Phytohormone Crosstalk of Cytokinin Biosynthesis and Signaling Family Genes in Moso Bamboo (Phyllostachys edulis)
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization of Key Family Genes Acting on the CK Pathway in Moso Bamboo
2.2. Phylogenetic Analysis of CK Pathway-Related Family Genes in Moso Bamboo
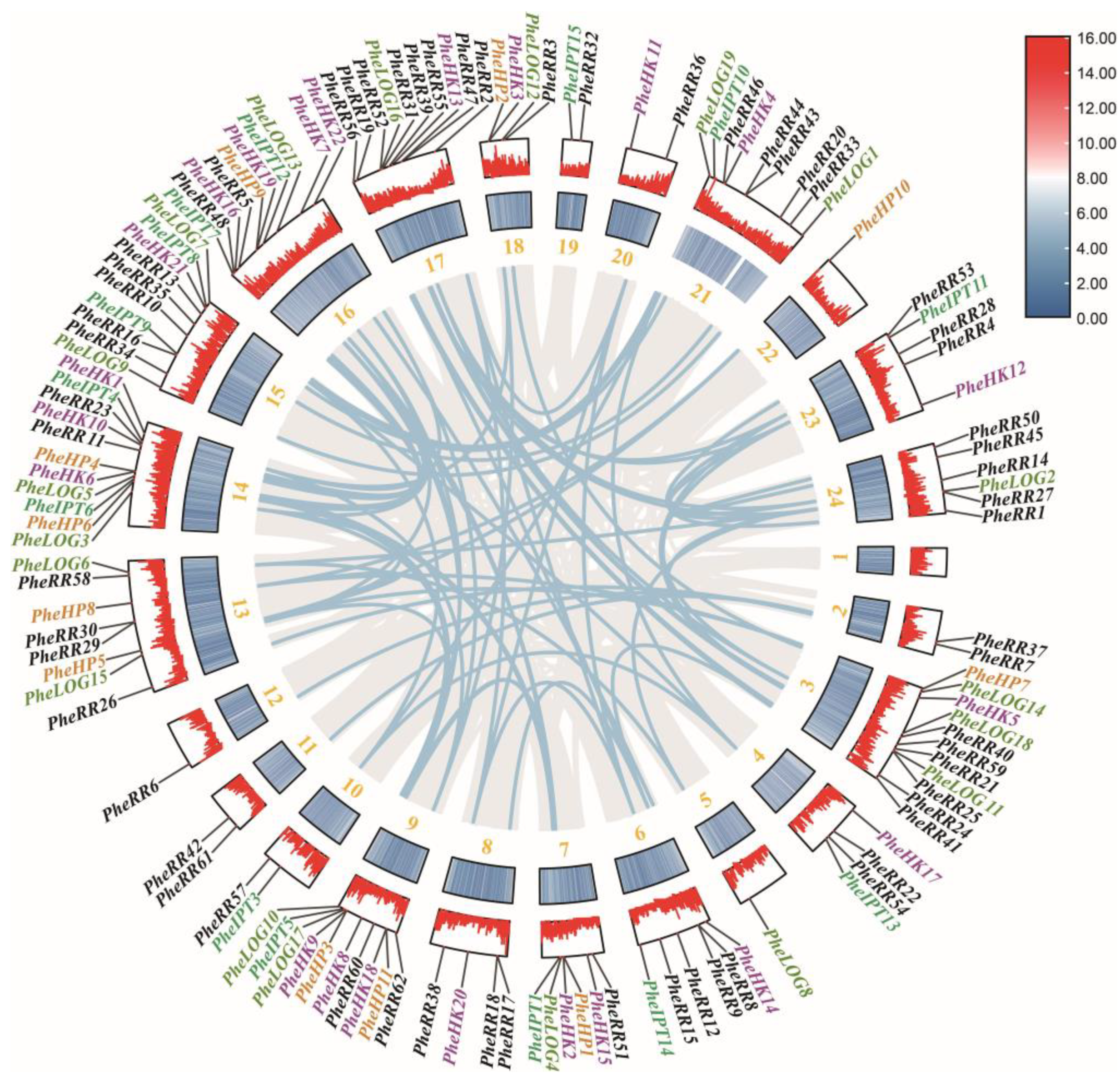
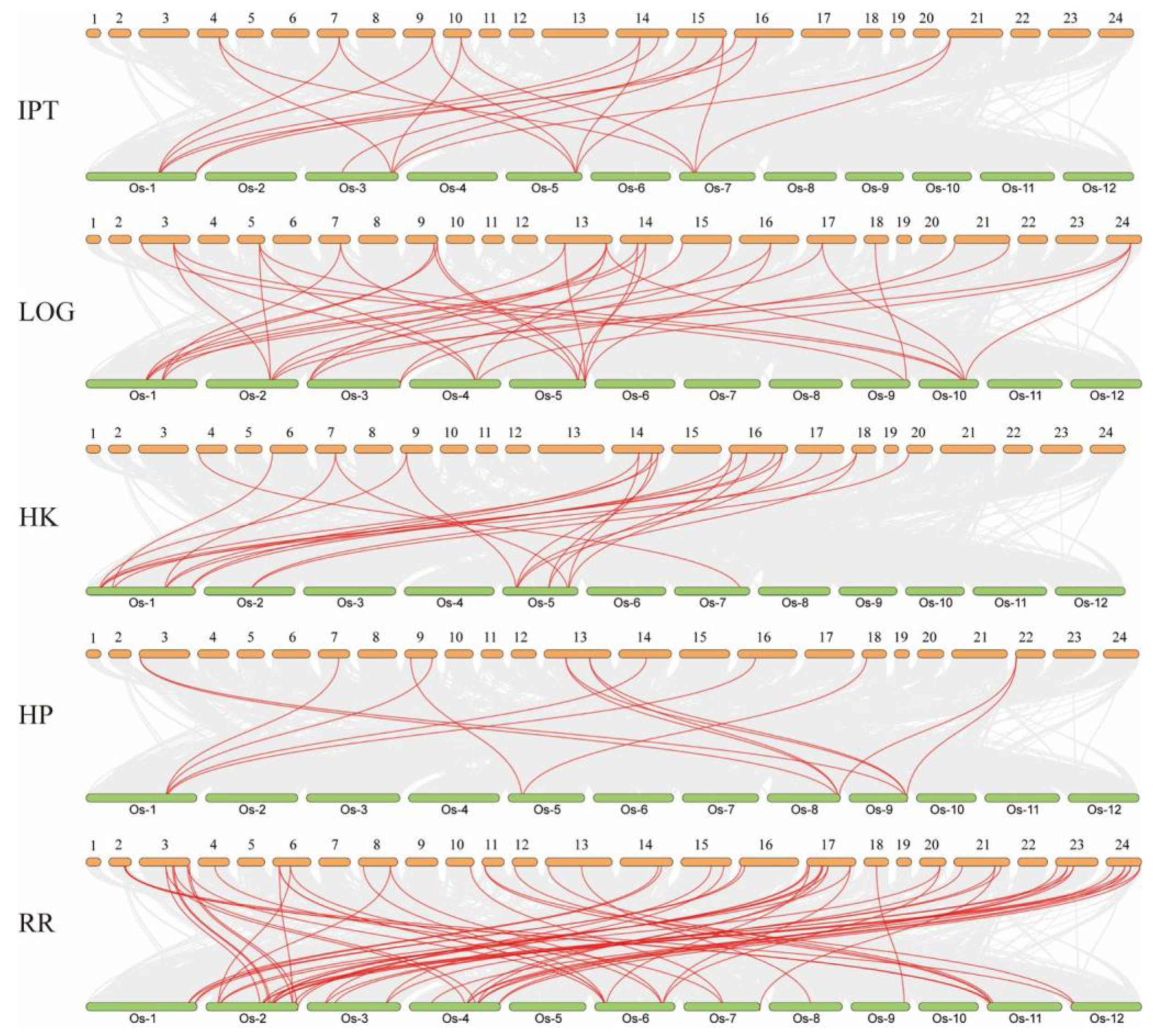
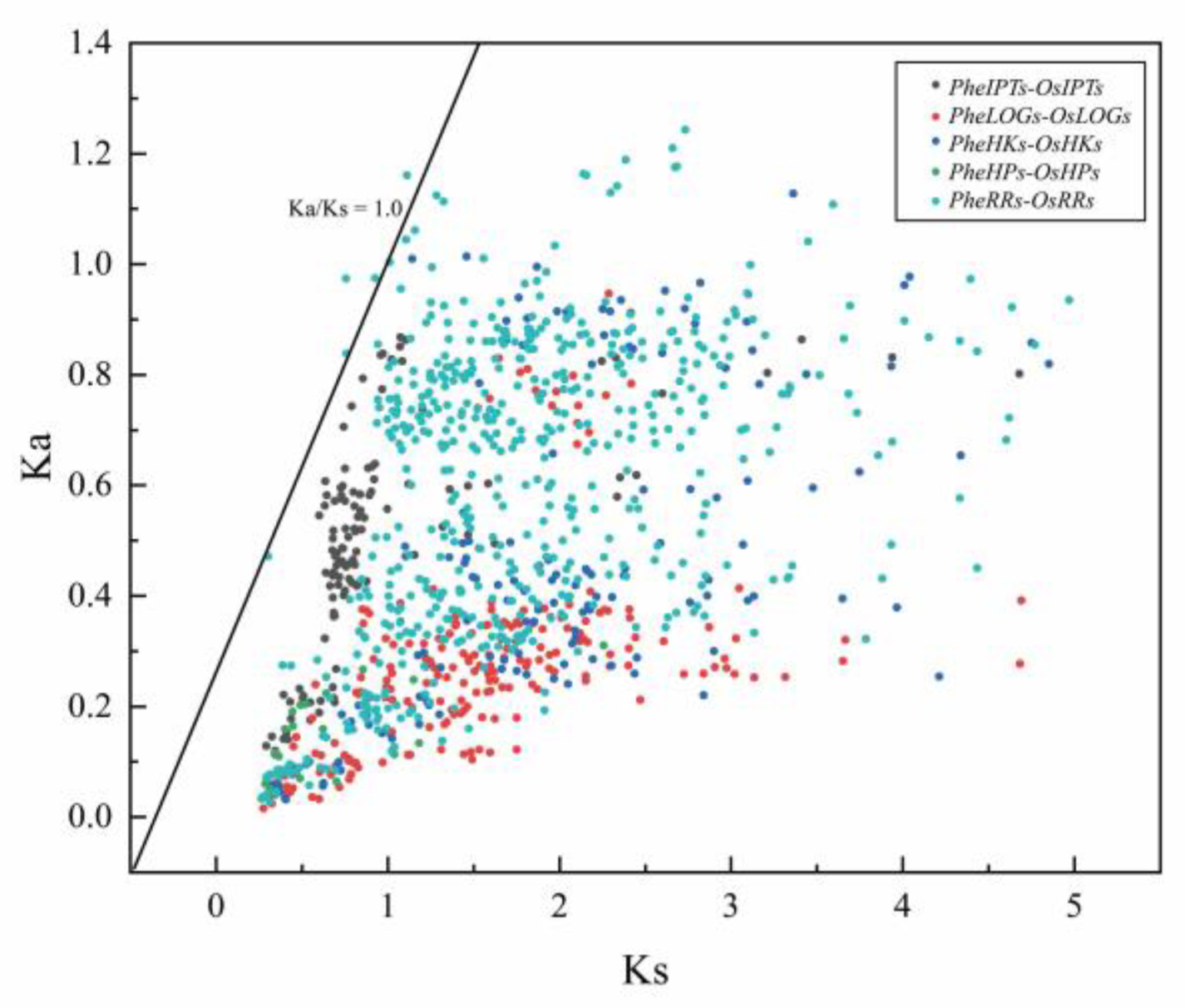
2.3. Analysis of the Evolutionary Pattern of the CK Pathway in Moso Bamboo
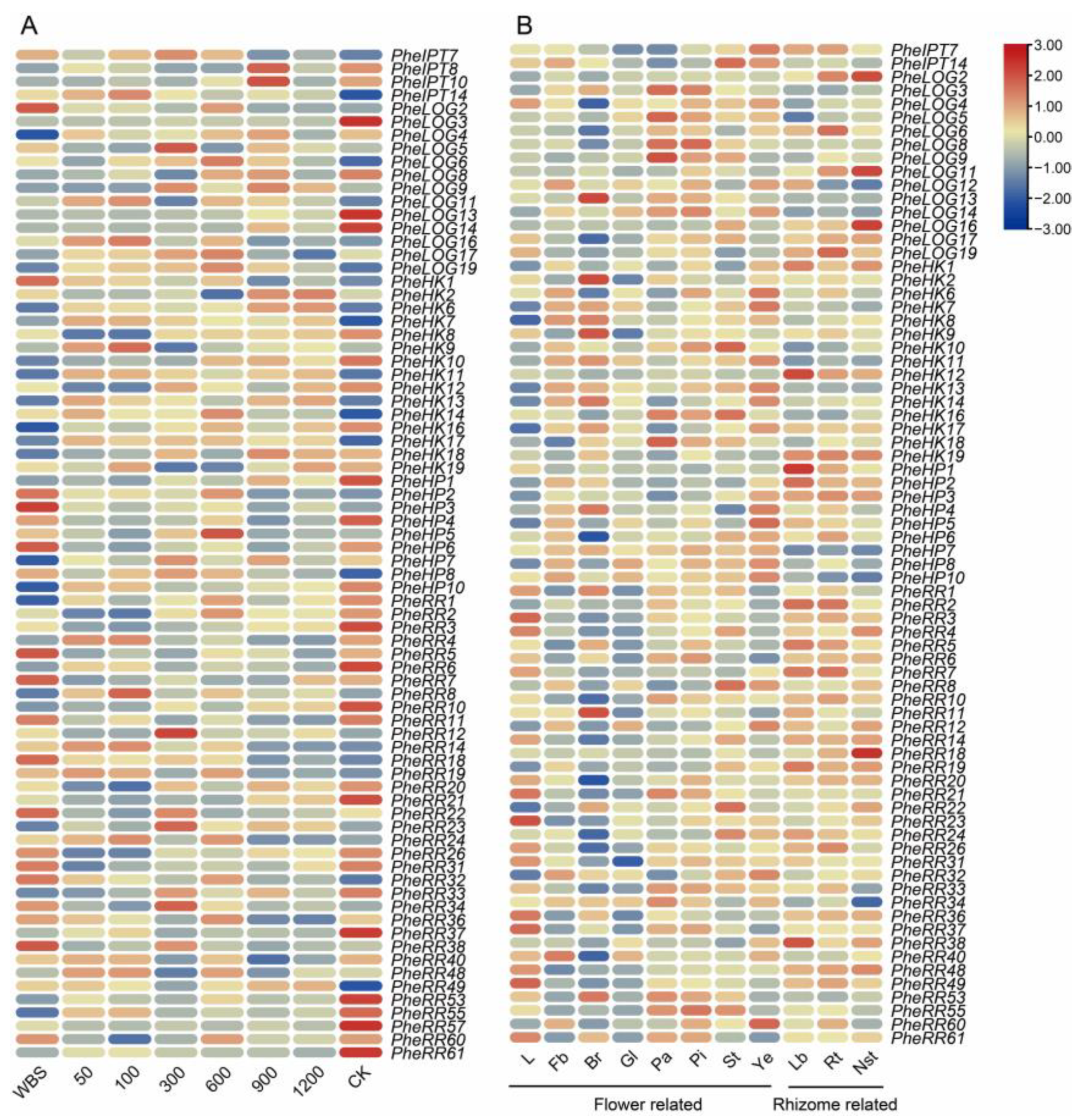
2.4. Tissue-Specific Expression of CK Pathway-Related Family Genes
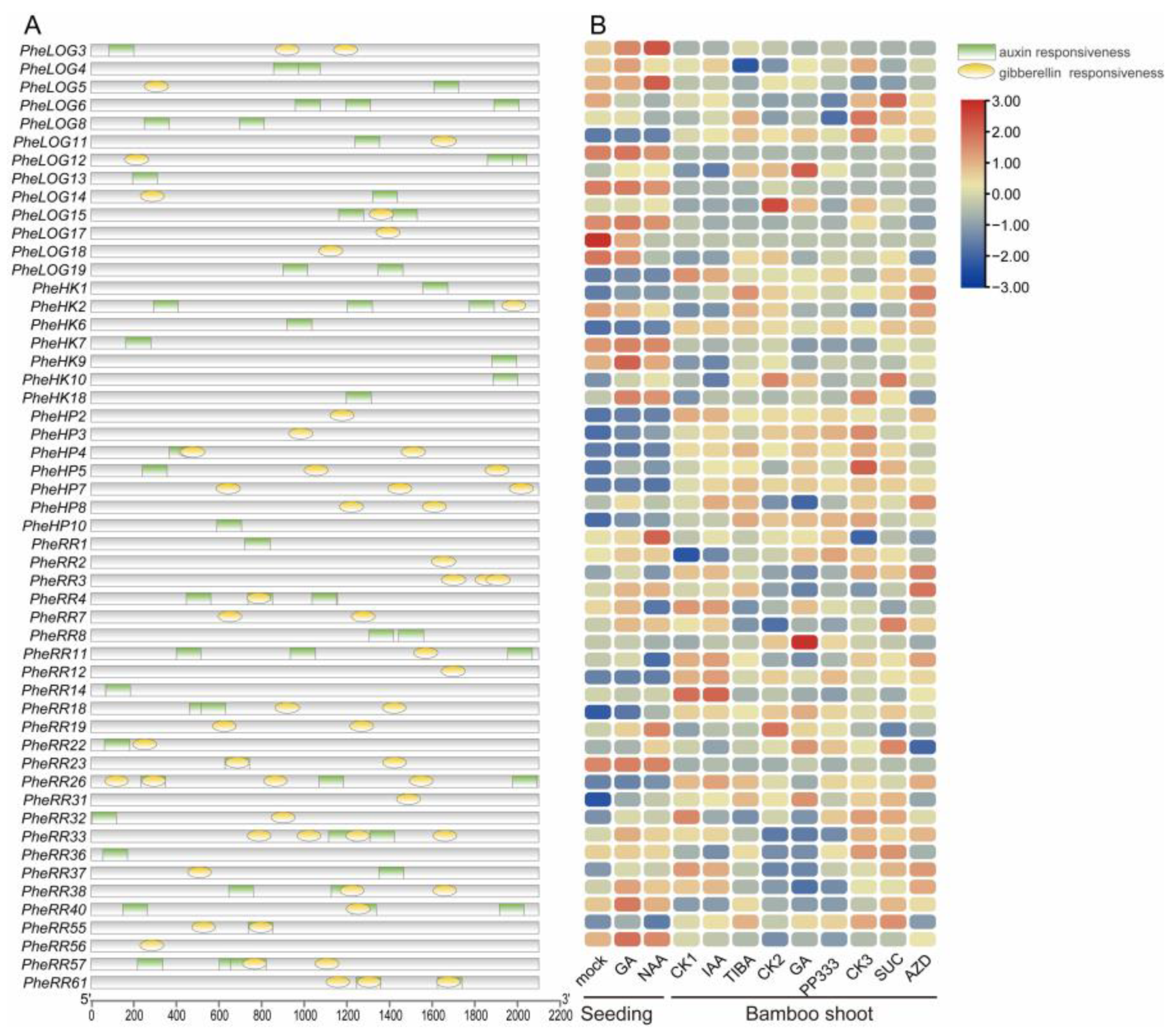
2.5. Analysis of Auxin and Gibberellin Response of CK Pathway-Related Family Genes
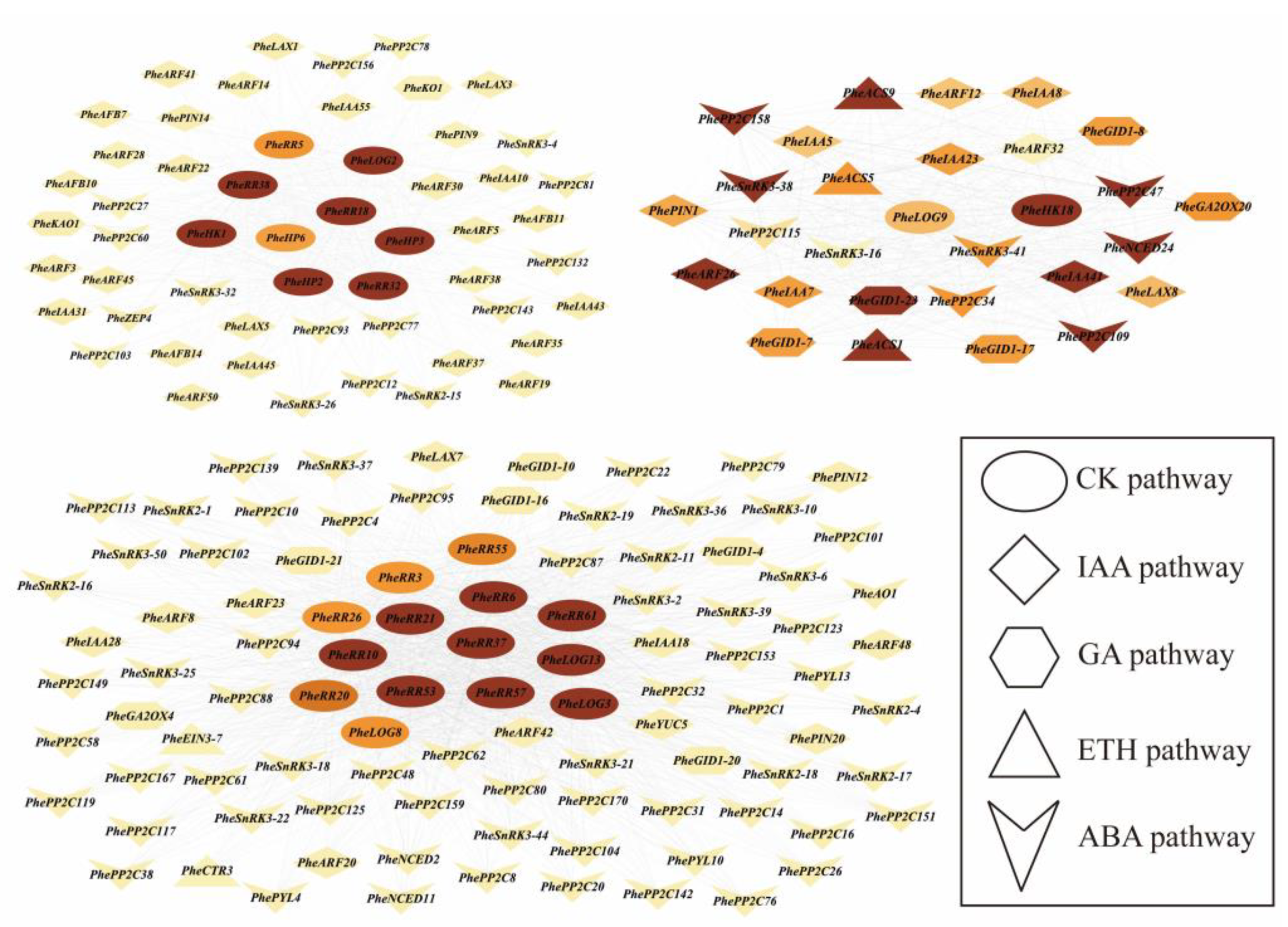
2.6. Analysis of Co-Expression Patterns of Key Gene Families of the Plant Hormone Pathway
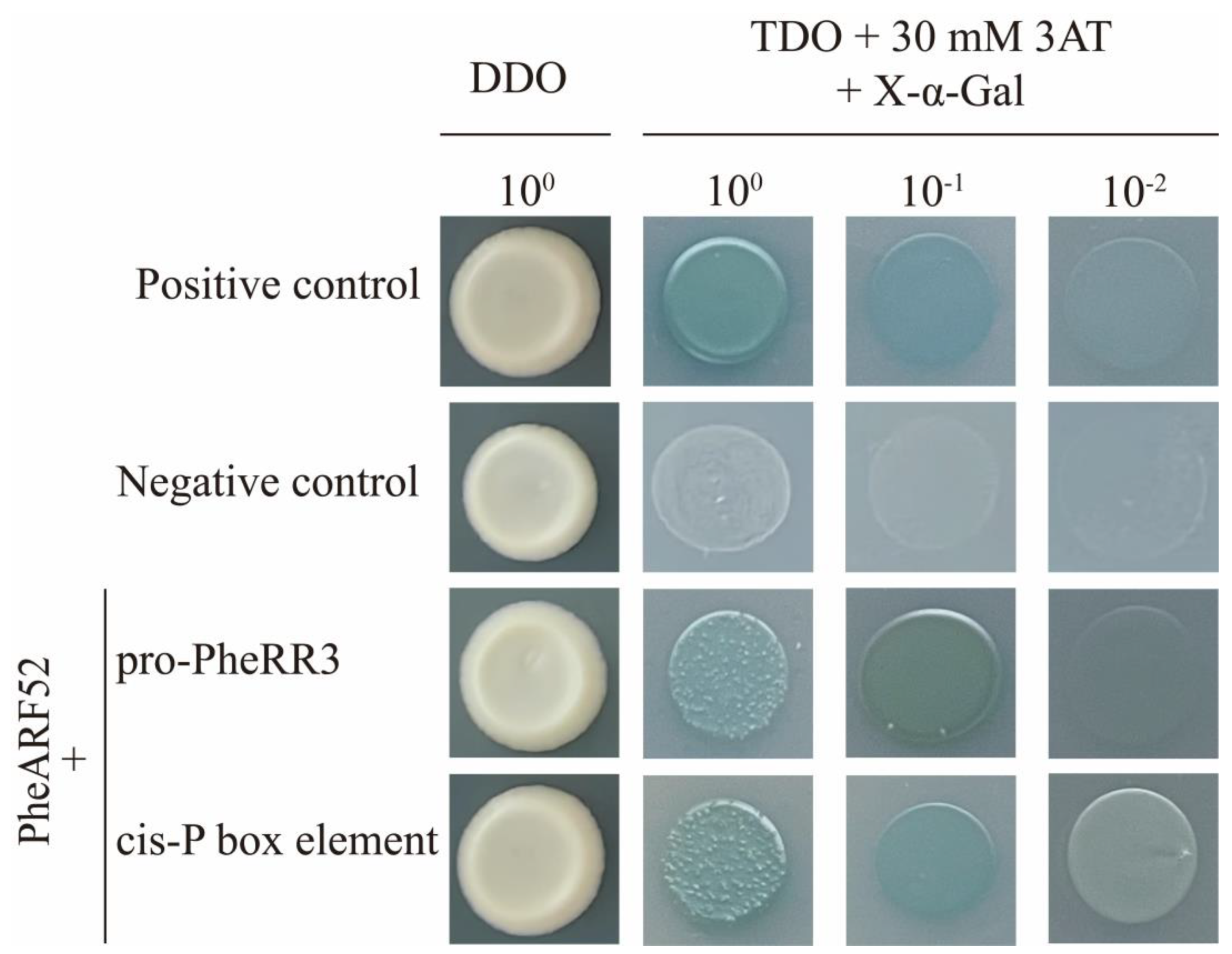
2.7. Validation of Upstream Regulators of PheRR3
3. Discussion
4. Materials and Methods
4.1. Identification and Physicochemical Property Analysis of Phytohormone Pathway Genes in Moso Bamboo
4.2. Phylogenetic Tree Construction and Evolutionary Pattern Analysis
4.3. Promoter Analysis and Transcriptome Data Acquisition
4.4. Co-Expression Analysis of Genes Related to the CK Pathway
4.5. Yeast One-Hybrid Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amasino, R. 1955: Kinetin arrives: The 50th anniversary of a new plant hormone. Plant. Physiol. 2005, 138, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Skoog, F.; Miller, C.O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–130. [Google Scholar] [PubMed]
