Abstract
Antibacterial chitosan films, versatile and eco-friendly materials, have garnered significant attention in both the food industry and medicine due to their unique properties, including biodegradability, biocompatibility, and antimicrobial activity. This review delves into the various types of chitosan films and their distinct applications. The categories of films discussed span from pure chitosan films to those enhanced with additives such as metal nanoparticles, metal oxide nanoparticles, graphene, fullerene and its derivatives, and plant extracts. Each type of film is examined in terms of its synthesis methods and unique properties, establishing a clear understanding of its potential utility. In the food industry, these films have shown promise in extending shelf life and maintaining food quality. In the medical field, they have been utilized for wound dressings, drug delivery systems, and as antibacterial coatings for medical devices. The review further suggests that the incorporation of different additives can significantly enhance the antibacterial properties of chitosan films. While the potential of antibacterial chitosan films is vast, the review underscores the need for future research focused on optimizing synthesis methods, understanding structure-property relationships, and rigorous evaluation of safety, biocompatibility, and long-term stability in real-world applications.
1. Introduction
Chitosan (Figure 1) is a versatile and promising biopolymer that has garnered significant interest in various fields due to its unique characteristics and properties. Derived from chitin, which is predominantly found in the exoskeleton of crustaceans, insects, and the cell walls of fungi, chitosan is the second most abundant natural polysaccharide after cellulose [1,2,3]. Its biodegradability, biocompatibility, non-toxicity, antimicrobial properties, antitumor effect and ability to sensitize tumor therapy [4,5] make it an attractive material for numerous applications, particularly in the food industry and medicine [6,7,8,9].
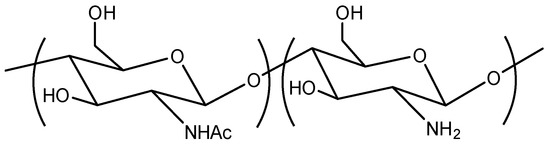
Figure 1.
Chemical structure of chitosan.
One of the most noteworthy applications of chitosan is the development of antibacterial films. These films are utilized as food packaging materials, which can extend the shelf life of food products by inhibiting the growth of spoilage-causing microorganisms [10,11,12,13]. Additionally, chitosan-based films are employed in the medical field as wound dressings, where they can prevent bacterial infections and facilitate healing. Chitosan films also can be utilized as drug delivery systems, enabling the controlled release of antibiotics or other therapeutic agents [14,15,16,17].
In the current review, we will delve into the following aspects of chitosan-based antibacterial films:
- Structure and synthesis of chitosan: we will provide an overview of chitosan’s chemical structure, including its main functional groups and degree of polymerization. Additionally, we will discuss the various methods of chitosan production, such as the deacetylation of chitin and the use of specific enzymes or microorganisms;
- Antibacterial properties and mechanisms of antibacterial action of chitosan: we will explore the various factors that contribute to chitosan’s antimicrobial activity, such as its molecular weight, degree of deacetylation, and environmental conditions. Furthermore, we will discuss the proposed mechanisms of action through which chitosan exerts its antibacterial effects, including cell membrane disruption, chelation of essential nutrients, and interference with microbial gene expression;
- Types of antibacterial chitosan films: we will examine different types of chitosan films, such as those composed of pure chitosan, chitosan-metal nanoparticles, chitosan-metal oxide nanoparticles, chitosan-graphene, chitosan-fullerene or its derivatives, and chitosan-plant extracts. For each type of film, we will discuss their synthesis methods, unique properties, and potential applications in both the food industry and medicine;
- Applications in the food industry: we will explore how chitosan-based films can be used to improve food safety and quality by preventing microbial contamination, reducing oxidation, and enhancing mechanical and barrier properties;
- Applications in medicine: we will discuss the potential medical applications of chitosan films, including their use as wound dressings, drug delivery systems, and tissue engineering scaffolds.
The analysis of the current state in the field of chitosan-based antibacterial films and discuss potential future directions for further development of these materials are given in the sections that follow below.
2. Structure and Synthesis of Chitosan
Chitosan is a natural linear biopolymer derived from chitin, a major structural component found in the exoskeleton of crustaceans, insects, and the cell walls of fungi. Chemically, chitosan consists of randomly distributed β-(1→4)-linked N-acetyl-D-glucosamine (GlcNAc) and D-glucosamine (GlcN) units [18]. The proportion of GlcNAc and GlcN units defines the degree of deacetylation (DD), a critical parameter that influences the physical, chemical, and biological properties of chitosan, such as solubility, viscosity, and antimicrobial activity.
Chitosan’s molecular weight varies widely, depending on the source material and synthesis method. Higher molecular weight chitosan typically exhibits increased viscosity and mechanical strength, whereas lower molecular weight chitosan demonstrates enhanced solubility and bioactivity [19].
Producing chitosan from chitin involves various methods, primarily focusing on deacetylation processes:
- Alkaline deacetylation: this method is the most widely employed for chitosan synthesis. It involves treating chitin with concentrated alkali solutions, such as sodium hydroxide, at elevated temperatures. This process removes acetyl groups, converting chitin into chitosan. The reaction conditions, such as alkali concentration, temperature, and reaction time, influence the degree of deacetylation and the molecular weight of the resulting chitosan [20,21]. The undoubted advantage of alkaline deacetylation is its cheapness, while the main disadvantages of this method are associated with its environmental damage (the use of large amounts of aggressive reagents, i.e., alkalis);
- Enzymatic deacetylation: this method uses specific chitinase enzymes to selectively remove acetyl groups from chitin. The enzymatic process offers advantages such as specificity, milder reaction conditions, and reduced environmental impact compared to alkaline deacetylation. However, this method is less popular due to higher costs and lower yields. Ongoing research focuses on improving enzyme efficiency and lowering production costs [22];
- Microbial fermentation: this emerging method employs specific microorganisms, such as Streptomyces or Bacillus strains, to convert chitin into chitosan. The process holds the potential to be more sustainable and environmentally friendly compared to chemical methods. However, some challenges, including optimizing reaction conditions, scalability, and cost-effectiveness, remain to be addressed [23].
We want to point out that enzymatic deacetylation provides the highest degree of deacetylation (up to almost 100%) and preserves the chitosan backbone to the maximum. At the same time, for alkaline deacetylation, a degree of deacetylation of 10–20% is considered a great success. In addition, alkaline deacetylation leads to partial depolymerization of the polysaccharide [24]. Once chitosan is synthesized, it can be further processed to form films through various techniques (Figure 2):
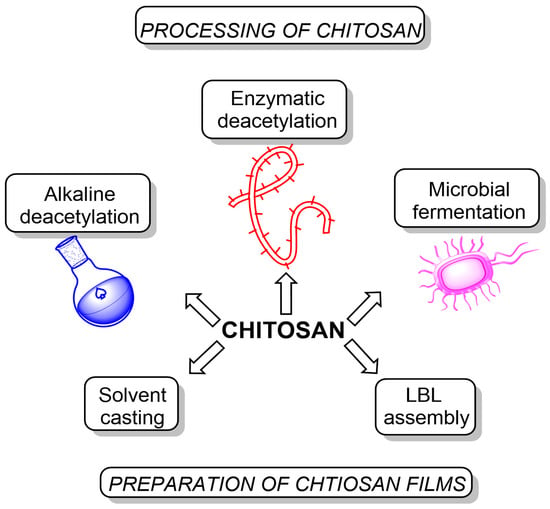
Figure 2.
Processing of chitosan and methods of preparation of chitosan-based films.
- Solvent casting: the most common method for producing chitosan films involves dissolving chitosan in a suitable solvent (typically acetic acid), casting the solution onto a flat surface, and evaporating the solvent to form a solid film. This process results in films with good mechanical and barrier properties, making them suitable for food packaging and medical applications [25];
- Layer-by-layer assembly: this method involves the alternate deposition of oppositely charged polyelectrolytes (including chitosan) onto a substrate to produce multilayer films with controlled thickness and composition. The properties of these films, such as permeability, mechanical strength, and antimicrobial activity, can be tailored by adjusting the number of layers, the choice of polyelectrolytes, and the assembly conditions. Layer-by-layer assembled chitosan films have been used in applications such as food packaging with controlled release of antimicrobial agents and medical devices with tunable drug release profiles [26,27].
