Abstract
Neutrophil gelatinase-associated lipocalin (NGAL) is a 25-kDa protein that is secreted mostly by immune cells such as neutrophils, macrophages, and dendritic cells. Its production is stimulated in response to inflammation. The concentrations of NGAL can be measured in plasma, urine, and biological fluids such as peritoneal effluent. NGAL is known mainly as a biomarker of acute kidney injury and is released after tubular damage and during renal regeneration processes. NGAL is also elevated in chronic kidney disease and dialysis patients. It may play a role as a predictor of the progression of renal function decreases with complications and mortality due to kidney failure. NGAL is also useful in the diagnostic processes of cardiovascular diseases. It is highly expressed in injured heart tissue and atherosclerostic plaque; its serum concentrations correlate with the severity of heart failure and coronary artery disease. NGAL increases inflammatory states and its levels rise in arterial hypertension, obesity, diabetes, and metabolic complications such as insulin resistance, and is also involved in carcinogenesis. In this review, we present the current knowledge on NGAL and its involvement in different pathologies, especially its role in renal and cardiovascular diseases.
1. Introduction
Neutrophil gelatinase-associated lipocalin (NGAL) is a protein that is secreted by activated neutrophils [1]. Although neutrophils are the main source of NGAL, their expression is also found in numerous human tissues, including tubular cells in the kidney, heart, lung, liver, stomach, colon, epithelial cells, macrophages, dendritic cells and adipocytes (Figure 1) [2,3,4,5,6,7,8]. NGAL is also known as lipocalin-2 and represents a family of lipocalin proteins [9]. Its structure is often described as a three-dimensional barrel [10]. It contains a ligand-binding site called calyx which allows receptors to attach to the surface of membranes and create bigger molecules [3]. Neutrophil chemoattractants, specifically N-formylated tripeptides, with leukotriene B4 and the platelet-activating factor, are the major group of ligands that connect to the binding side of NGAL [11]. There are three forms of NGAL: a 25 kDa monomer which is released by renal tubules, a 45 kDa homodimer secreted by neutrophils in an inflammatory response and a 135 kDa complex of NGAL with matrix metalloproteinase (MMP-9) [1,12,13]. The connection of NGAL to MMP-9 increases the activity of MMP-9 and protects against its degradation [14]. This results in enhanced proteolysis and the dissolution of collagen and contributes to fibrosis [15,16].
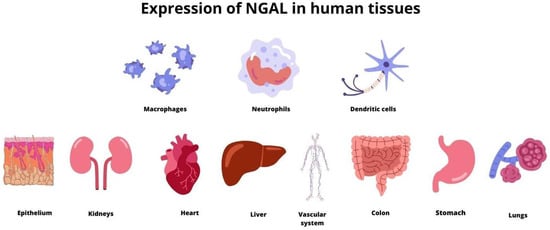
Figure 1.
The expression of NGAL in numerous human tissues.
Two receptors for NGAL can be found: lipocalin-2 receptor (24p3R) and megalin receptor. The role of the lipocalin-2 receptor is multifactorial. It regulates intracellular iron concentrations. It is also expressed in cardiomyocytes with up-regulation during myocarditis and is known to be involved in smooth muscle cell proliferation, cardiac remodeling, and cardiac fibrosis [17,18]. The role of the megalin receptor is not yet well understood. Studies have shown that it binds NGAL with high affinity: much higher than other lipocalins [19]. Its expression can be observed in cardiomyocytes, kidney and ileum epithelial cells, lung ependyma, epididymis, immune cells, and numerous types of cancer cells [20,21,22]. It is involved in various cancer processes such as proliferation, migration, angiogenesis, immunotolerance, and multidrug resistance [23].
2. The Biological Role of NGAL
NGAL is known to be a syderophoric protein that plays a role in regulating iron activity [24]. The molecule of NGAL-containing iron interacts with receptors on the cell surface. Then, it is transported into the cell and releases iron inside [25]. NGAL that is not bound to iron also interacts with the cell surface receptors, which results in an intracellular iron transfer out of the cell [26].
2.1. NGAL in Infections
NGAL plays a role as an acute phase protein and takes part in antibacterial immune processes. Inflammatory cytokines induce NGAL expression in neutrophils, epithelial cells, or hepatocytes [27,28,29]. The injury of epithelial cells in the intestine, stomach, liver, or lungs during infections results in an increase in plasma NGAL concentrations [30,31,32,33]. NGAL modulates iron transport as part of antibacterial immunity. During inflammatory processes, bacteria synthesize siderophores. The high affinity of siderophores for iron results in its dissociation from lactoferrin and transferrin and its transfer into the pathogen [34]. The macrophage of Toll-like receptor (TLR) stimulation up-regulates the NGAL gene and enhances NGAL synthesis. NGAL sequestrates siderophores, prevents bacteria from obtaining iron, and thus decreases bacteria growth and multiplication, as the pathogen’s ability to proliferate is often dependent on the bioavailability of iron (Figure 2) [6,34]. It has been proved that NGAL binds iron together with a metabolic product called catechol and creates complexes [35]. During experiments with mice, NGAL controlled bacterial infections by changing the iron transfer [36]. NGAL has been observed to prevent the production of siderophores by Escherichia coli, which could be involved in pneumonia. Moreover, NGAL expression in bronchial epithelium and alveolar type II pneumocytes is increased during respiratory infection with Escherichia coli [33]. NGAL also protects the respiratory system from other types of infections, such as Stapylococcus aureus, Klebsiella pneumoniae, or Mycobacterium tuberculosis [37,38,39]. The inflammation of mucosa during gastritis caused by Helicobacter pylori also increases the local expression of NGAL [31]. Additionally, NGAL up-regulates bacterial clearance from the urinary system [40]. Although the sequestration of bacterial siderophores is the major bacteriostatic function of NGAL, it is also involved in the activation and transformation of T- cells towards the Th1 type [41]. It has been found that the function of neutrophils is impaired in the state of an NGAL deficit. This may result in the dysfunction of chemotaxis and the adhesion and migration of inflammatory cells [42]. Studies have shown that patients with decreased NGAL levels are more prone to various infections [6].
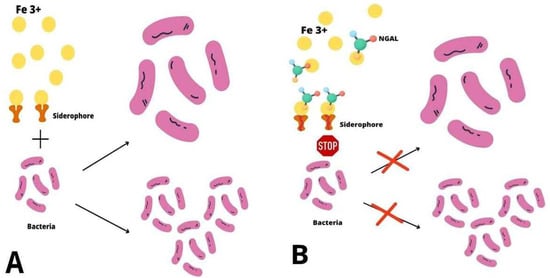
Figure 2.
The antibacterial mechanism of NGAL. (A) Bacterial iron uptake During inflammatory processes bacteria synthesize siderophores which scavenge iron ions (Fe 3+) and transfer them into the cell, which enables bacteria to grow and proliferate. (B) The role of NGAL in bacterial infections NGAL sequestrates siderophores, prevents bacteria from obtaining iron and thus decreases their growth and multiplication.
NGAL concentrations are increased in sepsis and correlate with inflammatory parameters such as interleukin-6 (IL-6), interleukin-10 (IL-10), vascular cellular adhesion molecule-1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), tumor necrosis factor-alpha (TNF-alpha), the C-reactive protein (CRP) and leukocytes account [43,44,45]. Additionally, plasma NGAL levels are higher in patients with septic shock and sepsis-related organ failure compared to those with a milder course of sepsis. Lentini found an association between the severity of a systemic inflammatory response and a higher plasma NGAL level in patients with sepsis and acute kidney injury [46]. However, further studies are necessary to assess if the risk of mortality is also higher in individuals with elevated plasma NGAL concentrations [43,47,48].
NGAL is also involved in fungal and viral infections. Despite the fact that the exact role of NGAL in fungal infection is not yet well known, a better expression of the NGAL gene was found in candidiasis [49]. The induction of NGAL gene expression has been documented in epithelial cells in patients with infections caused by human papillomavirus and rotavirus [50,51]. In children with dehydration caused by rotavirus infections, the NGAL level can be an early indicator of renal impairment [52]. By contrast, in patients with a human immunodeficiency virus (HIV), the serum NGAL level is low due to the decreased number and impaired function of neutrophils [53].
