Phage Targeting Neonatal Meningitis E. coli K1 In Vitro in the Intestinal Microbiota of Pregnant Donors and Impact on Bacterial Populations
Abstract
1. Introduction
2. Results
2.1. Determination of the Optimal Phage Concentration in the Galleria mellonella Model
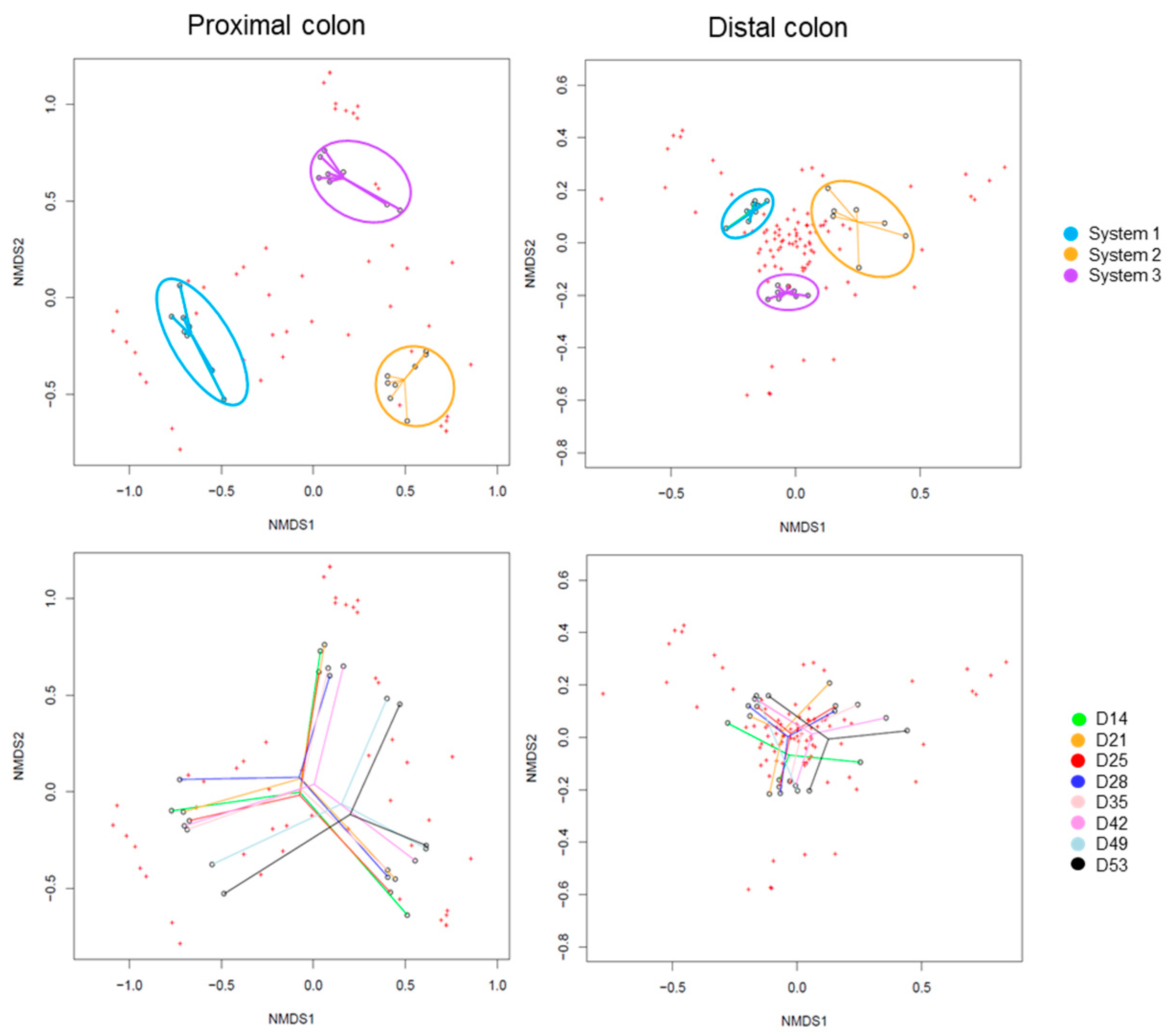
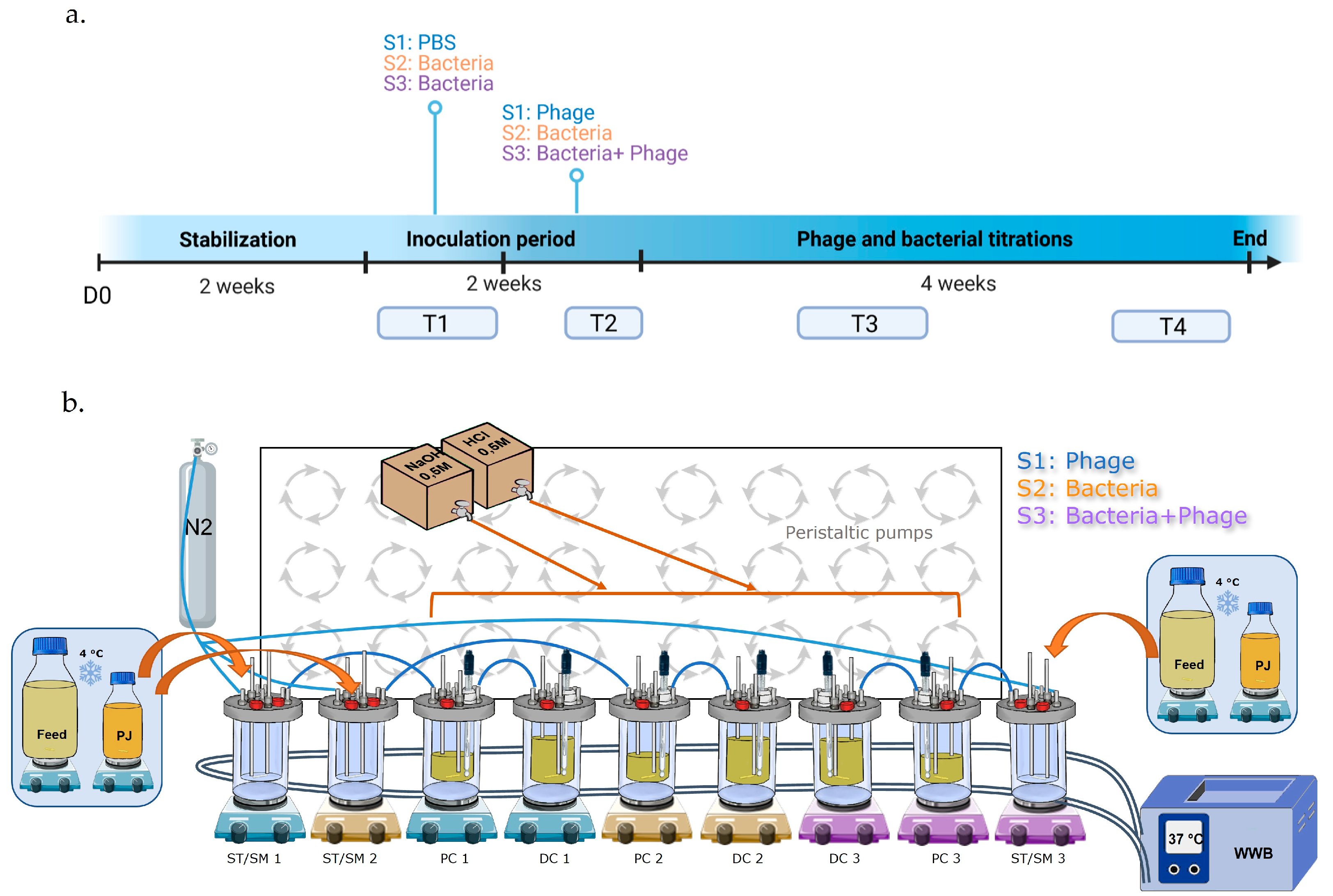
2.2. Triple-SHIME Experiments
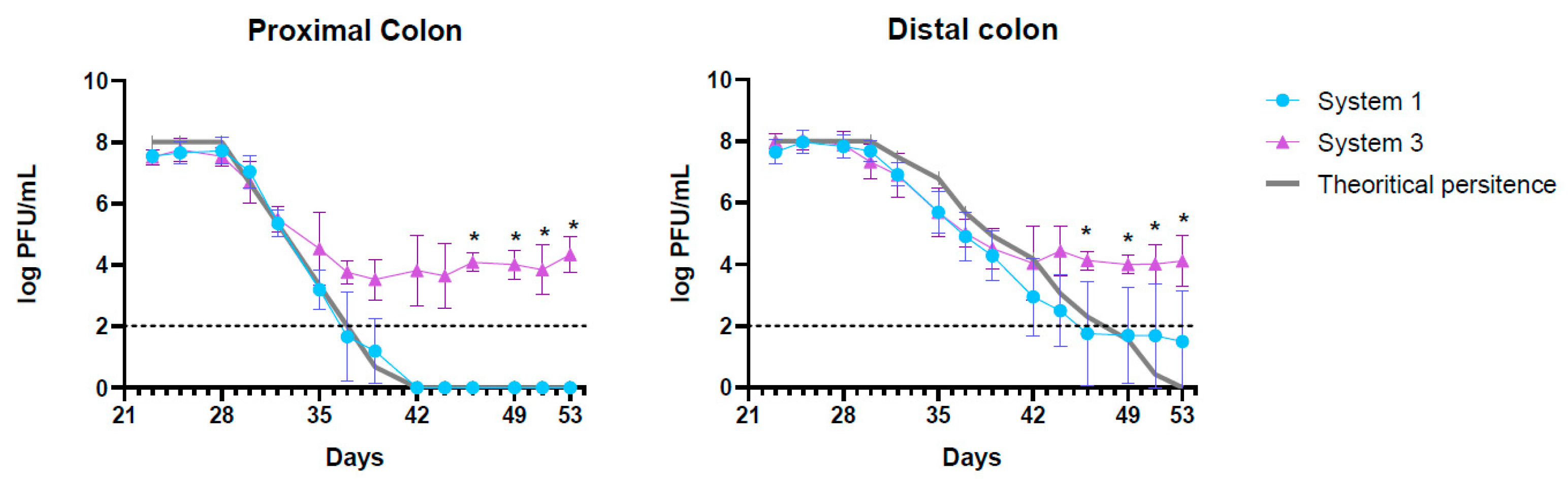
