M.; Ramakrishna, S.; Wolters, P.; Martin, S.; Delbrook, C.; Yates, B.; et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018, 24, 20–28. [Google Scholar] [CrossRef]
- Ślebioda, T.J.; Stanisławowski, M.; Kaszubowska, L.; Zaucha, J.M.; Żmijewski, M.A. Current and Future Perspectives for Chimeric Antigen Receptor T Cells Development in Poland. Biomedicines 2022, 10, 2912. [Google Scholar] [CrossRef]
- Kang, C.H.; Kim, Y.; Lee, H.K.; Lee, S.M.; Jeong, H.G.; Choi, S.U.; Park, C.H. Identification of Potent CD19 scFv for CAR T Cells through scFv Screening with NK/T-Cell Line. Int. J. Mol. Sci. 2020, 21, 9163. [Google Scholar] [CrossRef] [PubMed]
- Hamieh, M.; Dobrin, A.; Cabriolu, A.; Van Der Stegen, S.J.C.; Giavridis, T.; Mansilla-Soto, J.; Eyquem, J.; Zhao, Z.; Whitlock, B.M.; Miele, M.M.; et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 2019, 568, 112–116. [Google Scholar] [CrossRef]
- Olson, M.L.; Mause, E.R.V.; Radhakrishnan, S.V.; Brody, J.D.; Rapoport, A.P.; Welm, A.L.; Atanackovic, D.; Luetkens, T. Low-affinity CAR T cells exhibit reduced trogocytosis, preventing rapid antigen loss, and increasing CAR T cell expansion. Leukemia 2022, 36, 1943–1946. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Kong, W.; He, Y. The affinity of antigen-binding domain on the antitumor efficacy of CAR T cells: Moderate is better. Front. Immunol. 2022, 13, 1032403. [Google Scholar] [CrossRef] [PubMed]
- Klesmith, J.R.; Wu, L.; Lobb, R.R.; Rennert, P.D.; Hackel, B.J. Fine Epitope Mapping of the CD19 Extracellular Domain Promotes Design. Biochemistry 2019, 58, 4869–4881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, X.; Tian, Y.; Li, F.; Zhao, X.; Liu, J.; Yao, C.; Zhang, Y. Point mutation in CD19 facilitates immune escape of B cell lymphoma from CAR-T cell therapy. J. Immunother. Cancer 2020, 8, e001150. [Google Scholar] [CrossRef] [PubMed]