- Smart, C.M.; Scofield, S.R.; Bevan, M.W.; Dyer, T.A. Delayed Leaf Senescence in Tobacco Plants Transformed with tmr, a Gene for Cytokinin Production in Agrobacterium. Plant Cell 1991, 3, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under Stress: Involvement of Auxin and Cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef]
- Chandler, J.W.; Werr, W. Cytokinin-auxin crosstalk in cell type specification. Trends Plant Sci. 2015, 20, 291–300. [Google Scholar] [CrossRef]
- Yang, W.; Cortijo, S.; Korsbo, N.; Roszak, P.; Schiessl, K.; Gurzadyan, A.; Wightman, R.; Jonsson, H.; Meyerowitz, E. Molecular mechanism of cytokinin-activated cell division in Arabidopsis. Science 2021, 371, 1350–1355. [Google Scholar] [CrossRef]
- Svolacchia, N.; Sabatini, S. Cytokinins. Curr. Biol. 2023, 33, R10–R13. [Google Scholar] [CrossRef]
- Papon, N. Cytokinin receptor turns 20. Nat. Rev. Mol. Cell Biol. 2021, 22, 443. [Google Scholar] [CrossRef]
- Letham, D.S. Zeatin, a Factor Inducing Cell Division Isolated from Zea Mays. Life Sci. 1963, 8, 569–573. [Google Scholar] [CrossRef]
- Kakimoto, T. Biosynthesis of cytokinins. J. Plant Res. 2003, 116, 233–239. [Google Scholar] [CrossRef]
- Mok, D.W.; Mok, M.C. Cytokinin Metabolism and Action. Annu. Rev. Plant Physiol. Plant. Mol. Biol. 2001, 52, 89–118. [Google Scholar] [CrossRef]
- Hwang, I.; Sakakibara, H. Cytokinin biosynthesis and perception. Physiol. Plant. 2006, 126, 528–538. [Google Scholar] [CrossRef]