2.2. NGAL in Metabolic Complications
NGAL concentrations rise in many pathological states [51,52]. NGAL is expressed in numerous types of tissues, and its concentrations increase during injury. Inflammatory cytokines induce NGAL expression in neutrophils, epithelial cells, or hepatocytes [53,54,55]. Adipose tissue is also the source of NGAL, NGAL concentrations are higher in obesity, diabetes mellitus type 2, and nonalcoholic fatty liver disease [54,55]. There are studies that have proved that the estimation of NGAL concentrations in serum could be useful in predicting the metabolic complications caused by obesity [55,56]. NGAL is an independent risk factor for insulin resistance, systolic blood pressure, and lipid metabolism disorders [56,57,58,59,60]. The role of NGAL in the pathogenesis of obesity is not yet well understood. However, it is associated with the regulation of a proliferator-activated receptor gamma (PPAR gamma)—a molecule that participates in adipogenesis and lipogenesis [61]. A study by Zhang found that obese mice had high levels of NGAL mRNA, which induced PPARgamma [61]. The anti-inflammatory function of NGAL in adipose tissue is also possible by inhibiting nuclear factor κB (NFκB) activity, which antagonizes the effect of TNF-alpha on local inflammation in fat, and increases IL-6 and monocyte chemoattractant protein-1 (MCP-1) production or inhibits the secretion of leptin and adiponectin [61,62]. Numerous studies have shown a significant association between NGAL and the activity of 12-lipoxygenase and TNF-alpha in adipose tissue. The function of these molecules is crucial to the development of insulin resistance. It has been proved that NGAL stimulates its expression and that the deficit of NGAL protects from the progress of glucose metabolism disorders [56,57]. It was also documented that the synthesis of NGAL was stimulated by hyperglycemia [63]. An NGAL urinary excretion is a marker of diabetic nephropathy and is detected in urine early as a result of high serum glucose concentrations, even before kidney injury [64,65]. Urine NGAL concentrations correlate with the stage of renal damage in diabetic nephropathy [66]. Moreover, Hafez proved in their study that the level of urine NGAL in children with diabetes could be associated with an albumin-to-creatinine ratio, the duration of diabetes, glycated hemoglobin concentrations, and dyslipidemia [67].
2.3. NGAL in Carcinogenesis
NGAL is also known as a growth factor. It stimulates the proliferation and differentiation of epithelial cells [68]. Due to this fact, the role of NGAL has been assessed in different types of cancer. In the experiment with mouse mammary tumor cells, Hanai discovered that NGAL participated in the conversion of 4T1-Ras-transformed mesenchymal tumor cells to an epithelial phenotype. It increased E-cadherin expression, which resulted in a rise in cell invasiveness, tumor growth, and lung metastases [69]. Human studies have also demonstrated that NGAL promotes breast cancer progression through the induction of mesenchymal markers such as vimentin or fibronectin [70,71]. The deficit of NGAL also impaired the migration of tumor cells [72]. Conflicting data from this research might be caused by the activation of Ras oncogen in one setting but not in another [73]. In thyroid carcinoma, NGAL is responsible for the existence of tumor cells by regulating NFκB activity, which is connected with iron homeostasis [74]. The pro-metastatic role of the NGAL/MMP-9 complex in aggressive thyroid carcinomas was also considered [75]. In gastric cancer, the detection of this complex has been associated with a worse prognosis [76,77]. The increased activity of NGAL/MMP-9 accelerates the migration and invasion of malignant cells and promotes the metastasis of esophageal squamous cell carcinoma and prostate cancer [78,79,80,81]. Numerous studies have shown that NGAL has the ability to bind with MMP-9 and subsequently scavenge iron into cancer cells, thereby increasing the aggressiveness of the disease. Hopefully, NGAL could even be a biomarker of malignancy and the aim of anticancer therapies in the future, but further research is necessary [14,82,83,84,85].
3. NGAL in Kidney Diseases
3.1. NGAL in Acute Kidney Injury
In 2003 Mishra proposed that NGAL could be an early biomarker of acute kidney injury (AKI), initially in experimental and then clinical studies [86,87]. They found that in a mouse model of renal ischemia injury, NGAL was a very sensitive marker of ischemic AKI, and its urine concentrations were associated with the severity and duration of ischemia [86]. Many clinical studies have followed these observations [88,89,90]. Numerous reports have proved that NGAL is synthesized in kidney tissue following several mechanisms of kidney injury, such as ischemic, nephrotoxic, or septic [43,87,91,92,93,94]. NGAL can be detected in plasma within two hours of AKI, with a concentration peak after 6 h. Increased serum NGAL levels were observed for approximately five days after AKI before they decreased [95]. AKI also resulted in the elevation of urine NGAL levels. Elevated serum and urine NGAL, due to AKI, were observed 24 h earlier than the increase in creatinine [87,96]. There was no superiority of plasma NGAL over urine NGAL and urine NGAL over plasma NGAL in AKI, and these can be used according to laboratory preference [97].
It is nowadays known that NGAL is a marker of renal tubular damage as it is released from the distal tube [98]. The molecule is filtered through the glomerular membrane and is reabsorbed in the proximal tubule of the kidney. The NGAL observed in urine is caused by proximal tubular damage or originates from its up-regulated synthesis in the distal part of the nephron, especially in the ascending limb and Henle’s loop, and in the collecting duct [99]. The expression of NGAL in the regenerating tubular epithelial cells increases significantly after kidney injury [68,100]. Thus, NGAL in urine during AKI often originates from an impaired up-regulation in the proximal tubule segments and also from its intensified synthesis and secretion in the distal parts of the nephron. Increased NGAL synthesis in tubular epithelial cells, even in the early stages of AKI, often results from kidney regenerative processes. NGAL provides iron intracellular availability and, thus, promotes kidney regeneration. Increased plasma NGAL levels during AKI are multifactorial. One of the sources of NGAL in the serum is the activation process of neutrophils and monocytes during the acute phase of the reaction [101]. Additionally, the synthesis of NGAL in the liver and lungs during AKI is significantly elevated [99]. Moreover, a decrease in renal function results in the accumulation of NGAL in plasma, and an increase in its serum concentrations could be observed [98].
In conclusion, NGAL can be treated as a biomarker of acute kidney injury. However, its usefulness is limited in some diseases, especially when systemic inflammation occurs [102]. Some studies have indicated that NGAL levels may be preferable to serum creatinine concentrations for AKI prediction when measured at the same time [103,104]. The mechanisms of increased NGAL are presented in Table 1 and Figure 3.

Table 1.
The causes of increased NGAL in acute kidney injury.
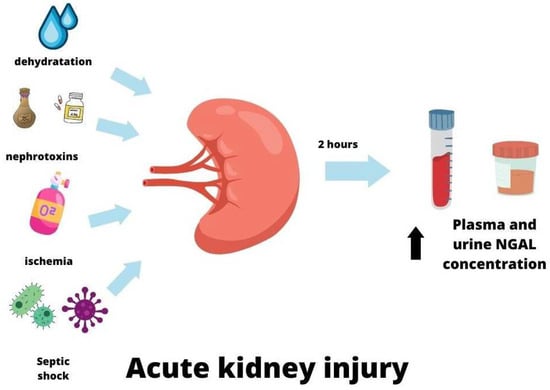
Figure 3.
NGAL in acute kidney injury.
3.2. NGAL in Chronic Kidney Disease
Patients with kidney function decrease have elevated serum NGAL concentrations compared to healthy controls, but the differences in serum NGAL levels between AKI and chronic kidney disease (CKD) patients are controversial. Some studies have proved that patients with AKI have higher plasma NGAL levels than patients with CKD. Other studies, however, have shown the opposite results [105,106]. The study of Gharishvandi found that NGAL was superior to cystatin C and creatinine in detecting a kidney function decrease in the early stages of CKD and in predicting its progression [107,108]. It has also been found that in patients with CKD, NGAL concentrations were negatively correlated with the eGFR value, which reflected the severity of kidney damage [99,108,109,110]. It is also known that NGAL can predict mortality in kidney function decreases [111]. The meta-analysis of Zhou revealed that NGAL could be an independent risk predictor of end-stage renal disease and mortality [112].
NGAL is secreted from injured kidney cells, and by taking part in proliferative processes and apoptosis, it is responsible for controlling tubular cells [113,114]. It mediates epidermal growth factor receptor (EGFR) signaling, whose activation stimulates hypoxia-inducible factor (HIF-1α) and finally enhances proliferation, cytogenesis, renal damage, and CKD progression [115,116,117]. Xiang found that NGAL can be associated with iron storage in CKD patients. They observed that NGAL was conversely correlated with hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), serum iron, and transferrin saturation (TSAT) [118]. Further research is needed, but plasma NGAL can hopefully become a more useful marker than serum ferritin for determining the iron status in CKD, including patients who require renal replacement therapy [119,120,121,122].
NGAL concentrations are elevated in different types of CKD. Its diagnostic value has been tested in the differentiation of primary inflammatory and non-inflammatory etiologies of CKD [123]. Ding compared the urinary levels of NGAL, creatinine, and N-acetyl-beta-D-glucosaminidase (NAG) in 40 healthy individuals and 70 patients with IgA nephropathy (IgAN) [124]. According to their findings, NGAL was the most sensitive marker of renal tubular injury in IgAN. This hypothesis was confirmed by Rhee, who also emphasized the prognostic value of NGAL in IgAN [125]. NGAL is also one of the best diagnostic markers in patients with SLE [126,127,128]. Some studies have found that NGAL acts as a nephroprotective agent. It probably modulates apoptosis in tubular cells and macrophages; however, additional studies are needed to clarify this mechanism [127,129]. Serum NGAL concentrations increase in diabetic nephropathy and correlate with the severity of kidney damage. Since an increase in the NGAL level of diabetic nephropathy occurs in the early stage of kidney injury, even in patients with normoalbuminuria, NGAL can play a role as a predictor of renal failure [66]. The role of NGAL in patients with autosomal dominant policystic kidney disease (ADPKD) has also been studied [130,131,132]. NGAL concentrations elevate with the impairment of renal function and with an increase in the number of cysts. Thus, NGAL has been suggested to be involved in the cyst growth process in ADPKD [130]. However, its role in ADPKD is not clear [133]. Some reports have shown that NGAL is able to inhibit cyst enlargement [134].