2.2.1. Persistence and Evolution of the Concentration of K1_ULINTec4
2.2.2. Evolution of E. coli K1 Concentrations
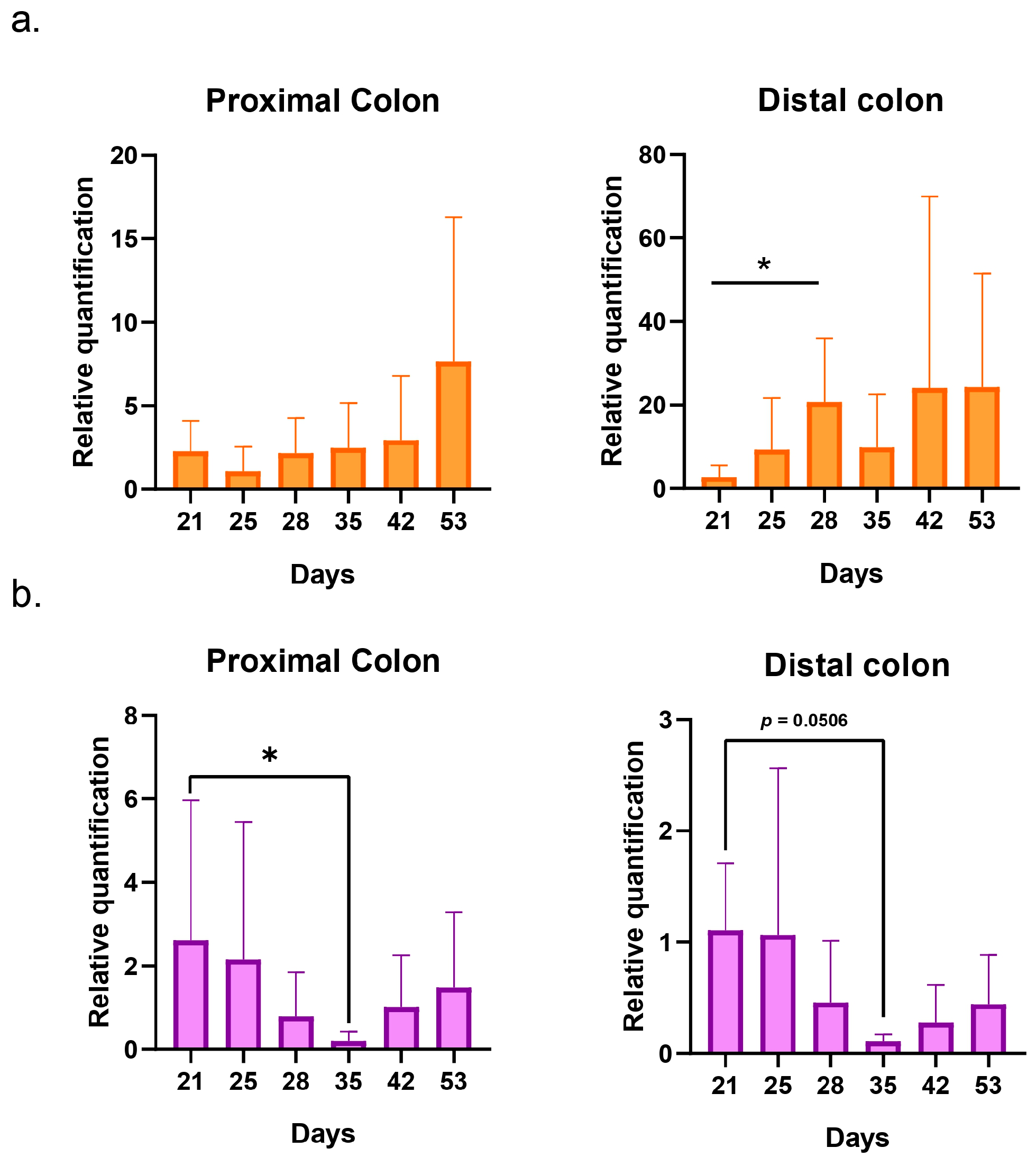
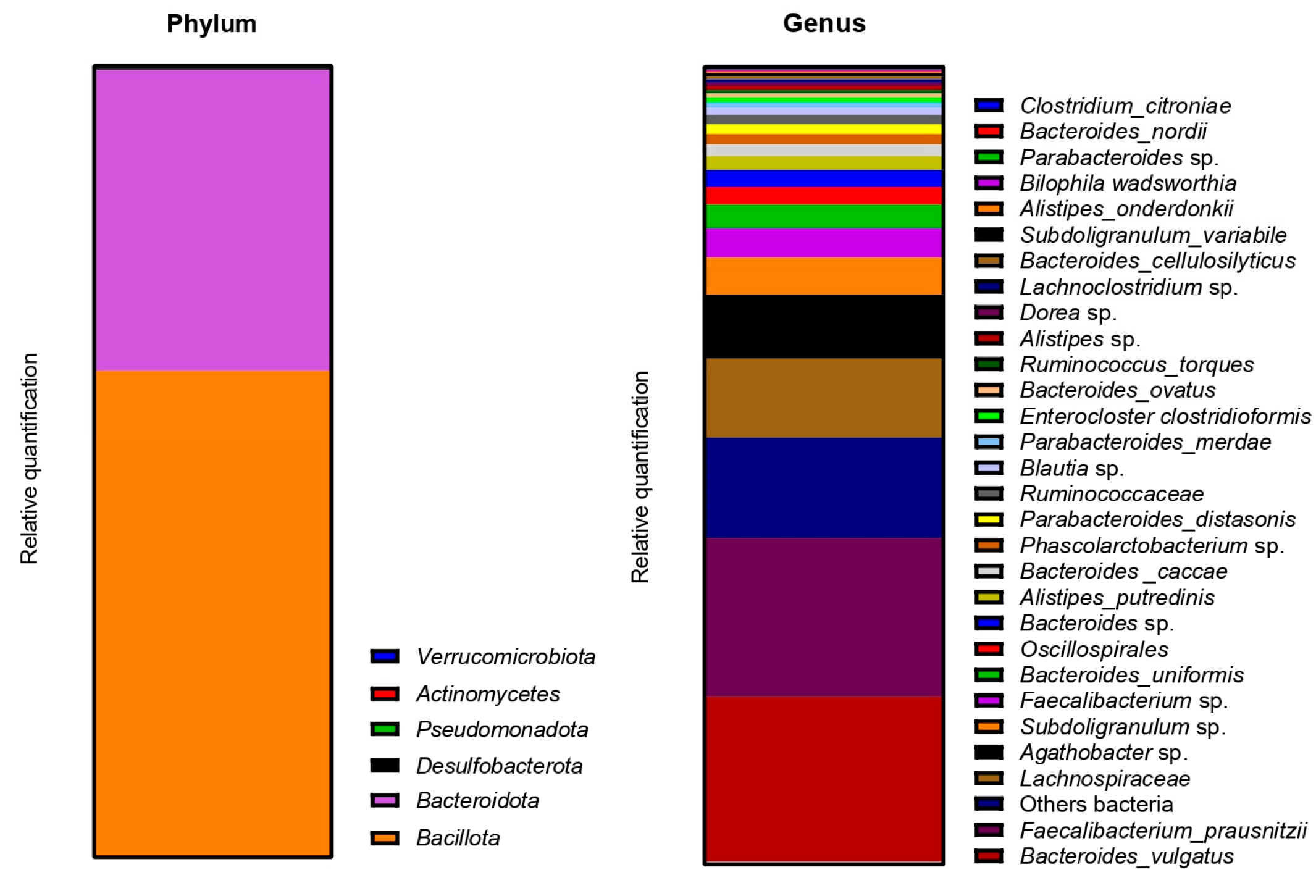