- Jaworek, P.; Kopecny, D.; Zalabak, D.; Sebela, M.; Kouril, S.; Hluska, T.; Koncitikova, R.; Podlesakova, K.; Tarkowski, P. Occurrence and biosynthesis of cytokinins in poplar. Planta 2019, 250, 229–244. [Google Scholar] [CrossRef]
- Takei, K.; Sakakibara, H.; Sugiyama, T. Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 26405–26410. [Google Scholar] [CrossRef]
- Kakimoto, T. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate: ATP/ADP isopentenyltransferases. Plant Cell Physiol. 2001, 42, 677–685. [Google Scholar] [CrossRef]
- Sakamoto, T.; Sakakibara, H.; Kojima, M.; Yamamoto, Y.; Nagasaki, H.; Inukai, Y.; Sato, Y.; Matsuoka, M. Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice. Plant Physiol. 2006, 142, 54–62. [Google Scholar] [CrossRef]
- Tokunaga, H.; Kojima, M.; Kuroha, T.; Ishida, T.; Sugimoto, K.; Kiba, T.; Sakakibara, H. Arabidopsis lonely guy (LOG) multiple mutants reveal a central role of the LOG-dependent pathway in cytokinin activation. Plant J. 2012, 69, 355–365. [Google Scholar] [CrossRef]
- Hwang, I.; Sheen, J.; Muller, B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef]
- Stolz, A.; Riefler, M.; Lomin, S.N.; Achazi, K.; Romanov, G.A.; Schmülling, T. The specificity of cytokinin signalling in Arabidopsis thaliana is mediated by differing ligand affinities and expression profiles of the receptors. Plant J. 2011, 67, 157–168. [Google Scholar] [CrossRef]
- Kakimoto, T. Perception and signal transduction of cytokinins. Annu. Rev. Plant Biol. 2003, 54, 605–627. [Google Scholar] [CrossRef]