3.3. NGAL and Dialysis
NGAL has been found to be an independent predictor of CKD progression [108]. HD patients with residual renal function have significantly lower serum NGAL concentrations compared to anuric individuals. Thus, NGAL levels can also reflect the degree of kidney function decreases in end-stage renal disease (ESRD). In the group of patients treated with HD, apart from renal dysfunction, low-grade inflammation, which is more intensified in anuric individuals, can contribute to increased serum NGAL levels [135]. Arteriovenous fistula (AVF) is the preferred vascular access for HD over an arterious graft (AVG) and a central venous permanent catheter (CVPC) due to a lower risk of infection episodes. Serum NGAL was found to be higher in CVPC patients compared to those with AVF and AVG. Moreover, its plasma concentrations correlated with the duration of the catheter and also with increased inflammatory parameters such as the high sensitivity of CRP (hs-CRP), IL-6, TNF-alpha, and ferritin. Thus, NGAL can act as an inflammatory marker in HD patients, especially when hemodialised with the use of CVPC, especially in those who are mostly susceptible to infections. The same study found an inverse relationship between serum NGAL and albumin in HD patients which could indicate the role of NGAL in the poor nutritional status of this group [45]. Imamaki also revealed that NGAL is related to malnutrition in patients treated with HD. They found a significant relationship between plasma NGAL and markers of nutrition, such as muscle mass and protein intake [136]. Apart from inflammation and residual renal function in ESRD, serum NGAL concentrations were also associated with iron deficiency anemia (IDA), which is higher in HD individuals with lower tranferrin saturation [137]. Very similar results were observed in the study of Aghsaeifard, which included 47 HD participants. The serum NGAL concentrations correlated significantly with IDA. They also found a negative relationship between serum NGAL and nutritional parameters such as albumin and total cholesterol, which could suggest the role of NGAL in the development of malnutrition in CKD patients treated with HD [138]. Jie investigated the association between NGAL and mineral bone disorders in HD patients, which are common complications of CKD. They found that plasma NGAL levels were associated with serum phosphatase, calcium, and phosphate, which suggests that NGAL could contribute to mineral bone disorders in HD individuals [139]. The study of Yigit with 61 HD participants found a positive relationship between serum NGAL and parathormone (PTH) in patients with severe hyperparathyroidism. They also revealed a significantly negative relationship between NGAL and hemoglobin in the group of HD patients with severe hyperparathyroidism [140].
NGAL reflects the status of the peritoneal membrane in peritoneal dialysis (PD) patients. Peritonitis, which is one of the complications of peritoneal dialysis, is associated with an increased risk of death, hospitalization rate, and peritoneal membrane failure. The diagnosis of peritonitis is made based on numerous clinical symptoms, including abdominal pain, nausea, vomiting, diarrhea, constipation, or a cloudy effluent. Laboratory measurements have revealed increased inflammatory parameters and a positive peritoneal fluid culture. However, there is often a lack of specific markers for peritonitis which could be useful in early diagnosis and enable the implementation of treatment in the initial stage of peritonitis. As NGAL was found in a peritoneal dialysis effluent, some studies have suggested that it may be considered a marker of perotonitis. The report of Virzì, which examined 27 episodes of peritonitis in 22 PD patients, found a positive relationship between NGAL concentrations and the WBC count in peritoneal dialysis effluent. This association was observed at each stage of peritonitis, with an increase in WBC and NGAL in the peritoneal dialysis effluent during the course of the disease, alongside their decrease in the recovery process. This study confirmed that peritoneal NGAL could be a reliable marker of peritonitis; moreover, it may be used in monitoring the course of the disease [141]. Previous studies have shown similar results. The report of Martino included 182 PD patients, among which 80 episodes of peritonitis were observed. They also found that NGAL in peritoneal dialysis effluent could be a marker of peritonitis episodes [142]. Lacquaniti proved that changes in peritoneal fluid NGAL concentrations in patients with peritonitis could play a diagnostic role in the process of treatment. They observed that the levels of NGAL in the peritoneal dialysis effluent fell at least 24 h earlier than the peritoneal WBC count [143].
3.4. NGAL in Kidney Transplantation
Numerous studies found that NGAL could be a marker of graft functions after kidney transplantation [144,145,146]. Higher plasma NGAL concentrations were observed before acute graft rejection [147]. Since serum NGAL levels were elevated in numerous pathologies, which could lead to graft dysfunction, increased NGAL concentrations and graft failure could be diagnosed by performing a biopsy and histopathology examination [148]. Additionally, the changes in NGAL levels could also indicate how fast the graft was going to assume its function [149,150]. Urinary NGAL concentrations in patients after kidney transplants with good graft function did not differ from healthy individuals [148,151].
4. The Role of NGAL in Cardiovascular Diseases
NGAL concentrations increased in patients with cardiovascular complications. NGAL could be expressed in heart tissue and in atherosclerotic plaques. Its plasma levels were elevated in coronary artery disease and also in acute and chronic heart failure [10,152,153,154,155]. Increased serum NGAL levels in patients with cardiovascular complications could be associated with renal dysfunction; however, numerous studies also found high NGAL concentrations in patients with cardiovascular events without kidney injury [156,157].
4.1. NGAL in Atheroslerosis
Inflammatory processes are often involved in the development of atherosclerosis from the onset of endothelial dysfunction through the formation of atherosclerotic plaque, which finally leads to plaque rupture with the formation of occlusive thrombus and the occurrence of acute coronary syndrome [158]. Activated neutrophils were found in atherosclerotic plaque [159]. In response to TNF-alpha, bone marrow-derived macrophages release NGAL and their stimulation by NGAL causes the up-regulation of M1 macrophage markers, the expression of scavenger receptor class A-1, and their conversion into foam cells [160]. NGAL may increase proteolytic activity inside the atherosclerotic plaque, which contributes to lower atherosclerotic plaque stability and, in consequence, also leads to cardiovascular events. It was assumed that the connection of NGAL with MMP-9, which potentiated the proteolytic activity of MMP-9, could result in plaque instability. The activation of MMP-9 is thought to be one of the mechanisms responsible for the development of inflammation and atheroslerosis [153,161]. The concentrations of the NGAL/MMP-9 complex were increased in the atherosclerotic plaque with central necrosis or hematoma, which are the states that make the atherosclerotic plaque more vulnerable to rupture [162]. Boekhorst found that the NGAL/MMP-9 complex was highly expressed in human atherosclerotic plaque and assumed that NGAL could be a novel tool for detecting patients with high-risk atherosclerotic plaques [163]. Patients with symptomatic carotid atherosclerosis had higher plasma NGAL concentrations compared to asymptomatic individuals. In addition, individuals with vulnerable plaque had higher levels of serum NGAL [164]. The study of Kafka, which included 140 patients with coronary artery disease, showed that serum NGAL concentrations increased with the severity of coronary artery disease—stable angina, unstable angina, non-ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation myocardial infarction (STEMI)—with the highest value in STEMI patients [165]. This correlation has also been confirmed in other studies [166,167]. Moreover, Sahinarslan found that patients with higher serum NGAL concentrations suffered from an increased incidence of acute myocardial infarction compared to individuals with stable coronary artery disease who had lower plasma NGAL levels [168]. The study of Lialso also revealed that patients with STEMI had significantly higher NGAL levels compared to those with stable angina or the control subjects [169]. However, even in the case of stable coronary artery disease, the concentrations of the NGAL/MMP-9 complex were higher than in healthy individuals [170]. The study of Chen, which included 177 participants with major adverse cardiovascular and cerebrovascular events (MACCE), proved that increased serum NGAL concentrations were significantly associated with the risk of MACCE [171]. Advanced atherosclerosis could be related to arterial stiffness and is known to be associated with worse outcomes [172]. In the study by Soylu, which included 101 patients, plasma NGAL concentrations negatively correlated with an aortic strain and distensibility but were positively associated with aortic stiffness [173].
4.2. NGAL in Myocardial Infarction
Plaque NGAL concentrations increased in patients with acute myocardial infarction (MI). Inflammation plays a crucial role in the ischaemia-reperfusion injury of the heart [174]. Neutrophils infiltrate the infarction area and initiate inflammatory processes which enable a reduction in the damaged area; however, on the other hand, their excessive infiltration may be unfavorable for myocardial healing. NGAL is one of the numerous glycoproteins that are secreted by neutrophils during myocardial infarction, and its concentrations in the left ventricle after one week of myocardial infarction are significantly elevated [175]. Increased serum NGAL levels in patients with MI are associated with negative outcomes [176]. The study of Avci, which included 235 patients with STEMI, showed that plasma NGAL concentrations were significantly higher in patients who died compared to participants who survived. Additionally, patients with a decreased ejection fraction (EF) after MI had elevated serum NGAL levels, unlike participants with preserved EF [177]. Echocardiographic analysis revealed that myocardial function after ischemic injury was better in individuals with lower plasma NGAL concentrations [178]. Additionally, the use of anti-NGAL antibodies in a study with mice resulted in a reduced number of macrophages and neutrophils in the ischemic zone and the limitation of cardiac lesions [174]. The report of Karetnikova showed that patients with increased serum NGAL concentrations after 12 days of STEMI had a higher incidence of adverse outcomes such as recurrent myocardial infarction, early post-infarction angina, an acute cerebrovascular event, or death [179]. Additionally, the meta-analysis of Fan found that patients with STEMI and higher plasma NGAL concentrations had a 47% to 52% greater risk of all-cause mortality and major adverse cardiovascular events (MACE). They assumed that the value of plasma NGAL levels in patients with STEMI could be a tool to stratify patients with STEMI in clinical practice [180]. NGAL is an independent predictor of both MACE frequency and mortality after STEMI [181,182].