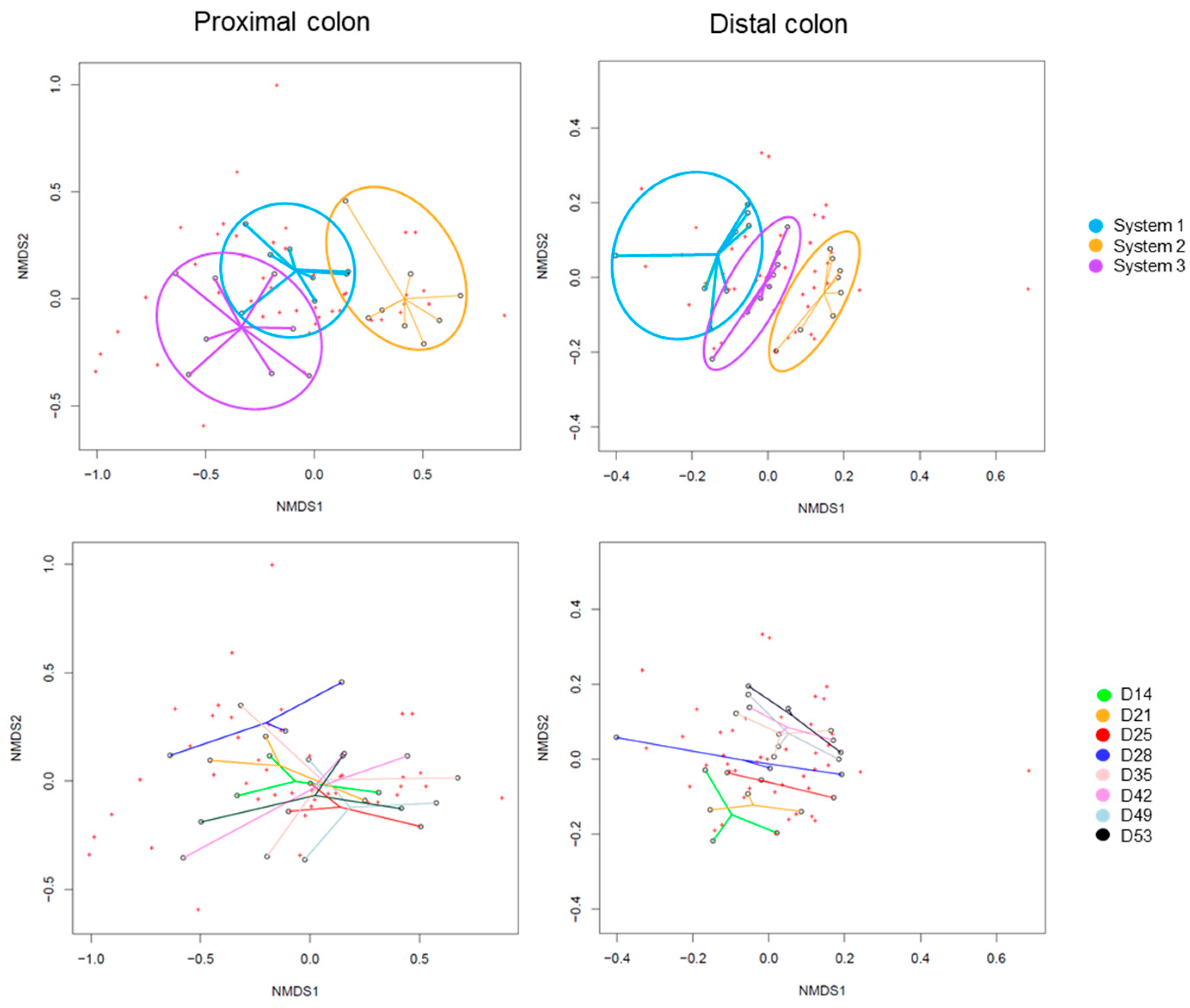
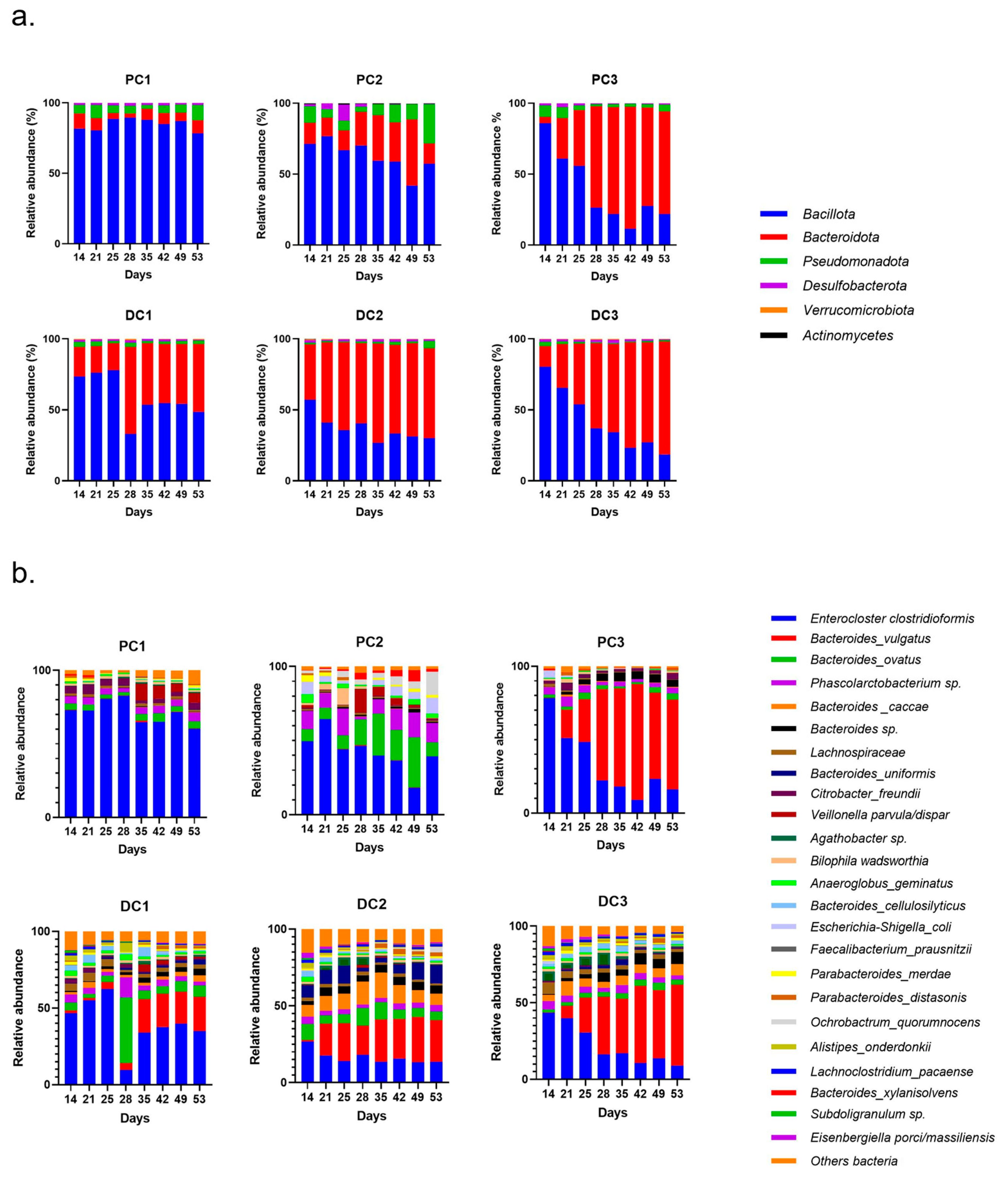
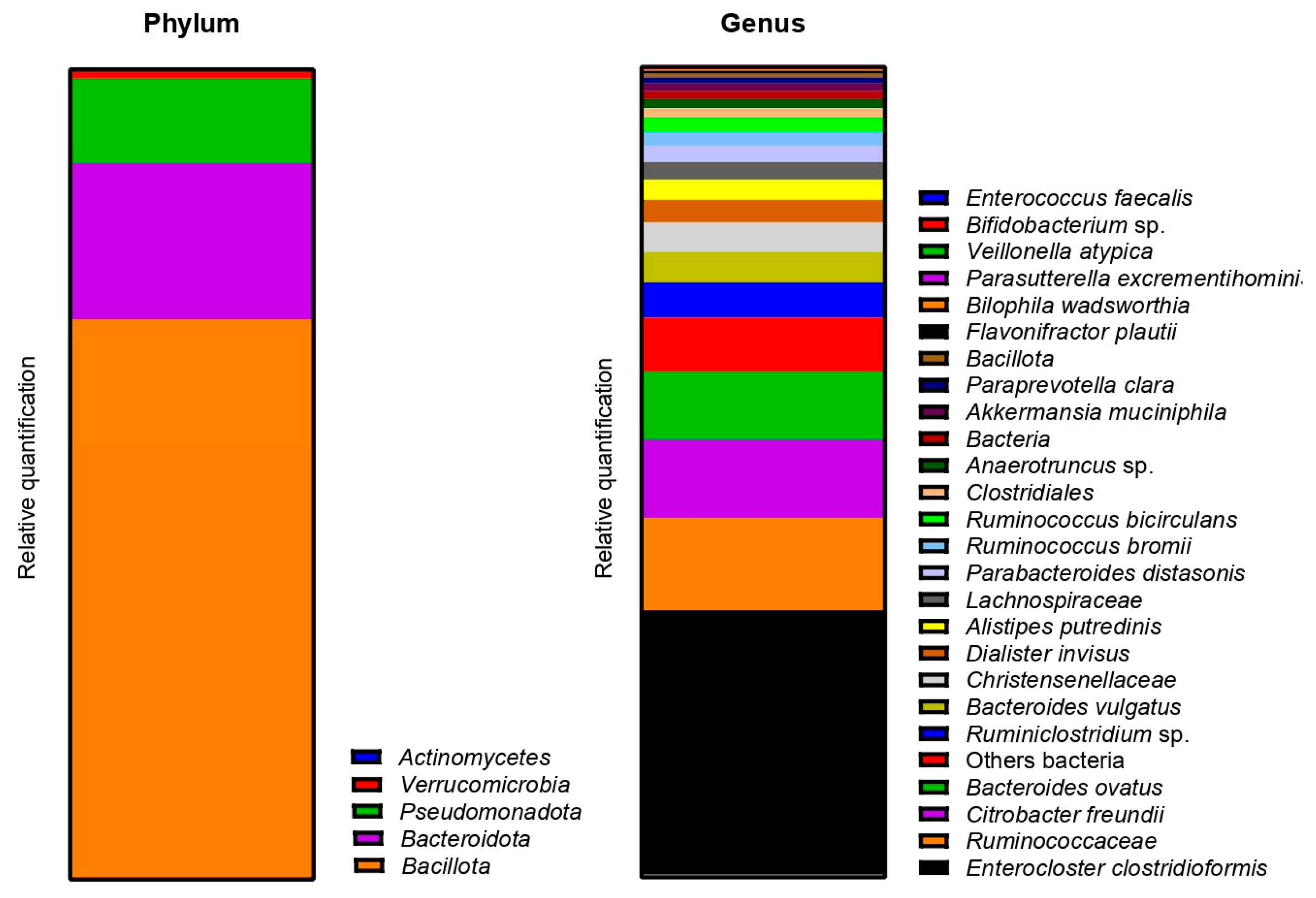
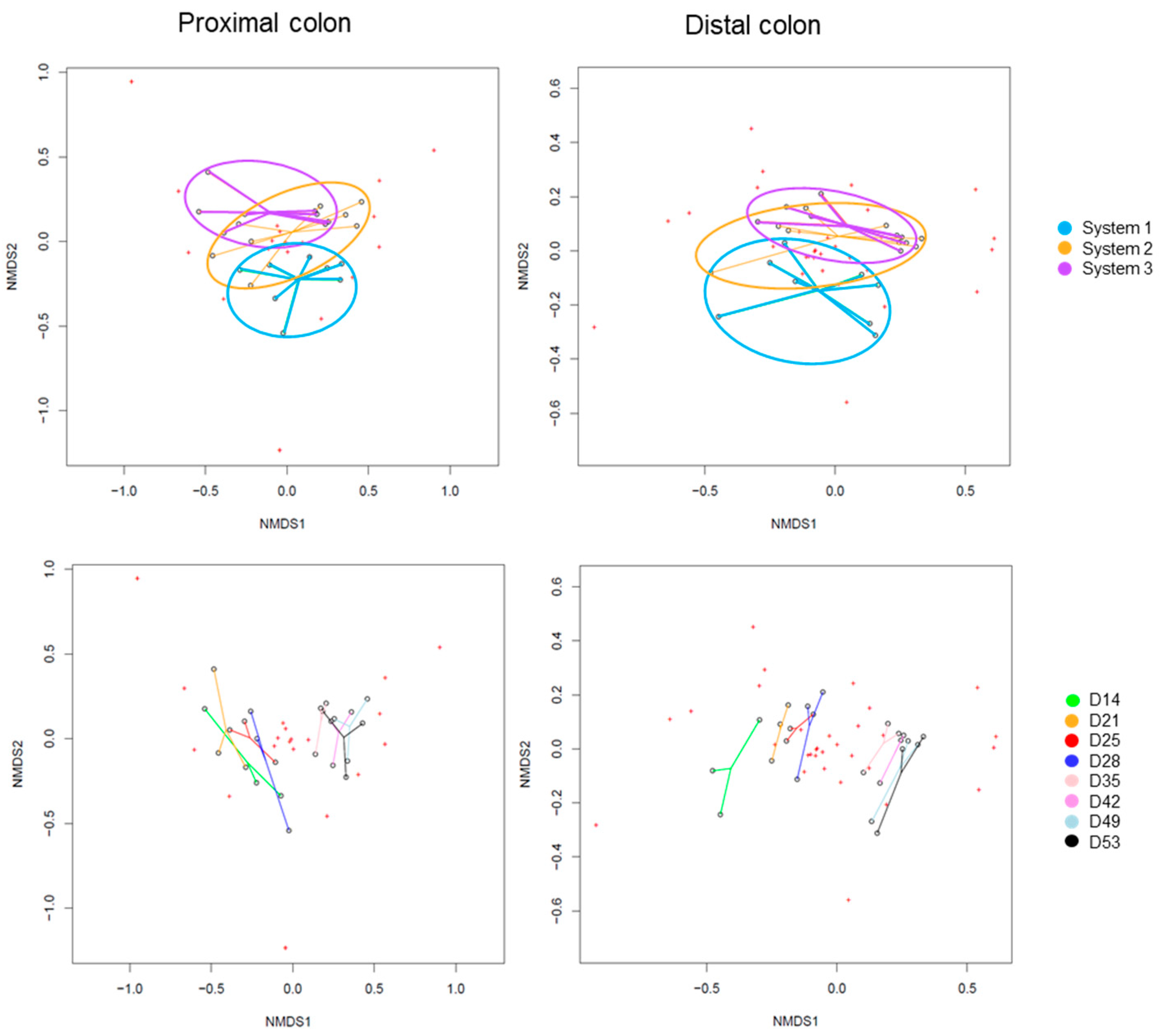
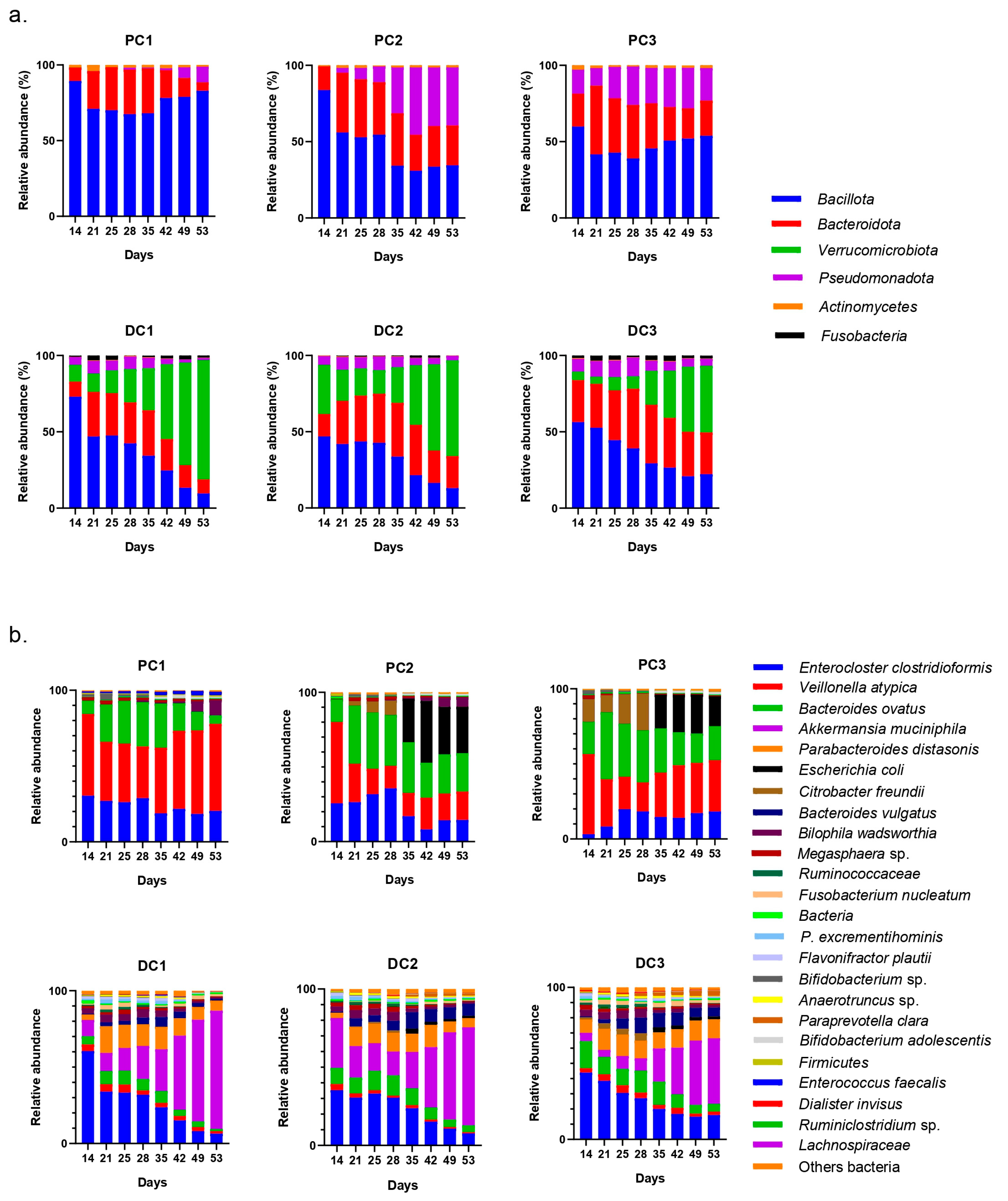