In the following sections, we will discuss the antibacterial properties of chitosan, the underlying mechanisms of its antibacterial action, and the various types of antibacterial chitosan films, along with their applications in the food industry and medicine.
3. Antibacterial Properties and Mechanism of Action of Chitosan
Chitosan, a natural linear biopolymer derived from chitin, has gained considerable attention due to its broad-spectrum antimicrobial activity against various microorganisms, including Gram-positive and Gram-negative bacteria, fungi, and yeasts [21]. This activity is influenced by several factors, such as molecular weight, degree of deacetylation (DD), pH, and the ionic strength of the environment.
The molecular weight of chitosan plays a critical role in determining its antimicrobial properties. Lower molecular weight chitosan tends to exhibit higher antimicrobial activity due to its increased solubility, which allows for better penetration of bacterial cell walls [19]. The DD of chitosan also significantly impacts its antimicrobial activity, with higher DD chitosan showing enhanced activity because of the increased density of protonated amino groups that interact with bacterial cell surfaces [28].
The antimicrobial action of chitosan involves several mechanisms, which can be summarized as follows (Figure 3):
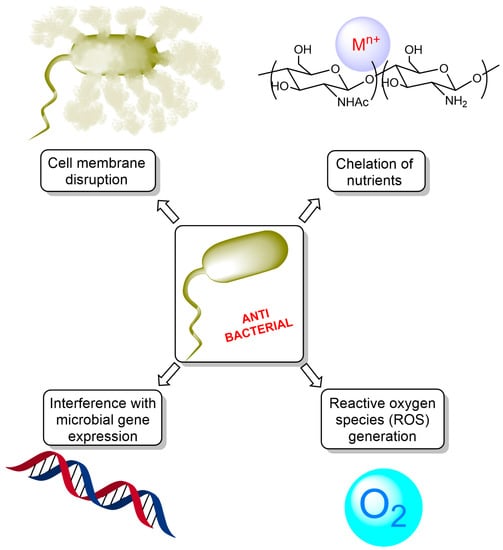
Figure 3.
Mechanisms of antibacterial action of chitosan.
- Cell membrane disruption: the cationic nature of chitosan, resulting from the protonation of its amino groups under acidic conditions, enables it to interact with the negatively charged bacterial cell surface. This interaction can cause changes in the cell membrane permeability, leading to the leakage of intracellular components, disruption of membrane integrity, and eventual cell death [29];
- Chelation of essential nutrients: chitosan possesses the ability to bind to essential metal ions such as calcium, magnesium, and iron, which are crucial for bacterial growth and metabolism. By chelating these essential nutrients, chitosan can inhibit bacterial growth and biofilm formation, impeding the ability of bacteria to colonize surfaces and cause infections [30];
- Interference with microbial gene expression: Chitosan can penetrate bacterial cells and interact with intracellular components such as DNA and RNA. This interaction can lead to the inhibition of bacterial gene expression and protein synthesis, ultimately resulting in bacterial growth inhibition and cell death [31];
- Reactive oxygen species (ROS) generation: Chitosan has been reported to induce the generation of ROS in bacterial cells, leading to oxidative stress, DNA damage, and cell death [32]. This mechanism contributes to the antimicrobial activity of chitosan and its derivatives.
In the upcoming sections, we will explore various types of antibacterial chitosan films, including pure chitosan films, chitosan films with metal nanoparticles, chitosan films with metal oxide nanoparticles, chitosan films with graphene, chitosan films with fullerene derivatives, and chitosan films with plant extracts. For each type of film, we will discuss their synthesis methods, unique properties, and potential applications in both the food industry and medicine.
4. Antibacterial Films from Pure Chitosan
Pure chitosan films have been extensively studied due to their inherent antimicrobial properties, biocompatibility, and biodegradability. These films can be used in various applications, such as food packaging, wound dressings, and medical device coatings. In this section, we will discuss the synthesis and properties of pure chitosan films, along with their applications in the food industry and medicine.
4.1. Use in the Food Industry
Chitosan films have been widely investigated for food packaging applications to prolong shelf life, maintain quality, and reduce spoilage caused by microbial contamination. These films can be synthesized using various methods, including solvent casting, electrospinning, and layer-by-layer assembly, as mentioned earlier. It should be noted that, due to the extreme rigidity of the chitosan macromolecule, the addition of a plasticizer is required to obtain a film with good mechanical properties. As a rule, glycerin is used as a plasticizer [33].
Chitosan films have been employed to preserve different types of food products, such as fruits, vegetables, meats, and fish. For example, chitosan films have been used to extend the shelf life of strawberries by reducing weight loss, maintaining firmness, and inhibiting fungal growth [34]. Similarly, chitosan-coated chicken breasts have shown reduced bacterial growth and lower lipid oxidation rates compared to uncoated samples [35]. It has also been found that chitosan films have similar oxygen permeability values to the commercially available food packaging ethylene-vinyl alcohol copolymer films or polyvinylidene chloride films [36].
4.2. Use in Medicine
In the medical field, pure chitosan films have been employed for wound dressings, drug delivery systems, and medical device coatings due to their antimicrobial, biocompatible, and biodegradable properties [24].
Chitosan films can promote wound healing by providing a moist environment, preventing bacterial infections, and stimulating tissue regeneration. These films have been shown to accelerate wound closure, reduce inflammation, and enhance re-epithelialization in animal models [37]. Chitosan films can be used for the improvement of the Schwann cell response in nerve regeneration [38], for the tissue engineering of bone, supporting the bone growing in the attaching parts as well as preserving the integrity of the structure during in vivo remodeling of tissue [39]. Chitosan-based dressings have been extensively applied for the preparation of wound healing materials due to their excellent properties, such as good gas permeation, high porosity, and high surface area. Exudate removal, moisture retention, skin regeneration, cell respiration, and hemostasis were all aided by using chitosan films [40]. Yang et al. developed a film-forming solution for the treatment of MRSA infections in wounds. The film-forming solution was more suitable for the treatment of MRSA than a wide range of previously described systems based on synthetic polymers [41].
Chitosan films can also potentially be used as drug delivery and sustained release systems. The release of a drug from chitosan films is followed by polymer degradation and a complex diffusion process. Drug release behavior can be influenced by a variety of factors, including drug state, surface functionalization, and polymer properties [42,43]. Chitosan-based films can be used for the transdermal delivery of proteins and peptides [44] and antibiotics [45].
The main disadvantages of films based on pure chitosan are associated with their meager mechanical properties and moderate antibacterial activity. The most important way to expand the mechanical and antibacterial properties of chitosan films is the introduction of various active additives into the polymer matrix of the film. In the following sections, we will discuss various types of antibacterial chitosan films, including chitosan films with metal nanoparticles, chitosan films with metal oxide nanoparticles, graphene, fullerene and its derivatives, and also chitosan-based films with plant extracts. For each type of film, we will discuss their preparation methods and potential applications in both the food industry and medicine.
5. Antibacterial Films from Chitosan with Metal Nanoparticles
The incorporation of metal nanoparticles into chitosan films can enhance their antimicrobial properties, making them more effective in various applications. In this section, we will discuss the synthesis and properties of chitosan films with metal nanoparticles, such as silver, gold, and copper, and their applications in the food industry and medicine.
5.1. Use in the Food Industry
Chitosan films containing metal nanoparticles can be synthesized using methods like in situ reduction, electrospinning, and solvent casting. Among the different metal nanoparticles, silver nanoparticles (AgNPs) have been widely studied due to their strong antimicrobial activity against a broad spectrum of microorganisms [46].