4.3. NGAL in Heart Failure
The inflammation and degradation of the matrix are the processes involved in the pathogenesis of heart failure (HF). It has been found that NGAL is involved in cardiac remodeling and, therefore, could be considered as biomarker for HF [161,183,184,185]. The NGAL/MMP-9 complex increases MMP-9’s enzymatic activity and intensifies cardiac remodeling. NGAL also participates in immune cell migration processes, which acts as a growth factor and promotes the proliferation and differentiation of vascular smooth muscle cells or cardiac fibroblasts [10]. Serum NGAL levels were increased in patients with acute and chronic heart failure due to myocardial infarction [186]. It has been proved in both clinical and experimental studies that the increase in plasma NGAL is the result of myocardial injury, which confirms that immune processes participate in the pathogenesis of HF [186]. Patients with chronic HF and increased serum NGAL serum concentrations have a higher mortality rate; therefore, NGAL may be a prognostic marker of survival in this group [187]. Serum plasma NGAL levels in individuals with chronic HF have been observed to be negatively correlated with EF [188]. Additionally, patients with chronic HF have higher serum NGAL concentrations compared to healthy subjects. Moreover, plasma NGAL levels increased with the severity of HF, with the highest in class IV of the NYHA (New York Heart Association) classification [187].
NGAL may also be considered a biomarker of acute HF. Individuals with acute HF and increased serum NGAL concentrations experienced a higher mortality rate, as well as a higher incidence of cardiovascular complications and hospital readmission [189]. Therefore, serum NGAL levels could help to stratify patients with acute HF. Plasma NGAL concentrations at the time of discharge from the hospital were found to be a predictor of 30-day outcomes in patients with acute HF [190].
4.4. NGAL in Cardiorenal Syndrome
The estimation of NGAL concentrations could predict AKI during hospitalization due to HF [189]. NGAL, as a marker of renal dysfunction, is useful in identifying HF patients in the early stages of kidney function decreases who are at high risk of developing cardiorenal syndrome (CRS). CRS is defined as a multi-organ disease, which is caused by an interaction between the heart and kidney when the acute or chronic dysfunction of one organ leads to acute or chronic dysfunction in the other. There are five types of cardiorenal syndrome that are based on primary organ failure, causing the derangements of the second one—cardiorenal or renocardiac, acute, or chronic. Type 5 cardiorenal syndrome can be diagnosed if systemic diseases such as sepsis, diabetes mellitus, or amyloidosis lead simultaneously to kidney and heart dysfunctions [191]. Among numerous classical biomarkers of acute kidney failure, such as serum creatinine, eGFR, albuminuria, and cystatin C, NGAL was found to be one of the earliest markers of renal injury. The study by Alvelos reported that the determination of serum NGAL concentrations in patients with acute HF and preserved renal function at admission to the hospital may be a helpful tool in diagnosing an early stage of type 1 CRS [192]. Numerous recent studies have confirmed that NGAL might be a predictive marker of acute kidney injury due to heart failure. The report by Nasonova proved that increased urine NGAL concentrations were prognostic markers of kidney function decreases in patients with acute decompensation of chronic heart failure [193]. The study of Dankova also proved elevated urine NGAL levels at admission to the hospital due to acute HF and predicted the following development of AKI [194]. Moreover, the report by Thai, which included 139 patients with acute heart failure, revealed that increased plasma NGAL concentrations along with elevated creatinine levels could predict the development of CRS 1 [195]. Since the rise in plasma NGAL exceeded an increase in serum creatinine, this also played a predictive role in the onset of acute kidney failure and thus allowed us to implement preventive therapeutic procedures [196]. As NGAL alone may be an independent predictive marker of CRS, the combination of serum NGAL with NT-proBNP is helpful in the early diagnosis of CRS 1 [197]. In an experimental model of chronic heart failure, an increase in NGAL concentrations could aggravate heart and renal dysfunctions by elevating the enzymatic activity of MMP-9 and intensifying extracellular matrix degradation. Thus, NGAL may also take part in the pathogenesis of type 2 CRS [198].
4.5. NGAL in Arrhythmias
As NGAL participates in cardiac remodeling, it could also be hypothesized that it is also involved in the occurrence of atrial fibrillation (AF) episodes [199]. The maintenance of sinus rhythm after an episode of atrial fibrillation and electrical cardioversion depends on various factors, e.g., the left atrium size, age, or the longevity of atrial fibrillation. The study of Mlodawska found that plasma concentrations of the NGAL/MMP complex could predict AF recurrence after successful electrical cardioversion in obese patients. The increased concentrations of serum NGAL/MMP levels were positively associated with the recurrence of AF [200].
4.6. NGAL in Hypertension
Hypertensive patients had higher plasma NGAL concentrations compared to normotensive individuals [201]. Primary hyperaldosteronism is one of the main causes of secondary hypertension. It has been proved that NGAL is involved in the development of hypertension, which is induced by increased aldosterone concentrations. The exact mechanism is still not entirely known. It is assumed that it may be associated with the activation of immune cells [202]. After the stimulation of dendritic cells and macrophages, which took place during aldosterone excess, the synthesis of NGAL increased and led to the fibrosis of blood vessels and the development of hypertension [203,204,205]. Moreover, as NGAL is an important biomarker of renal dysfunction, it may be helpful to detect kidney damage in the early stages of kidney failure in hypertensive individuals [206]. Serum NGAL concentrations correlate with ambulatory blood pressure values; thus, it is possible to diagnose a group of patients with a higher risk of cardiovascular diseases and mortality due to increased plasma NGAL [207].
4.7. NGAL in Cardiac Surgery
There are numerous studies that have shown that plasma and urine NGAL concentrations increase in patients undergoing cardiac surgery [208,209]. Elevated serum NGAL is associated with acute kidney injury, which is a frequent complication of heart surgery. Thus, NGAL is a marker of renal insufficiency in patients undergoing cardiac surgery [210,211].
4.8. NGAL in Abdominal Aortic Aneurysm
NGAL is also considered a promising marker for pathogenesis and the progression of abdominal aortic aneurysms (AAA) [212]. Polymorphonuclear neutrophils isolated from patients with AAA were able to secrete more NGAL. In a mouse model with NGAL depletion or anti-NGAL treatment, it was proved that the formation of AAA was limited, possibly due to the depletion of neutrophils [213]. Ramos-Mozo discovered that luminal thrombus released large amounts of NGAL in comparison with abluminal AAA thrombus, the AAA wall, and healthy aortic media [214].
5. NGAL in Biological Fluids and Additional Functions of NGAL
NGAL is also present in biological fluids. Agrawi studied NGAL expression in the saliva and tears of patients with primary Sjögren’s syndrome (pSS) and found that NGAL was up-regulated in both of them [215]. The continuation of this study was the research, which for the first time, explored NGAL expression at the site of inflammation in the pSS target organ. The results of this report were very promising. They found that the expression of NGAL in the acinar and ductal epithelium of the salivatory gland of pSS was significantly higher compared to patients without pSS [216]. Thus, NGAL may hopefully become, in the future, a promising marker for diagnostic procedures of pSS and monitoring the disease in the future.
NGAL has also been observed in human breast milk postpartum. Metallinou investigated the changes in NGAL concentrations in human breast milk in normal pregnancies and pregnancies that developed insulin-dependent gestational diabetes mellitus (iGDM). They found that the concentrations of NGAL were the highest on the first day of colostrum, with a fall after two days in both groups. The milk levels of the NGAL/MMP-9 complex also decreased in normal pregnancies as well as iGDM. However, in the second sample, the concentrations of the NGAL/MMP-9 complex were significantly higher in diabetic women. The physiological role of milk NGAL needs further study. The researchers of NGAL in human milk speculate that if NGAL plays a bacteriostatic role and takes part in mucosal healing, it may exert a protective influence on the neonatal gastrointestinal tract [217].
