Comprehensive Metabolite Profile Uncovers the Bioactive Components, Antioxidant and Antibacterial Activities in Wild Tree Peony Leaves
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic Content of the Leaves of Nine WTPS
2.2. Total Flavonoid Content of the Leaves of Nine WTPS
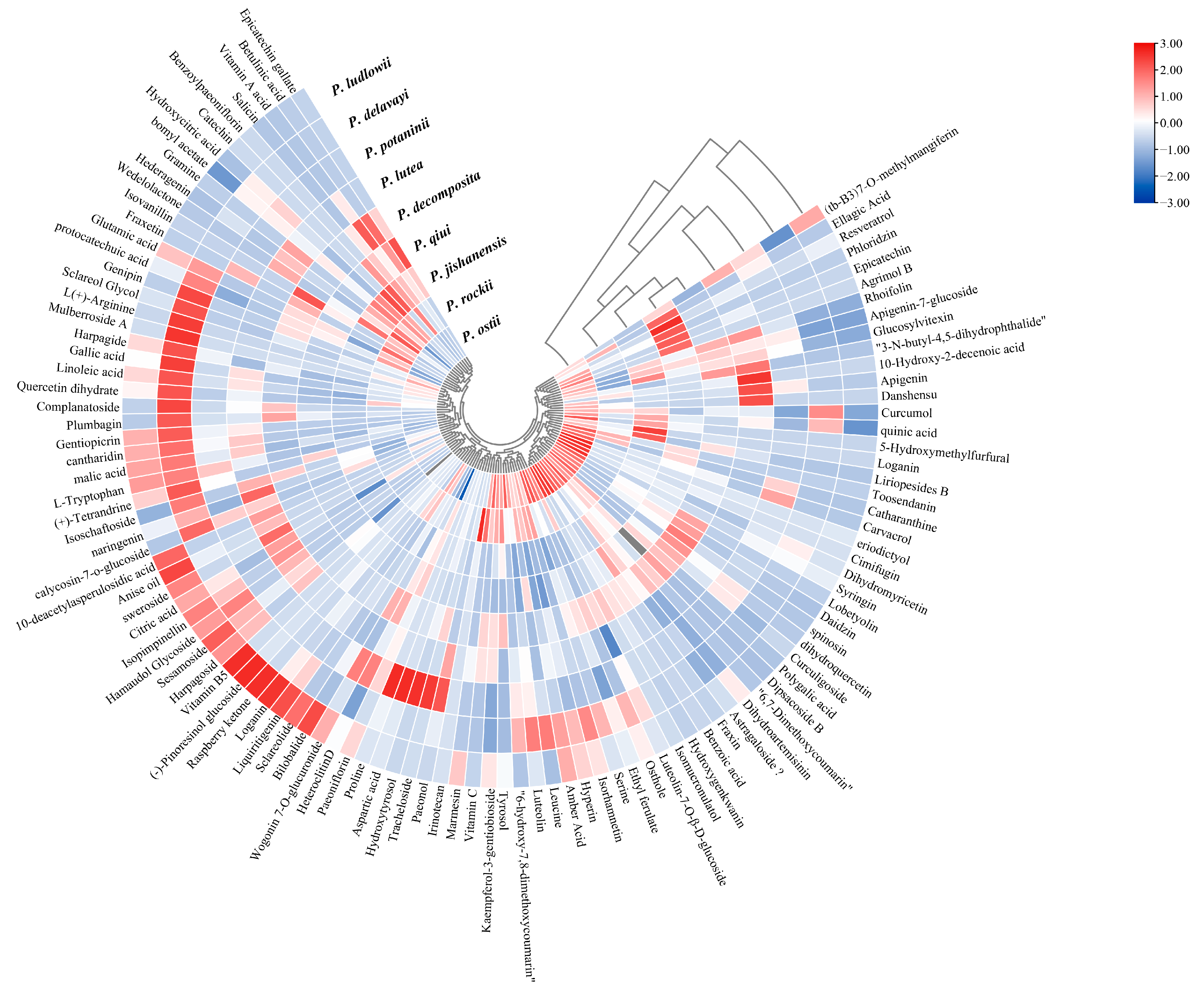
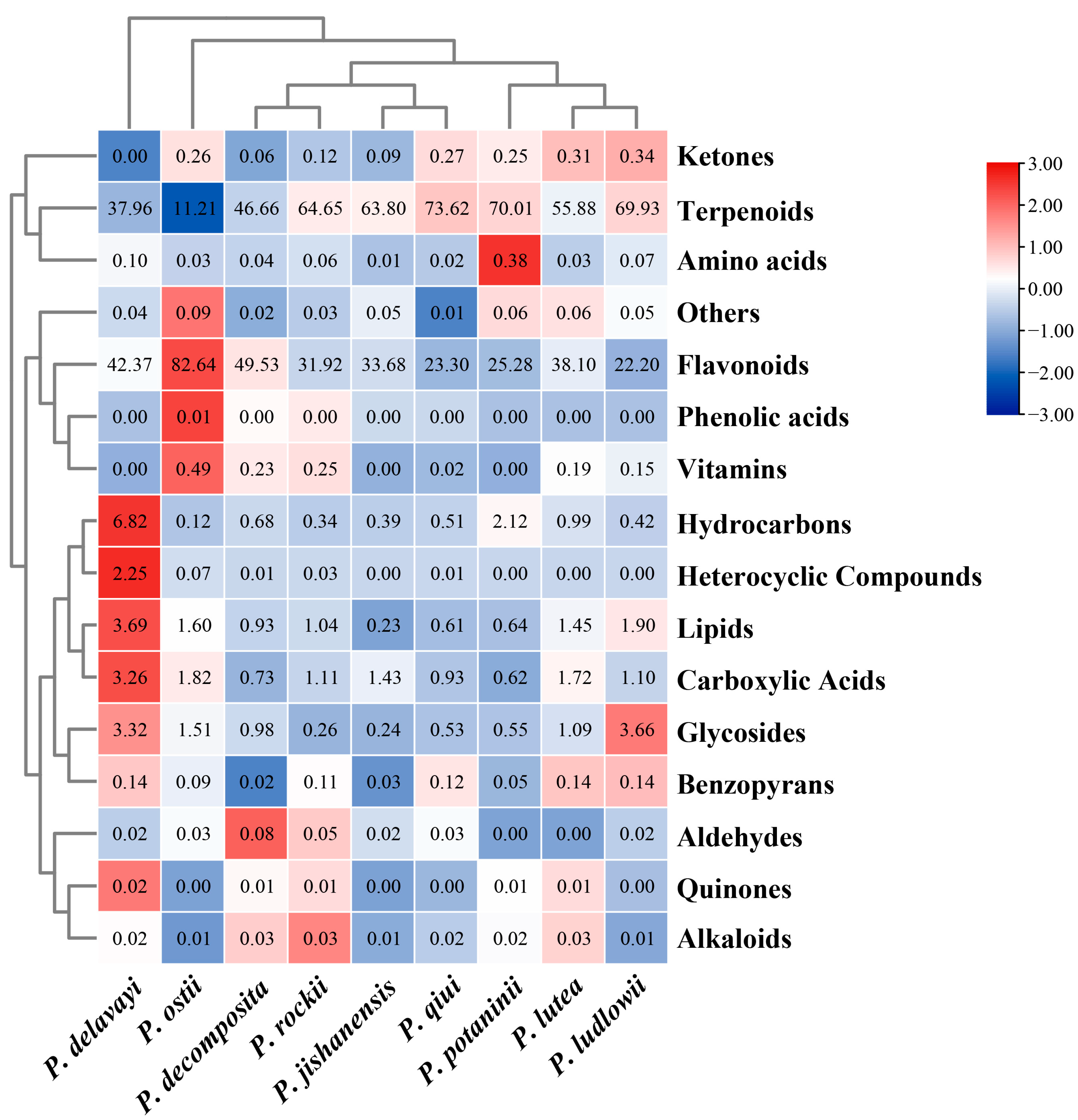
2.3. LC-MS Non-Targeted Metabolome Analysis of the Leaves of Nine WTPS
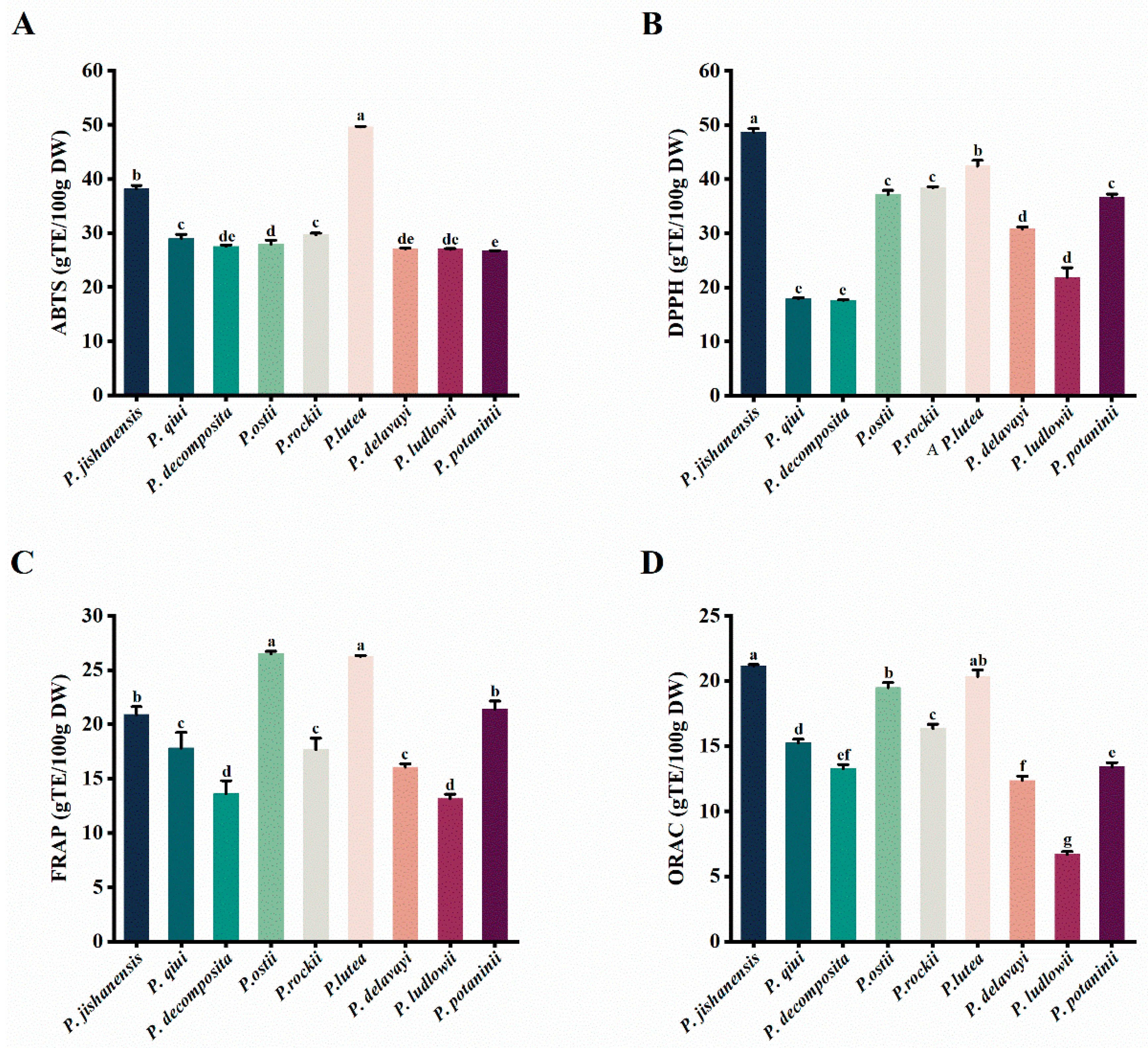
2.4. Evaluation of Antioxidant Activity of the Leaves of Nine WTPS
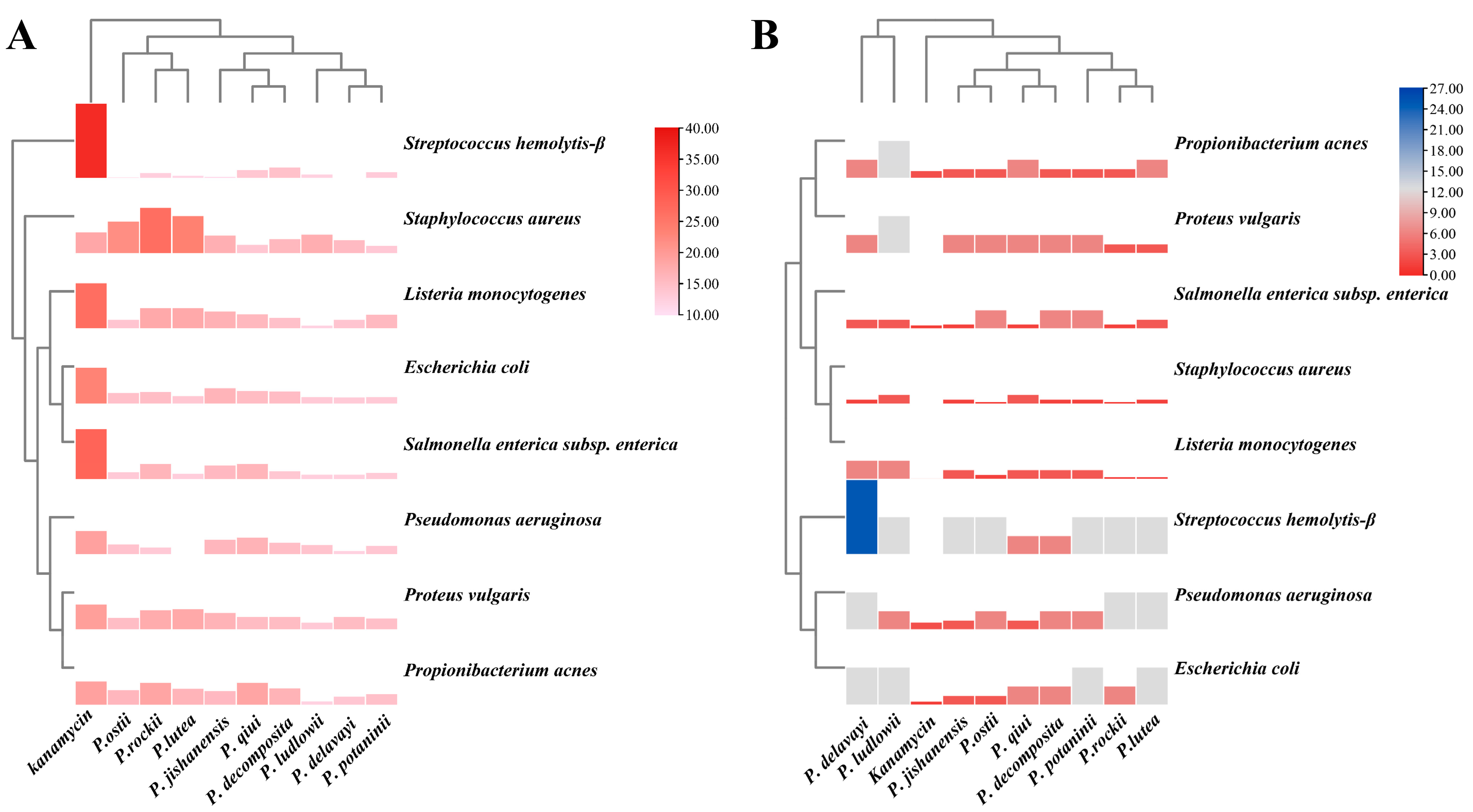
2.5. Evaluation of the Antibacterial Activity of the Leaves of Nine WTPS
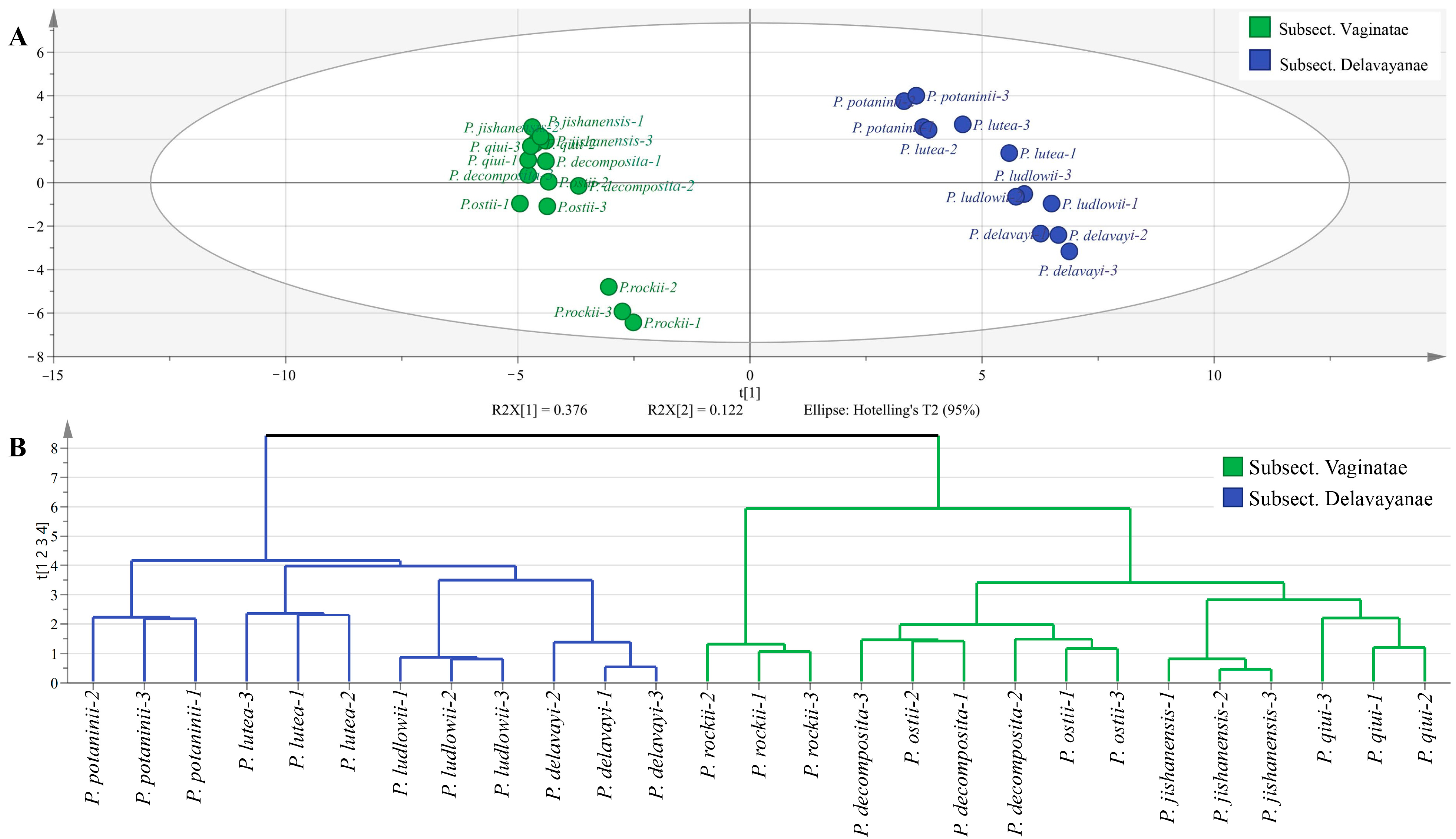
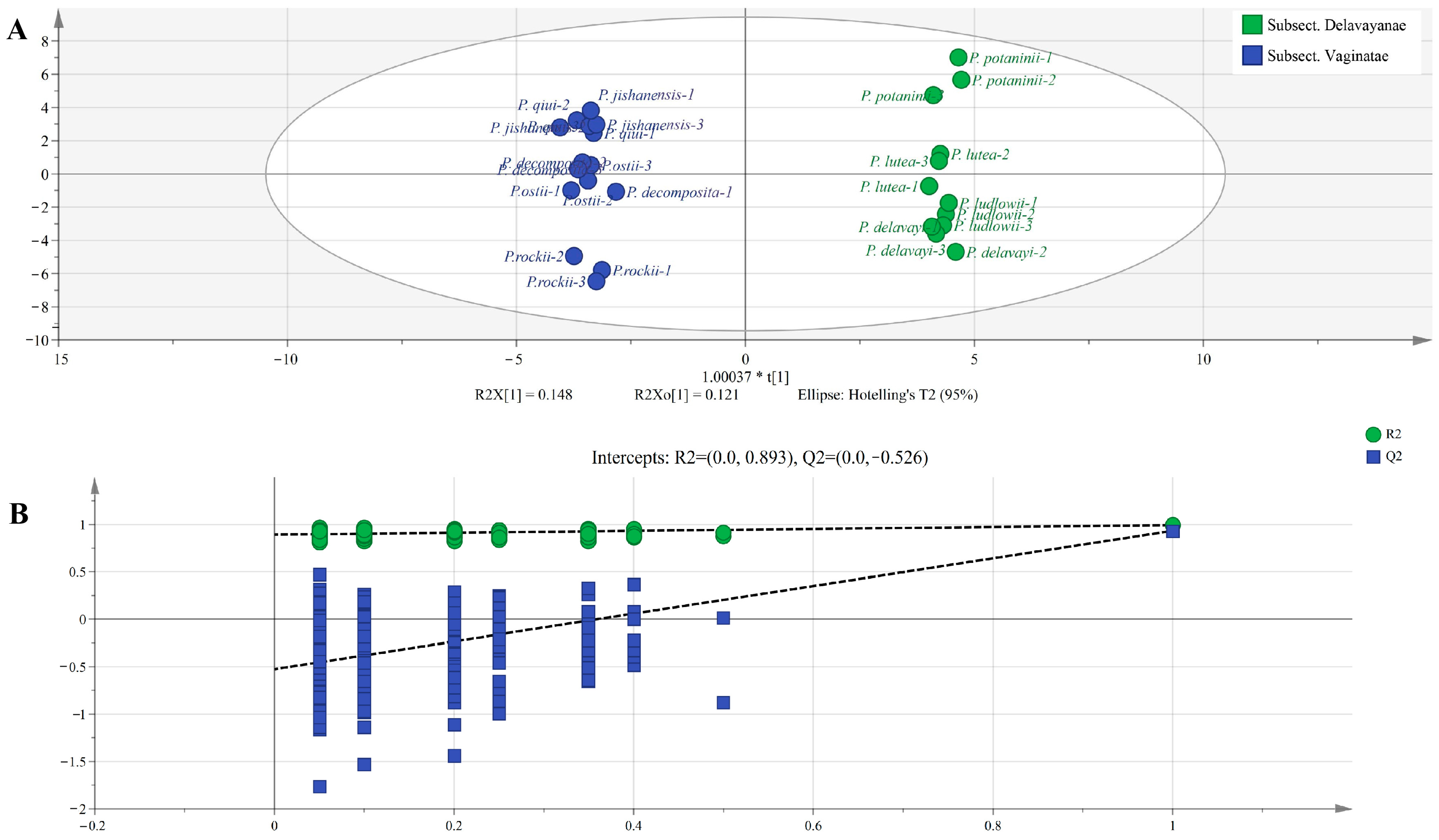
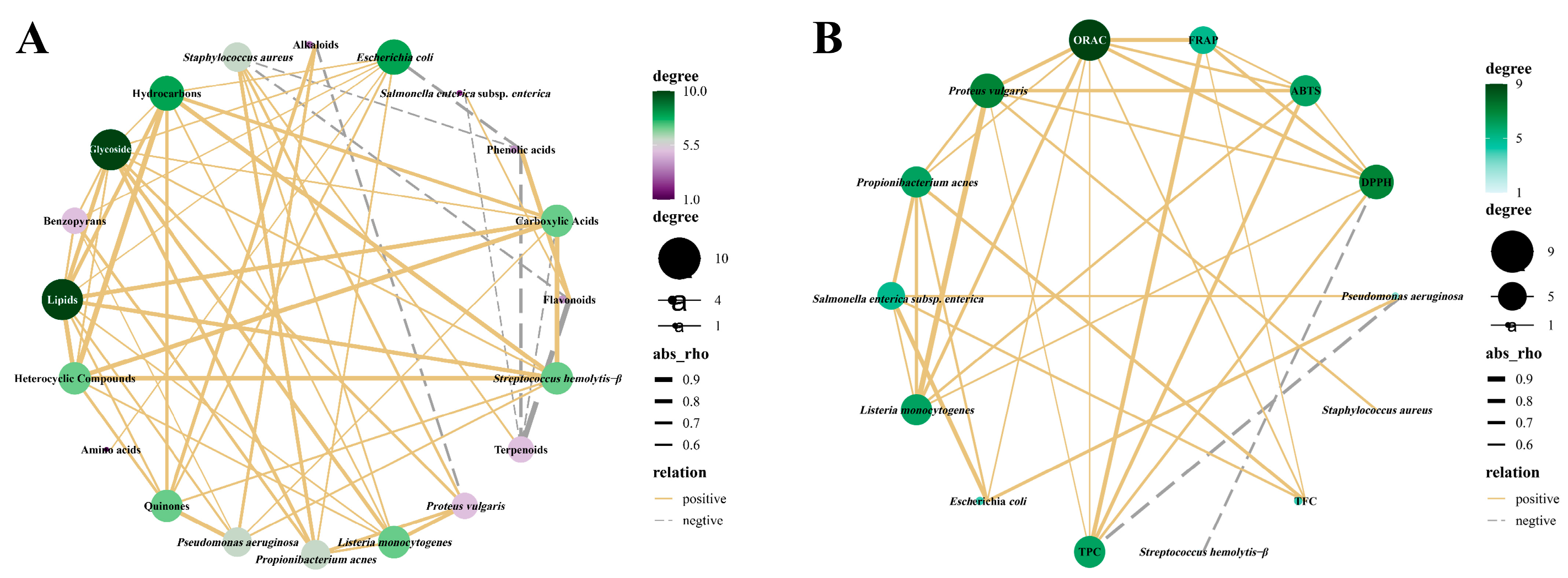
2.6. Multivariate Statistical Analysis
3. Materials and Methods
3.1. Plant Materials
3.2. Sample Extraction
3.3. Chemicals
3.4. Determination of the Total Phenolic Content
3.5. Determination of the Total Flavonoid Content
3.6. LC-QTOF-MS/MS Analysis
3.7. Assessment of Antioxidant Activity with ABTS+•, DPPH•, FRAP, and ORAC Assays
3.8. Assessment of Antibacterial Activity through Agar Diffusion Method
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, D.Y.; Pan, K.Y. Taxonomical history and revision of Paeonia sect. Moutan (Paeoniaceae). Acta Phytotaxon. Sin. 1999, 37, 351–368. [Google Scholar]
- Hong, D.Y. Peonies of the World: Taxonomy and Phytogeography; Royal Botanic Gardens, Kew: London, UK, 2010; pp. 12–15. [Google Scholar]
- Ekiert, H.; Klimek-Szczykutowicz, M.; Szopa, A. Paeonia × suffruticosa (Moutan Peony)—A Review of the Chemical Composition, Traditional and Professional Use in Medicine, Position in Cosmetics Industries, and Biotechnological Studies. Plants 2022, 11, 3379. [Google Scholar] [CrossRef]