Chitosan films incorporating AgNPs have been used in food packaging to prolong shelf life and maintain quality. For example, chitosan films containing AgNPs have been shown to effectively inhibit the growth of spoilage bacteria on chicken breast fillets, extending their shelf life [47]. As compared to the chitosan film, various properties were enhanced in the chitosan/AgNPs blend films, including light barrier, opacity, elongation at break, as well as bioactivities, thus suggesting that films could be used as novel alternative food packaging application [48]. Chitosan films containing covalent organic frameworks (COFs) immobilized AgNPs showed that the tensile strength of the nanocomposite films enhanced dramatically with the increase of the COFs-AgNPs content, while the UV–visible light barrier property, water swelling and solubility properties, and water vapor permeability decreased significantly. Furthermore, the CS/COFs-AgNPs nanocomposite films also showed outstanding antibacterial activity and effectively prolonged the storage time of white crucian carp (Carassius auratus) [49]. Recently, Padil et al. demonstrated that the addition of AgNPs to the polymer blend matrix results in significant improvement of the mechanical characteristics of chitosan-based films. The cross-linking motion of the silver nanoparticles leads to a decrease in swelling degree, moisture retention capability, and water vapor permeability. The addition of 0.0075% of the nanoparticles dramatically increases the tensile strength of the films. The real-time application of the films was tested by evaluating the shelf-life existence of carrot pieces covered with the composite films. The composite film containing AgNPs becomes effective in lowering bacterial contamination when compared to plastic polyethylene films [50].
Similarly, chitosan films with gold nanoparticles (AuNPs) and copper nanoparticles (CuNPs) have also demonstrated enhanced antimicrobial activity and potential applications in food packaging [51] Chitosan-based films with copper nanoparticles are characterized by enhanced antimicrobial activity against Salmonella and S. typhimurium as well as improved mechanical behavior in comparison with the corresponding pure chitosan films [52]. Cardenas et al. obtained a film based on chitosan and colloidal copper nanoparticles by casting with microwave heating. The film-forming solution has good dispersion and film-forming properties due to the high density of amino and hydroxyl groups of chitosan, which avoids the aggregation of metal particles. The elaborate composite film has distinct advantages over chitosan film in terms of oxygen permeability, vapor permeability and light transmission. In addition, the resulting film is characterized by a high antimicrobial effect and can be used to protect food products and extend their shelf life [53].
5.2. Use in Medicine
Chitosan-based films reinforced by metal nanoparticles are increasingly being used in the biomedical field. This is mainly due to the antibacterial effect of chitosan films, which in many cases is significantly enhanced by the introduction of metal nanoparticles into the polymer matrix [54]. In addition, in some instances, metal nanoparticles and chitosan macromolecules have a symbiotic positive effect on the stimulation of tissue growth and regeneration, which is important for wound healing [55].
Research has demonstrated that chitosan films imbued with AgNPs foster the wound recovery process by creating a damp milieu, lessening inflammatory responses, and invigorating the renewal of impaired tissues [56]. These films also exhibit a protective quality against wound contaminations, effectively halting the proliferation of harmful microbes, including Staphylococcus aureus, Pseudomonas aeruginosa [56] and E. Coli in vitro and in vivo [57]. Recently elaborated chitosan films with silver nanoparticles demonstrated high antibacterial activity against E. coli. At the same time, the prepared multilayers showed mild activity against S. aureus predominantly due to the antiadhesive effect, and this material looks promising as a prospective material for covering the surface of medical implants in order to reduce their bacterial contamination [58]. Chitosan-based dressing hydrogel films with AgNPs, elaborated Karami et al. showed strong antibacterial activity against Bacillus cereus and Staphylococcus aureus and a positive effect on tissue regeneration [59].
In drug delivery systems, chitosan films containing metal nanoparticles can be used as carriers for various bioactive agents, including antibiotics, anti-inflammatory drugs, and growth factors. The release of these agents can be controlled by modifying the film’s composition, thickness, and degree of cross-linking [60].
Chitosan films with metal nanoparticles have also been used as coatings for medical devices, such as catheters, implants, and prosthetics, to prevent bacterial adhesion and biofilm formation. These coatings can minimize the risk of device-related infections, which are a significant concern in healthcare settings [61].
6. Antibacterial Films from Chitosan with Metal Oxide Nanoparticles
The incorporation of metal oxide nanoparticles into chitosan films can also improve their antimicrobial properties and broaden their applications. In this section, we will discuss the synthesis and properties of chitosan films with metal oxide nanoparticles, such as zinc oxide (ZnO) and titanium dioxide (TiO2) as the most used oxides in chitosan films, and their applications in the food industry and medicine.
6.1. Use in the Food Industry
Chitosan films containing metal oxide nanoparticles can be synthesized using methods like in situ oxide generation, direct introduction of metal oxide into a polymer matrix, electrospinning, and solvent casting. Zinc oxide nanoparticles (ZnONPs) have been widely investigated due to their antimicrobial properties against a broad range of microorganisms [62].
Chitosan films incorporating ZnONPs have been used in food packaging to extend shelf life and maintain quality. For example, chitosan films containing ZnONPs have been shown to inhibit the growth of spoilage bacteria on fresh-cut fruits, such as melons and apples [63]. Chitosan and zinc oxide nanoparticles loaded gallic-acid films showed good physical properties such as oxygen and water vapor permeability, swelling, UV-vis light transmittance and significant antibacterial potential [64]. Hamideskar et al. obtained a composite film based on chitosan as a polymer matrix and zinc oxide nanoparticles as a filler. The nanocomposite had a good porous structure with interconnection. Evaluation of the antibacterial activity of the produced films showed that the synergistic effect between chitosan and zinc oxide improves the antibacterial activity of the nanocomposite. The potential of the nanocomposite for chicken fillet and cheese packaging has been explored. Microbiological analysis of food samples during storage showed that the most effective inhibition of inoculating bacteria was obtained for chicken fillet and cheese samples wrapped with a nanocomposite film. The use of the film protected the physicochemical quality of the chicken fillet and cheese samples during storage. At the same time, the weight loss of chicken fillet and cheese samples wrapped with a nanocomposite was lower than that of samples wrapped with pure chitosan or even polyethylene film. Therefore, antibacterial nanocomposite film is suitable for poultry and cheese packaging, which holds great promise for improving the quality and shelf life of the mentioned food products [65]. Chitosan-ZnO coating prepared by Nabulsi et al. demonstrated a reduction in the initial count of E. coli during 28 days with a constant dispersal of nanoparticles within the polymer medium. The elaborated coatings showed excellent effectiveness for white-brined cheese shelf-life prolongation [66]. Similarly, chitosan films with titanium dioxide nanoparticles (TiO2NPs) have also demonstrated enhanced antimicrobial activity and potential applications in food packaging [67]. Chitosan-alginate nanocomposite film with nanoparticles of TiO2 showed enhanced tensile strength and elongation at break as well as antimicrobial activity against foodborne pathogens E. coli, S. aureus, S. typhi, and L. monocytogene. The film with 0.1% of TiO2 demonstrated the complete killing of gram-positive bacteria. Moreover, the film was completely biodegraded during the 3 months [68]. The composite membrane of chitosan and TiO2NPs enhanced the firmness of the mango. The peroxidase and polyphenol oxidase activity of the composite coated fruits, as well as the total phenol and flavonoid content was also higher than in control group, that indicated that the chitosan/nano-TiO2 composite coating could maintain the nutrient composition of mangoes [69]. Mannaa et al. stated that TiO2 incorporated in chitosan film enhanced it mechanical properties and antimicrobial activity compared to blank film, that suggest they could be a viable alternative to non-biodegradable food-packaging material [70].