NGAL concentration changes in cerebrospinal fluid (CSF) were also observed in patients with cognitive impairment. Naudé found that concentrations of NGAL in CSF were significantly lower in participants with mild cognitive impairment compared to patients with subjective cognitive decline. Moreover, lower CSF NGAL concentrations predicted a conversion from mild cognitive impairment to Alzheimer’s disease. Therefore, CSF NGAL may possibly become a marker of cognitive impairment worsening [218]. The study of Wu found that peripheral blood concentrations of NGAL were higher in mild cognitive impairments and Alzheimer’s disease compared to the healthy controls, which allowed them to assume that increased neutrophil activation expressed in elevated blood NGAL levels could take part in cognitive impairments due to Alzheimer’s disease [219]. Patients with central nervous system (CNS) infections had significantly higher NGAL concentrations in CSF compared to healthy individuals. Moreover, patients with electroencephalography (EEG) abnormalities also revealed higher CSF NGAL levels. Thus, increased NGAL CSF could suggest EEG abnormalities in patients with CNS infections [220]. The CSF NGAL concentrations of patients with a subarachnoid hemorrhage (SAH) increased significantly up to the fifth day after SAH, and then they began to decrease. As NGAL was released by neutrophils, this indicated their important role in inflammatory processes in the brain after SAH [221].
NGAL was also estimated as a marker of prosthetic joint infection. NGAL in synovial fluid was found to be a marker of inflammatory processes in the prosthatic joint with promising diagnostic performance [222]. Very similar results have also been observed in recent numerous studies [223,224,225].
The complex of NGAL/MMP-9 was also evaluated in the urine and CSF of pediatric patients with moyamoya disease. The study of Sesen provided the hypothesis that the NGAL/MMP-9 complex may be useful as a predictor of the presence of moyamoya disease and may enable the monitoring of the process of treatment [226].
The expression of NGAL in the lung tissue of patients with chronic obstructive pulmonary disease (COPD) is higher compared to the healthy controls, which is the result of an increased number of neutrophils in COPD airways. NGAL may participate in the progression of COPD by enhancing airway remodeling. NGAL intensifies the proliferation and migration of human bronchial smooth muscle cells. NGAL also intensifies the epithelial-mesenchymal transition through the downregulation of E-cadherin expression and up-regulation of the expression of α-smooth muscle actin, which results in increased organ fibrosis. Wang suggested that NGAL could become a target for the novel treatment of airway remodeling and obstruction in COPD patients [227].
NGAL may also act as a novel marker in ventilator-associated lung injury. Chen conducted an experimental model of ventilator-associated lung injury. NGAL expression in bronchoalveolar lavage fluid was elevated in all methods of mechanical ventilation treatment, which increased with the degree of lung injury and was highest in the most severe lung damage [228].
The concentrations of NGAL may be measured in pleural fluid. The report by Wu found a positive correlation between serum and pleural fluid NGAL in complicated parapneumonic effusion (CPPE) and empyema; hence, serum NGAL may be a biological marker of these two pathological states [229]. NGAL may also be useful in discriminating pleural and peritoneal malignant effusion and tuberculous pleural effusion [230].
NGAL is also present in vaginal fluid. Beghini studied the relationship between NGAL and the bacterial vaginosis of reproductive-age women. They stated that the derangements of vaginal NGAL concentrations could be due to a decrease in Lactobacilli. This may result in an overgrowth of anaerobic or facultative Gram-negative bacteria, which are usually associated with bacterial vaginosis [231]. The report of Beghini also found that the concentrations of NGAL in the vaginal fluid were higher in women with vaginal inflammation [232].
The levels of NGAL were also assessed in amniotic fluid with significantly increased values in the microbial invasion of the amniotic cavity and histological chorioamnionitis in women with the pre-labor rupture of membranes [233].
6. Summary
NGAL is a protein that is secreted mainly by neutrophils, with its expression found in a wide variety of human tissues such as the kidney, heart, lung, liver, stomach, and colon. NGAL is an acute-phase protein and plays a role in inflammation. Its serum concentrations positively correlated with inflammatory cytokines, which are very high in septic shock and sepsis-related organ failure. Its main antibacterial mechanism is the regulation of iron metabolism, but it may also have an influence on the chemotaxis, adhesion, and migration of inflammatory cells. NGAL levels could be estimated in plasma, urine, and biological fluids such as peritoneal effluent, cerebrospinal fluid, saliva, tears, and human breast milk. Since plasma and urine NGAL concentrations increase rapidly after kidney injury, this could play a role as a biomarker in renal failure. Elevated NGAL concentrations were found in both acute and chronic kidney disease. In PD patients, NGAL could be a reliable marker of peritonitis. High serum NGAL concentrations were observed in atherosclerosis, hypertension, myocardial infarction, and heart failure. NGAL levels rose with the severity of atherosclerosis and coronary artery disease. Increased NGAL could also indicate the instability of atherosclerotic plaque. NGAL may be a predictor of the development of cardiorenal syndrome. NGAL also plays a role in metabolic complications. NGAL plasma levels have been found to be increased in obese individuals where it is likely to perform an anti-inflammatory function. The synthesis of NGAL is stimulated by increased serum glucose concentrations; therefore, NGAL levels are also elevated in diabetes and insulin resistance. NGAL is also involved in tumor processes such as cell proliferation, migration, multidrug resistance, and immunotolerance, which enhance the aggressiveness of the disease and promote metastases. Additionally, NGAL may possibly participate in the pathogenesis of primary Sjögren syndrome, cognitive impairment, and Alzheimer’s disease, as well as chronic obstructive pulmonary disease. However, this requires further study. The role of NGAL is summarized in Figure 4.
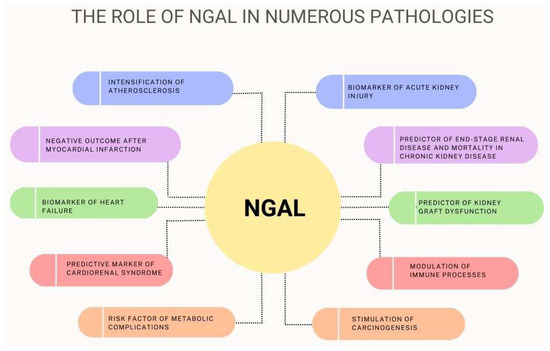
Figure 4.
The role of NGAL in numerous pathological states.
There is a lot of research on the role of NGAL in a variety of pathological situations. The knowledge of the different functions of this protein has been extended. Its role as a biomarker is promising; however, NGAL may also be a target for therapeutic procedures. Although numerous studies have been conducted, the exact mechanisms behind various activities of NGAL have not yet been completely understood.
Author Contributions
Writing—original draft preperation, K.R., M.M.; Writing—review and editing, S.N.; Visualization—K.R., M.M.; Supervision—S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kjeldsen, L.; Johnsen, A.H.; Sengeløv, H.; Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, H.; Chen, H.; Zhang, M.; Ma, Q. High expression of neutrophil gelatinase-associated lipocalin (NGAL) in the kidney proximal tubules of diabetic rats. Adv. Med. Sci. 2015, 60, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, S.A.; Cohen, A.; D’Souza, C.; Abdulrazzaq, Y.M.; Ojha, S.; Bastaki, S.; Adeghate, E.A. Lipocalin-2: Structure, function, distribution and role in metabolic disorders. Biomed. Pharmacother. 2021, 142, 112002. [Google Scholar] [CrossRef] [PubMed]
- Latouche, C.; El Moghrabi, S.; Messaoudi, S.; Nguyen Dinh Cat, A.; Hernandez-Diaz, I.; Alvarez de la Rosa, D.; Perret, C.; López Andrés, N.; Rossignol, P.; Zannad, F.; et al. Neutrophil gelatinase-associated lipocalin is a novel mineralocorticoid target in the cardiovascular system. Hypertension 2012, 59, 966–972. [Google Scholar] [CrossRef]