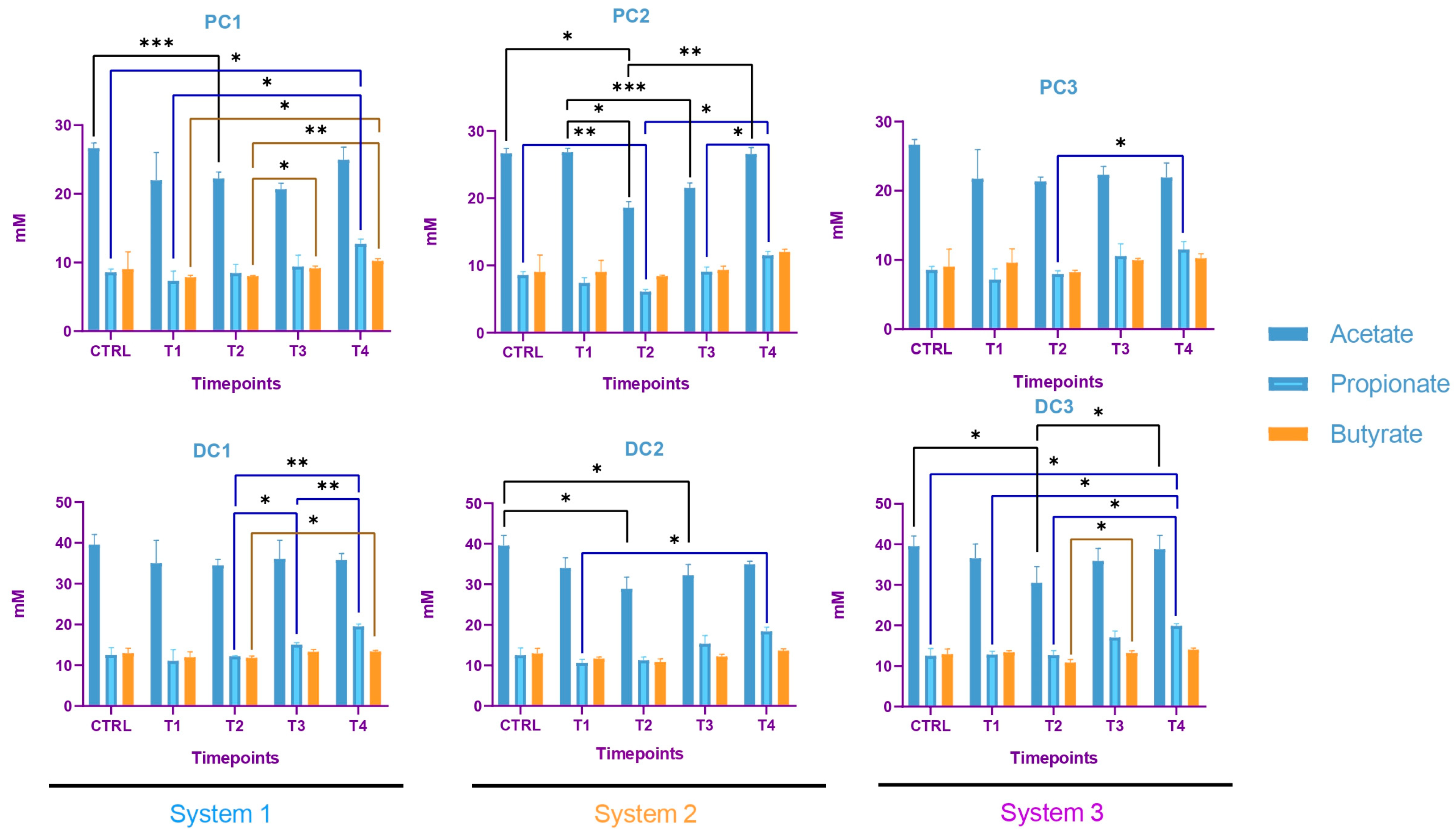
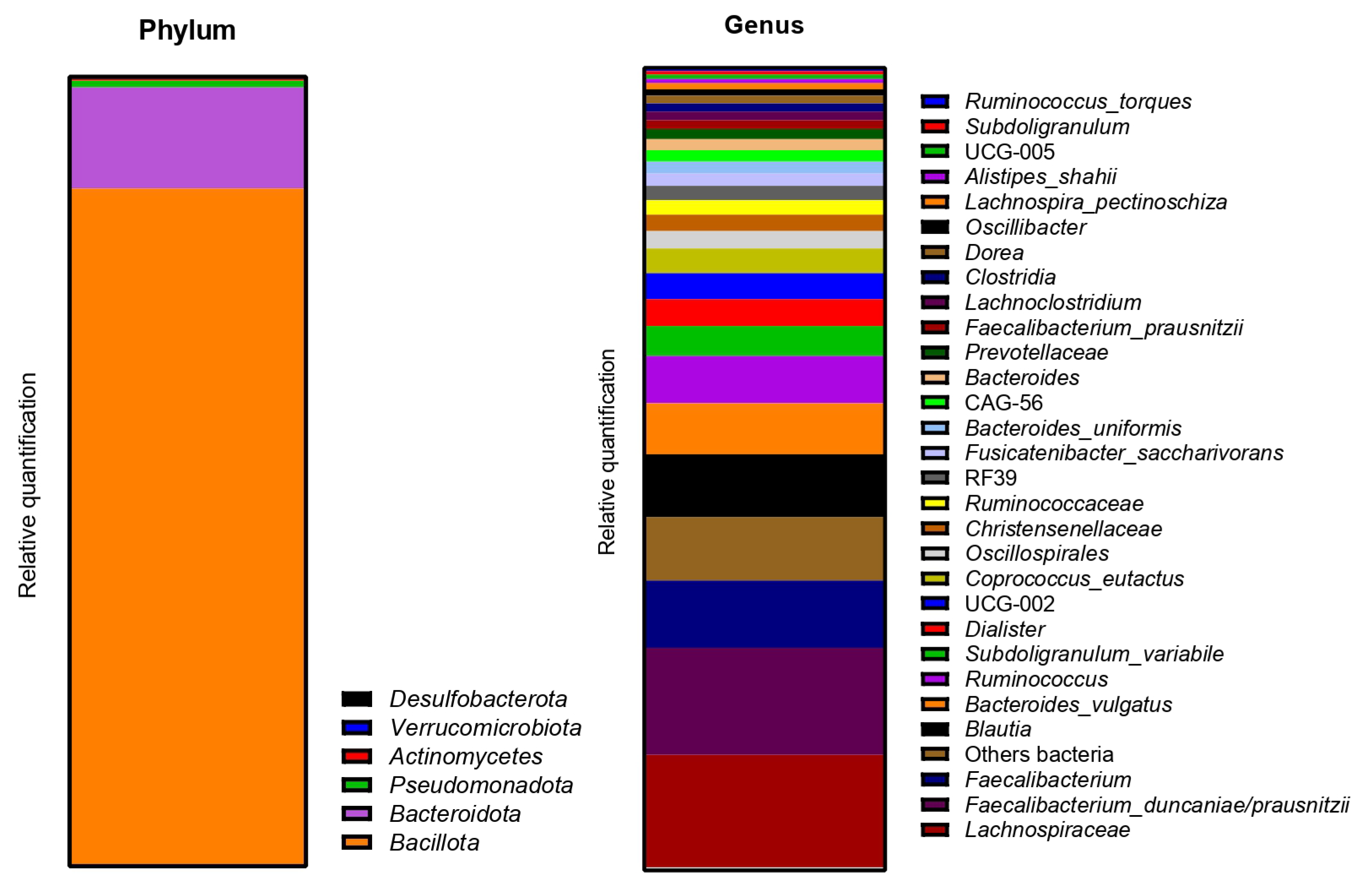
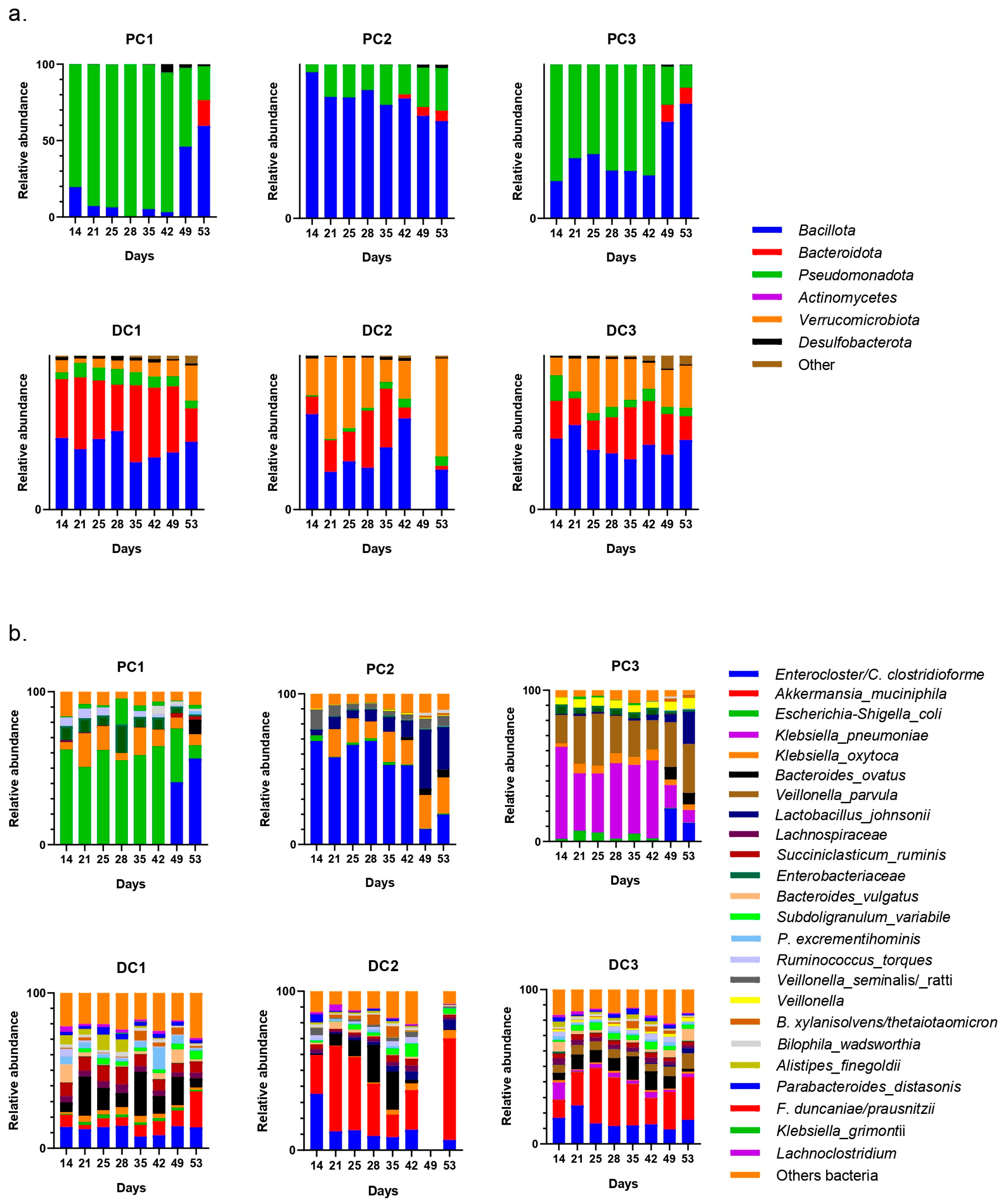
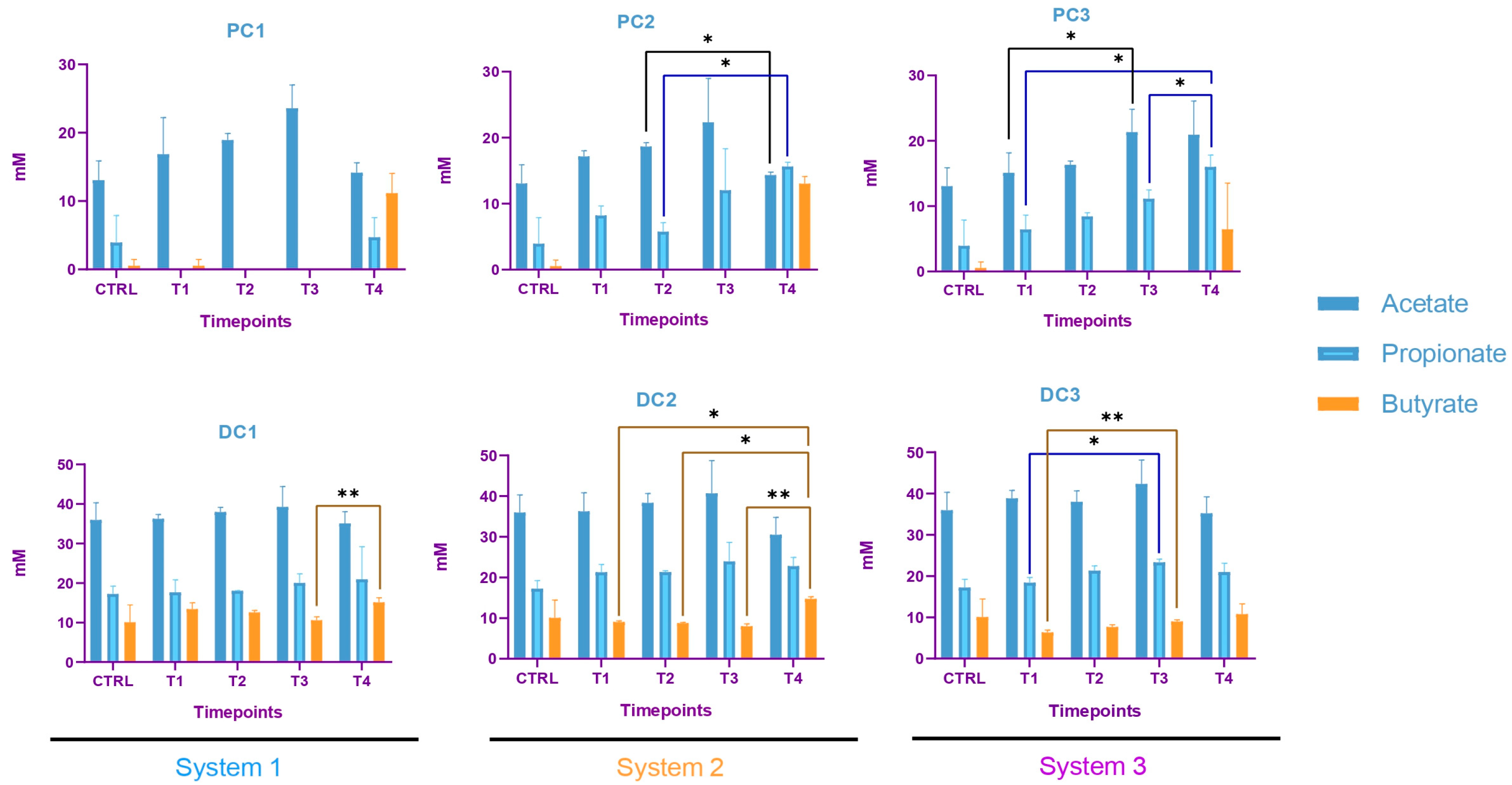
2.2.3. Impact on Pregnant Donors’ Microbiota
- Donor 1
- 2.