6.2. Use in Medicine
In medical applications, chitosan films with metal oxide nanoparticles have been used mainly for wound dressings. Chitosan films with ZnONPs have been shown to promote wound healing by providing a moist environment, reducing inflammation, and stimulating the regeneration of damaged tissue [62]. In addition, zinc oxide has a pronounced antibacterial effect, including against many strains of microorganisms that are resistant to conventional antibiotics [71]. Recently, Teplea et al. prepared novel chitosan-based films with ZnO nanoparticles for wound healing. The film has significant activity against S. aureus and C. albicans being promising materials for antimicrobial wound dressing, especially to protect against infections. The authors suppose that symbatic action of chitosan and ZnO nanoparticles is beneficial in healing and tissue regeneration [72]. A similar film with zinc oxide nanoparticles was elaborated by Sodagar, who noted the excellent antibacterial effect of the film on Streptococcus mutans, Streptococcus sanguis, and Lactobacillus acidophilus and demonstrated its potential for the treatment of diseases of the oral cavity (dentistry) [73].
7. Antibacterial Films from Chitosan with Graphene
Graphene is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure [74]. Incorporating graphene into chitosan films can enhance their antibacterial properties and expand their applications. First, graphene is an extremely strong material, and it greatly enhances the strength of chitosan films [75]. It is also worth noting that graphene has the biocompatibility with mammalian cells required for use as a scaffold structure for tissue engineering [76,77].
7.1. Use in the Food Industry
The introduction of certain amounts of graphene into the polymer matrix of chitosan films can significantly improve their physicochemical and biological properties, thereby expanding the scope of these films [78]. Chitosan films incorporating graphene have been used in food packaging to extend shelf life and maintain quality. Recently, high-performance graphene-containing films based on chitosan have been developed for potential applications such as food packaging. The introduction of small amounts of graphene oxide significantly increases the Young’s modulus, as well as the elasticity of the obtained films; in addition, the introduction of the mentioned carbon nanostructures significantly improves the barrier properties of the material. It should also be noted the high antibacterial activity of chitosan-graphene films in comparison with blank chitosan films [79]. These studies are in agreement with previous data on the antibacterial activity of graphene [80].
7.2. Use in Medicine
And yet, most of the examples described in the literature of the use of chitosan films with graphene developed for the biomedical field. In particular, graphene-containing systems are proposed as film-like scaffolds and coatings for tissue engineering [81,82,83], systems with enhanced loading capacity and releasing profile for drug delivery [84,85], antibacterial films and hydrogel films for wound healing [86,87].
The most widespread application of graphene-containing films based on chitosan concerns drug delivery. In these cases, graphene structures often act as the core, which is the main force in absorbing the pharmacological substance or genetic material via π–π stacking interactions, hydrophobic interactions, van der Waals forces, and in some instances, hydrogen bonds [88]. Chitosan plays the role of a polymer matrix, which is characterized by biocompatibility, biodegradability and non-toxicity. In addition, the characteristic features of the polymer matrix also largely determine the kinetics of drug release [89].
8. Antibacterial Films from Chitosan with Fullerene and Its Derivatives
Fullerene, a type of carbon substance characterized by its substantial electron density and extensive volumetric surface area, shows immense potential. Its bonding energy is somewhat low, generally resulting in physisorption. This structure offers a platform for diverse modifications, potential electrical uses, transporting interstitial atoms, as well as functioning as a substance for adsorption and storage. There are two known forms of adsorption in fullerene: one weaker form on its upper surface and another stronger one in the gaps between its spherical molecules. Fullerene’s uses are numerous, including serving as an antiviral, an antioxidant and neuroprotectant, an X-ray contrast agent, an antimicrobial, a tool in genetic manipulation, an implant prosthesis, and a diagnostic and treatment agent for oral cancer [90,91]. Incorporating fullerene and its derivatives into chitosan films is often able to give the material new properties. In this section, we will discuss the synthesis and properties of chitosan films with fullerene and its derivatives and their applications in the food industry and medicine.
Fullerene’s unique properties also include antimicrobial activity against a variety of microorganisms [92]. In addition, fullerene reinforcement of chitosan films can be used to improve their strength, elasticity, and barrier characteristics [93]. All these parameters of food films and coatings are of paramount importance. In particular, the introduction of C60 fullerene in the chitosan matrix enhances the thermal, viscoelastic, and optical properties of the biodegradable chitosan-based composite film [94]. The authors of this paper recommend this elaborated film for use in food biotechnology. Similarly, there are a few examples of the introduction of fullerenol into the polymer matrix of chitosan films. Fullerenol-60 is a water-soluble hydroxylic derivative of C60 fullerene, i.e., C60(OH)24 [95]. Fullerenol in chitosan-based films potentiates the antibacterial effect of chitosan and promotes tissue regeneration on wound surfaces, which is of interest to medicine [96].
9. Antibacterial Films from Chitosan with Plant Extracts
Incorporating plant extracts into chitosan films can improve their antibacterial properties and expand their applications. In this section, we will discuss the synthesis and properties of chitosan films with plant extracts and their applications in the food industry and medicine.
9.1. Use in the Food Industry
Chitosan films containing plant extracts can be synthesized using methods like solvent casting and electrospinning. Plant extracts, such as essential oils, phenolic compounds, and flavonoids, have been widely investigated for their antimicrobial properties [13].
Chitosan films incorporating plant extracts have been used in food packaging to extend shelf life and maintain quality. For example, chitosan films containing thyme essential oil have been shown to inhibit the growth of spoilage bacteria on fresh-cut fruits and vegetables [97]. Chitosan film with Boldo extract showed great protection against lipid oxidation and inhibition of microorganisms on cheese slices at 4 °C [98]. Extracts of sage and rosemary in chitosan film decrease the percentage of swelling and water vapor permeability values and increase antibacterial activity, and the authors propose to use the resulting films for active food packaging [99]. Extracts of turmeric and green tea increased the antioxidant capacity and antifungal activity of chitosan film used to preserve postharvest strawberries [100]. An edible chitosan film loaded with Nigella sativa L. extract was used to cover grapes. It was shown that the uncovered grapes spoiled, and the grapes covered with films were not spoiled. [101]. Xie et al. prepared a pH-sensitive colorimetric film with red cabbage anthocyanin extract. The elaborated film exhibited good mechanical properties, high barrier ability, excellent thermal stability, significant antioxidant and antimicrobial activity, and an especially sensitive response to pH and ammonia, which can be used to monitor shrimp freshness [102]. A chitosan-based film with poly (vinyl alcohol) and Phyllanthus reticulatus fruit extract was prepared in Tilak Gasti’s research group. The obtained new chitosan-based film showed a superior soil degradation rate; this indicates the films are biodegradable in nature. Further, the films demonstrated extensive antimicrobial properties in comparison with the blank chitosan-poly(vinyl alcohol) film. The elaborated film is recommended for application in food biotechnology [103]. Similarly, a chitosan-based film with anthocyanin-rich extract from black rice had strong antioxidant activity, antimicrobial effect, and different color responses to various conditions. The introduction of anthocyanins enables the composite film’s color to change from red to blue with the degree of meat spoilage increased. This circumstance was used as the indicative property of the composite films with regard to meat rotting [104]. Green pea (Pisum sativum L.) extract included in chitosan film improved antioxidant and antibacterial activity, tensile strength, and elongation percentage. Moreover, compared to control samples, packing corn oil in green pea pod extract films slowed the rise of thiobarbituric acid and peroxide values [105]. A film based on chitosan and Arabic gum enriched with grape seed extract showed significantly increased thickness and resistance to water vapor while reducing water solubility, visible light transmission, and rotational force in comparison to films without grape seed extract. Cookies with this edible coating were harder and tougher but with much better sensory properties than control during 6 months of storage, and it was confirmed that the coated cookies with the edible film were safe and of acceptable quality after 6 months of storage [106].
9.2. Use in Medicine
Plant extracts are widely used in medicine due to their rich pharmacological effects (antimicrobial, anti-inflammatory, antianginal, antispasmodic, regenerative, and much more), which are due to the presence of natural biologically active compounds in these extracts (flavonoids, tannins, saponins, alkaloids, etc.) [107]. Not surprisingly, these circumstances have contributed to the medical studies of polymer films loaded with plant extracts. Among these, chitosan-based films can also be loaded with plant extracts, and this is interesting for biomedical applications.