- Eilenberg, W.; Stojkovic, S.; Piechota-Polanczyk, A.; Kaun, C.; Rauscher, S.; Gröger, M.; Klinger, M.; Wojta, J.; Neumayer, C.; Huk, I.; et al. Neutrophil Gelatinase-Associated Lipocalin (NGAL) is Associated with Symptomatic Carotid Atherosclerosis and Drives Pro-inflammatory State In Vitro. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 623–631. [Google Scholar] [CrossRef]
- Flo, T.H.; Smith, K.D.; Sato, S.; Rodriguez, D.J.; Holmes, M.A.; Strong, R.K.; Akira, S.; Aderem, A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004, 432, 917–921. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin—An emerging troponin for kidney injury. Nephrol. Dial. Transplant. 2008, 23, 3737–3743. [Google Scholar] [CrossRef]
- Cowland, J.B.; Borregaard, N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics 1997, 45, 17–23. [Google Scholar] [CrossRef]
- Chen, J.J.; Lee, T.H.; Lee, C.C.; Chang, C.H. Using lipocalin as a prognostic biomarker in acute kidney injury. Expert. Rev. Mol. Diagn. 2021, 21, 455–464. [Google Scholar] [CrossRef]
- Buonafine, M.; Martinez-Martinez, E.; Jaisser, F. More than a simple biomarker: The role of NGAL in cardiovascular and renal diseases. Clin. Sci. 2018, 132, 909–923. [Google Scholar] [CrossRef]
- Goetz, D.H.; Willie, S.T.; Armen, R.S.; Bratt, T.; Borregaard, N.; Strong, R.K. Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochemistry 2000, 39, 1935–1941. [Google Scholar] [CrossRef]
- Nasioudis, D.; Witkin, S.S. Neutrophil gelatinase-associated lipocalin and innate immune responses to bacterial infections. Med. Microbiol. Immunol. 2015, 204, 471–479. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Cowland, J.B.; Borregaard, N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim. Biophys. Acta 2000, 1482, 272–283. [Google Scholar] [CrossRef]
- Candido, S.; Abrams, S.L.; Steelman, L.S.; Lertpiriyapong, K.; Fitzgerald, T.L.; Martelli, A.M.; Cocco, L.; Montalto, G.; Cervello, M.; Polesel, J.; et al. Roles of NGAL and MMP-9 in the tumor microenvironment and sensitivity to targeted therapy. Biochim. Biophys. Acta 2016, 1863, 438–448. [Google Scholar] [CrossRef]
- Yan, L.; Borregaard, N.; Kjeldsen, L.; Moses, M.A. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J. Biol. Chem. 2001, 276, 37258–37265. [Google Scholar] [CrossRef]
- Gupta, K.; Shukla, M.; Cowland, J.B.; Malemud, C.J.; Haqqi, T.M. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007, 56, 3326–3335. [Google Scholar] [CrossRef]
- Ding, L.; Hanawa, H.; Ota, Y.; Hasegawa, G.; Hao, K.; Asami, F.; Watanabe, R.; Yoshida, T.; Toba, K.; Yoshida, K.; et al. Lipocalin-2/neutrophil gelatinase-B associated lipocalin is strongly induced in hearts of rats with autoimmune myocarditis and in human myocarditis. Circ. J. 2010, 74, 523–530. [Google Scholar] [CrossRef]
- Buonafine, M.; Martínez-Martínez, E.; Amador, C.; Gravez, B.; Ibarrola, J.; Fernández-Celis, A.; El Moghrabi, S.; Rossignol, P.; López-Andrés, N.; Jaisser, F. Neutrophil Gelatinase-Associated Lipocalin from immune cells is mandatory for aldosterone-induced cardiac remodeling and inflammation. J. Mol. Cell. Cardiol. 2018, 115, 32–38. [Google Scholar] [CrossRef]
- Hvidberg, V.; Jacobsen, C.; Strong, R.K.; Cowland, J.B.; Moestrup, S.K.; Borregaard, N. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005, 579, 773–777. [Google Scholar] [CrossRef]
- Van Dijk, A.; Vermond, R.A.; Krijnen, P.A.; Juffermans, L.J.; Hahn, N.E.; Makker, S.P.; Aarden, L.A.; Hack, E.; Spreeuwenberg, M.; van Rossum, B.C.; et al. Intravenous clusterin administration reduces myocardial infarct size in rats. Eur. J. Clin Investig. 2010, 40, 893–902. [Google Scholar] [CrossRef]
- Borregaard, N.; Cowland, J.B. Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals 2006, 19, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Moestrup, S.K.; Verroust, P.J. Megalin- and cubilin-mediated endocytosis of protein-bound vitamins, lipids, and hormones in polarized epithelia. Annu. Rev. Nutr. 2001, 21, 407–428. [Google Scholar] [CrossRef] [PubMed]
- Bauvois, B.; Susin, S.A. Revisiting Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Cancer: Saint or Sinner? Cancers 2018, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yeoh, B.S.; Vijay-Kumar, M. Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation. Annu. Rev. Nutr. 2017, 37, 103–130. [Google Scholar] [CrossRef]
- Goetz, D.; Holmes, M.A.; Borregaard, N.; Bluhm, M.E.; Raymond, K.N.; Strong, R.K. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 2002, 10, 1033–1043. [Google Scholar] [CrossRef]
- Marakala, V. Neutrophil gelatinase-associated lipocalin (NGAL) in kidney injury—A systematic review. Clin. Chim. Acta 2022, 536, 135–141. [Google Scholar] [CrossRef]
- Luchtefeld, M.; Preuss, C.; Rühle, F.; Bogalle, E.P.; Sietmann, A.; Figura, S.; Müller, W.; Grote, K.; Schieffer, B.; Stoll, M. Gp130-dependent release of acute phase proteins is linked to the activation of innate immune signaling pathways. PLoS ONE 2011, 6, e19427. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Bainton, D.F.; Sengeløv, H.; Borregaard, N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 1994, 83, 799–807. [Google Scholar] [CrossRef]
- Cowland, J.B.; Sørensen, O.E.; Sehested, M.; Borregaard, N. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1 beta, but not by TNF-alpha. J. Immunol. 2003, 171, 6630–6639. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Ko, S.H.; Jung, J.; Kim, Y.J.; Kim, J.S.; Kim, J.M. Bacteroides fragilis enterotoxin upregulates lipocalin-2 expression in intestinal epithelial cells. Lab. Investig. 2013, 93, 384–396. [Google Scholar] [CrossRef]
- Alpízar-Alpízar, W.; Laerum, O.D.; Illemann, M.; Ramírez, J.A.; Arias, A.; Malespín-Bendaña, W.; Ramírez, V.; Lund, L.R.; Borregaard, N.; Nielsen, B.S. Neutrophil gelatinase-associated lipocalin (NGAL/Lcn2) is upregulated in gastric mucosa infected with Helicobacter pylori . Virchows Arch. 2009, 455, 225–233. [Google Scholar] [CrossRef]
- Xu, M.J.; Feng, D.; Wu, H.; Wang, H.; Chan, Y.; Kolls, J.; Borregaard, N.; Porse, B.; Berger, T.; Mak, T.W.; et al. Liver is the major source of elevated serum lipocalin-2 levels after bacterial infection or partial hepatectomy: A critical role for IL-6/STAT3. Hepatology 2015, 61, 692–702. [Google Scholar] [CrossRef]
- Wu, H.; Santoni-Rugiu, E.; Ralfkiaer, E.; Porse, B.T.; Moser, C.; Høiby, N.; Borregaard, N.; Cowland, J.B. Lipocalin 2 is protective against E. coli pneumonia. Respir. Res. 2010, 11, 96. [Google Scholar] [CrossRef]
- Parrow, N.L.; Fleming, R.E.; Minnick, M.F. Sequestration and scavenging of iron in infection. Infect. Immun. 2013, 81, 3503–3514. [Google Scholar] [CrossRef]
- Bao, G.; Clifton, M.; Hoette, T.M.; Mori, K.; Deng, S.X.; Qiu, A.; Viltard, M.; Williams, D.; Paragas, N.; Leete, T.; et al. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat. Chem. Biol. 2010, 6, 602–609. [Google Scholar] [CrossRef]
- Barasch, J.; Mori, K. Cell biology: Iron thievery. Nature 2004, 432, 811–813. [Google Scholar] [CrossRef]
- Robinson, K.M.; McHugh, K.J.; Mandalapu, S.; Clay, M.E.; Lee, B.; Scheller, E.V.; Enelow, R.I.; Chan, Y.R.; Kolls, J.K.; Alcorn, J.F. Influenza A virus exacerbates Staphylococcus aureus pneumonia in mice by attenuating antimicrobial peptide production. J. Infect. Dis. 2014, 209, 865–875. [Google Scholar] [CrossRef]
- Bachman, M.A.; Miller, V.L.; Weiser, J.N. Mucosal lipocalin 2 has pro-inflammatory and iron-sequestering effects in response to bacterial enterobactin. PLoS Pathog. 2009, 5, e1000622. [Google Scholar] [CrossRef]
- Ratledge, C. Iron, mycobacteria and tuberculosis. Tuberculosis 2004, 84, 110–130. [Google Scholar] [CrossRef]
- Paragas, N.; Kulkarni, R.; Werth, M.; Schmidt-Ott, K.M.; Forster, C.; Deng, R.; Zhang, Q.; Singer, E.; Klose, A.D.; Shen, T.H.; et al. α-Intercalated cells defend the urinary system from bacterial infection. J. Clin. Investig. 2014, 124, 2963–2976. [Google Scholar] [CrossRef]