- Donor 2
- 3.
- Donor 3
3. Discussion
4. Materials and Methods
4.1. Strains and Culture
4.2. Determination of the Optimal Phage Concentration in the Galleria mellonella Model
4.3. Fecal Material Collection and Screening
4.4. Human Gastrointestinal Model Set-Up and Experimental Design
4.5. 16S rDNA Gene Sequencing and qPCR of Selected Bacterial Groups
4.6. Short-Chain Fatty Acids Analysis
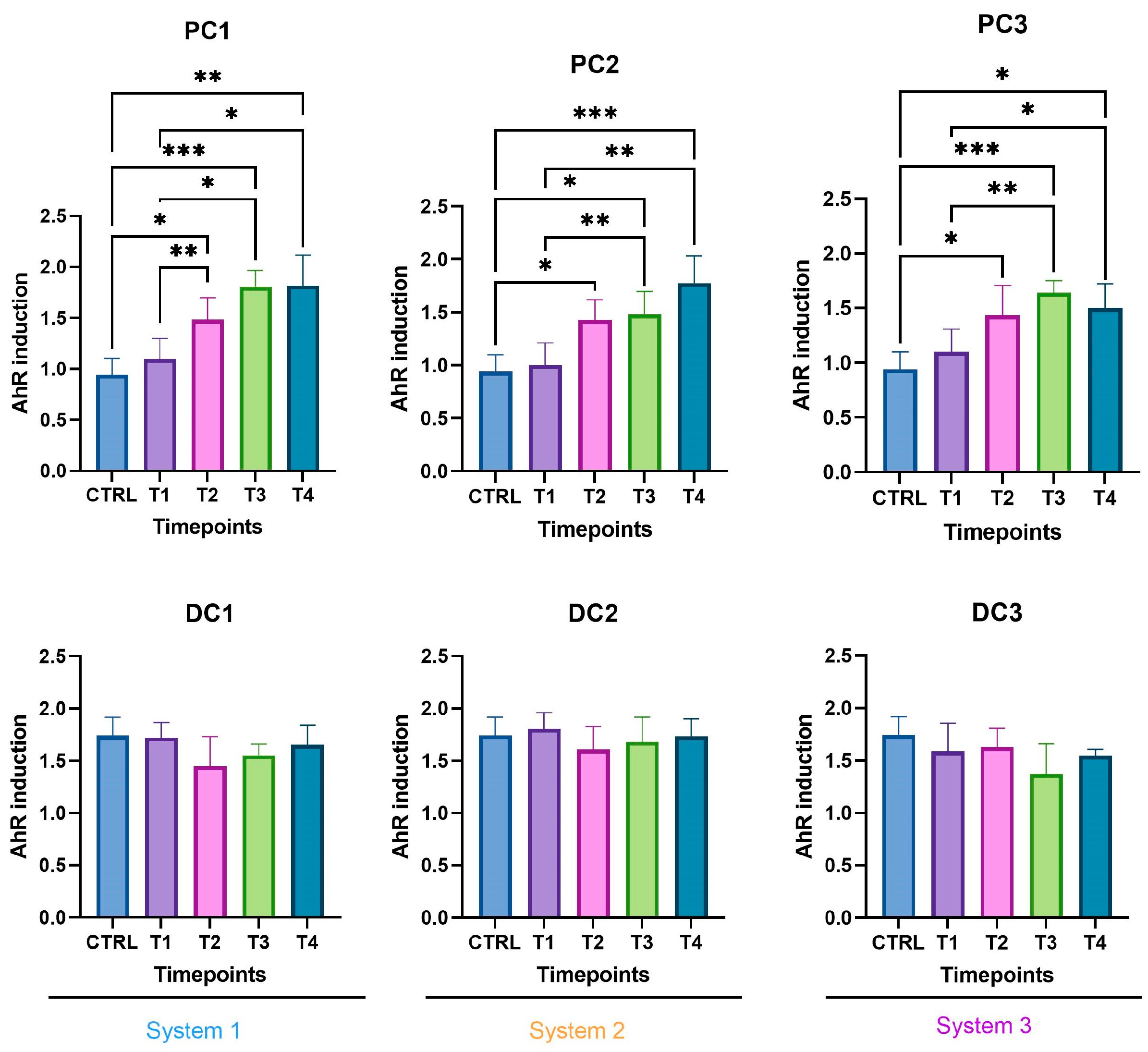
4.7. AhR Activity and Cytotoxicity Assays
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaschignard, J.; Levy, C.; Romain, O.; Cohen, R.; Bingen, E.; Aujard, Y.; Boileau, P. Neonatal Bacterial Meningitis: 444 Cases in 7 Years. Pediatr. Infect. Dis. J. 2011, 30, 212. [Google Scholar] [CrossRef] [PubMed]
- Zainel, A.; Mitchell, H.; Sadarangani, M. Bacterial Meningitis in Children: Neurological Complications, Associated Risk Factors, and Prevention. Microorganisms 2021, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Ku, L.C.; Boggess, K.A.; Cohen-Wolkowiez, M. Bacterial Meningitis in the Infant. Clin. Perinatol. 2015, 42, 29–45. [Google Scholar] [CrossRef]
- Klinger, G.; Levy, I.; Sirota, L.; Boyko, V.; Reichman, B.; Lerner-Geva, L. Epidemiology and Risk Factors for Early Onset Sepsis among Very-Low-Birthweight Infants. Am. J. Obstet. Gynecol. 2009, 201, 38.e1–38.e6. [Google Scholar] [CrossRef] [PubMed]
- Furyk, J.S.; Swann, O.; Molyneux, E. Systematic Review: Neonatal Meningitis in the Developing World. Trop. Med. Int. Health 2011, 16, 672–679. [Google Scholar] [CrossRef]
- Doran, K.S.; Fulde, M.; Gratz, N.; Kim, B.J.; Nau, R.; Prasadarao, N.; Schubert-Unkmeir, A.; Tuomanen, E.I.; Valentin-Weigand, P. Host–Pathogen Interactions in Bacterial Meningitis. Acta Neuropathol. 2016, 131, 185–209. [Google Scholar] [CrossRef]
- Houdouin, V.; Bonacorsi, S.; Bidet, P.; Blanco, J.; De La Rocque, F.; Cohen, R.; Aujard, Y.; Bingen, E. Association between Mortality of Escherichia coli Meningitis in Young Infants and Non-Virulent Clonal Groups of Strains. Clin. Microbiol. Infect. 2008, 14, 685–690. [Google Scholar] [CrossRef]
- King, J.E.; Aal Owaif, H.A.; Jia, J.; Roberts, I.S. Phenotypic Heterogeneity in Expression of the K1 Polysaccharide Capsule of Uropathogenic Escherichia coli and Downregulation of the Capsule Genes during Growth in Urine. Infect. Immun. 2015, 83, 2605–2613. [Google Scholar] [CrossRef]
- Kathayat, D.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Avian Pathogenic Escherichia coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies. Pathogens 2021, 10, 467. [Google Scholar] [CrossRef]
- Sikias, P.; Biran, V.; Foix-L’Hélias, L.; Plainvert, C.; Boileau, P.; Bonacorsi, S. EOS study group Early-Onset Neonatal Sepsis in the Paris Area: A Population-Based Surveillance Study from 2019 to 2021. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 114–120. [Google Scholar] [CrossRef]
- Dubois, D.; Prasadarao, N.V.; Mittal, R.; Bret, L.; Roujou-Gris, M.; Bonnet, R. CTX-M β-Lactamase Production and Virulence of Escherichia coli K1. Emerg. Infect. Dis. 2009, 15, 1988–1990. [Google Scholar] [CrossRef] [PubMed]
- Wijetunge, D.S.S.; Gongati, S.; DebRoy, C.; Kim, K.S.; Couraud, P.O.; Romero, I.A.; Weksler, B.; Kariyawasam, S. Characterizing the Pathotype of Neonatal Meningitis Causing Escherichia coli (NMEC). BMC Microbiol. 2015, 15, 211. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Birchenough, G.M.H.; Taylor, P.W. Loss of Trefoil Factor 2 Sensitizes Rat Pups to Systemic Infection with the Neonatal Pathogen Escherichia coli K1. Infect. Immun. 2019, 87, e00878-18. [Google Scholar] [CrossRef] [PubMed]
- Witcomb, L.A.; Czupryna, J.; Francis, K.P.; Frankel, G.; Taylor, P.W. Non-Invasive Three-Dimensional Imaging of Escherichia coli K1 Infection Using Diffuse Light Imaging Tomography Combined with Micro-Computed Tomography. Methods 2017, 127, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Herold, R.; Schroten, H.; Schwerk, C. Virulence Factors of Meningitis-Causing Bacteria: Enabling Brain Entry across the Blood–Brain Barrier. Int. J. Mol. Sci. 2019, 20, 5393. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Elliott, S.J.; Di Cello, F.; Stins, M.F.; Kim, K.S. The K1 Capsule Modulates Trafficking of E. Coli-Containing Vacuoles and Enhances Intracellular Bacterial Survival in Human Brain Microvascular Endothelial Cells. Cell. Microbiol. 2003, 5, 245–252. [Google Scholar] [CrossRef]
- Basmaci, R.; Bonacorsi, S.; Bidet, P.; Biran, V.; Aujard, Y.; Bingen, E.; Béchet, S.; Cohen, R.; Levy, C. Escherichia coli Meningitis Features in 325 Children From 2001 to 2013 in France. Clin. Infect. Dis. 2015, 61, 779–786. [Google Scholar] [CrossRef]
- Gu, H.; Liao, Y.; Zhang, J.; Wang, Y.; Liu, Z.; Cheng, P.; Wang, X.; Zou, Q.; Gu, J. Rational Design and Evaluation of an Artificial Escherichia coli K1 Protein Vaccine Candidate Based on the Structure of OmpA. Front. Cell. Infect. Microbiol. 2018, 8, 172. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Gao, C.; Wang, Y.; Cheng, X.; Yang, Y.; Gou, Q.; Lei, L.; Chen, Y.; Wang, X.; et al. Development of a Chitosan-modified PLGA Nanoparticle Vaccine for Protection against Escherichia coli K1 Caused Meningitis in Mice. J. Nanobiotechnol. 2021, 19, 69. [Google Scholar] [CrossRef]
- Westphal, N.; Theis, T.; Loers, G.; Schachner, M.; Kleene, R. Nuclear Fragments of the Neural Cell Adhesion Molecule NCAM with or without Polysialic Acid Differentially Regulate Gene Expression. Sci. Rep. 2017, 7, 13631. [Google Scholar] [CrossRef]
- Birchenough, G.M.H.; Dalgakiran, F.; Witcomb, L.A.; Johansson, M.E.V.; McCarthy, A.J.; Hansson, G.C.; Taylor, P.W. Postnatal Development of the Small Intestinal Mucosa Drives Age-Dependent, Regio-Selective Susceptibility to Escherichia coli K1 Infection. Sci. Rep. 2017, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.J.; Negus, D.; Martin, P.; Pechincha, C.; Oswald, E.; Stabler, R.A.; Taylor, P.W. Pathoadaptive Mutations of Escherichia coli K1 in Experimental Neonatal Systemic Infection. PLoS ONE 2016, 11, e0166793. [Google Scholar] [CrossRef] [PubMed]
