Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options
Abstract
1. Introduction
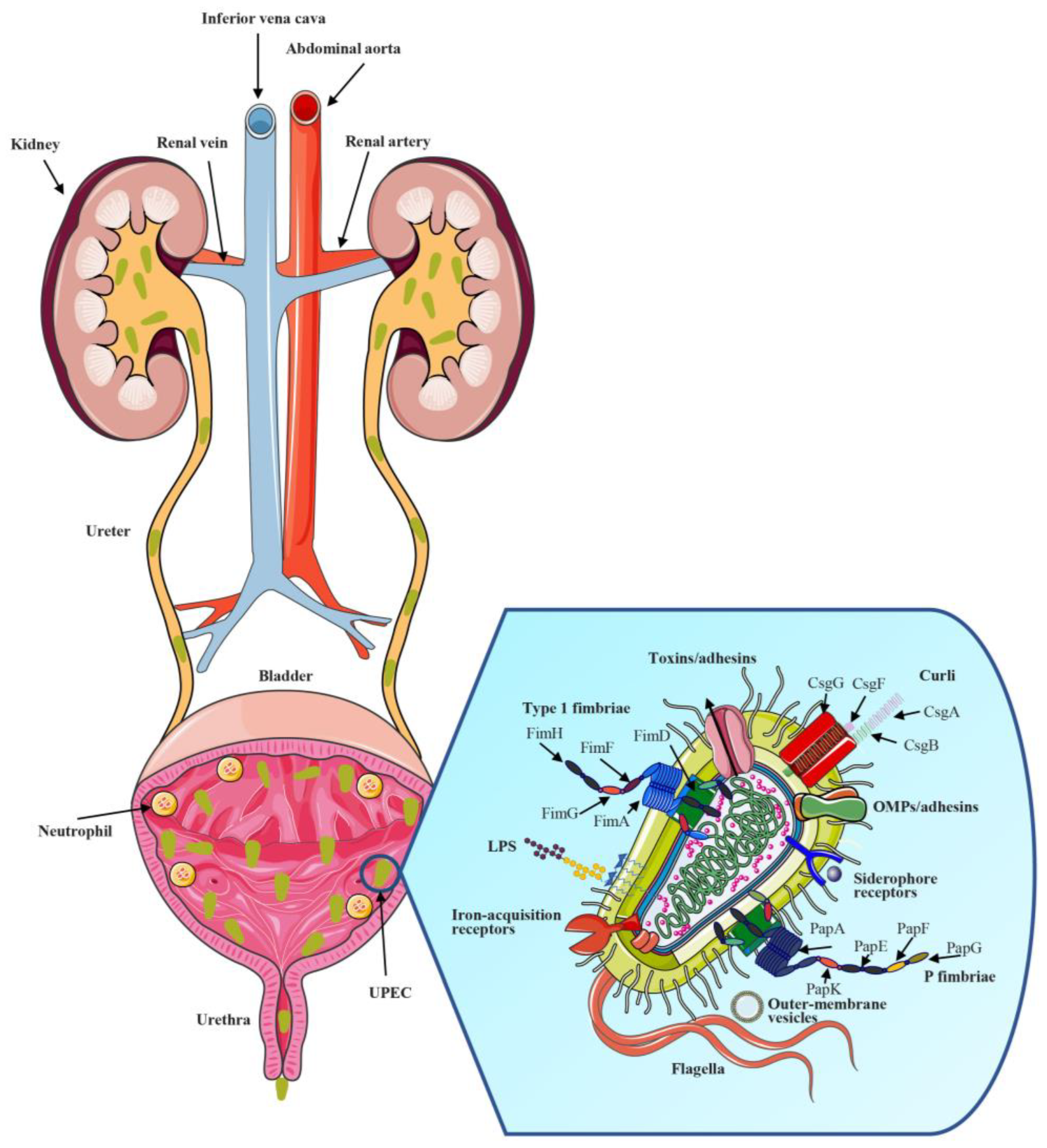
2. Mechanisms of UPEC UTIs
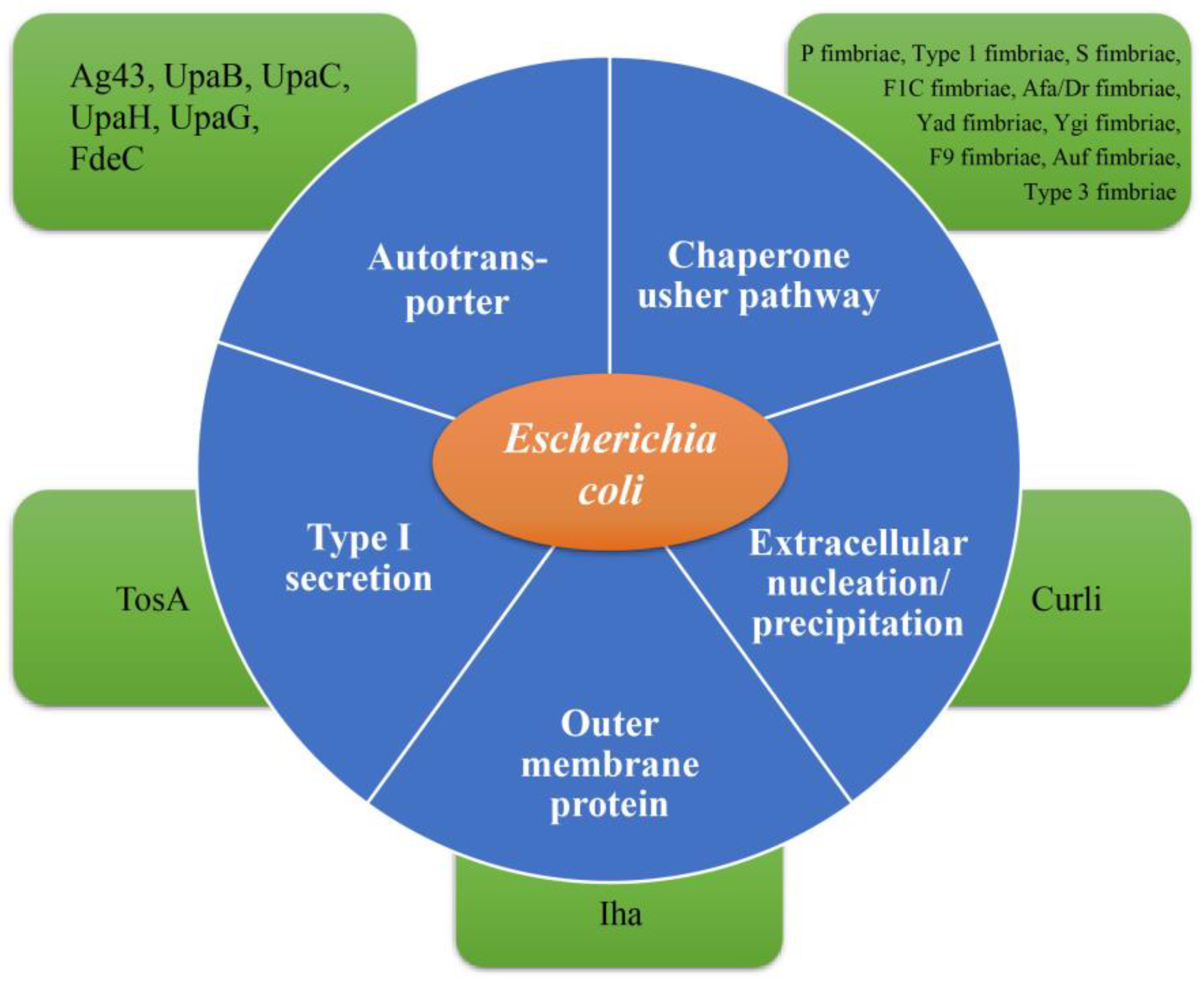
2.1. UPEC Adhesins
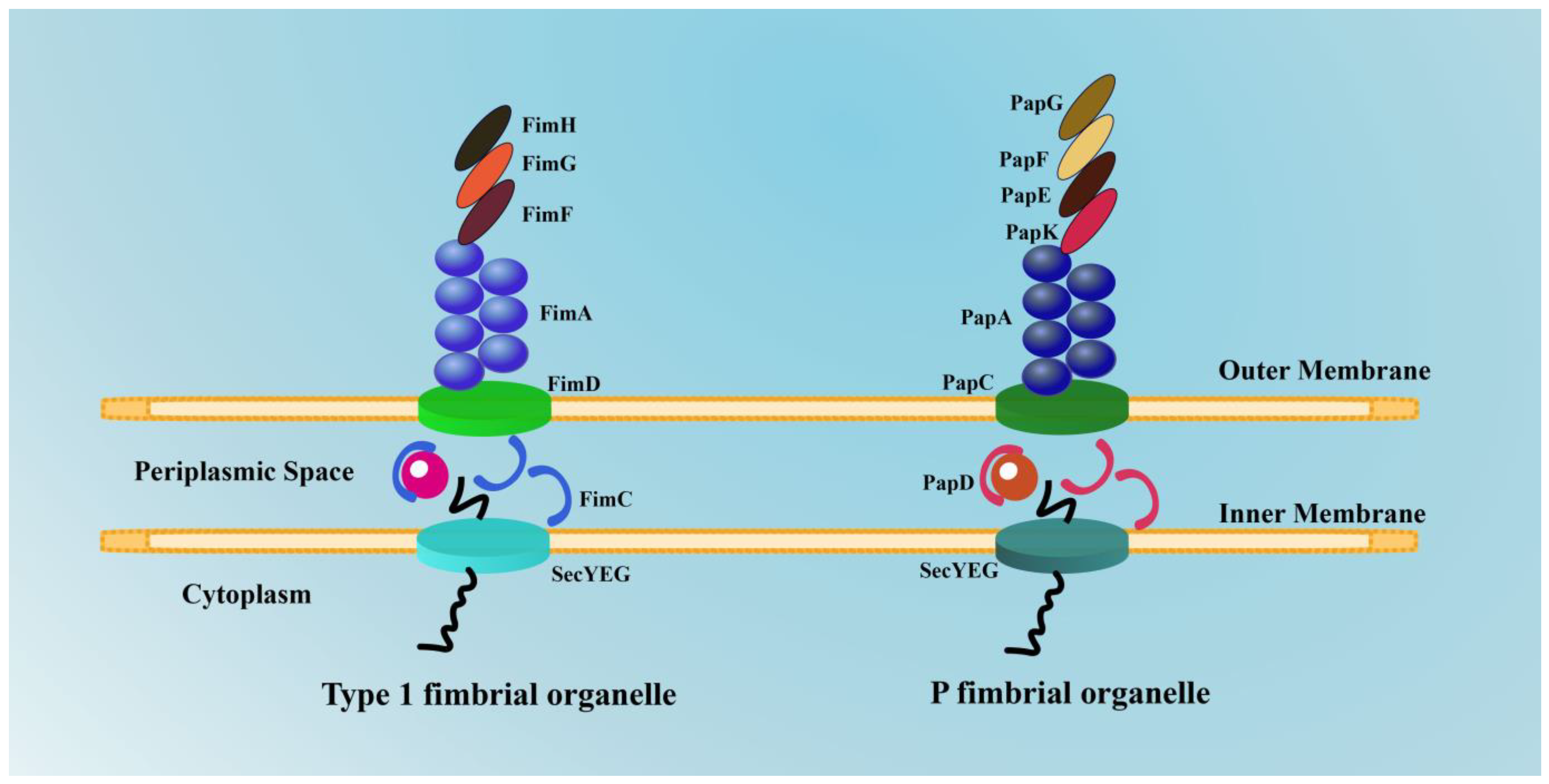
2.1.1. Type 1 Fimbriae and FimH
2.1.2. P Fimbriae and PapG
2.1.3. Other Fimbriae and Non-Fimbrial Adhesins
2.2. Flagella-Mediated Motility
2.3. Toxins
2.4. Metal Acquisition
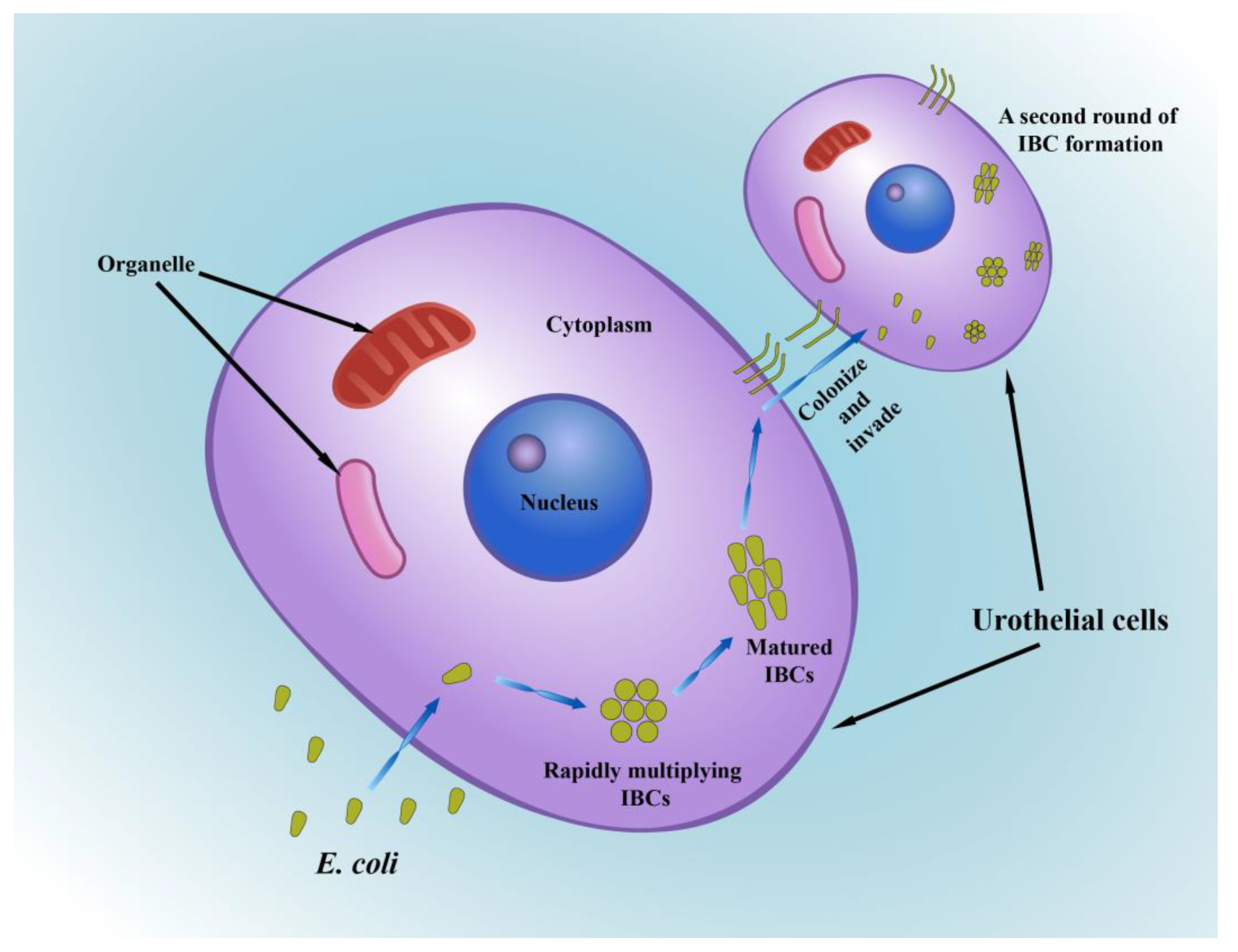
2.5. Intracellular Bacterial Communities (IBCs)
2.6. Strategies for Evading Host Defenses
3. Treatment of Urinary Tract Infections
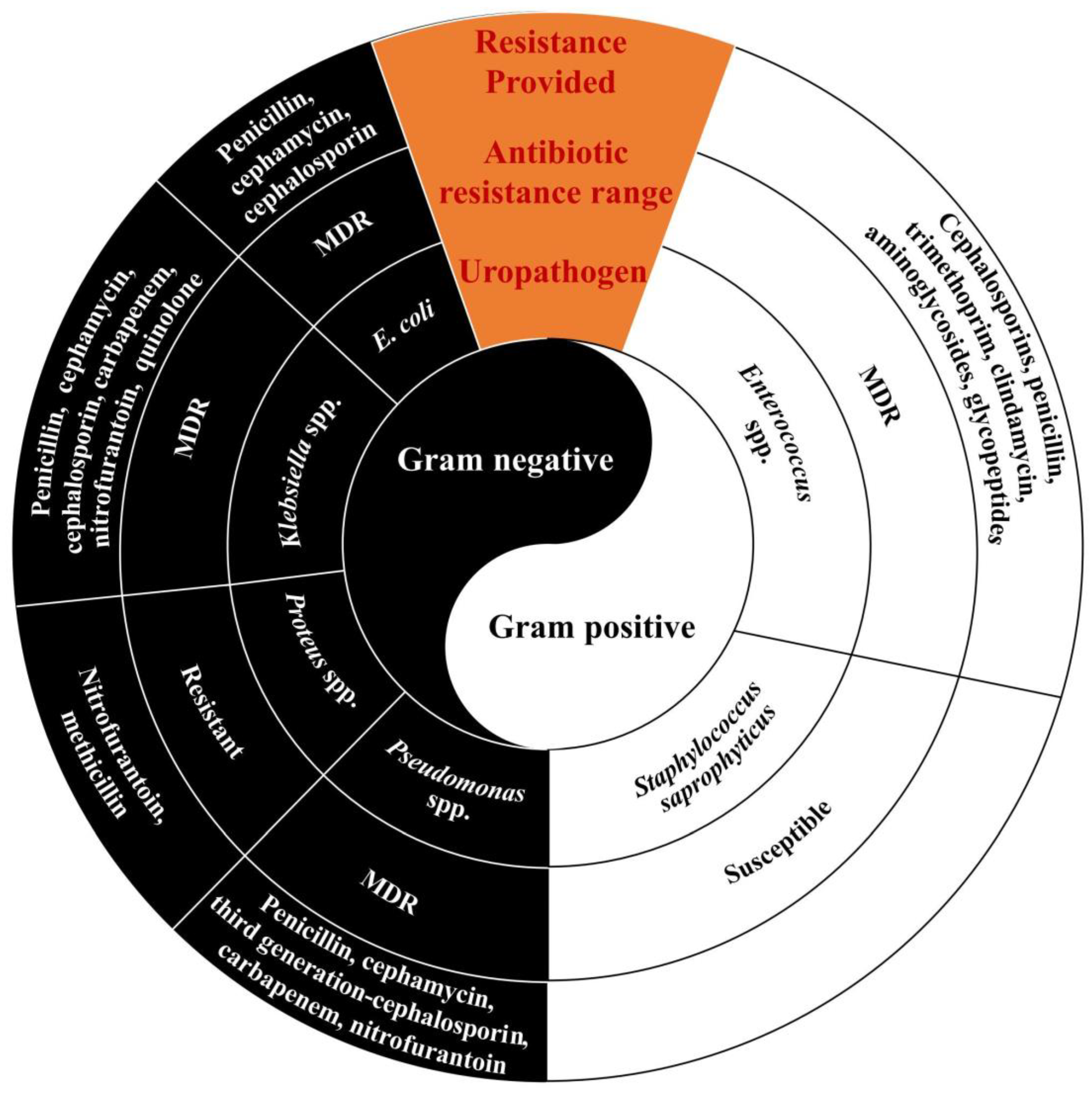
3.1. Antibiotics and Multidrug Resistance
3.2. Preventative Strategies and Alternative Therapeutics for Urinary Tract Infections
3.2.1. Vaccines
3.2.2. Medicinal Plants for the Management and Treatment of Urinary Tract Infections
4. Conclusion and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, V.P.; Hannan, T.J.; Nielsen, H.V.; Hultgren, S.J. Drug and Vaccine Development for the Treatment and Prevention of Urinary Tract Infections. Microbiol. Spectr. 2016, 4, 589–646. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tandogdu, Z.; Wagenlehner, F.M. Global epidemiology of urinary tract infections. Curr. Opin. Infect. Dis. 2016, 29, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Tullus, K.; Shaikh, N. Urinary tract infections in children. Lancet 2020, 395, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Liska, D.; Talan, D.; Chung, M. Cranberry Reduces the Risk of Urinary Tract Infection Recurrence in Otherwise Healthy Women: A Systematic Review and Meta-Analysis. J. Nutr. 2017, 147, 2282–2288. [Google Scholar] [CrossRef]
- Anger, J.; Lee, U.; Ackerman, A.L.; Chou, R.; Chughtai, B.; Clemens, J.Q.; Hickling, D.; Kapoor, A.; Kenton, K.S.; Kaufman, M.R.; et al. Recurrent Uncomplicated Urinary Tract Infections in Women: AUA/CUA/SUFU Guideline. J. Urol. 2019, 202, 282–289. [Google Scholar] [CrossRef]
- Schneeberger, C.; Geerlings, S.E.; Middleton, P.; Crowther, C.A. Interventions for preventing recurrent urinary tract infection during pregnancy. Cochrane Database Syst. Rev. 2015, 2015, Cd009279. [Google Scholar] [CrossRef]
- Matthews, S.J.; Lancaster, J.W. Urinary tract infections in the elderly population. Am. J. Geriatr. Pharmacother. 2011, 9, 286–309. [Google Scholar] [CrossRef]
- Jung, C.; Brubaker, L. The etiology and management of recurrent urinary tract infections in postmenopausal women. Climacteric 2019, 22, 242–249. [Google Scholar] [CrossRef]
- Becknell, B.; Schober, M.; Korbel, L.; Spencer, J.D. The diagnosis, evaluation and treatment of acute and recurrent pediatric urinary tract infections. Expert Rev. Anti-Infect. Ther. 2015, 13, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.; Silva, A.C.; Oliveira, E.A.; Mak, R.H. Urinary tract infection in pediatrics: An overview. J. Pediatr. (Rio. J.) 2020, 96, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Keren, R.; Shaikh, N.; Pohl, H.; Gravens-Mueller, L.; Ivanova, A.; Zaoutis, L.; Patel, M.; de Berardinis, R.; Parker, A.; Bhatnagar, S.; et al. Risk Factors for Recurrent Urinary Tract Infection and Renal Scarring. Pediatrics 2015, 136, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, T.K.; Shaikh, N.; Nelson, C.P. Contemporary Management of Urinary Tract Infection in Children. Pediatrics 2021, 147, e2020012138. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Schreiber, H.L.T.; Hooton, T.M.; Hultgren, S.J. From physiology to pharmacy: Developments in the pathogenesis and treatment of recurrent urinary tract infections. Curr. Urol. Rep. 2013, 14, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Hannan, T.J.; Totsika, M.; Mansfield, K.J.; Moore, K.H.; Schembri, M.A.; Hultgren, S.J. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol. Rev. 2012, 36, 616–648. [Google Scholar] [CrossRef] [PubMed]
- Nielubowicz, G.R.; Mobley, H.L. Host-pathogen interactions in urinary tract infection. Nat. Rev. Urol. 2010, 7, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Dhakal, B.K.; Kulesus, R.R.; Mulvey, M.A. Mechanisms and consequences of bladder cell invasion by uropathogenic Escherichia coli. Eur. J. Clin. Investig. 2008, 38, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, C.N.; Hultgren, S.J. Adhesive Pili in UTI Pathogenesis and Drug Development. Pathogens 2016, 5, 30. [Google Scholar] [CrossRef]
- Totsika, M.; Moriel, D.G.; Idris, A.; Rogers, B.A.; Wurpel, D.J.; Phan, M.D.; Paterson, D.L.; Schembri, M.A. Uropathogenic Escherichia coli mediated urinary tract infection. Curr. Drug Targets 2012, 13, 1386–1399. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, J.; Srivastava, S.; Singh, M. Pathogenomics of uropathogenic Escherichia coli. Indian J. Med. Microbiol. 2012, 30, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ribić, R.; Meštrović, T.; Neuberg, M.; Kozina, G. Effective anti-adhesives of uropathogenic Escherichia coli. Acta Pharm. 2018, 68, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pakharukova, N.; McKenna, S.; Tuittila, M.; Paavilainen, S.; Malmi, H.; Xu, Y.; Parilova, O.; Matthews, S.; Zavialov, A.V. Archaic and alternative chaperones preserve pilin folding energy by providing incomplete structural information. J. Biol. Chem. 2018, 293, 17070–17080. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, M.J.; Heuser, J.; Normark, S.; Hultgren, S.J. P pili in uropathogenic E. coli are composite fibres with distinct fibrillar adhesive tips. Nature 1992, 356, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Phan, G.; Remaut, H.; Wang, T.; Allen, W.J.; Pirker, K.F.; Lebedev, A.; Henderson, N.S.; Geibel, S.; Volkan, E.; Yan, J.; et al. Crystal structure of the FimD usher bound to its cognate FimC-FimH substrate. Nature 2011, 474, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Waksman, G. Structural and Molecular Biology of a Protein-Polymerizing Nanomachine for Pilus Biogenesis. J. Mol. Biol. 2017, 429, 2654–2666. [Google Scholar] [CrossRef]
- Chen, S.L.; Hung, C.S.; Xu, J.; Reigstad, C.S.; Magrini, V.; Sabo, A.; Blasiar, D.; Bieri, T.; Meyer, R.R.; Ozersky, P.; et al. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: A comparative genomics approach. Proc. Natl. Acad. Sci. USA 2006, 103, 5977–5982. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P. Classical chaperone-usher (CU) adhesive fimbriome: Uropathogenic Escherichia coli (UPEC) and urinary tract infections (UTIs). Folia Microbiol. 2020, 65, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.L.; Beatson, S.A.; Totsika, M.; Forestier, C.; McEwan, A.G.; Schembri, M.A. Molecular analysis of type 3 fimbrial genes from Escherichia coli, Klebsiella and Citrobacter species. BMC Microbiol. 2010, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Zavialov, A.; Zav’yalova, G.; Korpela, T.; Zav’yalov, V. FGL chaperone-assembled fimbrial polyadhesins: Anti-immune armament of Gram-negative bacterial pathogens. FEMS Microbiol. Rev. 2007, 31, 478–514. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Mo, W.J.; Sebbel, P.; Min, G.; Neubert, T.A.; Glockshuber, R.; Wu, X.R.; Sun, T.T.; Kong, X.P. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: Evidence from in vitro FimH binding. J. Cell Sci. 2001, 114, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Eto, D.S.; Jones, T.A.; Sundsbak, J.L.; Mulvey, M.A. Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog. 2007, 3, e100. [Google Scholar] [CrossRef] [PubMed]
- Mossman, K.L.; Mian, M.F.; Lauzon, N.M.; Gyles, C.L.; Lichty, B.; Mackenzie, R.; Gill, N.; Ashkar, A.A. Cutting edge: FimH adhesin of type 1 fimbriae is a novel TLR4 ligand. J. Immunol. 2008, 181, 6702–6706. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, E.; Behzadi, P. The role of toll-like receptors (TLRs) in urinary tract infections (UTIs). Cent. Eur. J. Urol. 2016, 69, 404–410. [Google Scholar]
- Spaulding, C.N.; Klein, R.D.; Ruer, S.; Kau, A.L.; Schreiber, H.L.; Cusumano, Z.T.; Dodson, K.W.; Pinkner, J.S.; Fremont, D.H.; Janetka, J.W.; et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 2017, 546, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Lund, B.; Lindberg, F.; Marklund, B.I.; Normark, S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 1987, 84, 5898–5902. [Google Scholar] [CrossRef]
- Klemm, P.; Hancock, V.; Schembri, M.A. Fimbrial adhesins from extraintestinal Escherichia coli. Environ. Microbiol. Rep. 2010, 2, 628–640. [Google Scholar] [CrossRef]
- Langermann, S.; Möllby, R.; Burlein, J.E.; Palaszynski, S.R.; Auguste, C.G.; DeFusco, A.; Strouse, R.; Schenerman, M.A.; Hultgren, S.J.; Pinkner, J.S.; et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 2000, 181, 774–778. [Google Scholar] [CrossRef]
- Wright, K.J.; Seed, P.C.; Hultgren, S.J. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol. 2007, 9, 2230–2241. [Google Scholar] [CrossRef]
- Schaeffer, A.J. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. J. Urol. 2003, 170, 335. [Google Scholar] [CrossRef]
- Martinez, J.J.; Mulvey, M.A.; Schilling, J.D.; Pinkner, J.S.; Hultgren, S.J. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000, 19, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.J.; Hultgren, S.J. Sticky fibers and uropathogenesis: Bacterial adhesins in the urinary tract. Future Microbiol. 2006, 1, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Sarowar, S.; Hu, O.J.; Werneburg, G.T.; Thanassi, D.G.; Li, H. The Escherichia coli P and Type 1 Pilus Assembly Chaperones PapD and FimC Are Monomeric in Solution. J. Bacteriol. 2016, 198, 2360–2369. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, Z.; De la Cruz, M.A.; Carrillo-Casas, E.M.; Durán, L.; Zhang, Y.; Hernández-Castro, R.; Puente, J.L.; Daaka, Y.; Girón, J.A. Production of the Escherichia coli common pilus by uropathogenic E. coli is associated with adherence to HeLa and HTB-4 cells and invasion of mouse bladder urothelium. PLoS ONE 2014, 9, e101200. [Google Scholar] [CrossRef] [PubMed]