- Zhai, J.; Guo, Y. Paeoniflorin attenuates cardiac dysfunction in endotoxemic mice via the inhibition of nuclear factor-κB. Biomed. Pharmacother. 2016, 80, 200–206. [Google Scholar] [CrossRef]
- Ma, X.; Guo, J.; Ma, Y.; Jin, B.; Zhan, Z.; Yuan, Y.; Huang, L. Characterization of a monoterpene synthase from Paeonia lactiflora producing alpha-pinene as its single product. Biotechnol. Lett. 2016, 38, 1213–1219. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Wu, L.Y.; Lv, Y.Q.; Ye, Y.; Liang, Y.R.; Ye, J.H. Transcriptomic and Translatomic Analyses Reveal Insights into the Developmental Regulation of Secondary Metabolism in the Young Shoots of Tea Plants (Camellia sinensis L.). J. Agric. Food Chem. 2020, 68, 10750–10762. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Garrido, M.; Pariente, J.A.; Espino, J.; Delgado-Adámez, J. Bioavailability of Bioactive Molecules from Olive Leaf Extracts and its Functional Value. Phytother. Res. 2016, 30, 1172–1179. [Google Scholar] [CrossRef]
- Skinnider, M.A.; Johnston, C.W.; Gunabalasingam, M.; Merwin, N.J.; Kieliszek, A.M.; MacLellan, R.J.; Li, H.X.; Ranieri, M.R.M.; Webster, A.L.H.; Cao, M.P.T.; et al. Comprehensive prediction of secondary metabolite structure and biological activity from microbial genome sequences. Nat. Commun. 2020, 11, 6058. [Google Scholar] [CrossRef]
- Gericke, S.; Lubken, T.; Wolf, D.; Kaiser, M.; Hannig, C.; Speer, K. Identification of New Compounds from Sage Flowers (Salvia officinalis L.) as Markers for Quality Control and the Influence of the Manufacturing Technology on the Chemical Composition and Antibacterial Activity of Sage Flower Extracts. J. Agric. Food Chem. 2018, 66, 1843–1853. [Google Scholar] [CrossRef]