Ganesan et al. elaborated new attractive chitosan films with Aloe barbadensis Mill, Cissus quadrangularis and Curcuma longa extracts. This film possesses significant antibacterial activity against Escherichia coli and Staphylococcus aureus. Furthermore, the film had no cytotoxic reactivity to fibroblast cells and test silk fibroin blended film showed slight cytotoxic reactivity to fibroblast cells after 24 h contact [108]. Poly(vinyl alcohol)/Chitosan film loaded by Basella alba stem extract is also interesting due to its good antibacterial activity against the foremost infectious bacterial strains, S. aureus and E. coli. Additionally, Basella alba stem extract integrated chitosan/poly(vinyl alcohol) film showed anti-inflammatory properties, hemocompatibility, and excellent biocompatibility, and the in vitro scratch assay and cell adhesion test results illustrated prominent wound healing and adhesion [109]. Chitosan films embedded with bud poplar extract demonstrated good antimicrobial and antibiofilm properties against Gram-positive bacteria and the yeast Candida albicans. Bud poplar extract induced an immunomodulatory effect on human macrophages, and this film induced a good regenerative effect in human fibroblasts by in vitro cell migration assay [110].
10. Conclusions
This review has provided a comprehensive overview of antibacterial chitosan films and their applications in the food industry and medicine. We have discussed various types of chitosan films, including pure chitosan films, films with metal nanoparticles, films with metal oxide nanoparticles, films with graphene, films with fullerene and its derivatives, and films with plant extracts. We have discussed the properties and potential applications of these films and highlighted the significant role that chitosan films play in extending shelf life and maintaining the quality of food products. In addition, we have discussed their use as wound dressings, drug delivery systems, and medical device coatings in medical applications.
The incorporation of different additives, such as metal nanoparticles, metal oxide nanoparticles, graphene, fullerene derivatives, and plant extracts, can further enhance the antibacterial properties of chitosan films. These additives can help to overcome the limitations of pure chitosan films, such as low solubility and limited antimicrobial activity against specific microorganisms.
The continued development of chitosan films with improved properties will undoubtedly expand their potential applications in various industries. Future research should focus on optimizing the synthesis methods and understanding the structure-property relationships of these materials. Additionally, more studies are needed to evaluate the safety, biocompatibility, and long-term stability of chitosan films in both food and medical applications.
Overall, antibacterial chitosan films hold great promise as a versatile and eco-friendly material for a wide range of applications. Their unique properties, including biodegradability, biocompatibility, and antimicrobial activity, make them an attractive choice for researchers and industry professionals alike. As for the further prospects for the widespread introduction of antimicrobial films of chitosan into clinical practice, the authors of the current review believe that these prospects await chitosan and its derivatives in the near future. This is primarily due to the wide availability of chitosan, a wide variety of its positive biological and pharmacological properties, as well as the convenience of chemical modification, unlike other polysaccharides (due to the presence of primary amine functionality in the macromolecule). The same limitations as the difficulty of standardizing chitosan in the pharmacopoeial analysis will undoubtedly be overcome, and, in the opinion of the authors, they are not a serious obstacle.
Author Contributions
Conceptualization, O.M.K. and A.S.K.; methodology, O.M.K.; software, A.R.E.; validation, A.A.K., A.G.T. and A.S.K.; formal analysis, A.A.K.; investigation, O.M.K.; resources, V.N.K.; data curation, A.G.T.; writing—review and editing, A.S.K.; visualization, O.M.K.; supervision, A.S.K.; project administration, A.S.K.; funding acquisition, V.N.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Russian Science Foundation grant № 23-23-00021, www.rscf.ru/project/23-23-00021/ (accessed on 24 June 2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yanat, M.; Schroën, K. Advances in chitin-based nanoparticle use in biodegradable polymers: A review. Carbohydr. Polym. 2023, 312, 120789. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.S.; Beg, S.; Nayak, A.K. Chitosan in Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–405. [Google Scholar]
- Crini, G. Chitin and Chitosan: Discoveries and Applications for Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–257. [Google Scholar]
- Zhou, Z.; Zheng, C.; Liu, Y.; Luo, W.; Deng, H.; Shen, J. Chitosan biguanide induced mitochondrial inhibition to amplify the efficacy of oxygen-sensitive tumor therapies. Carbohydr. Polym. 2022, 295, 119878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Y.; Jiang, X.; Zheng, C.; Luo, W.; Xiang, X.; Qi, X.; Shen, J. Metformin modified chitosan as a multi-functional adjuvant to enhance cisplatin-based tumor chemotherapy efficacy. Int. J. Biol. Macromol. 2023, 224, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Volkova, O.V.; Kritchenkov, I.S.; Kurliuk, A.V.; Shakola, T.V.; Khrustalev, V.N. Ultrasound-assisted catalyst-free phenol-yne reaction for the synthesis of new water-soluble chitosan derivatives and their nanoparticles with enhanced antibacterial properties. Int. J. Biol. Macromol. 2019, 139, 103–113. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Zhaliazniak, N.V.; Egorov, A.R.; Lobanov, N.N.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Kurliuk, A.V.; Shakola, T.V.; Rubanik, V.V.; et al. Chitosan derivatives and their based nanoparticles: Ultrasonic approach to the synthesis, antimicrobial and transfection properties. Carbohydr. Polym. 2020, 242, 116478. [Google Scholar] [CrossRef]
- Thirugnanasambandan, T.; Gopinath, S.C.B. Laboratory to industrial scale synthesis of chitosan-based nanomaterials: A review. Process Biochem. 2023, 130, 147–155. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Dysin, A.P.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Kurliuk, A.V.; Shakola, T.V. Ultrasound-assisted Cu(I)-catalyzed azide-alkyne click cycloaddition as polymer-analogous transformation in chitosan chemistry. High antibacterial and transfection activity of novel triazol betaine chitosan derivatives and their nanoparticles. Int. J. Biol. Macromol. 2019, 137, 592–603. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Vatankhah, M.; Hassanisaadi, M.; Kennedy, J.F. Chitosan-based nanocomposites as coatings and packaging materials for the postharvest improvement of agricultural product: A review. Carbohydr. Polym. 2023, 309, 120666. [Google Scholar] [CrossRef]