- Sidaway, P. Infection: Exposure to human urine decreases type 1 pili expression in uropathogenic Escherichia coli. Nat. Rev. Urol. 2015, 12, 422. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.E.; Hibbing, M.E.; Janetka, J.; Chen, S.L.; Hultgren, S.J. Human Urine Decreases Function and Expression of Type 1 Pili in Uropathogenic Escherichia coli. mBio 2015, 6, e00820. [Google Scholar] [CrossRef] [PubMed]
- Zav’yalov, V.; Zavialov, A.; Zav’yalova, G.; Korpela, T. Adhesive organelles of Gram-negative pathogens assembled with the classical chaperone/usher machinery: Structure and function from a clinical standpoint. FEMS Microbiol. Rev. 2010, 34, 317–378. [Google Scholar] [CrossRef] [PubMed]
- Conover, M.S.; Ruer, S.; Taganna, J.; Kalas, V.; De Greve, H.; Pinkner, J.S.; Dodson, K.W.; Remaut, H.; Hultgren, S.J. Inflammation-Induced Adhesin-Receptor Interaction Provides a Fitness Advantage to Uropathogenic, E. coli during Chronic Infection. Cell Host Microbe 2016, 20, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Chahales, P.; Thanassi, D.G. Structure, Function, and Assembly of Adhesive Organelles by Uropathogenic Bacteria. Microbiol. Spectr. 2015, 3, 277–329. [Google Scholar] [CrossRef]
- Thumbikat, P.; Berry, R.E.; Zhou, G.; Billips, B.K.; Yaggie, R.E.; Zaichuk, T.; Sun, T.T.; Schaeffer, A.J.; Klumpp, D.J. Bacteria-induced uroplakin signaling mediates bladder response to infection. PLoS Pathog. 2009, 5, e1000415. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Min, G.; Glockshuber, R.; Sun, T.T.; Kong, X.P. Uropathogenic, E. coli adhesin-induced host cell receptor conformational changes: Implications in transmembrane signaling transduction. J. Mol. Biol. 2009, 392, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Eto, D.S.; Gordon, H.B.; Dhakal, B.K.; Jones, T.A.; Mulvey, M.A. Clathrin, AP-2, and the NPXY-binding subset of alternate endocytic adaptors facilitate FimH-mediated bacterial invasion of host cells. Cell Microbiol. 2008, 10, 2553–2567. [Google Scholar] [CrossRef] [PubMed]
- Schembri, M.A.; Sokurenko, E.V.; Klemm, P. Functional flexibility of the FimH adhesin: Insights from a random mutant library. Infect. Immun. 2000, 68, 2638–2646. [Google Scholar] [CrossRef] [PubMed]
- Sokurenko, E.V.; Courtney, H.S.; Maslow, J.; Siitonen, A.; Hasty, D.L. Quantitative differences in adhesiveness of type 1 fimbriated Escherichia coli due to structural differences in fimH genes. J. Bacteriol. 1995, 177, 3680–3686. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Hung, C.S.; Pinkner, J.S.; Walker, J.N.; Cusumano, C.K.; Li, Z.; Bouckaert, J.; Gordon, J.I.; Hultgren, S.J. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc. Natl. Acad. Sci. USA 2009, 106, 22439–22444. [Google Scholar] [CrossRef]
- Biggel, M.; Xavier, B.B.; Johnson, J.R.; Nielsen, K.L.; Frimodt-Møller, N.; Matheeussen, V.; Goossens, H.; Moons, P.; Van Puyvelde, S. Horizontally acquired papGII-containing pathogenicity islands underlie the emergence of invasive uropathogenic Escherichia coli lineages. Nat. Commun. 2020, 11, 5968. [Google Scholar] [CrossRef]
- Bessaiah, H.; Pokharel, P.; Loucif, H.; Kulbay, M.; Sasseville, C.; Habouria, H.; Houle, S.; Bernier, J.; Massé, É.; Van Grevenynghe, J.; et al. The RyfA small RNA regulates oxidative and osmotic stress responses and virulence in uropathogenic Escherichia coli. PLoS Pathog. 2021, 17, e1009617. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.B. Urinary tract infection: Clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011, 128, 595–610. [Google Scholar] [CrossRef]
- Norinder, B.S.; Köves, B.; Yadav, M.; Brauner, A.; Svanborg, C. Do Escherichia coli strains causing acute cystitis have a distinct virulence repertoire? Microb. Pathog. 2012, 52, 10–16. [Google Scholar] [CrossRef]
- O’Brien, V.P.; Hannan, T.J.; Schaeffer, A.J.; Hultgren, S.J. Are you experienced? Understanding bladder innate immunity in the context of recurrent urinary tract infection. Curr. Opin. Infect. Dis. 2015, 28, 97–105. [Google Scholar] [CrossRef]
- Ishitoya, S.; Yamamoto, S.; Mitsumori, K.; Ogawa, O.; Terai, A. Non-secretor status is associated with female acute uncomplicated pyelonephritis. BJU Int. 2002, 89, 851–854. [Google Scholar] [CrossRef]
- Sauer, F.G.; Mulvey, M.A.; Schilling, J.D.; Martinez, J.J.; Hultgren, S.J. Bacterial pili: Molecular mechanisms of pathogenesis. Curr. Opin. Microbiol. 2000, 3, 65–72. [Google Scholar] [CrossRef]
- La Combe, B.; Clermont, O.; Messika, J.; Eveillard, M.; Kouatchet, A.; Lasocki, S.; Corvec, S.; Lakhal, K.; Billard-Pomares, T.; Fernandes, R.; et al. Pneumonia-Specific Escherichia coli with Distinct Phylogenetic and Virulence Profiles, France, 2012–2014. Emerg. Infect. Dis. 2019, 25, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, G.T.; Thanassi, D.G. Pili Assembled by the Chaperone/Usher Pathway in Escherichia coli and Salmonella. EcoSal Plus 2018, 8, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.C.; Mobley, H.L. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 2007, 72, 19–25. [Google Scholar] [CrossRef]
- Mobley, H.L.; Jarvis, K.G.; Elwood, J.P.; Whittle, D.I.; Lockatell, C.V.; Russell, R.G.; Johnson, D.E.; Donnenberg, M.S.; Warren, J.W. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: The role of alpha Gal(1-4) beta Gal binding in virulence of a wild-type strain. Mol. Microbiol. 1993, 10, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.P.; Yu, M.; Xu, H.; Haslam, D.B. Forssman synthetase expression results in diminished shiga toxin susceptibility: A role for glycolipids in determining host-microbe interactions. Infect. Immun. 2003, 71, 6543–6552. [Google Scholar] [CrossRef] [PubMed]
- Melican, K.; Sandoval, R.M.; Kader, A.; Josefsson, L.; Tanner, G.A.; Molitoris, B.A.; Richter-Dahlfors, A. Uropathogenic Escherichia coli P and Type 1 fimbriae act in synergy in a living host to facilitate renal colonization leading to nephron obstruction. PLoS Pathog. 2011, 7, e1001298. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Alsén, B.; Roche, N.; Angström, J.; von Euler, A.; Breimer, M.E.; Westerlund-Wikström, B.; Teneberg, S.; Richter-Dahlfors, A. Identification of target tissue glycosphingolipid receptors for uropathogenic, F1C-fimbriated Escherichia coli and its role in mucosal inflammation. J. Biol. Chem. 2002, 277, 18198–18205. [Google Scholar] [CrossRef] [PubMed]
- Selvarangan, R.; Goluszko, P.; Singhal, J.; Carnoy, C.; Moseley, S.; Hudson, B.; Nowicki, S.; Nowicki, B. Interaction of Dr adhesin with collagen type IV is a critical step in Escherichia coli renal persistence. Infect. Immun. 2004, 72, 4827–4835. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Hu, F.; Wu, S.; Ye, X.; Zhu, D.; Zhang, Y.; Wang, M. Comparison of adhesin genes and antimicrobial susceptibilities between uropathogenic and intestinal commensal Escherichia coli strains. PLoS ONE 2013, 8, e61169. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.; Mobley, H.L.T. Virulence and Fitness Determinants of Uropathogenic Escherichia coli. Microbiol. Spectr. 2015, 3, 235–261. [Google Scholar] [CrossRef] [PubMed]
- Wurpel, D.J.; Totsika, M.; Allsopp, L.P.; Hartley-Tassell, L.E.; Day, C.J.; Peters, K.M.; Sarkar, S.; Ulett, G.C.; Yang, J.; Tiralongo, J.; et al. F9 fimbriae of uropathogenic Escherichia coli are expressed at low temperature and recognise Galβ1-3GlcNAc-containing glycans. PLoS ONE 2014, 9, e93177. [Google Scholar] [CrossRef] [PubMed]
- Spurbeck, R.R.; Stapleton, A.E.; Johnson, J.R.; Walk, S.T.; Hooton, T.M.; Mobley, H.L. Fimbrial profiles predict virulence of uropathogenic Escherichia coli strains: Contribution of ygi and yad fimbriae. Infect. Immun. 2011, 79, 4753–4763. [Google Scholar] [CrossRef]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef]
- Wiles, T.J.; Kulesus, R.R.; Mulvey, M.A. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp. Mol. Pathol. 2008, 85, 11–19. [Google Scholar] [CrossRef]
- Vigil, P.D.; Alteri, C.J.; Mobley, H.L. Identification of in vivo-induced antigens including an RTX family exoprotein required for uropathogenic Escherichia coli virulence. Infect. Immun. 2011, 79, 2335–2344. [Google Scholar] [CrossRef]
- Vigil, P.D.; Stapleton, A.E.; Johnson, J.R.; Hooton, T.M.; Hodges, A.P.; He, Y.; Mobley, H.L. Presence of putative repeat-in-toxin gene tosA in Escherichia coli predicts successful colonization of the urinary tract. mBio 2011, 2, e00066-11. [Google Scholar] [CrossRef]
- Engstrom, M.D.; Alteri, C.J.; Mobley, H.L. A conserved PapB family member, TosR, regulates expression of the uropathogenic Escherichia coli RTX nonfimbrial adhesin TosA while conserved LuxR family members TosE and TosF suppress motility. Infect. Immun. 2014, 82, 3644–3656. [Google Scholar] [CrossRef] [PubMed]
- Nesta, B.; Spraggon, G.; Alteri, C.; Moriel, D.G.; Rosini, R.; Veggi, D.; Smith, S.; Bertoldi, I.; Pastorello, I.; Ferlenghi, I.; et al. FdeC, a novel broadly conserved Escherichia coli adhesin eliciting protection against urinary tract infections. mBio 2012, 3, e00010-12. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Mabbett, A.N.; Ulett, G.C.; Toledo-Arana, A.; Wecker, K.; Totsika, M.; Schembri, M.A.; Ghigo, J.M.; Beloin, C. UpaG, a new member of the trimeric autotransporter family of adhesins in uropathogenic Escherichia coli. J. Bacteriol. 2008, 190, 4147–4161. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Jelacic, S.; Schoening, L.M.; Clabots, C.; Shaikh, N.; Mobley, H.L.; Tarr, P.I. The IrgA homologue adhesin Iha is an Escherichia coli virulence factor in murine urinary tract infection. Infect. Immun. 2005, 73, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, M.M.; Chapman, M.R. Curli biogenesis and function. Annu. Rev. Microbiol. 2006, 60, 131–147. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, H.W.; Choi, Y.W.; Kim, S.H.; Lee, J.C.; Lee, Y.C.; Seol, S.Y.; Cho, D.T.; Kim, J. Involvement of curli fimbriae in the biofilm formation of Enterobacter cloacae. J. Microbiol. 2012, 50, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, L.K.; de Anda, J.; Jain, N.; Grando, K.C.M.; Miller, A.L.; Bessho, S.; Gallucci, S.; Wong, G.C.L.; Tükel, Ç. Assembly of ordered DNA-curli fibril complexes during Salmonella biofilm formation correlates with strengths of the type I interferon and autoimmune responses. PLoS Pathog. 2022, 18, e1010742. [Google Scholar] [CrossRef]