- Nemzer, B.; Abshiru, N.; Al-Taher, F. Identification of Phytochemical Compounds in Coffea arabica Whole Coffee Cherries and Their Extracts by LC-MS/MS. J. Agric. Food Chem. 2021, 69, 3430–3438. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alias, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Kumar, L.; Patel, S.K.S.; Kharga, K.; Kumar, R.; Kumar, P.; Pandohee, J.; Kulshresha, S.; Harjai, K.; Chhibber, S. Molecular Mechanisms and Applications of N-Acyl Homoserine Lactone-Mediated Quorum Sensing in Bacteria. Molecules 2022, 27, 7584. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic Effects Between Metal Nanoparticles and Commercial Antimicrobial Agents: A Review. Acs Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef]
- Kharga, K.; Kumar, L.; Patel, S.K.S. Recent Advances in Monoclonal Antibody-Based Approaches in the Management of Bacterial Sepsis. Biomedicines 2023, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Kon, K.V. Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Elsevier Academic Press: Amsterdam, The Netherlands, 2013; pp. 65–94. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.Y.; Li, N.N.; Wu, L.Y.; Huang, Z.; Hu, Z.Y.; Li, X.J.; Qu, Z.C. Phytochemicals and anti-tyrosinase activities of Paeonia ostii leaves and roots. Plant Physiol. Biochem. 2022, 181, 50–60. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.S.; da Silva, J.A.T.; Yu, X.N.; Wang, L.S. Characterization of phytochemicals in the roots of wild herbaceous peonies from China and screening for medicinal resources. Phytochemistry 2020, 174, 112331. [Google Scholar] [CrossRef]
- Yan, Z.G.; Xie, L.H.; Li, M.C.; Yuan, M.; Tian, Y.; Sun, D.Y.; Zhang, Y.L.; Niu, L.X. Phytochemical components and bioactivities of novel medicinal food-Peony roots. Food Res. Int. 2021, 140, 109902. [Google Scholar] [CrossRef]