- Bioluminescent Imaging Reveals Novel Patterns of Colonization and Invasion in Systemic Escherichia coli K1 Experimental Infection in the Neonatal Rat. Available online: https://journals.asm.org/doi/epdf/10.1128/IAI.00953-15?src=getftr (accessed on 25 March 2023).
- Martindale, J.; Stroud, D.; Moxon, E.R.; Tang, C.M. Genetic Analysis of Escherichia coli K1 Gastrointestinal Colonization. Mol. Microbiol. 2000, 37, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Glode, M.P.; Sutton, A.; Moxon, E.R.; Robbins, J.B. Pathogenesis of Neonatal Escherichia coli Meningitis: Induction of Bacteremia and Meningitis in Infant Rats Fed E. coli K1. Infect. Immun. 1977, 16, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Pluschke, G.; Mercer, A.; Kusećek, B.; Pohl, A.; Achtman, M. Induction of Bacteremia in Newborn Rats by Escherichia coli K1 Is Correlated with Only Certain O (Lipopolysaccharide) Antigen Types. Infect. Immun. 1983, 39, 599–608. [Google Scholar] [CrossRef]
- Huang, S.-H.; He, L.; Zhou, Y.; Wu, C.-H.; Jong, A. Lactobacillus rhamnosus GG Suppresses Meningitic E. coli K1 Penetration across Human Intestinal Epithelial Cells In Vitro and Protects Neonatal Rats against Experimental Hematogenous Meningitis. Int. J. Microbiol. 2008, 2009, e647862. [Google Scholar] [CrossRef]
- Mushtaq, N.; Redpath, M.B.; Luzio, J.P.; Taylor, P.W. Prevention and Cure of Systemic Escherichia coli K1 Infection by Modification of the Bacterial Phenotype. Antimicrob. Agents Chemother. 2004, 48, 1503–1508. [Google Scholar] [CrossRef]
- Laforêt, F.; Antoine, C.; Lebrun, S.; Gonza, I.; Goya-Jorge, E.; Douny, C.; Duprez, J.-N.; Scippo, M.-L.; Taminiau, B.; Daube, G.; et al. Impact Assessment of VB_KpnP_K1-ULIP33 Bacteriophage on the Human Gut Microbiota Using a Dynamic In Vitro Model. Viruses 2023, 15, 719. [Google Scholar] [CrossRef]
- Verthé, K.; Possemiers, S.; Boon, N.; Vaneechoutte, M.; Verstraete, W. Stability and Activity of an Enterobacter Aerogenes-Specific Bacteriophage under Simulated Gastro-Intestinal Conditions. Appl. Microbiol. Biotechnol. 2004, 65, 465–472. [Google Scholar] [CrossRef]
- Federici, S.; Kredo-Russo, S.; Valdés-Mas, R.; Kviatcovsky, D.; Weinstock, E.; Matiuhin, Y.; Silberberg, Y.; Atarashi, K.; Furuichi, M.; Oka, A.; et al. Targeted Suppression of Human IBD-Associated Gut Microbiota Commensals by Phage Consortia for Treatment of Intestinal Inflammation. Cell 2022, 185, 2879–2898.e24. [Google Scholar] [CrossRef]
- Moye, Z.D.; Woolston, J.; Abbeele, P.V.D.; Duysburgh, C.; Verstrepen, L.; Das, C.R.; Marzorati, M.; Sulakvelidze, A. A Bacteriophage Cocktail Eliminates Salmonella Typhimurium from the Human Colonic Microbiome While Preserving Cytokine Signaling and Preventing Attachment to and Invasion of Human Cells by Salmonella In Vitro. J. Food Prot. 2019, 82, 1336–1349. [Google Scholar] [CrossRef]
- Antoine, C.; Laforêt, F.; Blasdel, B.; Fall, A.; Duprez, J.-N.; Mainil, J.; Delcenserie, V.; Thiry, D. In Vitro Characterization and In Vivo Efficacy Assessment in Galleria Mellonella Larvae of Newly Isolated Bacteriophages against Escherichia coli K1. Viruses 2021, 13, 2005. [Google Scholar] [CrossRef]
- Smith, H.W.; Huggins, M.B. Successful Treatment of Experimental Escherichia coli Infections in Mice Using Phage: Its General Superiority over Antibiotics. Microbiology 1982, 128, 307–318. [Google Scholar] [CrossRef]
- Schneider, G.; Szentes, N.; Horváth, M.; Dorn, Á.; Cox, A.; Nagy, G.; Doffkay, Z.; Maróti, G.; Rákhely, G.; Kovács, T. Kinetics of Targeted Phage Rescue in a Mouse Model of Systemic Escherichia coli K1. BioMed Res. Int. 2018, 2018, e7569645. [Google Scholar] [CrossRef]
- Alkeskas, A.; Ogrodzki, P.; Saad, M.; Masood, N.; Rhoma, N.R.; Moore, K.; Farbos, A.; Paszkiewicz, K.; Forsythe, S. The Molecular Characterisation of Escherichia coli K1 Isolated from Neonatal Nasogastric Feeding Tubes. BMC Infect. Dis. 2015, 15, 449. [Google Scholar] [CrossRef]
- Alos, J.I.; Lambert, T.; Courvalin, P. Comparison of Two Molecular Methods for Tracing Nosocomial Transmission of Escherichia coli K1 in a Neonatal Unit. J. Clin. Microbiol. 1993, 31, 1704–1709. [Google Scholar] [CrossRef]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e02071-21. [Google Scholar] [CrossRef]
- Scanlan, J.G.; Hall, A.R.; Scanlan, P.D. Impact of Bile Salts on Coevolutionary Dynamics between the Gut Bacterium Escherichia coli and Its Lytic Phage PP01. Infect. Genet. Evol. 2019, 73, 425–432. [Google Scholar] [CrossRef]
- Mangalea, M.R.; Duerkop, B.A. Fitness Trade-Offs Resulting from Bacteriophage Resistance Potentiate Synergistic Antibacterial Strategies. Infect. Immun. 2020, 88, e00926-19. [Google Scholar] [CrossRef]
- Burmeister, A.R.; Fortier, A.; Roush, C.; Lessing, A.J.; Bender, R.G.; Barahman, R.; Grant, R.; Chan, B.K.; Turner, P.E. Pleiotropy Complicates a Trade-off between Phage Resistance and Antibiotic Resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 11207–11216. [Google Scholar] [CrossRef]
- McGee, L.W.; Barhoush, Y.; Shima, R.; Hennessy, M. Phage-Resistant Mutations Impact Bacteria Susceptibility to Future Phage Infections and Antibiotic Response. Ecol. Evol. 2023, 13, e9712. [Google Scholar] [CrossRef] [PubMed]
- Costea, P.I.; Hildebrand, F.; Arumugam, M.; Bäckhed, F.; Blaser, M.J.; Bushman, F.D.; de Vos, W.M.; Ehrlich, S.D.; Fraser, C.M.; Hattori, M.; et al. Enterotypes in the Landscape of Gut Microbial Community Composition. Nat. Microbiol. 2018, 3, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, N.; Garrido, D. Species Deletions from Microbiome Consortia Reveal Key Metabolic Interactions between Gut Microbes. mSystems 2019, 4, e00185-19. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Goya-Jorge, E.; Gonza, I.; Bondue, P.; Douny, C.; Taminiau, B.; Daube, G.; Scippo, M.-L.; Delcenserie, V. Human Adult Microbiota in a Static Colon Model: AhR Transcriptional Activity at the Crossroads of Host–Microbe Interaction. Foods 2022, 11, 1946. [Google Scholar] [CrossRef]
- Marinelli, L.; Martin-Gallausiaux, C.; Bourhis, J.-M.; Béguet-Crespel, F.; Blottière, H.M.; Lapaque, N. Identification of the Novel Role of Butyrate as AhR Ligand in Human Intestinal Epithelial Cells. Sci. Rep. 2019, 9, 643. [Google Scholar] [CrossRef]