- Tükel, C.; Raffatellu, M.; Humphries, A.D.; Wilson, R.P.; Andrews-Polymenis, H.L.; Gull, T.; Figueiredo, J.F.; Wong, M.H.; Michelsen, K.S.; Akçelik, M.; et al. CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol. Microbiol. 2005, 58, 289–304. [Google Scholar] [CrossRef]
- Bian, Z.; Brauner, A.; Li, Y.; Normark, S. Expression of and cytokine activation by Escherichia coli curli fibers in human sepsis. J. Infect. Dis. 2000, 181, 602–612. [Google Scholar] [CrossRef]
- Kai-Larsen, Y.; Lüthje, P.; Chromek, M.; Peters, V.; Wang, X.; Holm, A.; Kádas, L.; Hedlund, K.O.; Johansson, J.; Chapman, M.R.; et al. Uropathogenic Escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37. PLoS Pathog. 2010, 6, e1001010. [Google Scholar] [CrossRef]
- Lane, M.C.; Lockatell, V.; Monterosso, G.; Lamphier, D.; Weinert, J.; Hebel, J.R.; Johnson, D.E.; Mobley, H.L. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect. Immun. 2005, 73, 7644–7656. [Google Scholar] [CrossRef]
- Wright, K.J.; Seed, P.C.; Hultgren, S.J. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 2005, 73, 7657–7668. [Google Scholar] [CrossRef] [PubMed]
- Simms, A.N.; Mobley, H.L. PapX, a P fimbrial operon-encoded inhibitor of motility in uropathogenic Escherichia coli. Infect. Immun. 2008, 76, 4833–4841. [Google Scholar] [CrossRef] [PubMed]
- Girón, J.A.; Torres, A.G.; Freer, E.; Kaper, J.B. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 2002, 44, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yamamoto, N.; Kino, Y.; Yamamoto, N.; Kamei, S.; Mori, H.; Kurokawa, K.; Nakashima, N. Establishment of a multi-species biofilm model and metatranscriptomic analysis of biofilm and planktonic cell communities. Appl. Microbiol. Biotechnol. 2016, 100, 7263–7279. [Google Scholar] [CrossRef]
- Lane, M.C.; Alteri, C.J.; Smith, S.N.; Mobley, H.L. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc. Natl. Acad. Sci. USA 2007, 104, 16669–16674. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.A.; Pinkner, J.S.; Jones, J.M.; Walker, J.N.; Clegg, S.; Hultgren, S.J. Utilization of an intracellular bacterial community pathway in Klebsiella pneumoniae urinary tract infection and the effects of FimK on type 1 pilus expression. Infect. Immun. 2008, 76, 3337–3345. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, P.; Laestadius, A.; Jahnukainen, T.; Söderblom, T.; Bäckhed, F.; Celsi, G.; Brismar, H.; Normark, S.; Aperia, A.; Richter-Dahlfors, A. Alpha-haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature 2000, 405, 694–697. [Google Scholar] [CrossRef]
- Nagamatsu, K.; Hannan, T.J.; Guest, R.L.; Kostakioti, M.; Hadjifrangiskou, M.; Binkley, J.; Dodson, K.; Raivio, T.L.; Hultgren, S.J. Dysregulation of Escherichia coli α-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc. Natl. Acad. Sci. USA 2015, 112, E871–E880. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, M.; Landraud, L.; Lemichez, E. Rho GTPase-activating bacterial toxins: From bacterial virulence regulation to eukaryotic cell biology. FEMS Microbiol. Rev. 2007, 31, 515–534. [Google Scholar] [CrossRef]
- Petracchini, S.; Hamaoui, D.; Doye, A.; Asnacios, A.; Fage, F.; Vitiello, E.; Balland, M.; Janel, S.; Lafont, F.; Gupta, M.; et al. Optineurin links Hace1-dependent Rac ubiquitylation to integrin-mediated mechanotransduction to control bacterial invasion and cell division. Nat. Commun. 2022, 13, 6059. [Google Scholar] [CrossRef]
- Hofman, P.; Le Negrate, G.; Mograbi, B.; Hofman, V.; Brest, P.; Alliana-Schmid, A.; Flatau, G.; Boquet, P.; Rossi, B. Escherichia coli cytotoxic necrotizing factor-1 (CNF-1) increases the adherence to epithelia and the oxidative burst of human polymorphonuclear leukocytes but decreases bacteria phagocytosis. J. Leukoc. Biol. 2000, 68, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The Diversity of Escherichia coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Heimer, S.R.; Rasko, D.A.; Lockatell, C.V.; Johnson, D.E.; Mobley, H.L. Autotransporter genes pic and tsh are associated with Escherichia coli strains that cause acute pyelonephritis and are expressed during urinary tract infection. Infect. Immun. 2004, 72, 593–597. [Google Scholar] [CrossRef]
- Guyer, D.M.; Radulovic, S.; Jones, F.E.; Mobley, H.L. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect. Immun. 2002, 70, 4539–4546. [Google Scholar] [CrossRef] [PubMed]
- Guyer, D.M.; Henderson, I.R.; Nataro, J.P.; Mobley, H.L. Identification of sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 2000, 38, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.R.; Cappello, R.; Navarro-García, F.; Nataro, J.P. Functional comparison of serine protease autotransporters of enterobacteriaceae. Infect. Immun. 2002, 70, 7105–7113. [Google Scholar] [CrossRef] [PubMed]
- Liévin-Le Moal, V.; Comenge, Y.; Ruby, V.; Amsellem, R.; Nicolas, V.; Servin, A.L. Secreted autotransporter toxin (Sat) triggers autophagy in epithelial cells that relies on cell detachment. Cell Microbiol. 2011, 13, 992–1013. [Google Scholar] [CrossRef] [PubMed]
- Parham, N.J.; Srinivasan, U.; Desvaux, M.; Foxman, B.; Marrs, C.F.; Henderson, I.R. PicU, a second serine protease autotransporter of uropathogenic Escherichia coli. FEMS Microbiol. Lett. 2004, 230, 73–83. [Google Scholar] [CrossRef]
- Nichols, K.B.; Totsika, M.; Moriel, D.G.; Lo, A.W.; Yang, J.; Wurpel, D.J.; Rossiter, A.E.; Strugnell, R.A.; Henderson, I.R.; Ulett, G.C.; et al. Molecular Characterization of the Vacuolating Autotransporter Toxin in Uropathogenic Escherichia coli. J. Bacteriol. 2016, 198, 1487–1498. [Google Scholar] [CrossRef]
- Díaz, J.M.; Dozois, C.M.; Avelar-González, F.J.; Hernández-Cuellar, E.; Pokharel, P.; de Santiago, A.S.; Guerrero-Barrera, A.L. The Vacuolating Autotransporter Toxin (Vat) of Escherichia coli Causes Cell Cytoskeleton Changes and Produces Non-lysosomal Vacuole Formation in Bladder Epithelial Cells. Front. Cell Infect. Microbiol. 2020, 10, 299. [Google Scholar] [CrossRef]
- Skaar, E.P. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 2010, 6, e1000949. [Google Scholar] [CrossRef] [PubMed]
- Valdebenito, M.; Bister, B.; Reissbrodt, R.; Hantke, K.; Winkelmann, G. The detection of salmochelin and yersiniabactin in uropathogenic Escherichia coli strains by a novel hydrolysis-fluorescence-detection (HFD) method. Int. J. Med. Microbiol. 2005, 295, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Peterson, C.M.; Grady, R.W.; Cerami, A. Low molecular weight iron-binding factor from mammalian tissue that potentiates bacterial growth. J. Exp. Med. 1980, 151, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Porcheron, G.; Habib, R.; Houle, S.; Caza, M.; Lépine, F.; Daigle, F.; Massé, E.; Dozois, C.M. The small RNA RyhB contributes to siderophore production and virulence of uropathogenic Escherichia coli. Infect. Immun. 2014, 82, 5056–5068. [Google Scholar] [CrossRef] [PubMed]
- Hagan, E.C.; Mobley, H.L. Haem acquisition is facilitated by a novel receptor Hma and required by uropathogenic Escherichia coli for kidney infection. Mol. Microbiol. 2009, 71, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, I.; Perkins-Balding, D. Processing of heme and heme-containing proteins by bacteria. DNA Cell Biol. 2002, 21, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.P.; Crowley, J.R.; Pinkner, J.S.; Walker, J.N.; Tsukayama, P.; Stamm, W.E.; Hooton, T.M.; Hultgren, S.J. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 2009, 5, e1000305. [Google Scholar] [CrossRef] [PubMed]
- Raffatellu, M.; George, M.D.; Akiyama, Y.; Hornsby, M.J.; Nuccio, S.P.; Paixao, T.A.; Butler, B.P.; Chu, H.; Santos, R.L.; Berger, T.; et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 2009, 5, 476–486. [Google Scholar] [CrossRef]
- Valdebenito, M.; Crumbliss, A.L.; Winkelmann, G.; Hantke, K. Environmental factors influence the production of enterobactin, salmochelin, aerobactin, and yersiniabactin in Escherichia coli strain Nissle 1917. Int. J. Med. Microbiol. 2006, 296, 513–520. [Google Scholar] [CrossRef]
- Feldmann, F.; Sorsa, L.J.; Hildinger, K.; Schubert, S. The salmochelin siderophore receptor IroN contributes to invasion of urothelial cells by extraintestinal pathogenic Escherichia coli in vitro. Infect. Immun. 2007, 75, 3183–3187. [Google Scholar] [CrossRef]
- Russo, T.A.; Mcfadden, C.D.; Carlino-Macdonald, U.B.; Beanan, J.M.; Olson, R.; Wilding, G.E. The Siderophore Receptor IroN of Extraintestinal Pathogenic Escherichia coli Is a Potential Vaccine Candidate. Infect. Immun. 2003, 71, 7164–7169. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.S.; Hung, C.S.; Crowley, J.R.; Stapleton, A.E.; Henderson, J.P. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat. Chem. Biol. 2012, 8, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, C.S.; Hultgren, S.J.; Gordon, J.I. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J. Biol. Chem. 2007, 282, 21259–21267. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.E.; Totsika, M.; Challinor, V.L.; Mabbett, A.N.; Ulett, G.C.; De Voss, J.J.; Schembri, M.A. Contribution of siderophore systems to growth and urinary tract colonization of asymptomatic bacteriuria Escherichia coli. Infect. Immun. 2012, 80, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.C.; Brumbaugh, A.R.; Mobley, H.L. Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect. Immun. 2011, 79, 1225–1235. [Google Scholar] [CrossRef]