- Zhang, X.X.; Shi, Q.Q.; Ji, D.; Niu, L.X.; Zhang, Y.L. Determination of the phenolic content, profile, and antioxidant activity of seeds from nine tree peony (Paeonia section Moutan DC.) species native to China. Food Res. Int. 2017, 97, 141–148. [Google Scholar] [CrossRef]
- Ye, Q.; Zheng, D.; Chen, Z. Rapid determination of paeonol in traditional Chinese medicinal preparations by microwave-assisted extraction followed by headspace solid-phase microextraction and gas chromatography-mass spectrometry. J. Anal. Chem. 2011, 66, 285–289. [Google Scholar] [CrossRef]
- Bai, Z.Z.; Tang, J.M.; Ni, J.; Zheng, T.T.; Zhou, Y.; Sun, D.Y.; Li, G.N.; Liu, P.; Niu, L.X.; Zhang, Y.L. Comprehensive metabolite profile of multi-bioactive extract from tree peony (Paeonia ostii and Paeonia rockii) fruits based on MS/MS molecular networking. Food Res. Int. 2021, 148, 110609. [Google Scholar] [CrossRef]
- Yang, W.Z.; Hu, J.Y.; Behera, J.R.; Kilaru, A.; Yuan, Y.P.; Zhai, Y.H.; Xu, Y.F.; Xie, L.H.; Zhang, Y.L.; Zhang, Q.Y.; et al. A Tree Peony Trihelix Transcription Factor PrASIL1 Represses Seed Oil Accumulation. Front. Plant Sci. 2021, 12, 796181. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Xin, Z.W.; Hu, J.Y.; Zhang, Y.L.; Zhang, Q.Y.; Niu, L.X. The tree peony nuclear factor Y transcription factor PrNF-YC2 promotes seed oil accumulation. Plant J. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Xin, Z.; Yang, W.; Duan, Y.; Wang, W.; Niu, L.; Sun, D.; Zhang, Y. Bioactive components and antibacterial activities of hydrolate extracts by optimization conditions from Paeonia ostii T. Hong & J. X. Zhang. Ind. Crops Prod. 2022, 188, 115737. [Google Scholar] [CrossRef]