- Modoux, M.; Rolhion, N.; Lefevre, J.H.; Oeuvray, C.; Nádvorník, P.; Illes, P.; Emond, P.; Parc, Y.; Mani, S.; Dvorak, Z.; et al. Butyrate Acts through HDAC Inhibition to Enhance Aryl Hydrocarbon Receptor Activation by Gut Microbiota-Derived Ligands. Gut Microbes 2022, 14, 2105637. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic Distribution of Three Pathways for Propionate Production within the Human Gut Microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef]
- Horvath, T.D.; Ihekweazu, F.D.; Haidacher, S.J.; Ruan, W.; Engevik, K.A.; Fultz, R.; Hoch, K.M.; Luna, R.A.; Oezguen, N.; Spinler, J.K.; et al. Bacteroides Ovatus Colonization Influences the Abundance of Intestinal Short Chain Fatty Acids and Neurotransmitters. iScience 2022, 25, 104158. [Google Scholar] [CrossRef]
- Bortolussi, R.; Ferrier, P. Protection against Escherichia coli K1 Infection in Newborn Rats by Antibody to K1 Capsular Polysaccharide Antigen. Infect. Immun. 1980, 28, 111–117. [Google Scholar] [CrossRef]
- Delannoy, S.; Beutin, L.; Mariani-Kurkdjian, P.; Fleiss, A.; Bonacorsi, S.; Fach, P. The Escherichia coli Serogroup O1 and O2 Lipopolysaccharides Are Encoded by Multiple O-Antigen Gene Clusters. Front. Cell. Infect. Microbiol. 2017, 7, 30. [Google Scholar] [CrossRef]
- Van Twest, R.; Kropinski, A.M. Bacteriophage Enrichment from Water and Soil. In Bacteriophages: Methods and Protocols, Volume 1: Isolation, Characterization, and Interactions; Clokie, M.R.J., Kropinski, A.M., Eds.; Methods in Molecular BiologyTM; Humana Press: Totowa, NJ, USA, 2009; pp. 15–21. ISBN 978-1-60327-164-6. [Google Scholar]
- Van den Abbeele, P.; Grootaert, C.; Marzorati, M.; Possemiers, S.; Verstraete, W.; Gérard, P.; Rabot, S.; Bruneau, A.; El Aidy, S.; Derrien, M.; et al. Microbial Community Development in a Dynamic Gut Model Is Reproducible, Colon Region Specific, and Selective for Bacteroidetes and Clostridium Cluster IX. Appl. Environ. Microbiol. 2010, 76, 5237–5246. [Google Scholar] [CrossRef]
- Van de Wiele, T.; den Abbeele, P.V.; Ossieur, W.; Possemiers, S.; Marzorati, M. The Simulator of the Human Intestinal Microbial Ecosystem (SHIME®). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models [Internet]; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Bacchetti De Gregoris, T.; Aldred, N.; Clare, A.S.; Burgess, J.G. Improvement of Phylum- and Class-Specific Primers for Real-Time PCR Quantification of Bacterial Taxa. J. Microbiol. Methods 2011, 86, 351–356. [Google Scholar] [CrossRef]
- Penders, J.; Vink, C.; Driessen, C.; London, N.; Thijs, C.; Stobberingh, E.E. Quantification of Bifidobacterium Spp., Escherichia coli and Clostridium Difficile in Faecal Samples of Breast-Fed and Formula-Fed Infants by Real-Time PCR. FEMS Microbiol. Lett. 2005, 243, 141–147. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Luo, Y.; Lan, C.; Li, H.; Ouyang, Q.; Kong, F.; Wu, A.; Ren, Z.; Tian, G.; Cai, J.; Yu, B.; et al. Rational Consideration of Akkermansia Muciniphila Targeting Intestinal Health: Advantages and Challenges. npj Biofilms Microbiomes 2022, 8, 81. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Gu, X.; Su, Y.; Zheng, Q.; Yuan, X.; Bao, Z.; Lu, J.; Li, L. Health and Disease: Akkermansia Muciniphila, the Shining Star of the Gut Flora. Research 2023, 6, 0107. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Douny, C.; Dufourny, S.; Brose, F.; Verachtert, P.; Rondia, P.; Lebrun, S.; Marzorati, M.; Everaert, N.; Delcenserie, V.; Scippo, M.-L. Development of an Analytical Method to Detect Short-Chain Fatty Acids by SPME-GC-MS in Samples Coming from an in Vitro Gastrointestinal Model. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1124, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Goya-Jorge, E.; Rampal, C.; Loones, N.; Barigye, S.J.; Carpio, L.E.; Gozalbes, R.; Ferroud, C.; Sylla-Iyarreta Veitía, M.; Giner, R.M. Targeting the Aryl Hydrocarbon Receptor with a Novel Set of Triarylmethanes. Eur. J. Med. Chem. 2020, 207, 112777. [Google Scholar] [CrossRef] [PubMed]




| Groups | 1st Injection | 2nd Injection | |
|---|---|---|---|
| 1 | C5 + ULINTec4 MOI 100 | C5: 106 CFU/10 µL | K1_ULINTec4: 108 PFU/10 µL |
| 2 | C5 + ULINTec4 MOI 10 | C5: 106 CFU/10 µL | K1_ULINTec4: 107 PFU/10 µL |
| 3 | C5 + ULINTec4 MOI 1 | C5: 106 CFU/10 µL | K1_ULINTec4: 106 PFU/10 µL |
| 4 | C5 + PBS | C5: 106 CFU/10 µL | PBS: 10 µL |
| 5 | PBS + ULINTec4 MOI 100 | PBS: 10 µL | K1_ULINTec4: 108 PFU/10 µL |
| 6 | PBS + PBS | PBS: 10 µL | PBS: 10 µL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoine, C.; Laforêt, F.; Goya-Jorge, E.; Gonza, I.; Lebrun, S.; Douny, C.; Duprez, J.-N.; Fall, A.; Taminiau, B.; Scippo, M.-L.; et al. Phage Targeting Neonatal Meningitis E. coli K1 In Vitro in the Intestinal Microbiota of Pregnant Donors and Impact on Bacterial Populations. Int. J. Mol. Sci. 2023, 24, 10580. https://doi.org/10.3390/ijms241310580
Antoine C, Laforêt F, Goya-Jorge E, Gonza I, Lebrun S, Douny C, Duprez J-N, Fall A, Taminiau B, Scippo M-L, et al. Phage Targeting Neonatal Meningitis E. coli K1 In Vitro in the Intestinal Microbiota of Pregnant Donors and Impact on Bacterial Populations. International Journal of Molecular Sciences. 2023; 24(13):10580. https://doi.org/10.3390/ijms241310580
Chicago/Turabian StyleAntoine, Céline, Fanny Laforêt, Elizabeth Goya-Jorge, Irma Gonza, Sarah Lebrun, Caroline Douny, Jean-Noël Duprez, Abdoulaye Fall, Bernard Taminiau, Marie-Louise Scippo, and et al. 2023. "Phage Targeting Neonatal Meningitis E. coli K1 In Vitro in the Intestinal Microbiota of Pregnant Donors and Impact on Bacterial Populations" International Journal of Molecular Sciences 24, no. 13: 10580. https://doi.org/10.3390/ijms241310580
APA StyleAntoine, C., Laforêt, F., Goya-Jorge, E., Gonza, I., Lebrun, S., Douny, C., Duprez, J.-N., Fall, A., Taminiau, B., Scippo, M.-L., Daube, G., Thiry, D., & Delcenserie, V. (2023). Phage Targeting Neonatal Meningitis E. coli K1 In Vitro in the Intestinal Microbiota of Pregnant Donors and Impact on Bacterial Populations. International Journal of Molecular Sciences, 24(13), 10580. https://doi.org/10.3390/ijms241310580