- Torres, A.G.; Redford, P.; Welch, R.A.; Payne, S.M. TonB-dependent systems of uropathogenic Escherichia coli: Aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 2001, 69, 6179–6185. [Google Scholar] [CrossRef] [PubMed]
- Justice, S.S.; Hung, C.; Theriot, J.A.; Fletcher, D.A.; Anderson, G.G.; Footer, M.J.; Hultgren, S.J. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 1333–1338. [Google Scholar] [CrossRef]
- Rosen, D.A.; Hooton, T.M.; Stamm, W.E.; Humphrey, P.A.; Hultgren, S.J. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007, 4, e329. [Google Scholar] [CrossRef]
- Anderson, G.G.; Martin, S.M.; Hultgren, S.J. Host subversion by formation of intracellular bacterial communities in the urinary tract. Microbes Infect. 2004, 6, 1094–1101. [Google Scholar] [CrossRef]
- Eto, D.S.; Sundsbak, J.L.; Mulvey, M.A. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell Microbiol. 2006, 8, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Leatham-Jensen, M.P.; Mokszycki, M.E.; Rowley, D.C.; Deering, R.; Camberg, J.L.; Sokurenko, E.V.; Tchesnokova, V.L.; Frimodt-Møller, J.; Krogfelt, K.A.; Leth Nielsen, K.; et al. Uropathogenic Escherichia coli Metabolite-Dependent Quiescence and Persistence May Explain Antibiotic Tolerance during Urinary Tract Infection. mSphere 2016, 1, e00055-15. [Google Scholar] [CrossRef] [PubMed]
- Goetz, D.H.; Holmes, M.A.; Borregaard, N.; Bluhm, M.E.; Raymond, K.N.; Strong, R.K. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 2002, 10, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Lin, H.; Zhou, L.; Yu, Y.; Abergel, R.J.; Liu, D.R.; Raymond, K.N.; Wanner, B.L.; Strong, R.K.; Walsh, C.T.; et al. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc. Natl. Acad. Sci. USA 2006, 103, 16502–16507. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.D. Iron metabolism at the host pathogen interface: Lipocalin 2 and the pathogen-associated iroA gene cluster. Int. J. Biochem. Cell Biol. 2007, 39, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.W.; May, J.M.; Sherman, D.J.; Kahne, D.; Ruiz, N. Lipopolysaccharide transport to the cell surface: Biosynthesis and extraction from the inner membrane. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20150029. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Billips, B.K.; Schaeffer, A.J.; Klumpp, D.J. Molecular basis of uropathogenic Escherichia coli evasion of the innate immune response in the bladder. Infect. Immun. 2008, 76, 3891–3900. [Google Scholar] [CrossRef]
- Hunstad, D.A.; Justice, S.S.; Hung, C.S.; Lauer, S.R.; Hultgren, S.J. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect. Immun. 2005, 73, 3999–4006. [Google Scholar] [CrossRef]
- Jacobson, S.H.; Ostenson, C.G.; Tullus, K.; Brauner, A. Serum resistance in Escherichia coli strains causing acute pyelonephritis and bacteraemia. Apmis 1992, 100, 147–153. [Google Scholar] [CrossRef]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Ejrnæs, K.; Stegger, M.; Reisner, A.; Ferry, S.; Monsen, T.; Holm, S.E.; Lundgren, B.; Frimodt-Møller, N. Characteristics of Escherichia coli causing persistence or relapse of urinary tract infections: Phylogenetic groups, virulence factors and biofilm formation. Virulence 2011, 2, 528–537. [Google Scholar] [CrossRef]
- Norinder, B.S.; Lüthje, P.; Yadav, M.; Kadas, L.; Fang, H.; Nord, C.E.; Brauner, A. Cellulose and PapG are important for Escherichia coli causing recurrent urinary tract infection in women. Infection 2011, 39, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Salo, J.; Sevander, J.J.; Tapiainen, T.; Ikäheimo, I.; Pokka, T.; Koskela, M.; Uhari, M. Biofilm formation by Escherichia coli isolated from patients with urinary tract infections. Clin. Nephrol. 2009, 71, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Tapiainen, T.; Hanni, A.M.; Salo, J.; Ikäheimo, I.; Uhari, M. Escherichia coli biofilm formation and recurrences of urinary tract infections in children. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Bahrani-Mougeot, F.K.; Buckles, E.L.; Lockatell, C.V.; Hebel, J.R.; Johnson, D.E.; Tang, C.M.; Donnenberg, M.S. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 2002, 45, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.; Smith, S.N.; Spurbeck, R.R.; Kole, M.M.; Mobley, H.L. Genome-wide detection of fitness genes in uropathogenic Escherichia coli during systemic infection. PLoS Pathog. 2013, 9, e1003788. [Google Scholar] [CrossRef] [PubMed]
- Conway, C.; Beckett, M.C.; Dorman, C.J. The DNA relaxation-dependent OFF-to-ON biasing of the type 1 fimbrial genetic switch requires the Fis nucleoid-associated protein. Microbiology 2023, 169, 001283. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.; Roesch, P.; Davis, L.; Moritz, R.; Pellett, S.; Welch, R.A. Regulation of type 1 fimbriae by unlinked FimB- and FimE-like recombinases in uropathogenic Escherichia coli strain CFT073. Infect. Immun. 2006, 74, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Grigoryan, L.; Trautner, B. Urinary Tract Infection. Ann. Intern. Med. 2017, 167, Itc49–Itc64. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.M.; Lowder, J.L. Diagnosis and treatment of urinary tract infections across age groups. Am. J. Obstet. Gynecol. 2018, 219, 40–51. [Google Scholar] [CrossRef]
- Muller, A.E.; Verhaegh, E.M.; Harbarth, S.; Mouton, J.W.; Huttner, A. Nitrofurantoin’s efficacy and safety as prophylaxis for urinary tract infections: A systematic review of the literature and meta-analysis of controlled trials. Clin. Microbiol. Infect. 2017, 23, 355–362. [Google Scholar] [CrossRef]
- Davis, W.H.; Magee, M.R.; Monks, S.M.; Geno, K.A.; Crawford, S.B. Assessment of nationally recommended antibiotics for treatment of UTI in U.S.-Mexico border emergency departments. Am. J. Emerg. Med. 2022, 61, 12–17. [Google Scholar] [CrossRef]
- Gupta, K.; Hooton, T.M.; Roberts, P.L.; Stamm, W.E. Short-course nitrofurantoin for the treatment of acute uncomplicated cystitis in women. Arch. Intern. Med. 2007, 167, 2207–2212. [Google Scholar] [CrossRef]
- Moya-Dionisio, V.; Díaz-Zabala, M.; Ibáñez-Fernández, A.; Suárez-Leiva, P.; Martínez-Suárez, V.; Ordóñez-Álvarez, F.A.; Santos-Rodríguez, F. Uropathogen pattern and antimicrobial susceptibility in positive urinary cultures isolates from paediatric patients. Rev. Esp. Quimioter. 2016, 29, 146–150. [Google Scholar]
- Nicolle, L.E. Pivmecillinam in the treatment of urinary tract infections. J. Antimicrob. Chemother. 2000, 46, 35–39; discussion 63–65. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.S.; Loeb, M.; Leto, D.; Brooks, A.A. Treatment of urinary tract infections in the era of antimicrobial resistance and new antimicrobial agents. Postgrad. Med. 2020, 132, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Quek, W.M.; Teng, C.B.; Tan, Y.Z.; Chong, K.; Lye, D.C.; Ng, T.M. Outcomes of Fosfomycin Use in Ceftriaxone-Resistant Enterobacteriaceae Urinary Tract Infection in the Elderly. Int. J. Antimicrob. Agents 2019, 53, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Peretz, A.; Naamneh, B.; Tkhawkho, L.; Nitzan, O. High Rates of Fosfomycin Resistance in Gram-Negative Urinary Isolates from Israel. Microb. Drug. Resist. 2019, 25, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Ko, W.C.; Hsueh, P.R. Emerging resistance problems and future perspectives in pharmacotherapy for complicated urinary tract infections. Expert Opin. Pharm. 2013, 14, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L. Resistance in gram-negative bacteria: Enterobacteriaceae. Am. J. Med. 2006, 34, S20–S28; discussion S62–S70. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti. Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Bhadelia, N. Management of urinary tract infections from multidrug-resistant organisms. Infect. Dis. Clin. N. Am. 2014, 28, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.S.; Loeb, M.; Brooks, A.A. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad. Med. 2017, 129, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Garau, J. Other antimicrobials of interest in the era of extended-spectrum beta-lactamases: Fosfomycin, nitrofurantoin and tigecycline. Clin. Microbiol. Infect. 2008, 14, 198–202. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef]
- Bradford, P.A. Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Chang, U.I.; Kim, H.W.; Wie, S.H. Use of cefuroxime for women with community-onset acute pyelonephritis caused by cefuroxime-susceptible or -resistant Escherichia coli. Korean J. Intern. Med. 2016, 31, 145–155. [Google Scholar] [CrossRef]
- Whelan, S.; O’Grady, M.C.; Corcoran, G.D.; Finn, K.; Lucey, B. Effect of Sub-Inhibitory Concentrations of Nitrofurantoin, Ciprofloxacin, and Trimethoprim on In Vitro Biofilm Formation in Uropathogenic Escherichia coli (UPEC). Med. Sci 2022, 11, 1. [Google Scholar] [CrossRef]
- Walker, G.K.; Suyemoto, M.M.; Hull, D.M.; Gall, S.; Jimenez, F.; Chen, L.R.; Thakur, S.; Crespo, R.; Borst, L.B. Genomic Characterization of a Nalidixic Acid-Resistant Salmonella Enteritidis Strain Causing Persistent Infections in Broiler Chickens. Front. Vet. Sci. 2021, 8, 725737. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jabeen, H.; Ahmad, T.; Rehman, N.U.; Khan, S.S.; Shareef, H.; Sarwar, R.; Yahya, S.; Hussain, N.; Uddin, J.; et al. Comparative efficacy of cephradine-loaded silver and gold nanoparticles against resistant human pathogens. Artif. Cells Nanomed. Biotechnol. 2022, 50, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Millner, R.; Becknell, B. Urinary Tract Infections. Pediatr. Clin. N. Am. 2019, 66, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zalewska-Piątek, B.M.; Piątek, R.J. Alternative treatment approaches of urinary tract infections caused by uropathogenic Escherichia coli strains. Acta Biochim. Pol. 2019, 66, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Saatchi, A.; Yoo, J.W.; Schwartz, K.L.; Silverman, M.; Morris, A.M.; Patrick, D.M.; McCormack, J.; Marra, F. Quantifying the Gap between Expected and Actual Rates of Antibiotic Prescribing in British Columbia, Canada. Antibiotics 2021, 10, 1428. [Google Scholar] [CrossRef]