- Tang, D.D.; Li, H.H.; Zhang, Y.L.; Luo, J.R.; Yu, H.; Yuan, L.X. Analysis on pigments and antioxidant activity in red-leaf of Paeonia rockii var. purpurea. North. Hortic. 2015, 23, 79–86. [Google Scholar] [CrossRef]
- Xiang, J.; Yang, C.; Beta, T.; Liu, S.; Yang, R. Phenolic Profile and Antioxidant Activity of the Edible Tree Peony Flower and Underlying Mechanisms of Preventive Effect on H2O2-Induced Oxidative Damage in Caco-2 Cells. Foods 2019, 8, 471. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, M.; Shan, X.F.; Wang, C.D.; Yang, L.F.; Fan, Q.; Zhang, B.H.; Wu, M.Y. Extraction and Antioxidant Activity of Polysaccharides from Peony Stamen by Steam Explosion. Food Sci. Technol. 2021, 46, 5. [Google Scholar] [CrossRef]
- Bai, Z.Z.; Ni, J.; Tang, J.M.; Sun, D.Y.; Yan, Z.G.; Zhang, J.; Niu, L.X.; Zhang, Y.L. Bioactive components, antioxidant and antimicrobial activities of Paeonia rockii fruit during development. Food Chem. 2021, 343, 128444. [Google Scholar] [CrossRef]
- Liu, L.; Yuan, Y.D.; Tao, J. Antioxidant and antibacterial activities of 13 ornamental herbaceous peony cultivars: A comparative study with stems and leaves. N. Z. J. Crop Hortic. Sci. 2022, 50, 326–340. [Google Scholar] [CrossRef]
- Tong, N.N.; Zhou, X.Y.; Peng, L.P.; Liu, Z.A.; Shu, Q.Y. A comprehensive study of three species of Paeonia stem and leaf phytochemicals, and their antioxidant activities. J. Ethnopharmacol. 2021, 273, 113985. [Google Scholar] [CrossRef]
- Luo, X.N.; Yuan, M.; Li, B.J.; Li, C.Y.; Zhang, Y.L.; Shi, Q.Q. Variation of floral volatiles and fragrance reveals the phylogenetic relationship among nine wild tree peony species. Flavour Fragr. J. 2020, 35, 227–241. [Google Scholar] [CrossRef]