- Shahbaz, U.; Basharat, S.; Javed, U.; Bibi, A.; Yu, X.-B. Chitosan: A multipurpose polymer in food industry. Polym. Bull. 2022, 80, 3547–3569. [Google Scholar] [CrossRef]
- Muñoz-Tebar, N.; Pérez-Álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Chitosan Edible Films and Coatings with Added Bioactive Compounds: Antibacterial and Antioxidant Properties and Their Application to Food Products: A. Review. Polymers 2023, 15, 396. [Google Scholar] [CrossRef]
- Tang, W.; Wang, J.; Hou, H.; Li, Y.; Wang, J.; Fu, J.; Lu, L.; Gao, D.; Liu, Z.; Zhao, F.; et al. Review: Application of chitosan and its derivatives in medical materials. Int. J. Biol. Macromol. 2023, 240, 124398. [Google Scholar] [CrossRef]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef]
- Ying, T.-H.; Huang, C.-J.; Hsieh, C.-J.; Wu, P.-J.; Yeh, C.-C.; Hung, P.-K.; Chang, W.-H.; Wu, M.-H.; Hung, H.; Chang, J.-W.; et al. Potential Factors Associated with the Blood Metal Concentrations of Reproductive-Age Women in Taiwan. Expos. Health 2023, 1–17, in press. [Google Scholar] [CrossRef]
- Tavakoli, M.; Labbaf, S.; Mirhaj, M.; Salehi, S.; Seifalian, A.M.; Firuzeh, M. Natural polymers in wound healing: From academic studies to commercial products. J. Appl. Polym. Sci. 2023, 140, e53910. [Google Scholar] [CrossRef]
- Badar, R.; Zahid, A.A.; Yar, M. 11—Chitin-based nanomaterials. In Biopolymeric Nanomaterials; Kanwar, S., Kumar, A., Nguyen, T.A., Sharma, S., Slimani, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 249–275. [Google Scholar]
- Minh, N.C.; Van Hoa, N.; Trung, T.S. Chapter 15—Preparation, properties, and application of low-molecular-weight chitosan. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 453–471. [Google Scholar]
- Singh, A.; Jaiswal, S.; Kumar, S.; Dutta, P.K. Chitin—A Natural Bio-feedstock and Its Derivatives. In High-Performance Materials from Bio-Based Feedstocks; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 207–233. [Google Scholar]
- El Knidri, H.; Laajeb, A.; Lahsini, A. Chapter 2—Chitin and chitosan: Chemistry, solubility, fiber formation, and their potential applications. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–57. [Google Scholar]
- Kudan, S.; Pongchaisirikul, N.; Dantragoon, N.; Pichyangkura, R. Enzymatic determination of the degree of chitosan deacetylation by family 18 chitinase. Anal. Biochem. 2023, 28, 1. [Google Scholar]
- Jayakumar, R.; Prabaharan, M. Chitosan for Biomaterials III; Springer: New York, NY, USA, 2021. [Google Scholar]
- Yao, K.; Li, J.; Yao, F.; Yin, Y. Chitosan-Based Hydrogels; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Hasan, S.; Boddu, V.M.; Viswanath, D.S.; Ghosh, T.K. Application of Chitosan in the Medical and Biomedical Field. In Chitin and Chitosan: Science and Engineering; Hasan, S., Boddu, V.M., Viswanath, D.S., Ghosh, T.K., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 291–321. [Google Scholar]
- Li, K.; Zhu, J.; Guan, G.; Wu, H. Preparation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: Film properties, characterization, and formation mechanism. Int. J. Biol. Macromol. 2019, 122, 485–492. [Google Scholar] [CrossRef]
- Antunes, J.C.; Pereira, C.L.; Molinos, M.; Ferreira-da-Silva, F.; Dessì, M.; Gloria, A.; Ambrosio, L.; Gonçalves, R.M.; Barbosa, M.A. Layer-by-Layer Self-Assembly of Chitosan and Poly(γ-glutamic acid) into Polyelectrolyte Complexes. Biomacromolecules 2011, 12, 4183–4195. [Google Scholar] [CrossRef]
- Akpan, E.I.; Gbenebor, O.P.; Adeosun, S.O.; Cletus, O. Chapter 5—Solubility, degree of acetylation, and distribution of acetyl groups in chitosan. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–164. [Google Scholar]
- Goy, R.C.; Britto, D.d.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. [Google Scholar]
- Xia, Q.; Wang, X.; Zeng, Q.; Guo, D.; Zhu, Z.; Chen, H.; Dong, H. Mechanisms of Enhanced Antibacterial Activity by Reduced Chitosan-Intercalated Nontronite. Environ. Sci. Technol. 2020, 54, 5207–5217. [Google Scholar] [CrossRef] [PubMed]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: New York, NY, USA, 2019; pp. 81–123. [Google Scholar]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Ueno, H.; Yamada, H.; Tanaka, I.; Kaba, N.; Matsuura, M.; Okumura, M.; Kadosawa, T.; Fujinaga, T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials 1999, 20, 1407–1414. [Google Scholar] [CrossRef]
- Scaccini, L.; Mezzena, R.; De Masi, A.; Gagliardi, M.; Gambarotta, G.; Cecchini, M.; Tonazzini, I. Chitosan Micro-Grooved Membranes with Increased Asymmetry for the Improvement of the Schwann Cell Response in Nerve Regeneration. Int. J. Mol. Sci. 2021, 22, 7901. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Yang, S.; Yang, Y.; Cui, S.; Feng, Z.; Du, Y.; Song, Z.; Tong, Y.; Yang, L.; Wang, Z.; Zeng, H.; et al. Chitosan-polyvinyl alcohol nanoscale liquid film-forming system facilitates MRSA-infected wound healing by enhancing antibacterial and antibiofilm properties. Int. J. Nanomed. 2018, 13, 4987–5002. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.d.C.C.; Bi, H.; Tedesco, A.C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Khalilpour, H.; Shafiee, P.; Darbandi, A.; Yusuf, M.; Mahmoudi, S.; Goudarzi, Z.; Mirzamohammadi, S. Application of Polyoxometalate-based composites for sensor systems: A review. J. Compos. Compd. 2021, 3, 129–139. [Google Scholar] [CrossRef]
- Malviya, R.; Tyagi, A.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.; Sundram, S.; Karupiah, S.; Chakravarthi, S.; Meenakshi, D.U.; Gupta, N.; et al. Fabrication and Characterization of Chitosan—Tamarind Seed Polysaccharide Composite Film for Transdermal Delivery of Protein/Peptide. Polymers 2021, 13, 1531. [Google Scholar] [CrossRef]
- Qureshi, D.; Pattanaik, S.; Mohanty, B.; Anis, A.; Kulikouskaya, V.; Hileuskaya, K.; Agabekov, V.; Sarkar, P.; Maji, S.; Pal, K. Preparation of novel poly(vinyl alcohol)/chitosan lactate-based phase-separated composite films for UV-shielding and drug delivery applications. Polym. Bull. 2022, 79, 3253–3290. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; de Carvalho, A.P.A.d.; Conte-Junior, C.A. Recent advances in biobased and biodegradable polymer nanocomposites, nanoparticles, and natural antioxidants for antibacterial and antioxidant food packaging applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3673–3716. [Google Scholar] [CrossRef]
- Pal, K.; Sarkar, P.; Anis, A.; Wiszumirska, K.; Jarzębski, M. Polysaccharide-Based Nanocomposites for Food Packaging Applications. Materials 2021, 14, 5549. [Google Scholar] [CrossRef]
- Affes, S.; Maalej, H.; Aranaz, I.; Kchaou, H.; Acosta, N.; Heras, Á.; Nasri, M. Controlled size green synthesis of bioactive silver nanoparticles assisted by chitosan and its derivatives and their application in biofilm preparation. Carbohydr. Polym. 2020, 236, 116063. [Google Scholar] [CrossRef]