- McLellan, L.K.; Hunstad, D.A. Urinary Tract Infection: Pathogenesis and Outlook. Trends Mol. Med. 2016, 22, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Grischke, E.M.; Rüttgers, H. Treatment of bacterial infections of the female urinary tract by immunization of the patients. Urol. Int. 1987, 42, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.J.; Elkahwaji, J.; Beierle, L.M.; Leverson, G.E.; Uehling, D.T. Vaginal mucosal vaccine for recurrent urinary tract infections in women: Results of a phase 2 clinical trial. J. Urol. 2007, 177, 1349–1353. [Google Scholar] [CrossRef]
- Beerepoot, M.A.; Geerlings, S.E.; van Haarst, E.P.; van Charante, N.M.; ter Riet, G. Nonantibiotic prophylaxis for recurrent urinary tract infections: A systematic review and meta-analysis of randomized controlled trials. J. Urol. 2013, 190, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Kochiashvili, D.; Khuskivadze, A.; Kochiashvili, G.; Koberidze, G.; Kvakhajelidze, V. Role of the bacterial vaccine Solco-Urovac® in treatment and prevention of recurrent urinary tract infections of bacterial origin. Georgian. Med. News 2014, 231, 11–16. [Google Scholar]
- Wade, D.; Cooper, J.; Derry, F.; Taylor, J. Uro-Vaxom® versus placebo for the prevention of recurrent symptomatic urinary tract infections in participants with chronic neurogenic bladder dysfunction: A randomised controlled feasibility study. Trials 2019, 20, 223. [Google Scholar] [CrossRef] [PubMed]
- Magasi, P.; Pánovics, J.; Illés, A.; Nagy, M. Uro-Vaxom and the management of recurrent urinary tract infection in adults: A randomized multicenter double-blind trial. Eur. Urol. 1994, 26, 137–140. [Google Scholar] [CrossRef]
- Cruz, F.; Dambros, M.; Naber, K.G.; Bauer, H.W.; Cozma, G. Recurrent Urinary Tract Infections: Uro-Vaxom, a New Alternative. Eur. Urol. Suppl. 2009, 8, 762–768. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.Y.; Jeong, I.G.; Paick, J.S.; Son, H.; Lim, D.J.; Shim, H.B.; Park, W.H.; Jung, H.C.; Choo, M.S. A prospective multi-center trial of Escherichia coli extract for the prophylactic treatment of patients with chronically recurrent cystitis. J. Korean Med. Sci. 2010, 25, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Magistro, G.; Stief, C.G. Vaccine Development for Urinary Tract Infections: Where Do We Stand? Eur. Urol. Focus. 2019, 5, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Marinova, S.; Nenkov, P.; Markova, R.; Nikolaeva, S.; Kostadinova, R.; Mitov, I.; Vretenarska, M. Cellular and humoral systemic and mucosal immune responses stimulated by an oral polybacterial immunomodulator in patients with chronic urinary tract infections. Int. J. Immunopathol. Pharmacol. 2005, 18, 457–473. [Google Scholar] [CrossRef]
- Del Bino, L.; Østerlid, K.E.; Wu, D.Y.; Nonne, F.; Romano, M.R.; Codée, J.; Adamo, R. Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance. Chem. Rev. 2022, 122, 15672–15716. [Google Scholar] [CrossRef]
- Russo, T.A.; Beanan, J.M.; Olson, R.; MacDonald, U.; Cope, J.J. Capsular polysaccharide and the O-specific antigen impede antibody binding: A potential obstacle for the successful development of an extraintestinal pathogenic Escherichia coli vaccine. Vaccine 2009, 27, 388–395. [Google Scholar] [CrossRef]
- Billips, B.K.; Yaggie, R.E.; Cashy, J.P.; Schaeffer, A.J.; Klumpp, D.J. A live-attenuated vaccine for the treatment of urinary tract infection by uropathogenic Escherichia coli. J. Infect. Dis. 2009, 200, 263–272. [Google Scholar] [CrossRef]
- Horvath, D.J., Jr.; Patel, A.S.; Mohamed, A.; Storm, D.W.; Singh, C.; Li, B.; Zhang, J.; Koff, S.A.; Jayanthi, V.R.; Mason, K.M.; et al. Association of O-Antigen Serotype with the Magnitude of Initial Systemic Cytokine Responses and Persistence in the Urinary Tract. J. Bacteriol. 2016, 198, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Uehling, D.T.; Hopkins, W.J.; Dahmer, L.A.; Balish, E. Phase I clinical trial of vaginal mucosal immunization for recurrent urinary tract infection. J. Urol. 1994, 152, 2308–2311. [Google Scholar] [CrossRef] [PubMed]
- Uehling, D.T.; Hopkins, W.J.; Elkahwaji, J.E.; Schmidt, D.M.; Leverson, G.E. Phase 2 clinical trial of a vaginal mucosal vaccine for urinary tract infections. J. Urol. 2003, 170, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Beanan, J.M.; Olson, R.; Genagon, S.A.; MacDonald, U.; Cope, J.J.; Davidson, B.A.; Johnston, B.; Johnson, J.R. A killed, genetically engineered derivative of a wild-type extraintestinal pathogenic E. coli strain is a vaccine candidate. Vaccine 2007, 25, 3859–3870. [Google Scholar] [CrossRef] [PubMed]
- Billips, B.K.; Forrestal, S.G.; Rycyk, M.T.; Johnson, J.R.; Klumpp, D.J.; Schaeffer, A.J. Modulation of host innate immune response in the bladder by uropathogenic Escherichia coli. Infect Immun. 2007, 75, 5353–5360. [Google Scholar] [CrossRef]
- William, B.S.; Darren, A.; Bart, S.; Oscar, G.; Wouter, H.; Tiziano, D.R.; Kellen, F.; Jan, P.; Stefan, T.; Ibarra, D. 2712. Safety and Immunogenicity of two Doses of ExPEC4V Vaccine Against Extraintestinal Pathogenic Escherichia coli Disease in Healthy Adult Participants. Open Forum Infect. Dis. 2019, 6, S954. [Google Scholar]
- Frenck, R.W., Jr.; Ervin, J.; Chu, L.; Abbanat, D.; Spiessens, B.; Go, O.; Haazen, W.; van den Dobbelsteen, G.; Poolman, J.; Thoelen, S.; et al. Safety and immunogenicity of a vaccine for extra-intestinal pathogenic Escherichia coli (ESTELLA): A phase 2 randomised controlled trial. Lancet Infect. Dis. 2019, 19, 631–640. [Google Scholar] [CrossRef]
- Asadi Karam, M.R.; Oloomi, M.; Mahdavi, M.; Habibi, M.; Bouzari, S. Vaccination with recombinant FimH fused with flagellin enhances cellular and humoral immunity against urinary tract infection in mice. Vaccine 2013, 31, 1210–1216. [Google Scholar] [CrossRef]
- Hasanzadeh, S.; Habibi, M.; Shokrgozar, M.A.; Ahangari Cohan, R.; Ahmadi, K.; Asadi Karam, M.R.; Bouzari, S. In silico analysis and in vivo assessment of a novel epitope-based vaccine candidate against uropathogenic Escherichia coli. Sci. Rep. 2020, 10, 16258. [Google Scholar] [CrossRef]
- Asadi Karam, M.R.; Habibi, M.; Bouzari, S. Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli. Mol. Immunol. 2019, 108, 56–67. [Google Scholar] [CrossRef]
- Savar, N.S.; Jahanian-Najafabadi, A.; Mahdavi, M.; Shokrgozar, M.A.; Jafari, A.; Bouzari, S. In silico and in vivo studies of truncated forms of flagellin (FliC) of enteroaggregative Escherichia coli fused to FimH from uropathogenic Escherichia coli as a vaccine candidate against urinary tract infections. J. Biotechnol. 2014, 175, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Asadi Karam, M.R.; Habibi, M.; Bouzari, S. Use of flagellin and cholera toxin as adjuvants in intranasal vaccination of mice to enhance protective immune responses against uropathogenic Escherichia coli antigens. Biologicals 2016, 44, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Asadi Karam, M.R.; Bouzari, S. Evaluation of prevalence, immunogenicity and efficacy of FyuA iron receptor in uropathogenic Escherichia coli isolates as a vaccine target against urinary tract infection. Microb. Pathog. 2017, 110, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Brumbaugh, A.R.; Smith, S.N.; Mobley, H.L. Immunization with the yersiniabactin receptor, FyuA, protects against pyelonephritis in a murine model of urinary tract infection. Infect. Immun. 2013, 81, 3309–3316. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Hatz, C.; van den Dobbelsteen, G.; Abbanat, D.; Hornacek, A.; Frölich, R.; Dreyer, A.M.; Martin, P.; Davies, T.; Fae, K.; et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: A randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect. Dis. 2017, 17, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Langermann, S.; Palaszynski, S.; Barnhart, M.; Auguste, G.; Pinkner, J.S.; Burlein, J.; Barren, P.; Koenig, S.; Leath, S.; Jones, C.H.; et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 1997, 276, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Poggio, T.V.; La Torre, J.L.; Scodeller, E.A. Intranasal immunization with a recombinant truncated FimH adhesin adjuvanted with CpG oligodeoxynucleotides protects mice against uropathogenic Escherichia coli challenge. Can. J. Microbiol. 2006, 52, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Karam, M.R.; Oloomi, M.; Mahdavi, M.; Habibi, M.; Bouzari, S. Assessment of immune responses of the flagellin (FliC) fused to FimH adhesin of Uropathogenic Escherichia coli. Mol. Immunol. 2013, 54, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Bagherpour, G.; Fooladi, A.A.; Mehrabadi, J.F.; Nourani, M.R.; Einollahi, B. Evaluation of mammalian codon usage of fimH in DNA vaccine design. Acta Microbiol. Immunol. Hung. 2011, 58, 259–271. [Google Scholar] [CrossRef]
- Ferraro, B.; Morrow, M.P.; Hutnick, N.A.; Shin, T.H.; Lucke, C.E.; Weiner, D.B. Clinical applications of DNA vaccines: Current progress. Clin. Infect. Dis. 2011, 53, 296–302. [Google Scholar] [CrossRef]
- Imani Fooladi, A.A.; Bagherpour, G.; Khoramabadi, N.; Fallah Mehrabadi, J.; Mahdavi, M.; Halabian, R.; Amin, M.; Izadi Mobarakeh, J.; Einollahi, B. Cellular immunity survey against urinary tract infection using pVAX/fimH cassette with mammalian and wild type codon usage as a DNA vaccine. Clin. Exp. Vaccine Res. 2014, 3, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Kaack, M.B.; Baskin, G.; Chapman, M.R.; Hunstad, D.A.; Pinkner, J.S.; Hultgren, S.J. Antibody responses and protection from pyelonephritis following vaccination with purified Escherichia coli PapDG protein. J. Urol. 2004, 171, 1682–1685. [Google Scholar] [CrossRef] [PubMed]