- Heyl, A.; Schmulling, T. Cytokinin signal perception and transduction. Curr. Opin. Plant Biol. 2003, 6, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Lomin, S.N.; Krivosheev, D.M.; Steklov, M.Y.; Arkhipov, D.V.; Osolodkin, D.I.; Schmülling, T.; Romanov, G.A. Plant membrane assays with cytokinin receptors underpin the unique role of free cytokinin bases as biologically active ligands. J. Exp. Bot. 2015, 66, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Zubo, Y.O.; Schaller, G.E. Role of the Cytokinin-Activated Type-B Response Regulators in Hormone Crosstalk. Plants 2020, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Taniguchi, M.; Imamura, A.; Ueguchi, C.; Mizuno, T.; Sugiyama, T. Differential expression of genes for response regulators in response to cytokinins and nitrate in Arabidopsis thaliana. Plant Cell Physiol. 1999, 40, 767–771. [Google Scholar] [CrossRef]
- Hosoda, K.; Imamura, A.; Katoh, E.; Hatta, T.; Tachiki, M.; Yamada, H.; Mizuno, T.; Yamazaki, T. Molecular structure of the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators. Plant Cell 2002, 14, 2015–2029. [Google Scholar] [CrossRef]
- Bai, Y.; Cai, M.; Mu, C.; Cheng, W.; Zheng, H.; Cheng, Z.; Li, J.; Mu, S.; Gao, J. New insights into the local auxin biosynthesis and its effects on the rapid growth of Moso Bamboo (Phyllostachys edulis). Front. Plant Sci. 2022, 13, 11. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Ding, Y.; Fei, Z.; Jiao, C.; Fan, M.; Yao, B.; Xin, P.; Chu, J.; Wei, Q. Cellular and molecular characterization of a thick-walled variant reveal a pivotal role of shoot apical meristem in transverse development of bamboo culm. J. Exp. Bot. 2019, 70, 3911–3926. [Google Scholar] [CrossRef]