- Dai, X.; Li, S.; Li, S.; Ke, K.; Pang, J.; Wu, C.; Yan, Z. High antibacterial activity of chitosan films with covalent organic frameworks immobilized silver nanoparticles. Int. J. Biol. Macromol. 2022, 202, 407–417. [Google Scholar] [CrossRef]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennis, T.T.; Wacławek, S.; Černík, M.; Padil, V.V.T. Chitosan/Gelatin/Silver Nanoparticles Composites Films for Biodegradable Food Packaging Applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef]
- Virgili, A.H.; Laranja, D.C.; Malheiros, P.S.; Pereira, M.B.; Costa, T.M.H.; de Menezes, E.W. Nanocomposite film with antimicrobial activity based on gold nanoparticles, chitosan and aminopropylsilane. Surf. Coat. Technol. 2021, 415, 127086. [Google Scholar] [CrossRef]
- Gamboa-Solana, C.D.; Chuc-Gamboa, M.G.; Aguilar-Pérez, F.J.; Cauich-Rodríguez, J.V.; Vargas-Coronado, R.F.; Aguilar-Pérez, D.A.; Herrera-Atoche, J.R.; Pacheco, N. Zinc Oxide and Copper Chitosan Composite Films with Antimicrobial Activity. Polymers 2021, 13, 3861. [Google Scholar] [CrossRef]
- Cardenas, G.; Diaz, V.J.; Melendrez, M.; Cruzat, C.; Cancino, A. Colloidal Cu nanoparticles/chitosan composite film obtained by microwave heating for food package applications. Polym. Bull. 2009, 62, 511–524. [Google Scholar] [CrossRef]
- Mohamed, N.; Madian, N.G. Evaluation of the mechanical, physical and antimicrobial properties of chitosan thin films doped with greenly synthesized silver nanoparticles. Mater. Today Commun. 2020, 25, 101372. [Google Scholar] [CrossRef]
- Constantin, M.; Lupei, M.; Bucatariu, S.-M.; Pelin, I.M.; Doroftei, F.; Ichim, D.L.; Daraba, O.M.; Fundueanu, G. PVA/Chitosan Thin Films Containing Silver Nanoparticles and Ibuprofen for the Treatment of Periodontal Disease. Polymers 2023, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.S.; Sharifianjazi, F.; Esmaeilkhanian, A.; Salehi, E. Chitosan films and scaffolds for regenerative medicine applications: A review. Carbohydr. Polym. 2021, 273, 118631. [Google Scholar] [CrossRef]
- Pansara, C.; Mishra, R.; Mehta, T.; Parikh, A.; Garg, S. Formulation of Chitosan Stabilized Silver Nanoparticle-Containing Wound Healing Film: In Vitro and In Vivo Characterization. J. Pharm. Sci. 2020, 109, 2196–2205. [Google Scholar] [CrossRef]
- Kulikouskaya, V.; Zhdanko, T.; Hileuskaya, K.; Kraskouski, A.; Zhura, A.; Skorohod, H.; Butkevich, V.; Pal, K.; Tratsyak, S.; Agabekov, V. Physicochemical aspects of design of ultrathin films based on chitosan, pectin, and their silver nanocomposites with antiadhesive and bactericidal potential. J. Biomed. Mater. Res. A 2022, 110, 217–228. [Google Scholar] [CrossRef]
- Samadi, A.; Azandeh, S.; Orazizadeh, M.; Bayati, V.; Rafienia, M.; Karami, M.A. Fabrication and Characterization of Glycerol/Chitosan/Polyvinyl Alcohol-Based Transparent Hydrogel Films Loaded with Silver Nanoparticles for Antibacterial Wound Dressing Applications. Adv. Biomed. Res. 2021, 10, 4. [Google Scholar]
- Noel, S.P.; Courtney, H.; Bumgardner, J.D.; Haggard, W.O. Chitosan films: A potential local drug delivery system for antibiotics. Clin. Orthop. Relat. Res. 2008, 466, 1377–1382. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Pino, P.; Bosco, F.; Mollea, C.; Onida, B. Antimicrobial Nano-Zinc Oxide Biocomposites for Wound Healing Applications: A Review. Pharmaceutics 2023, 15, 970. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2022, 38, 537–565. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan based ZnO nanoparticles loaded gallic-acid films for active food packaging. Food Chem. 2021, 334, 127605. [Google Scholar] [CrossRef]
- Amjadi, S.; Emaminia, S.; Nazari, M.; Davudian, S.H.; Roufegarinejad, L.; Hamishehkar, H. Application of Reinforced ZnO Nanoparticle-Incorporated Gelatin Bionanocomposite Film with Chitosan Nanofiber for Packaging of Chicken Fillet and Cheese as Food Models. Food Bioproc. Technol. 2019, 12, 1205–1219. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.; Osaili, T.; Sawalha, A.; Olaimat, A.N.; Albiss, B.A.; Mehyar, G.; Ayyash, M.; Holley, R. Antimicrobial activity of chitosan coating containing ZnO nanoparticles against E. coli O157:H7 on the surface of white brined cheese. Int. J. Food Microbiol. 2020, 334, 108838. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J.-W. Titanium dioxide (TiO2) for the manufacture of multifunctional active food packaging films. Food Packag. Shelf Life 2022, 31, 100806. [Google Scholar] [CrossRef]
- Perera, K.Y.; Sharma, S.; Duffy, B.; Pathania, S.; Jaiswal, A.K.; Jaiswal, S. An active biodegradable layer-by-layer film based on chitosan-alginate-TiO2 for the enhanced shelf life of tomatoes. Food Packag. Shelf Life 2022, 34, 100971. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, H.; Guo, X.; Bi, X.; Liu, X.; Xu, Q.; Wang, Q.; Li, W.; Li, X.; Shui, Y.; et al. Effect of chitosan/Nano-TiO2 composite coatings on the postharvest quality and physicochemical characteristics of mango fruits. Sci. Hortic. 2020, 263, 109135. [Google Scholar] [CrossRef]
- Alghamdi, H.; Abutalib, M.; Rajeh, A.; Mannaa, M.; Nur, O.; Abdelrazek, E.M. Effect of the Fe2O3/TiO2 Nanoparticles on the Structural, Mechanical, Electrical Properties and Antibacterial Activity of the Biodegradable Chitosan/Polyvinyl Alcohol Blend for Food Packaging. J. Polym. Environ. 2022, 30, 3865–3874. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Tiplea, R.E.; Lemnaru, G.M.; Trusca, R.; Holban, A.; Kaya, M.G.A.; Dragu, D.; Ficai, D.; Ficai, A.; Bleotu, C. Antimicrobial Films based on Chitosan, Collagen, and ZnO for Skin Tissue Regeneration. Biointerface Res. Appl. Chem. 2021, 11, 11985–11995. [Google Scholar]
- Amir Hossein, M.; Abbas, B.; Mohammad Zaman, K.; Ghazaleh, D.; Mohammad Sadegh, A.-A.; Ahmad, S. Antimicrobial Effect of Nano-Zinc Oxide and Nano-Chitosan Particles in Dental Composite Used in Orthodontics. J. Med. Bacteriol. 2015, 2, 1–10. [Google Scholar]
- Urade, A.R.; Lahiri, I.; Suresh, K.S. Graphene Properties, Synthesis and Applications: A Review. JOM 2023, 75, 614–630. [Google Scholar] [CrossRef] [PubMed]
- Ghuge, A.D.; Shirode, A.R.; Kadam, V.J. Graphene: A Comprehensive Review. Curr. Drug Targets 2017, 18, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; Khan, A.u. Graphene synthesis, characterization and its applications: A review. Results Phys. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- Chen, W.; Weimin, H.; Li, D.; Chen, S.; Dai, Z. A critical review on the development and performance of polymer/graphene nanocomposites. Sci. Eng. Compos. Mater. 2018, 25, 1059–1073. [Google Scholar] [CrossRef]
- Grande, C.D.; Mangadlao, J.; Fan, J.; De Leon, A.; Delgado-Ospina, J.; Rojas, J.G.; Rodrigues, D.F.; Advincula, R. Chitosan Cross-Linked Graphene Oxide Nanocomposite Films with Antimicrobial Activity for Application in Food Industry. Macromol. Symp. 2017, 374, 1600114. [Google Scholar] [CrossRef]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotechnol. 2020, 8, 465. [Google Scholar] [CrossRef]
- Arnaldi, P.; Carosio, F.; Di Lisa, D.; Muzzi, L.; Monticelli, O.; Pastorino, L. Assembly of chitosan-graphite oxide nanoplatelets core shell microparticles for advanced 3D scaffolds supporting neuronal networks growth. Colloids Surf. B 2020, 196, 111295. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, F.; Wang, Q.; Wu, X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Mater. Sci. Eng. C 2017, 79, 697–701. [Google Scholar] [CrossRef]