- Li, C.H.; Du, H.; Wang, L.S.; Shu, Q.Y.; Zheng, Y.R.; Xu, Y.J.; Zhang, J.J.; Zhang, J.; Yang, R.Z.; Ge, Y.X. Flavonoid Composition and Antioxidant Activity of Tree Peony (Paeonia Section Moutan) Yellow Flowers. J. Agric. Food Chem. 2009, 57, 8496–8503. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Nang, S.C.; Chan, H.K.; Li, J. Novel antimicrobial agents for combating antibiotic-resistant bacteria. Adv. Drug Deliv. Rev. 2022, 187, 114378. [Google Scholar] [CrossRef]
- Han, C.V.; Bhat, R. In vitro control of food-borne pathogenic bacteria by essential oils and solvent extracts of underutilized flower buds of Paeonia suffruticosa (Andr.). Ind. Crops Prod. 2014, 54, 203–208. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Yu, S.-H.; Cheng, K.-W.; Liou, Y.-W.; Hsu, C.-C.; Hsieh, C.-W.; Kuo, C.-H.; Cheng, K.-C. Production and analysis of metabolites from solid-state fermentation of Chenopodium formosanum (Djulis) sprouts in a bioreactor. Food Res. Int. 2023, 168, 112707. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Sumczynski, D.; Bubelova, Z.; Sneyd, J.; Erb-Weber, S.; Mlcek, J. Total phenolics, flavonoids, antioxidant activity, crude fibre and digestibility in non-traditional wheat flakes and muesli. Food Chem. 2015, 174, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Stachniuk, A.; Sumara, A.; Montowska, M.; Fornal, E. LC-QTOF-MS identification of rabbit-specific peptides for authenticating the species composition of meat products. Food Chem. 2020, 329, 127185. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Garcia, E.J.; Oldoni, T.L.C.; Alencar, S.M.d.; Reis, A.; Loguercio, A.D.; Grande, R.H.M. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz. Dent. J. 2012, 23, 22–27. [Google Scholar] [CrossRef]
- Nguyen, A.T.L.; Akanbi, T.O.; Tawiah, N.A.; Aryee, A.N.A. Valorization of seed and kernel marcs and evaluation of their antioxidant potential. Food Chem. 2022, 390, 113168. [Google Scholar] [CrossRef]