- Li, L.; Cheng, Z.; Ma, Y.; Bai, Q.; Li, X.; Cao, Z.; Wu, Z.; Gao, J. The association of hormone signalling genes, transcription and changes in shoot anatomy during moso bamboo growth. Plant Biotechnol. J. 2018, 16, 72–85. [Google Scholar] [CrossRef]
- Bai, Y.; Cai, M.; Mu, C.; Zheng, H.; Cheng, Z.; Xie, Y.; Gao, J. Integrative analysis of exogenous auxin mediated plant height regulation in Moso bamboo (Phyllostachys edulis). Ind. Crops Prod. 2023, 200, 116852. [Google Scholar] [CrossRef]
- Chen, M.; Guo, L.; Ramakrishnan, M.; Fei, Z.; Vinod, K.K.; Ding, Y.; Jiao, C.; Gao, Z.; Zha, R.; Wang, C.; et al. Rapid growth of Moso bamboo (Phyllostachys edulis): Cellular roadmaps, transcriptome dynamics, and environmental factors. Plant Cell 2022, 34, 3577–3610. [Google Scholar] [CrossRef]
- Chen, C.M.; Ertl, J.R.; Leisner, S.M.; Chang, C.C. Localization of cytokinin biosynthetic sites in pea plants and carrot roots. Plant Physiol. 1985, 78, 510–513. [Google Scholar] [CrossRef]
- Zhao, J.; Ding, B.; Zhu, E.; Deng, X.; Zhang, M.; Zhang, P.; Wang, L.; Dai, Y.; Xiao, S.; Zhang, C.; et al. Phloem unloading via the apoplastic pathway is essential for shoot distribution of root-synthesized cytokinins. Plant Physiol. 2021, 186, 2111–2123. [Google Scholar] [CrossRef]
- Petrasek, J.; Hoyerova, K.; Motyka, V.; Hejatko, J.; Dobrev, P.; Kaminek, M.; Vankova, R. Auxins and Cytokinins in Plant Development 2018. Int. J. Mol. Sci. 2019, 20, 909. [Google Scholar] [CrossRef]