- Karimi, N.; Kharaziha, M.; Raeissi, K. Electrophoretic deposition of chitosan reinforced graphene oxide-hydroxyapatite on the anodized titanium to improve biological and electrochemical characteristics. Mater. Sci. Eng. C 2019, 98, 140–152. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Chen, B.; Gu, L.; Li, Y.; Yu, S. Design and evaluation of galactosylated chitosan/graphene oxide nanoparticles as a drug delivery system. J. Colloid Interface Sci. 2018, 516, 332–341. [Google Scholar] [CrossRef]
- Zhao, X.; Zou, X.; Ye, L. Controlled pH- and glucose-responsive drug release behavior of cationic chitosan based nano-composite hydrogels by using graphene oxide as drug nanocarrier. J. Ind. Eng. Chem. 2017, 49, 36–45. [Google Scholar] [CrossRef]
- Najafabadi, S.A.A.; Mohammadi, A.; Kharazi, A.Z. Polyurethane nanocomposite impregnated with chitosan-modified graphene oxide as a potential antibacterial wound dressing. Mater. Sci. Eng. C 2020, 115, 110899. [Google Scholar] [CrossRef]
- Yang, M.-C.; Tseng, Y.-Q.; Liu, K.-H.; Cheng, Y.-W.; Chen, W.-T.; Chen, W.-T.; Hsiao, C.-W.; Yung, M.-C.; Hsu, C.-C.; Liu, T.-Y. Preparation of Amphiphilic Chitosan–Graphene Oxide–Cellulose Nanocrystalline Composite Hydrogels and Their Biocompatibility and Antibacterial Properties. Appl. Sci. 2019, 9, 3051. [Google Scholar] [CrossRef]
- Hoseini-Ghahfarokhi, M.; Mirkiani, S.; Mozaffari, N.; Abdolahi Sadatlu, M.A.; Ghasemi, A.; Abbaspour, S.; Akbarian, M.; Farjadian, F.; Karimi, M. Applications of Graphene and Graphene Oxide in Smart Drug/Gene Delivery: Is the World Still Flat? Int. J. Nanomed. 2020, 15, 9469–9496. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, N.K. Hybrid Nanomaterials for Drug Delivery; Woodhead Publishing: Soston, UK, 2022; p. 384. [Google Scholar]
- Bhakta, P.; Barthunia, B. Fullerene and its applications: A review. J. Indian Acad. Oral Med. Radiol. 2020, 32, 159–163. [Google Scholar] [CrossRef]
- Agrawal, P.S.; Belkhode, P.N.; Brijpuriya, D.S.; Gouda, S.P.; Rokhum, S.L. Stimulation in fullerene for adsorbing pollutant gases: A review. Chem. Phys. Impact 2023, 6, 100156. [Google Scholar] [CrossRef]
- Lyon, D.Y.; Adams, L.K.; Falkner, J.C.; Alvarez, P.J.J. Antibacterial Activity of Fullerene Water Suspensions: Effects of Preparation Method and Particle Size. Environ. Sci. Technol. 2006, 40, 4360–4366. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Jacob, M.; Vignesh, N.S.; Varalakshmi, P. Pristine and modified chitosan as solid catalysts for catalysis and biodiesel production: A minireview. Int. J. Biol. Macromol. 2021, 167, 807–833. [Google Scholar] [CrossRef]
- Ahmed, E.M. Enhancing thermal, viscoelastic, and optical properties of biodegradable fullerene(C60)/agarose/chitosan composite films for biotechnology. Appl. Phys. 2021, 127, 481. [Google Scholar] [CrossRef]
- Semenov, K.N.; Charykov, N.A.; Keskinov, V.A.; Kritchenkov, A.S.; Murin, I.V. Fullerenol-d Solubility in Fullerenol-d–Inorganic Salt–Water Ternary Systems at 25 °C. Ind. Eng. Chem. Res. 2013, 52, 16095–16100. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Yagafarov, N.Z.; Khrustalev, V.N. Chitosan Derivatives: Synthesis, Antibacterial and Transfection Activity; RUDN University: Moscow, Russia, 2020; p. 207. [Google Scholar]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Sobral, P.J.A. Gelatin-chitosan edible film activated with Boldo extract for improving microbiological and antioxidant stability of sliced Prato cheese. Int. J. Food Sci. Technol. 2019, 54, 1617–1624. [Google Scholar] [CrossRef]
- Kahya, N.; Kestir, S.M.; Öztürk, S.; Yolaç, A.; Torlak, E.; Kalaycıoğlu, Z.; Akın-Evingür, G.; Erim, F.B. Antioxidant and antimicrobial chitosan films enriched with aqueous sage and rosemary extracts as food coating materials: Characterization of the films and detection of rosmarinic acid release. Int. J. Biol. Macromol. 2022, 217, 470–480. [Google Scholar] [CrossRef]
- Yang, C.; Lu, J.-H.; Xu, M.-T.; Shi, X.-C.; Song, Z.-W.; Chen, T.-M.; Herrera-Balandrano, D.D.; Zhang, Y.-J.; Laborda, P.; Shahriar, M.; et al. Evaluation of chitosan coatings enriched with turmeric and green tea extracts on postharvest preservation of strawberries. LWT 2022, 163, 113551. [Google Scholar] [CrossRef]
- Karkar, B.; Patır, İ.; Eyüboğlu, S.; Şahin, S. Development of an edible active chitosan film loaded with Nigella sativa L. extract to extend the shelf life of grapes. Biocatal. Agric. Biotechnol. 2023, 50, 102708. [Google Scholar] [CrossRef]
- Ren, G.; He, Y.; Lv, J.; Zhu, Y.; Xue, Z.; Zhan, Y.; Sun, Y.; Luo, X.; Li, T.; Song, Y.; et al. Highly biologically active and pH-sensitive collagen hydrolysate-chitosan film loaded with red cabbage extracts realizing dynamic visualization and preservation of shrimp freshness. Int. J. Biol. Macromol. 2023, 233, 123414. [Google Scholar] [CrossRef]
- Hiremani, V.; Goudar, N.; Khanapure, S.; Gasti, T.; Eelager, M.; Narasgoudr, S.; Masti, S.; Chougale, R. Physicochemical and antimicrobial properties of Phyllanthus reticulatus fruit extract doped chitosan/poly (vinyl alcohol) blend films for food packaging applications. J. Food Meas. Charact. 2022, 17, 1548–1561. [Google Scholar] [CrossRef]
- Zeng, F.; Ye, Y.; Liu, J.; Fei, P. Intelligent pH indicator composite film based on pectin/chitosan incorporated with black rice anthocyanins for meat freshness monitoring. Food Chem. X 2023, 17, 100531. [Google Scholar] [CrossRef]
- Elsebaie, E.M.; Mousa, M.M.; Abulmeaty, S.A.; Shaat, H.A.; Elmeslamy, S.A.; Asker, G.A.; Faramawy, A.A.; Shaat, H.A.; Abd Elrahman, W.M.; Eldamaty, H.S.; et al. Chitosan-Based Green Pea (Pisum sativum L.) Pod Extract Gel Film: Characterization and Application in Food Packaging. Gels 2023, 9, 77. [Google Scholar] [CrossRef]
- Molnar, D.; Novotni, D.; Kurek, M.; Galić, K.; Iveković, D.; Bionda, H.; Ščetar, M. Characteristics of edible films enriched with fruit by-products and their application on cookies. Food Hydrocoll. 2023, 135, 108191. [Google Scholar] [CrossRef]
- Proestos, C. The Benefits of Plant Extracts for Human Health. Foods 2020, 9, 1653. [Google Scholar] [CrossRef]
- Ganesan, P. Biodegradable curative film for medical application. Indian J. Fibre Text. Res. 2023, 48, 52–60. [Google Scholar]
- D’Souza, O.J.; Gasti, T.; Hiremani, V.D.; Pinto, J.P.; Contractor, S.S.; Shettar, A.K.; Olivia, D.; Arakera, S.B.; Masti, S.P.; Chougale, R.B. Basella alba stem extract integrated poly (vinyl alcohol)/chitosan composite films: A promising bio-material for wound healing. Int. J. Biol. Macromol. 2023, 225, 673–686. [Google Scholar] [CrossRef]
- Russo, C.; Piccioni, M.; Lorenzini, M.L.; Catalano, C.; Ambrogi, V.; Pagiotti, R.; Pietrella, D. Bud-Poplar-Extract-Embedded Chitosan Films as Multifunctional Wound Healing Dressing. Molecules 2022, 27, 7757. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).