- Ou, B.X.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Davalos, A.; Gomez-Cordoves, C.; Bartolome, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Merillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Ozyurek, M.; Guclu, K.; Capanoglu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Henry, C.; Hérisse, M.; Denamur, E.; Barras, F. Commercial Lysogeny Broth culture media and oxidative stress: A cautious tale. Free Radic. Biol. Med. 2014, 74, 245–251. [Google Scholar] [CrossRef]
- Satti, L.; Abbasi, S.; Faiz, U. Evaluation of nutrient agar for the culture of Mycobacterium tuberculosis using the microcolony detection method. Int. J. Tuberc. Lung Dis. 2012, 16, 908–910. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.F.; Gao, J.Y.; Liu, Y.Q.; Hou, X.W.; Yin, W.P.; Deng, R.X. Two New Resveratrol Trimers with Antibacterial Activities from Seed Cake of Paeonia rockii. Chem. Nat. Compd. 2017, 53, 51–55. [Google Scholar] [CrossRef]
- Wang, X.; Chi, Z.; Yue, L.; Li, J. Purification and characterization of killer toxin from a marine yeast Pichia anomala YF07b against the pathogenic yeast in crab. Curr. Microbiol. 2007, 55, 396–401. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Z.X.; Wu, H.; Lv, F.X. Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int. J. Food Microbiol. 2009, 136, 71–74. [Google Scholar] [CrossRef]
- Zhai, Q.X.; Yin, R.J.; Yu, L.L.; Wang, G.; Tian, F.W.; Yu, R.P.; Zhao, J.X.; Liu, X.M.; Chen, Y.Q.; Zhang, H.; et al. Screening of lactic acid bacteria with potential protective effects against cadmium toxicity. Food Control 2015, 54, 23–30. [Google Scholar] [CrossRef]