- Cucinotta, M.; Manrique, S.; Guazzotti, A.; Quadrelli, N.E.; Mendes, M.A.; Benkova, E.; Colombo, L. Cytokinin response factors integrate auxin and cytokinin pathways for female reproductive organ development. Development 2016, 143, 4419–4424. [Google Scholar] [CrossRef]
- Israeli, A.; Burko, Y.; Shleizer-Burko, S.; Zelnik, I.D.; Sela, N.; Hajirezaei, M.R.; Fernie, A.R.; Tohge, T.; Ori, N.; Bar, M. Coordinating the morphogenesis-differentiation balance by tweaking the cytokinin-gibberellin equilibrium. PLoS Genet. 2021, 17, e1009537. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; He, Y.; Yan, Y.; Yu, X.; Ali, M.; Pan, C.; Lu, G. Cytokinin-inducible response regulator SlRR6 regulates plant height through gibberellin and auxin pathways in tomato. J. Exp. Bot. 2023, erad159. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.P.; Wu, H.Q.; Jin, H. Conversion of protocorm-like bodies of Dendrobium huoshanense to shoots: The role of polyamines in relation to the ratio of total cytokinins and indole-3-acetic acid. J. Plant Physiol. 2009, 166, 2013–2022. [Google Scholar] [CrossRef]
- Milhinhos, A.; Miguel, C.M. Hormone interactions in xylem development: A matter of signals. Plant Cell Rep. 2013, 32, 867–883. [Google Scholar] [CrossRef]
- Mandal, N.K.; Kumari, K.; Kundu, A.; Arora, A.; Bhowmick, P.K.; Iquebal, M.A.; Jaiswal, S.; Behera, T.K.; Munshi, A.D.; Dey, S.S. Cross-talk between the cytokinin, auxin, and gibberellin regulatory networks in determining parthenocarpy in cucumber. Front. Genet. 2022, 13, 957360. [Google Scholar] [CrossRef]