- Mike, L.A.; Smith, S.N.; Sumner, C.A.; Eaton, K.A.; Mobley, H.L. Siderophore vaccine conjugates protect against uropathogenic Escherichia coli urinary tract infection. Proc. Natl. Acad. Sci. USA 2016, 113, 13468–13473. [Google Scholar] [CrossRef]
- Wieser, A.; Romann, E.; Magistro, G.; Hoffmann, C.; Nörenberg, D.; Weinert, K.; Schubert, S. A multiepitope subunit vaccine conveys protection against extraintestinal pathogenic Escherichia coli in mice. Infect. Immun. 2010, 78, 3432–3442. [Google Scholar] [CrossRef]
- Wieser, A.; Magistro, G.; Nörenberg, D.; Hoffmann, C.; Schubert, S. First multi-epitope subunit vaccine against extraintestinal pathogenic Escherichia coli delivered by a bacterial type-3 secretion system (T3SS). Int. J. Med. Microbiol. 2012, 302, 10–18. [Google Scholar] [CrossRef]
- Alteri, C.J.; Hagan, E.C.; Sivick, K.E.; Smith, S.N.; Mobley, H.L. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 2009, 5, e1000586. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.T. Who will develop new antibacterial agents? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130430. [Google Scholar] [CrossRef]
- Marouf, R.S.; Mbarga, J.A.M.; Ermolaev, A.V.; Podoprigora, I.V.; Smirnova, I.P.; Yashina, N.V.; Zhigunova, A.V.; Martynenkova, A.V. Antibacterial Activity of Medicinal Plants against Uropathogenic Escherichia coli. J. Pharm. Bioallied. Sci. 2022, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, G.; Akram, M.; Jabeen, F.; Ali Shah, S.M.; Munir, N.; Daniyal, M.; Riaz, M.; Tahir, I.M.; Ghauri, A.O.; Sultana, S.; et al. Therapeutic potential of medicinal plants for the management of urinary tract infection: A systematic review. Clin. Exp. Pharmacol. Physiol. 2019, 46, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial Capacity of Plant Polyphenols against Gram-positive Bacteria: A Comprehensive Review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Rodríguez, J.C.; Borrás-Rocher, F.; Barrajón-Catalán, E.; Micol, V. The antimicrobial capacity of Cistus salviifolius and Punica granatum plant extracts against clinical pathogens is related to their polyphenolic composition. Sci. Rep. 2021, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Upadhyaya, I.; Kollanoor-Johny, A.; Venkitanarayanan, K. Combating pathogenic microorganisms using plant-derived antimicrobials: A minireview of the mechanistic basis. Biomed. Res. Int. 2014, 2014, 761741. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Deipenbrock, M.; Hensel, A. Polymethoxylated flavones from Orthosiphon stamineus leaves as antiadhesive compounds against uropathogenic E. coli. Fitoterapia 2019, 139, 104387. [Google Scholar] [CrossRef] [PubMed]
- Sarshar, S.; Sendker, J.; Qin, X.; Goycoolea, F.M.; Asadi Karam, M.R.; Habibi, M.; Bouzari, S.; Dobrindt, U.; Hensel, A. Antiadhesive hydroalcoholic extract from Apium graveolens fruits prevents bladder and kidney infection against uropathogenic E. coli. Fitoterapia 2018, 127, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Scharf, B.; Sendker, J.; Dobrindt, U.; Hensel, A. Influence of Cranberry Extract on Tamm-Horsfall Protein in Human Urine and its Antiadhesive Activity Against Uropathogenic Escherichia coli. Planta Med. 2019, 85, 126–138. [Google Scholar] [CrossRef]
- Mutters, N.T.; Mampel, A.; Kropidlowski, R.; Biehler, K.; Günther, F.; Bălu, I.; Malek, V.; Frank, U. Treating urinary tract infections due to MDR E. coli with Isothiocyanates—A phytotherapeutic alternative to antibiotics? Fitoterapia 2018, 129, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Sabadash, M.; Shulyak, A. Canephron® N in the treatment of recurrent cystitis in women of child-bearing Age: A randomised controlled study. Clin. Phyt. 2017, 3, 9. [Google Scholar] [CrossRef]
- Vacheva, A.; Mustafa, B.; Staneva, J.; Marhova, M.; Kostadinova, S.; Todorova, M.; Ivanova, R.; Stoitsova, S. Effects of Extracts from Medicinal Plants on Biofilm Formation by Escherichia coli Urinary Tract Isolates. Biotechnol. Biotechnol. Equip. 2011, 25, 92–97. [Google Scholar] [CrossRef]
- Žitek, T.; Postružnik, V.; Knez, Ž.; Golle, A.; Dariš, B.; Knez Marevci, M.; Arnica Montana, L. Supercritical Extraction Optimization for Antibiotic and Anticancer Activity. Front. Bioeng. Biotechnol. 2022, 10, 897185. [Google Scholar] [CrossRef]
- Hidalgo, G.; Chan, M.; Tufenkji, N. Inhibition of Escherichia coli CFT073 fliC expression and motility by cranberry materials. Appl. Environ. Microbiol. 2011, 77, 6852–6857. [Google Scholar] [CrossRef] [PubMed]
- Ranfaing, J.; Dunyach-Remy, C.; Lavigne, J.P.; Sotto, A. Propolis potentiates the effect of cranberry (Vaccinium macrocarpon) in reducing the motility and the biofilm formation of uropathogenic Escherichia coli. PLoS ONE 2018, 13, e0202609. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.; Horowitz, R.; Coppenhagen-Glazer, S.; Pizov, G.; Elia, A.; Gofrit, O.N.; Ginsburg, I.; Pode, D. Intravesical administration of green tea extract attenuates the inflammatory response of bacterial cystitis–a rat model. BJU Int. 2014, 114, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Fan, K.K.; Gu, L.F.; Yu, B.Y.; Chai, C.Z. Anti-inflammatory effects of Abelmoschus manihot (L.) Medik. on LPS-induced cystitis in mice: Potential candidate for cystitis treatment based on classic use. Chin. J. Nat. Med. 2022, 20, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Rafsanjany, N.; Lechtenberg, M.; Petereit, F.; Hensel, A. Antiadhesion as a functional concept for protection against uropathogenic Escherichia coli: In vitro studies with traditionally used plants with antiadhesive activity against uropathognic Escherichia coli. J. Ethnopharmacol. 2013, 145, 591–597. [Google Scholar] [CrossRef]
- Bortolami, M.; Di Matteo, P.; Rocco, D.; Feroci, M.; Petrucci, R. Metabolic Profile of Agropyron repens (L.) P. Beauv. Rhizome Herbal Tea by HPLC-PDA-ESI-MS/MS Analysis. Molecules 2022, 27, 4962. [Google Scholar] [CrossRef]
- Noundou, X.S.; Krause, R.W.; van Vuuren, S.F.; Ndinteh, D.T.; Olivier, D.K. Antibacterial effects of Alchornea cordifolia (Schumach. and Thonn.) Müll. Arg extracts and compounds on gastrointestinal, skin, respiratory and urinary tract pathogens. J. Ethnopharmacol. 2016, 179, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Chang, H.H.; Chung, Y.H.; Lee, T.Y. Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264.7 macrophages by inhibiting STAT3-mediated suppression of the NF-κB pathway. J. Ethnopharmacol. 2011, 135, 678–684. [Google Scholar] [CrossRef]
- de Arriba, S.G.; Naser, B.; Nolte, K.U. Risk assessment of free hydroquinone derived from Arctostaphylos Uva-ursi folium herbal preparations. Int. J. Toxicol. 2013, 32, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Dietz, B.M.; Hajirahimkhan, A.; Dunlap, T.L.; Bolton, J.L. Botanicals and Their Bioactive Phytochemicals for Women’s Health. Pharmacol. Rev. 2016, 68, 1026–1073. [Google Scholar] [CrossRef]
- Venkatadri, B.; Arunagirinathan, N.; Rameshkumar, M.R.; Ramesh, L.; Dhanasezhian, A.; Agastian, P. In vitro Antibacterial Activity of Aqueous and Ethanol Extracts of Aristolochia indica and Toddalia asiatica Against Multidrug-Resistant Bacteria. Indian J. Pharm. Sci. 2015, 77, 788–791. [Google Scholar] [PubMed]
- Michl, J.; Jennings, H.M.; Kite, G.C.; Ingrouille, M.J.; Simmonds, M.S.; Heinrich, M. Is aristolochic acid nephropathy a widespread problem in developing countries? A case study of Aristolochia indica L. in Bangladesh using an ethnobotanical-phytochemical approach. J. Ethnopharmacol. 2013, 149, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U.; Goos, K.H.; Schneider, B. A randomised, double-blind, placebo-controlled trial of a herbal medicinal product containing Tropaeoli majoris herba (Nasturtium) and Armoraciae rusticanae radix (Horseradish) for the prophylactic treatment of patients with chronically recurrent lower urinary tract infections. Curr. Med. Res. Opin. 2007, 23, 2415–2422. [Google Scholar] [PubMed]
- Conrad, A.; Biehler, D.; Nobis, T.; Richter, H.; Engels, I.; Biehler, K.; Frank, U. Broad spectrum antibacterial activity of a mixture of isothiocyanates from nasturtium (Tropaeoli majoris herba) and horseradish (Armoraciae rusticanae radix). Drug. Res. 2013, 63, 65–68. [Google Scholar] [CrossRef]
- Rajkumar, J.; Devi, A.S.; Beenish, T.K. Detection of Antibacterial Compound of Avicennia marina Against Pathogens Isolated from Urinary Tract Infected Patients. Asian J. Chem. 2014, 26, 458–460. [Google Scholar]
- Wojnicz, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kicia, M.; Tichaczek-Goska, D. Medicinal plants extracts affect virulence factors expression and biofilm formation by the uropathogenic Escherichia coli. Urol. Res. 2012, 40, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Sahu, L.; Jena, S.; Swain, S.S.; Sahoo, S.; Chand, P.K. Agrobacterium rhizogenes-mediated transformation of a multi-medicinal herb, Boerhaavia diffusa L.: Optimization of the process and anti-microbial activity against bacterial pathogens causing urinary tract infections. Front. Life Sci. 2013, 7, 197–209. [Google Scholar] [CrossRef]
- Ferreres, F.; Sousa, C.; Justin, M.; Valentão, P.; Andrade, P.B.; Llorach, R.; Rodrigues, A.; Seabra, R.M.; Leitão, A. Characterisation of the phenolic profile of Boerhaavia diffusa L. by HPLC-PAD-MS/MS as a tool for quality control. Phytochem. Anal. 2005, 16, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Adetutu, A.; Morgan, W.A.; Corcoran, O. Ethnopharmacological survey and in vitro evaluation of wound-healing plants used in South-western Nigeria. J. Ethnopharmacol. 2011, 137, 50–56. [Google Scholar] [CrossRef]
- Yeboah, G.N.; Owusu, F.W.A.; Archer, M.A.; Kyene, M.O.; Kumadoh, D.; Ayertey, F.; Mintah, S.O.; Atta-Adjei Junior, P.; Appiah, A.A. Bridelia ferruginea Benth.; An ethnomedicinal, phytochemical, pharmacological and toxicological review. Heliyon 2022, 8, e10366. [Google Scholar] [CrossRef]