- Sheng, Z.L.; Li, J.C.; Li, Y.H. Optimization of forsythoside extraction from Forsythia suspensa by Box-Behnken design. Afr. J. Biotechnol. 2011, 10, 11728–11737. [Google Scholar] [CrossRef]
- Ivanisevic, J.; Want, E.J. From Samples to Insights into Metabolism: Uncovering Biologically Relevant Information in LC-HRMS Metabolomics Data. Metabolites 2019, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xin, Z.; Xie, L.; Zhai, Y.; Zhang, Y.; Niu, L.; Zhang, Q. Integrative lipidomics profile uncovers the mechanisms underlying high-level α-linolenic acid accumulation in Paeonia rockii seeds. Hortic. Res. 2023, uhad106. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Z.; Yang, W.; Niu, L.; Zhang, Y. Comprehensive Metabolite Profile Uncovers the Bioactive Components, Antioxidant and Antibacterial Activities in Wild Tree Peony Leaves. Int. J. Mol. Sci. 2023, 24, 10609. https://doi.org/10.3390/ijms241310609
Xin Z, Yang W, Niu L, Zhang Y. Comprehensive Metabolite Profile Uncovers the Bioactive Components, Antioxidant and Antibacterial Activities in Wild Tree Peony Leaves. International Journal of Molecular Sciences. 2023; 24(13):10609. https://doi.org/10.3390/ijms241310609
Chicago/Turabian StyleXin, Ziwei, Weizong Yang, Lixin Niu, and Yanlong Zhang. 2023. "Comprehensive Metabolite Profile Uncovers the Bioactive Components, Antioxidant and Antibacterial Activities in Wild Tree Peony Leaves" International Journal of Molecular Sciences 24, no. 13: 10609. https://doi.org/10.3390/ijms241310609
APA StyleXin, Z., Yang, W., Niu, L., & Zhang, Y. (2023). Comprehensive Metabolite Profile Uncovers the Bioactive Components, Antioxidant and Antibacterial Activities in Wild Tree Peony Leaves. International Journal of Molecular Sciences, 24(13), 10609. https://doi.org/10.3390/ijms241310609