- Wang, C.; Gong, Z.; Han, G.Z. On the origins and evolution of phytohormone signaling and biosynthesis in plants. Mol. Plant 2023, 16, 511–513. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Zhang, S.; Lou, Y.; An, X.; Jiang, Z.; Gao, Z. Transcriptome and miRNAome analysis reveals components regulating tissue differentiation of bamboo shoots. Plant Physiol. 2022, 188, 2182–2198. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Dou, Y.; Xie, Y.; Zheng, H.; Gao, J. Phylogeny, transcriptional profile, and auxin-induced phosphorylation modification characteristics of conserved PIN proteins in Moso bamboo (Phyllostachys edulis). Int. J. Biol. Macromol. 2023, 234, 123671. [Google Scholar] [CrossRef] [PubMed]
- Kolachevskaya, O.O.; Myakushina, Y.A.; Getman, I.A.; Lomin, S.N.; Deyneko, I.V.; Deigraf, S.V.; Romanov, G.A. Hormonal regulation and crosstalk of auxin/cytokinin signaling pathways in potatoes in vitro and in relation to vegetation or tuberization stages. Int. J. Mol. Sci. 2021, 22, 8207. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, W.; Gu, L.; Ye, S.; Zhang, H.; Cai, C.; Xiang, M.; Gao, Y.; Wang, Q.; Lin, C.; Zhu, Q. Genome-wide analysis and transcriptomic profiling of the auxin biosynthesis, transport and signaling family genes in moso bamboo (Phyllostachys heterocycla). BMC Genom. 2017, 18, 870. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Cai, D.; Gao, Y.; Zhang, H.; Wang, Y.; Lin, C.; Ma, L.; Gu, L. Comprehensive profiling of rhizome-associated alternative splicing and alternative polyadenylation in moso bamboo (Phyllostachys edulis). Plant J. 2017, 91, 684–699. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Zhu, Q.; Gao, Y.; Wang, H.; Zhao, L.; Wang, Y.; Xi, F.; Wang, W.; Yang, Y. Transcriptome characterization of moso bamboo (Phyllostachys edulis) seedlings in response to exogenous gibberellin applications. BMC Plant Biol. 2018, 18, 125. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Cai, M.; Dou, Y.; Xie, Y.; Zheng, H.; Gao, J. Phytohormone Crosstalk of Cytokinin Biosynthesis and Signaling Family Genes in Moso Bamboo (Phyllostachys edulis). Int. J. Mol. Sci. 2023, 24, 10863. https://doi.org/10.3390/ijms241310863
Bai Y, Cai M, Dou Y, Xie Y, Zheng H, Gao J. Phytohormone Crosstalk of Cytokinin Biosynthesis and Signaling Family Genes in Moso Bamboo (Phyllostachys edulis). International Journal of Molecular Sciences. 2023; 24(13):10863. https://doi.org/10.3390/ijms241310863
Chicago/Turabian StyleBai, Yucong, Miaomiao Cai, Yuping Dou, Yali Xie, Huifang Zheng, and Jian Gao. 2023. "Phytohormone Crosstalk of Cytokinin Biosynthesis and Signaling Family Genes in Moso Bamboo (Phyllostachys edulis)" International Journal of Molecular Sciences 24, no. 13: 10863. https://doi.org/10.3390/ijms241310863
APA StyleBai, Y., Cai, M., Dou, Y., Xie, Y., Zheng, H., & Gao, J. (2023). Phytohormone Crosstalk of Cytokinin Biosynthesis and Signaling Family Genes in Moso Bamboo (Phyllostachys edulis). International Journal of Molecular Sciences, 24(13), 10863. https://doi.org/10.3390/ijms241310863