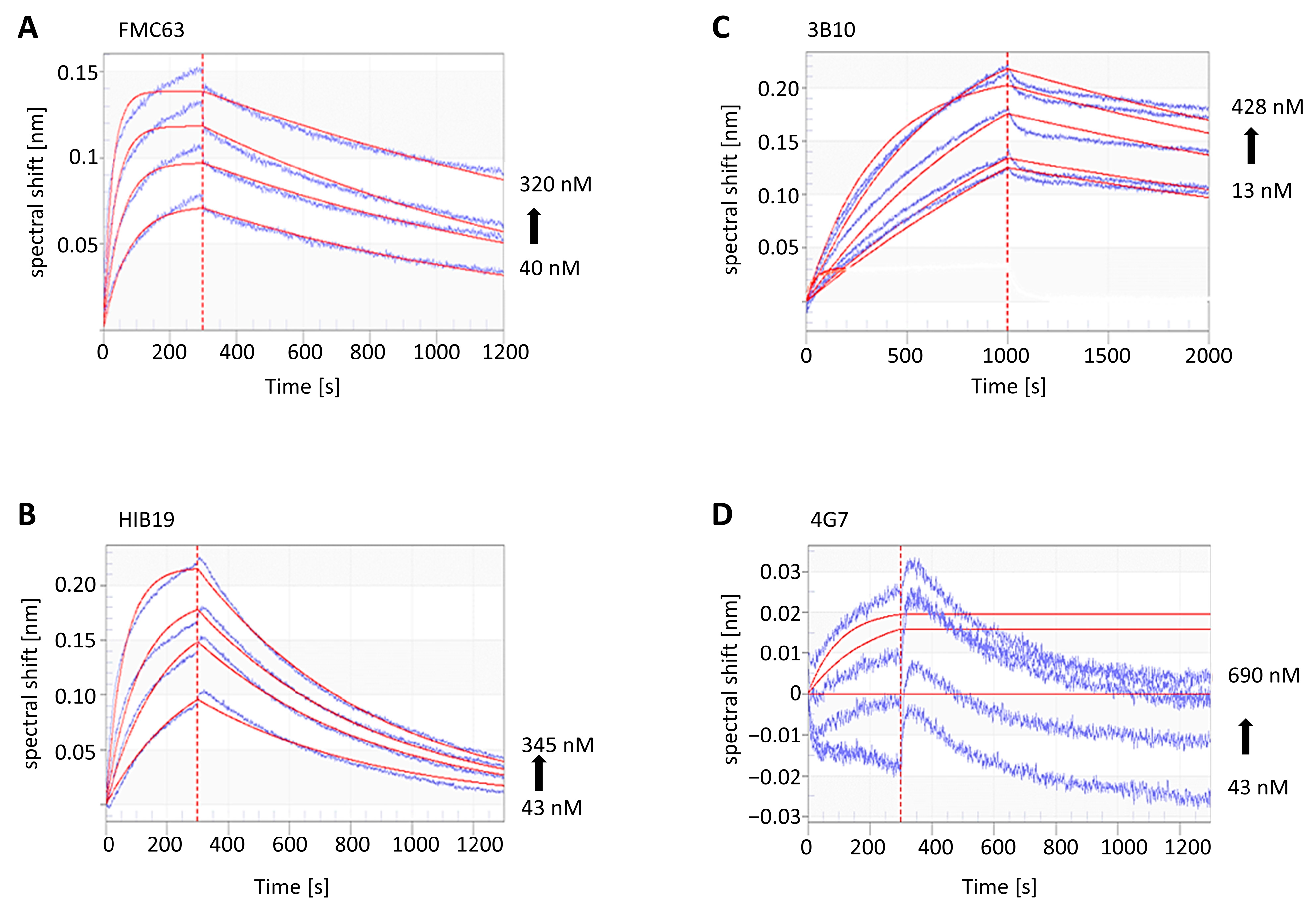

| Analyte in Solution | Immobilized Ligand ID | kon 105/Ms | koff [10−4/s] | KD (nM) |
|---|---|---|---|---|
| CD19-AD2 | FMC63 | 2.06 | 6.57 | 3.19 |
| HIB19 | 0.44 | 16.99 | 38.29 | |
| 3B10 | 0.11 | 2.16 | 19.12 | |
| 4G7 | no spec. binding | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billerhart, M.; Hunjadi, M.; Hawlin, V.; Grünwald-Gruber, C.; Maresch, D.; Mayrhofer, P.; Kunert, R. Recombinant Human CD19 in CHO-K1 Cells: Glycosylation Patterns as a Quality Attribute of High Yield Processes. Int. J. Mol. Sci. 2023, 24, 10891. https://doi.org/10.3390/ijms241310891
Billerhart M, Hunjadi M, Hawlin V, Grünwald-Gruber C, Maresch D, Mayrhofer P, Kunert R. Recombinant Human CD19 in CHO-K1 Cells: Glycosylation Patterns as a Quality Attribute of High Yield Processes. International Journal of Molecular Sciences. 2023; 24(13):10891. https://doi.org/10.3390/ijms241310891
Chicago/Turabian StyleBillerhart, Magdalena, Monika Hunjadi, Vanessa Hawlin, Clemens Grünwald-Gruber, Daniel Maresch, Patrick Mayrhofer, and Renate Kunert. 2023. "Recombinant Human CD19 in CHO-K1 Cells: Glycosylation Patterns as a Quality Attribute of High Yield Processes" International Journal of Molecular Sciences 24, no. 13: 10891. https://doi.org/10.3390/ijms241310891
APA StyleBillerhart, M., Hunjadi, M., Hawlin, V., Grünwald-Gruber, C., Maresch, D., Mayrhofer, P., & Kunert, R. (2023). Recombinant Human CD19 in CHO-K1 Cells: Glycosylation Patterns as a Quality Attribute of High Yield Processes. International Journal of Molecular Sciences, 24(13), 10891. https://doi.org/10.3390/ijms241310891