- Vučić, D.M.; Petković, M.R.; Rodić-Grabovac, B.B.; Stefanović, O.D.; Vasić, S.M.; Comić, L.R. In vitro activity of heather [Calluna vulgaris (L.) Hull] extracts on selected urinary tract pathogens. Bosn. J. Basic Med. Sci. 2014, 14, 234–238. [Google Scholar] [CrossRef]
- Vollmerhausen, T.L.; Ramos, N.L.; Dzung, D.T.; Brauner, A. Decoctions from Citrus reticulata Blanco seeds protect the uroepithelium against Escherichia coli invasion. J. Ethnopharmacol. 2013, 150, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Guo, L.; Dou, L.L.; Zhou, C.L.; Xu, F.G.; Zheng, G.D.; Li, P.; Liu, E.H. Discrimination of Citrus reticulata Blanco and Citrus reticulata ‘Chachi’ by gas chromatograph-mass spectrometry based metabolomics approach. Food Chem. 2016, 212, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Uliana, M.P.; Silva, A.G.D.; Fronza, M.; Scherer, R. In vitro Antioxidant and Antimicrobial Activities of Costus spicatus Swartz used in Folk Medicine for Urinary Tract Infection in Brazil. Latin. Am. J. Pharm. 2015, 34, 766–772. [Google Scholar]
- Chandra, S.; Gupta, C.P. Antibacterial activity of medicinal plant Crateava nurvala (bark) against bacterial strains causing urinary tract infection. Asian J. Chem. 2001, 13, 1181–1186. [Google Scholar]
- Bhattacharjee, A.; Shashidhara, S.C. Aswathanarayana, Phytochemical and ethno-pharmacological profile of Crataeva nurvala Buch-Hum (Varuna): A review. Asian Pac. J. Trop. Biomed. 2012, 2, S1162–S1168. [Google Scholar] [CrossRef]
- Packiavathy, I.A.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin—An anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.S.; Sumita, T.C.; Furlan, M.R.; Jorge, A.O.; Ueno, M. Antibacterial activity of essential oils on microorganisms isolated from urinary tract infection. Rev. Saude Publica 2004, 38, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Verma, R.; Ramteke, P. Cyperus rotundus: A potential novel source of therapeutic compound against urinary tract pathogens. J. Herb. Med. 2014, 4, 74–82. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Y.; Lv, Y.; Li, X.; Chen, L.; Huang, Z.; Zhou, J.; Wang, Y.; Wang, X.; Wang, X.; et al. Dendrobium officinale polysaccharides attenuate uropathogenic Escherichia coli (UPEC)-induced pyroptosis in macrophage cells. Biomed. Pharmacother. 2022, 151, 113098. [Google Scholar] [CrossRef]
- Lüthje, P.; Lokman, E.F.; Sandström, C.; Östenson, C.; Brauner, A. Gynostemma pentaphyllum exhibits anti-inflammatory properties and modulates antimicrobial peptide expression in the urinary bladder. J. Funct. Food. 2015, 17, 283–292. [Google Scholar] [CrossRef]
- Fazliana, M.; Ramos, N.L.; Lüthje, P.; Sekikubo, M.; Holm, A.; Wan Nazaimoon, W.M.; Brauner, A. Labisia pumila var. alata reduces bacterial load by inducing uroepithelial cell apoptosis. J. Ethnopharmacol. 2011, 136, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Lüthje, P.; Dzung, D.N.; Brauner, A. Lactuca indica extract interferes with uroepithelial infection by Escherichia coli. J. Ethnopharmacol. 2011, 135, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, K.H.; Choi, S.U.; Kim, Y.H.; Lee, K.R. Terpene and phenolic constituents of Lactuca indica L. Arch. Pharm. Res. 2008, 31, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, K.; Sultan, S.; Adam, A. Orthosiphon stamineus Benth. is an Outstanding Food Medicine: Review of Phytochemical and Pharmacological Activities. J. Pharm. Bioallied. Sci. 2018, 10, 109–118. [Google Scholar] [CrossRef]
- Elmahmood, A.M.; Ameh, J.M. In vitro antibacterial activity of Parkia biglobosa (Jacq.) root bark extract against some microorganisms associated with urinary tract infections. Afr. J. Biotechnol. 2007, 6, 1272–1275. [Google Scholar]
- Tamfu, A.N.; Roland, N.; Mfifen, A.M.; Kucukaydin, S.; Gaye, M.; Botezatu, A.V.; Duru, M.E.; Dinica, R.M. Phenolic composition, antioxidant and enzyme inhibitory activities of Parkia biglobosa (Jacq.) Benth., Tithonia diversifolia (Hemsl) A. Gray, and Crossopteryx febrifuga (Afzel.) Benth. Arab. J. Chem. 2022, 15, 103675. [Google Scholar] [CrossRef]
- Saeidi, S.; Amini Boroujeni, N.; Ahmadi, H.; Hassanshahian, M. Antibacterial Activity of Some Plant Extracts Against Extended- Spectrum Beta-Lactamase Producing Escherichia coli Isolates. Jundishapur. J. Microbiol. 2015, 8, e15434. [Google Scholar] [CrossRef]
- Mina, C.N.; Farzaei, M.H.; Gholamreza, A. Medicinal properties of Peganum harmala L. in traditional Iranian medicine and modern phytotherapy: A review. J. Tradit. Chin. Med. 2015, 35, 104–109. [Google Scholar] [PubMed]
- Friščić, M.; Jerković, I.; Marijanović, Z.; Dragović, S.; Hazler Pilepić, K.; Maleš, Ž. Essential Oil Composition of Different Plant Parts from Croatian Petasites albus (L.) Gaertn. and Petasites hybridus (L.) G.Gaertn., B.Mey. & Scherb. (Asteraceae). Chem. Biodivers 2019, 16, e1800531. [Google Scholar]
- Petrolini, F.V.; Lucarini, R.; de Souza, M.G.; Pires, R.H.; Cunha, W.R.; Martins, C.H. Evaluation of the antibacterial potential of Petroselinum crispum and Rosmarinus officinalis against bacteria that cause urinary tract infections. Braz. J. Microbiol. 2013, 44, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Tintino, S.R.; Souza, C.; Guedes, G.; Costa, J.; Duarte, F.M.; Chaves, M.; Silva, V.A.; Pessôa, H.; Lima, M.A.; Garcia, C.A. Modulatory antimicrobial activity of piper arboreum extracts. Acta Bot. Croat. 2014, 73, 281–289. [Google Scholar] [CrossRef]
- da Silva, A.C.A.; Matias, E.F.F.; Rocha, J.E.; Araújo, A.C.J.; de Freitas, T.S.; Campina, F.F.; Costa, M.D.S.; Silva, L.E.; Amaral, W.D.; Maia, B.; et al. Gas chromatography coupled to mass spectrometry (GC-MS) characterization and evaluation of antibacterial bioactivities of the essential oils from Piper arboreum Aubl., Piper aduncum L. e Piper gaudichaudianum Kunth. Z. Naturforsch. C J. Biosci. 2021, 76, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.G.; Zhang, L.J.; Sun, F.; Zhang, J.J.; Chen, A.Y.; Lan, Y.Y.; Li, Y.J.; Wang, A.M.; He, X.; Xiong, Y.; et al. Antibacterial and anti-inflammatory effects of extracts and fractions from Polygonum capitatum. J. Ethnopharmacol. 2011, 134, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Seifi, M.; Abbasalizadeh, S.; Mohammad-Alizadeh-Charandabi, S.; Khodaie, L.; Mirghafourvand, M. The effect of Rosa (L. Rosa canina) on the incidence of urinary tract infection in the puerperium: A randomized placebo-controlled trial. Phytother. Res. 2018, 32, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.M.; Fang, Y.; Hu, W.; Huang, Q. The pharmacological activities of Compound Salvia Plebeia Granules on treating urinary tract infection. J. Ethnopharmacol. 2010, 129, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-Y.; Wan, X.-H.; Niu, F.-J.; Xie, S.-M.; Guo, H.; Yang, Y.-Y.; Guo, L.-Y.; Zhou, C.-Z. Salvia plebeia R. Br.: An overview about its traditional uses, chemical constituents, pharmacology and modern applications. Biomed. Pharmacother. 2020, 121, 109589. [Google Scholar] [CrossRef] [PubMed]
- Sittiwet, C.; Puangpronpitag, D.; Niamsa, N. In vitro Antimicrobial Activity of Schefflera leucantha: The Potential of Respiratory Tract and Urinary Tract Infection Treatment. Int. J. Pharmacol. 2009, 5, 240–243. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, D.; Khan, F.-A.; Zhang, C.-L.; Liu, Y.-F.; Chen, R.-Y.; Choudhary, M.I.; Yu, D.-Q. Chemical constituents from Schefflera leucantha R.Vig. (Araliaceae). Biochem. Syst. Ecol. 2020, 91, 104076. [Google Scholar] [CrossRef]
- Karunai Raj, M.; Balachandran, C.; Duraipandiyan, V.; Agastian, P.; Ignacimuthu, S. Antimicrobial activity of Ulopterol isolated from Toddalia asiatica (L.) Lam.: A traditional medicinal plant. J. Ethnopharmacol. 2012, 140, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Guo, H.; Yao, R.; Bao, L.; Sun, J.; Bao, Y.; Guo, B.; Gao, Y.; Shi, Y.; Zhang, H.; et al. Crude polysaccharides from the seeds of Vaccaria segetalis prevent the urinary tract infection through the stimulation of kidney innate immunity. J. Ethnopharmacol. 2020, 260, 112578. [Google Scholar] [CrossRef] [PubMed]
- Uzoigwe, C.I.; Agwa, O.K. Antimicrobial activity of Vernonia amygdalina on selected urinary tract pathogens. Afr. J. Microbiol. Res. 2011, 5, 1467–1472. [Google Scholar] [CrossRef]
- Sharma, A.; Chandraker, S.; Patel, V.K.; Ramteke, P. Antibacterial Activity of Medicinal Plants Against Pathogens causing Complicated Urinary Tract Infections. Indian J. Pharm. Sci. 2009, 71, 136–139. [Google Scholar] [CrossRef] [PubMed]
| Drug | Dose and Duration | Common Adverse Effects | |
|---|---|---|---|
| Nitrofurantoin monohydrate macrocrystals | 100 mg twice daily for 5 d | Nausea, headache, gastrointestinal effects | |
| Pivmecillinam | 400 mg twice daily for 5 d | Rash and gastrointestinal upset, including nausea and vomiting | |
| Trimethoprim- sulfamethoxazole | 160/800 mg twice daily for 3 d | Rash, urticaria, nausea, vomiting, hematologic signs | |
| Fosfomycin tromethamine | 3 g single-dose sachet | Diarrhea, nausea, headache | |
| β-Lactams | The dose varies by agent from 5 to 7 d | Diarrhea, nausea, vomiting, rash, urticaria | |
| Fluoroquinolones | Ciprofloxacin | 500 mg twice daily or 1000 mg once daily for 3 d | Nausea, vomiting, diarrhea, headache, drowsiness, insomnia, tendon rupture, neuropathy |
| Norfloxacin | 400 mg twice daily for 3–7 d | ||
| Ofloxacin | 200–400 mg twice daily | ||
| Levofloxacin | 750 mg once daily for 3 d | ||
| Type of Vaccine | Component of Vaccine | Comments | References | |
|---|---|---|---|---|
| Whole-cell vaccines | Inactivated vaccines |
|
| [179,192,193] |
|
| [181] | ||
|
| [182,183,184] | ||
|
| [186,187] | ||
|
| [186,187] | ||
|
| [188] | ||
|
| [194] | ||
| Attenuated vaccines |
|
| [195] | |
| Antigen-specific vaccines | Capsular- or LPS-based vaccines |