- Floderer, M.; Prchal-Murphy, M.; Vizzardelli, C. Dendritic cell-secreted lipocalin2 induces CD8+ T-cell apoptosis, contributes to T-cell priming and leads to a TH1 phenotype. PLoS ONE 2014, 9, e101881. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Petersen, R.; Devireddy, L. Impaired neutrophil function in 24p3 null mice contributes to enhanced susceptibility to bacterial infections. J. Immunol. 2013, 190, 4692–4706. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, S.P.J.; Bosio, E.; Neil, C.; Arendts, G.; Burrows, S.; Smart, L.; Brown, S.G.A.; Fatovich, D.M. Resistin and NGAL are associated with inflammatory response, endothelial activation and clinical outcomes in sepsis. Inflamm. Res. 2017, 66, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S.; Jensen, J.S.; Hoffmann, S.; Iversen, A.Z.; Pedersen, S.H.; Biering-Sørensen, T.; Galatius, S.; Flyvbjerg, A.; Mogelvang, R.; Magnusson, N.E. Plasma Neutrophil Gelatinase-Associated Lipocalin Reflects Both Inflammation and Kidney Function in Patients with Myocardial Infarction. Cardiorenal Med. 2016, 6, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Yigit, I.P.; Celiker, H.; Dogukan, A.; Ilhan, N.; Gurel, A.; Ulu, R.; Aygen, B. Can serum NGAL levels be used as an inflammation marker on hemodialysis patients with permanent catheter? Ren. Fail. 2015, 37, 77–82. [Google Scholar] [CrossRef]
- Lentini, P.; de Cal, M.; Clementi, A.; D’Angelo, A.; Ronco, C. Sepsis and AKI in ICU Patients: The Role of Plasma Biomarkers. Crit. Care Res. Pract. 2012, 2012, 856401. [Google Scholar] [CrossRef]
- Macdonald, S.P.; Stone, S.F.; Neil, C.L.; van Eeden, P.E.; Fatovich, D.M.; Arendts, G.; Brown, S.G. Sustained elevation of resistin, NGAL and IL-8 are associated with severe sepsis/septic shock in the emergency department. PLoS ONE 2014, 9, e110678. [Google Scholar] [CrossRef]
- Zhang, A.; Cai, Y.; Wang, P.F.; Qu, J.N.; Luo, Z.C.; Chen, X.D.; Huang, B.; Liu, Y.; Huang, W.Q.; Wu, J.; et al. Diagnosis and prognosis of neutrophil gelatinase-associated lipocalin for acute kidney injury with sepsis: A systematic review and meta-analysis. Crit. Care 2016, 20, 41. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Whibley, N.; Mamo, A.J.; Siebenlist, U.; Chan, Y.R.; Gaffen, S.L. Interleukin-17-induced protein lipocalin 2 is dispensable for immunity to oral candidiasis. Infect. Immun. 2014, 82, 1030–1035. [Google Scholar] [CrossRef]
- Akgül, B.; Bauer, B.; Zigrino, P.; Storey, A.; Mauch, C.; Pfister, H. Upregulation of lipocalin-2 in human papillomavirus-positive keratinocytes and cutaneous squamous cell carcinomas. J. Gen. Virol. 2011, 92, 395–401. [Google Scholar] [CrossRef]
- Vijay-Kumar, M.; Gentsch, J.R.; Kaiser, W.J.; Borregaard, N.; Offermann, M.K.; Neish, A.S.; Gewirtz, A.T. Protein kinase R mediates intestinal epithelial gene remodeling in response to double-stranded RNA and live rotavirus. J. Immunol. 2005, 174, 6322–6331. [Google Scholar] [CrossRef]
- Çelik, T.; Altekin, E.; İşgüder, R.; Kenesari, Y.; Duman, M.; Arslan, N. Evaluation of neutrophil gelatinase-associated lipocalin in pediatric patients with acute rotavirus gastroenteritis and dehydration. Ital. J. Pediatr. 2013, 39, 52. [Google Scholar] [CrossRef]
- Landrø, L.; Damås, J.K.; Flo, T.H.; Heggelund, L.; Ueland, T.; Tjønnfjord, G.E.; Espevik, T.; Aukrust, P.; Frøland, S.S. Decreased serum lipocalin-2 levels in human immunodeficiency virus-infected patients: Increase during highly active anti-retroviral therapy. Clin. Exp. Immunol. 2008, 152, 57–63. [Google Scholar] [CrossRef]
- Wu, G.; Li, H.; Zhou, M.; Fang, Q.; Bao, Y.; Xu, A.; Jia, W. Mechanism and clinical evidence of lipocalin-2 and adipocyte fatty acid-binding protein linking obesity and atherosclerosis. Diabetes Metab. Res. Rev. 2014, 30, 447–456. [Google Scholar] [CrossRef]
- Wang, Y.; Lam, K.S.; Kraegen, E.W.; Sweeney, G.; Zhang, J.; Tso, A.W.; Chow, W.S.; Wat, N.M.; Xu, J.Y.; Hoo, R.L.; et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 2007, 53, 34–41. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, J.H.; Wang, Y.; Sweeney, G. Emerging clinical and experimental evidence for the role of lipocalin-2 in metabolic syndrome. Clin. Exp. Pharmacol. Physiol. 2012, 39, 194–199. [Google Scholar] [CrossRef]
- Law, I.K.; Xu, A.; Lam, K.S.; Berger, T.; Mak, T.W.; Vanhoutte, P.M.; Liu, J.T.; Sweeney, G.; Zhou, M.; Yang, B.; et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 2010, 59, 872–882. [Google Scholar] [CrossRef]
- Liu, J.T.; Song, E.; Xu, A.; Berger, T.; Mak, T.W.; Tse, H.F.; Law, I.K.; Huang, B.; Liang, Y.; Vanhoutte, P.M.; et al. Lipocalin-2 deficiency prevents endothelial dysfunction associated with dietary obesity: Role of cytochrome P450 2C inhibition. Br. J. Pharmacol. 2012, 165, 520–531. [Google Scholar] [CrossRef]
- Bhusal, A.; Rahman, M.H.; Lee, W.H.; Bae, Y.C.; Lee, I.K.; Suk, K. Paradoxical role of lipocalin-2 in metabolic disorders and neurological complications. Biochem. Pharmacol. 2019, 169, 113626. [Google Scholar] [CrossRef]
- Yan, Q.W.; Yang, Q.; Mody, N.; Graham, T.E.; Hsu, C.H.; Xu, Z.; Houstis, N.E.; Kahn, B.B.; Rosen, E.D. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 2007, 56, 2533–2540. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Zhang, Y.; Leroith, D.; Bernlohr, D.A.; Chen, X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol. Endocrinol. 2008, 22, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Ricote, M.; Glass, C.K. PPARs and molecular mechanisms of transrepression. Biochim. Biophys. Acta 2007, 1771, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Rajala, M.W.; Berger, J.P.; Moller, D.E.; Barzilai, N.; Scherer, P.E. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J. Biol. Chem. 2001, 276, 42077–42083. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Buendía, A.S.; García-Arroyo, F.E.; Cristóbal-García, M.; Loredo-Mendoza, M.L.; Tapia-Rodríguez, E.; Sánchez-Lozada, L.G.; Osorio-Alonso, H. Urinary excretion of neutrophil gelatinase-associated lipocalin in diabetic rats. Oxid. Med. Cell. Longev. 2014, 2014, 961326. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Hansen, H.P.; Jensen, B.R.; Parving, H.H.; Rossing, P. Urinary neutrophil gelatinase-associated lipocalin and progression of diabetic nephropathy in type 1 diabetic patients in a four-year follow-up study. Nephron. Clin. Pract. 2011, 118, 130–135. [Google Scholar] [CrossRef]
- Bolignano, D.; Lacquaniti, A.; Coppolino, G.; Donato, V.; Fazio, M.R.; Nicocia, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press. Res. 2009, 32, 91–98. [Google Scholar] [CrossRef]
- Hafez, M.H.; El-Mougy, F.A.; Makar, S.H.; Abd El Shaheed, S. Detection of an earlier tubulopathy in diabetic nephropathy among children with normoalbuminuria. Iran J. Kidney Dis. 2015, 9, 126–131. [Google Scholar]
- Schmidt-Ott, K.M.; Mori, K.; Li, J.Y.; Kalandadze, A.; Cohen, D.J.; Devarajan, P.; Barasch, J. Dual action of neutrophil gelatinase-associated lipocalin. J. Am. Soc. Nephrol. 2007, 18, 407–413. [Google Scholar] [CrossRef]
- Hanai, J.; Mammoto, T.; Seth, P.; Mori, K.; Karumanchi, S.A.; Barasch, J.; Sukhatme, V.P. Lipocalin 2 diminishes invasiveness and metastasis of Ras-transformed cells. J. Biol. Chem. 2005, 280, 13641–13647. [Google Scholar] [CrossRef]
- Fernández, C.A.; Yan, L.; Louis, G.; Yang, J.; Kutok, J.L.; Moses, M.A. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin. Cancer Res. 2005, 11, 5390–5395. [Google Scholar] [CrossRef]
- Leng, X.; Ding, T.; Lin, H.; Wang, Y.; Hu, L.; Hu, J.; Feig, B.; Zhang, W.; Pusztai, L.; Symmans, W.F.; et al. Inhibition of lipocalin 2 impairs breast tumorigenesis and metastasis. Cancer Res. 2009, 69, 8579–8584. [Google Scholar] [CrossRef]
- Yang, J.; Bielenberg, D.R.; Rodig, S.J.; Doiron, R.; Clifton, M.C.; Kung, A.L.; Strong, R.K.; Zurakowski, D.; Moses, M.A. Lipocalin 2 promotes breast cancer progression. Proc. Natl. Acad. Sci. USA 2009, 106, 3913–3918. [Google Scholar] [CrossRef]