|
| [196,197] |
| Fimbrial and non-fimbrial adhesin vaccines |
|
| [198,199,200] | |
|
| [198,201,202] | ||
|
| [20] | ||
| Iron-scavenger-receptor-based vaccines |
|
| [121,200,203,204] | |
| Toxin-based vaccines |
|
| [2,200] | |
| No. | Botanical Name | Part Used | Main Compound Class | Effect and Mechanism | Proof Obtained | References |
|---|---|---|---|---|---|---|
| 1 | Abelmoschus manihot (L.) Medik. | Flowers | Phenolics | Acts against LPS-induced cystitis; attributed to its anti-inflammatory profile by suppressing TLR4/MYD88/NF-κB pathways. | In vivo | [234] |
| 2 | Agropyron repens (L.) P. Beauv. | Rhizome | Phenolics, flavonoids | Decreased bacterial adhesion; interaction with bacterial outer-membrane proteins. | In vivo | [235,236] |
| 3 | Alchornea cordifolia (Schumach. and Thonn.) Müll.Arg. | Leaves, stem bark | Terpenoids, phenolics | Antibacterial activity on ESBL-producing E. coli isolates. | In vitro | [237] |
| 4 | Andrographis paniculate (Burm. F.) Nees | Leaves | Terpenoid | Inhibition of LPS-induced iNOS and COX-2 protein expression; negative regulation involving STAT3 phosphorylation and NF-κB activation. | In vitro | [238] |
| 5 | Arctostaphylos uva-ursi (L.) Spreng. | Leaves | Phenolics | UTI control; shrinking and tightening of mucous membranes. | In vitro | [239,240] |
| 6 | Aristolochia indica L. | Whole plant | Aristolochic acid analogs | Antibacterial activity against MDR UPEC. | In vitro | [241,242] |
| 7 | Armoracia rusticana (Lam.) P. Gaertner et Schreb. | Roots | Isothiocyanates | Possible damage to the cell membrane. | In vitro, in vivo, clinical | [243,244] |
| 8 | Arnica montana L. | Flowers | Terpenoids | Biofilm-modulating activity on UPEC. | In vitro | [229,230] |
| 9 | Avicennia marina (Forsk.) Vierh. | Leaves | Phenolics | Antibacterial activity. | In vitro | [245] |
| 10 | Betula pendula Roth. | Leaves | Phenolics | Bactericidal activity; modifications to the bacterial surface structures responsible for binding to the occupied surface. | In vitro | [246] |
| 11 | Boerhaavia diffusa L. | Hairy root, root | Phenolics | Active against UPEC MDR strains. | In vitro | [247,248] |
| 12 | Bridelia ferruginea Benth. | Leaves | Flavonoids, phenolics | Antibacterial activity. | In vitro | [249,250] |
| 13 | Calluna vulgaris Salisb. | Leaves, flowers | Phenolics | Antibacterial activity. | In vitro | [251] |
| 14 | Citrus reticulata Blanco | Seeds | Flavonoids, volatile oils | Reduction in UPEC invasion; decreased β1 integrin expression. | In vitro | [252,253] |
| 15 | Costus spicatus (Jacq.) Sw. | Leaves | Phenolics | Antimicrobial activity; correlation between the antioxidant and antimicrobial activity. | In vitro | [254] |
| 16 | Crateava nurvala Buch-Hum (Varuna) | Bark | Alkaloids, saponins | Growth inhibition. | In vitro, clinical | [255,256] |
| 17 | Curcuma longa L. | Rhizome | Phenolics | Antibiofilm activity; the inhibition of swimming and swarming behavior; the enhanced susceptibility of UPEC to antibiotics; alterations to biofilm morphology, including a reduction in thickness. | In vitro | [257] |
| 18 | Cymbopogon citratus (DC.) Stapf | Whole plant | Terpenoids | Antimicrobial activity. | In vitro | [258] |
| 19 | Cyperus rotundus L. | Rhizome | Terpenoids | Antibacterial activity. | In vitro | [259] |
| 20 | Dendrobium officinale Kimura et Migo | Rhizome | Polysaccharides | The mitigation of UPEC-promoted pyroptosis in macrophage cells; the inhibition of the NLRP3/caspase-1/GSDMD pathway and ROS signal activation. | In vitro | [260] |
| 21 | Equisetum arvense L. | Leaves | Phenolics | Antimicrobial activity; the inhibition of biofilm mass production; antiadhesive action; modifications to the bacterial surface structures responsible for binding to the occupied surface. | In vitro | [235,246] |
| 22 | Galium odoratum (L.) Scop. | Leaves | Phenolics | Modifications to the bacterial surface structures responsible for binding to the occupied surface. | In vitro | [246] |
| 23 | Gynostemma pentaphyllum (Thunb.) Makino | Leaves | Terpenoids, dammarane-type saponins | Reduction in pro-inflammatory response of BECs to UPEC; the modulation of antimicrobial peptides; NF-κB inhibition and ERK activation. | In vitro | [261] |
| 24 | Herniaria glabra Linnaeus. | Leaves | Phenolics | High bactericidal activity; the inhibition of biofilm mass production; modifications to the bacterial surface structures responsible for binding to the occupied surface. | In vitro | [246] |
| 25 | Labisia pumila var. alata (Scheff.) Mez | Herbal | Phenolics | Reduction in uroepithelial apoptosis and the number of intracellular UPEC cells in BECs; reduction in the expression of β1 integrin | In vitro | [262] |
| 26 | Lactuca indica L. | Leaves | Terpenoids, phenolics | Reduction in the bacterial colonization of bladder epithelial cells; the inhibition of FAK, significantly decreasing bacterial adherence. | In vitro | [263,264] |
| 27 | Lawsonia inermis L. | Leaves | Xanthones | Antimicrobial activity. | In vitro | [249] |
| 28 | Ocimum gratissimum L. | Leaves, flowers | Terpenoids | Antimicrobial activity. | In vitro | [258] |
| 29 | Orthosiphon stamineus Benth. | Leaves | Flavonoids, terpenoids, essential oils | Antiadhesive effects; direct interaction between compounds from the extract and the bacterial adhesins. | In vivo | [235,265] |
| 30 | Parkia biglobosa (Jacq.) Benth | Roots, bark | Phenolics | Antibacterial activity. | In vitro | [266,267] |
| 31 | Peganum. Harmala L. | Seeds | Alkaloids, quinazoline derivatives | Antibacterial Activity. | In vitro | [268,269] |
| 32 | Petasites albus (L.) Gaertn. | Leaves, flower stems, rhizomes | Terpenoids | Biofilm-modulating activity on UPEC. | In vitro | [229,270] |
| 33 | Petasites hybridus (L.) G.Gaertn., B.Mey. and Schreb. | Leaves, flower stems, rhizomes | Terpenoids | Biofilm-modulating activity on UPEC. | In vitro | [229,270] |
| 34 | Petroselinum crispum (Mill.) Hill | Leaves | Phenolics | Antibacterial activity. | In vitro | [271] |
| 35 | Piper arboreum Aubl. | Leaves | Terpenoids | Modulatory activity, synergistic activity with antibiotic drugs. | In vitro | [272,273] |
| 36 | Persicaria capitata (Buch.-Ham. ex D. Don) H. Gross | Whole plant | Terpenoids, phenolics | Anti-inflammatory and moderate antibacterial activity. | In vitro, in vivo | [274] |
| 37 | Punica granatum L. | Seed | Phenolics | Antibacterial activity. | In vitro | [259] |
| 38 | Rhodiola rosea L. | Roots, rhizomes | Phenolics | Biofilm-modulating activity on UPEC. | In vitro | [229] |
| 39 | Rosa canina L. | Fruit | Vitamins, minerals | The prevention of UTIs. | In vitro, clinical | [275] |
| 40 | Rosmarinus officinalis L. | Leaves | Phenolics | Antibacterial activity. | In vitro | [271] |
| 41 | Salvia officinalis L. | Leaves | Terpenoids | Antimicrobial activity. | In vitro | [258] |
| 42 | Salvia plebeia R. Br. | Whole plant | Flavonoids, terpenoids, Phenolic acids | Diuretic activity; UPEC susceptibility. | In vitro, in vivo | [276,277] |
| 43 | Schefflera leucantha R. Viguier | Leaves | terpenoids, saponins | Antibacterial activity. | In vitro | [278,279] |
| 44 | Toddalia asiatica (L.) Lam. | Whole plant, leaves | Phenolics alkaloids | Antibacterial activity against MDR UPEC. | In vitro | [241,280] |
| 45 | Tropaeoli majoris herba | Leaves | Isothiocyanates | Intermediate susceptibility; possible damage to the cell membrane. | In vitro, in vivo, clinical | [243,244] |
| 46 | Urtica dioica L. | Leaves | Phenolics | Antimicrobial activity; antiadhesive effects; modifications to the bacterial surface structures responsible for binding to the occupied surface, and the direct interaction between compounds from the extract and the bacterial adhesins. | In vitro, in vivo | [235,246] |
| 47 | Vaccaria segetalis (Neck.) Garcke | Seeds | Polysaccharides | The upregulation of innate immunity in the kidney. | In vivo | [281] |
| 48 | Vaccinium macrocarpon Aiton | Fruit | polyphenols | The obstruction of bacterial adhesion to bladder cells; downregulation or interference with several bacterial virulence factors. The repression of the inflammatory cascades triggered by the immune system; the inhibition of UPEC motility. | In vitro, in vivo | [218] |
| 49 | Vaccinium vitis-idaea L. | Leaves | Phenolics | High bactericidal activity; the inhibition of biofilm mass production; modifications to the bacterial surface structures responsible for binding to the occupied surface. | In vitro | [246] |
| 50 | Vernonia amygdalina L. | Leaves stems | Terpenoids | Antimicrobial activity. | In vitro | [249,282] |
| 51 | Zea mays L. | Stigmata | Phenolics | Decreased bacterial adhesion; interaction with bacterial outer-membrane proteins. | In vivo, in vitro | [235] |
| 52 | Zingiber officinale Roscoe | Rhizomes | Terpenoids | Antibacterial activity. | In vitro | [283] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhou, Z.; Zheng, L.; Gong, Z.; Li, Y.; Jin, Y.; Huang, Y.; Chi, M. Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options. Int. J. Mol. Sci. 2023, 24, 10537. https://doi.org/10.3390/ijms241310537
Zhou Y, Zhou Z, Zheng L, Gong Z, Li Y, Jin Y, Huang Y, Chi M. Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options. International Journal of Molecular Sciences. 2023; 24(13):10537. https://doi.org/10.3390/ijms241310537
Chicago/Turabian StyleZhou, Yang, Zuying Zhou, Lin Zheng, Zipeng Gong, Yueting Li, Yang Jin, Yong Huang, and Mingyan Chi. 2023. "Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options" International Journal of Molecular Sciences 24, no. 13: 10537. https://doi.org/10.3390/ijms241310537
APA StyleZhou, Y., Zhou, Z., Zheng, L., Gong, Z., Li, Y., Jin, Y., Huang, Y., & Chi, M. (2023). Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options. International Journal of Molecular Sciences, 24(13), 10537. https://doi.org/10.3390/ijms241310537