- Leng, X.; Wu, Y.; Arlinghaus, R.B. Relationships of lipocalin 2 with breast tumorigenesis and metastasis. J. Cell. Physiol. 2011, 226, 309–314. [Google Scholar] [CrossRef]
- Iannetti, A.; Pacifico, F.; Acquaviva, R.; Lavorgna, A.; Crescenzi, E.; Vascotto, C.; Tell, G.; Salzano, A.M.; Scaloni, A.; Vuttariello, E.; et al. The neutrophil gelatinase-associated lipocalin (NGAL), a NF-kappaB-regulated gene, is a survival factor for thyroid neoplastic cells. Proc. Natl. Acad. Sci. USA 2008, 105, 14058–14063. [Google Scholar] [CrossRef]
- Volpe, V.; Raia, Z.; Sanguigno, L.; Somma, D.; Mastrovito, P.; Moscato, F.; Mellone, S.; Leonardi, A.; Pacifico, F. NGAL controls the metastatic potential of anaplastic thyroid carcinoma cells. J. Clin. Endocrinol. Metab. 2013, 98, 228–235. [Google Scholar] [CrossRef]
- Kubben, F.J.; Sier, C.F.; Hawinkels, L.J.; Tschesche, H.; van Duijn, W.; Zuidwijk, K.; van der Reijden, J.J.; Hanemaaijer, R.; Griffioen, G.; Lamers, C.B.; et al. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Eur. J. Cancer 2007, 43, 1869–1876. [Google Scholar] [CrossRef]
- Koh, S.A.; Lee, K.H. HGF mediated upregulation of lipocalin 2 regulates MMP9 through nuclear factor-κB activation. Oncol. Rep. 2015, 34, 2179–2187. [Google Scholar] [CrossRef]
- Du, Z.P.; Wu, B.L.; Xie, Y.M.; Zhang, Y.L.; Liao, L.D.; Zhou, F.; Xie, J.J.; Zeng, F.M.; Xu, X.E.; Fang, W.K.; et al. Lipocalin 2 promotes the migration and invasion of esophageal squamous cell carcinoma cells through a novel positive feedback loop. Biochim. Biophys. Acta 2015, 1853, 2240–2250. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Xiao, D.; Xie, J.; Zeng, H.; Wang, Z.; Zhang, X.; Niu, Y.; Shen, Z.; Shen, J.; et al. Upregulation of neutrophil gelatinase-associated lipocalin in oesophageal squamous cell carcinoma: Significant correlation with cell differentiation and tumour invasion. J. Clin. Pathol. 2007, 60, 555–561. [Google Scholar] [CrossRef]
- Ding, G.; Fang, J.; Tong, S.; Qu, L.; Jiang, H.; Ding, Q.; Liu, J. Over-expression of lipocalin 2 promotes cell migration and invasion through activating ERK signaling to increase SLUG expression in prostate cancer. Prostate 2015, 75, 957–968. [Google Scholar] [CrossRef]
- Tung, M.C.; Hsieh, S.C.; Yang, S.F.; Cheng, C.W.; Tsai, R.T.; Wang, S.C.; Huang, M.H.; Hsieh, Y.H. Knockdown of lipocalin-2 suppresses the growth and invasion of prostate cancer cells. Prostate 2013, 73, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Meschi, T.; Nouvenne, A.; Mattiuzzi, C.; Borghi, L. Neutrophil gelatinase-associated lipocalin in cancer. Adv. Clin. Chem. 2014, 64, 179–219. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kaur, S.; Guha, S.; Batra, S.K. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta 2012, 1826, 129–169. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, J.J.; Mahadevan, N.R.; Zanetti, M. Lipocalin 2 in cancer: When good immunity goes bad. Cancer Lett. 2012, 316, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Candido, S.; Maestro, R.; Polesel, J.; Catania, A.; Maira, F.; Signorelli, S.S.; McCubrey, J.A.; Libra, M. Roles of neutrophil gelatinase-associated lipocalin (NGAL) in human cancer. Oncotarget 2014, 5, 1576–1594. [Google Scholar] [CrossRef]
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Barasch, J.; Devarajan, P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543. [Google Scholar] [CrossRef]
- Mishra, J.; Dent, C.; Tarabishi, R.; Mitsnefes, M.M.; Ma, Q.; Kelly, C.; Ruff, S.M.; Zahedi, K.; Shao, M.; Bean, J.; et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005, 365, 1231–1238. [Google Scholar] [CrossRef]
- Koyner, J.L.; Vaidya, V.S.; Bennett, M.R.; Ma, Q.; Worcester, E.; Akhter, S.A.; Raman, J.; Jeevanandam, V.; O’Connor, M.F.; Devarajan, P.; et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin. J. Am. Soc. Nephrol. 2010, 5, 2154–2165. [Google Scholar] [CrossRef]
- De Geus, H.R.; Bakker, J.; Lesaffre, E.M.; le Noble, J.L. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am. J. Respir. Crit. Care Med. 2011, 183, 907–914. [Google Scholar] [CrossRef]
- Devarajan, P. Review: Neutrophil gelatinase-associated lipocalin: A troponin-like biomarker for human acute kidney injury. Nephrology 2010, 15, 419–428. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Lee, M.J.; Seo, J.S.; Choi, D.; Park, J.B. Plasma neutrophil gelatinase-associated lipocalin as a predictive biomarker for the detection of acute kidney injury in adult poisoning. Clin. Toxicol. 2016, 54, 127–133. [Google Scholar] [CrossRef]
- Luo, Q.H.; Chen, M.L.; Sun, F.J.; Chen, Z.L.; Li, M.Y.; Zeng, W.; Gong, L.; Cheng, A.C.; Peng, X.; Fang, J.; et al. KIM-1 and NGAL as biomarkers of nephrotoxicity induced by gentamicin in rats. Mol. Cell. Biochem. 2014, 397, 53–60. [Google Scholar] [CrossRef]
- Vanmassenhove, J.; Vanholder, R.; Nagler, E.; Van Biesen, W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: An in-depth review of the literature. Nephrol. Dial. Transplant. 2013, 28, 254–273. [Google Scholar] [CrossRef]
- Di Grande, A.; Giuffrida, C.; Carpinteri, G.; Narbone, G.; Pirrone, G.; Di Mauro, A.; Calandra, S.; Noto, P.; Le Moli, C.; Alongi, B.; et al. Neutrophil gelatinase-associated lipocalin: A novel biomarker for the early diagnosis of acute kidney injury in the emergency department. Eur. Rev. Med. Pharmacol. Sci. 2009, 13, 197–200. [Google Scholar]
- Wasung, M.E.; Chawla, L.S.; Madero, M. Biomarkers of renal function, which and when? Clin. Chim. Acta 2015, 438, 350–357. [Google Scholar] [CrossRef]
- Antonucci, E.; Lippi, G.; Ticinesi, A.; Pigna, F.; Guida, L.; Morelli, I.; Nouvenne, A.; Borghi, L.; Meschi, T. Neutrophil gelatinase-associated lipocalin (NGAL): A promising biomarker for the early diagnosis of acute kidney injury (AKI). Acta Biomed. 2014, 85, 289–294. [Google Scholar]
- De Geus, H.R.; Ronco, C.; Haase, M.; Jacob, L.; Lewington, A.; Vincent, J.L. The cardiac surgery-associated neutrophil gelatinase-associated lipocalin (CSA-NGAL) score: A potential tool to monitor acute tubular damage. J. Thorac. Cardiovasc. Surg. 2016, 151, 1476–1481. [Google Scholar] [CrossRef]
- Wen, Y.; Parikh, C.R. Current concepts and advances in biomarkers of acute kidney injury. Crit. Rev. Clin. Lab. Sci. 2021, 58, 354–368. [Google Scholar] [CrossRef]
- Helanova, K.; Spinar, J.; Parenica, J. Diagnostic and prognostic utility of neutrophil gelatinase-associated lipocalin (NGAL) in patients with cardiovascular diseases—Review. Kidney Blood Press. Res. 2014, 39, 623–629. [Google Scholar] [CrossRef]
- Bolignano, D.; Donato, V.; Coppolino, G.; Campo, S.; Buemi, A.; Lacquaniti, A.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am. J. Kidney Dis. 2008, 52, 595–605. [Google Scholar] [CrossRef]
- Grigoryev, D.N.; Liu, M.; Hassoun, H.T.; Cheadle, C.; Barnes, K.C.; Rabb, H. The local and systemic inflammatory transcriptome after acute kidney injury. J. Am. Soc. Nephrol. 2008, 19, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P. The promise of biomarkers for personalized renal cancer care. Kidney Int. 2010, 77, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Haase-Fielitz, A.; Bellomo, R.; Devarajan, P.; Story, D.; Matalanis, G.; Dragun, D.; Haase, M. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery—A prospective cohort study. Crit. Care Med. 2009, 37, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Makris, K.; Markou, N.; Evodia, E.; Dimopoulou, E.; Drakopoulos, I.; Ntetsika, K.; Rizos, D.; Baltopoulos, G.; Haliassos, A. Urinary neutrophil gelatinase-associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin. Chem. Lab. Med. 2009, 47, 79–82. [Google Scholar] [CrossRef]
- Ozkan, S.; Durukan, P.; Kavalci, C.; Duman, A.; Sayhan, M.B.; Salt, O.; Ipekci, A. Importance of neutrophil gelatinase-associated lipocalin in differential diagnosis of acute and chronic renal failure. Iran Red. Crescent. Med. J. 2014, 16, e14133. [Google Scholar] [CrossRef]
- Corbacıoglu, S.K.; Cevik, Y.; Akinci, E.; Uzunosmanoglu, H.; Dagar, S.; Safak, T.; Oncul, V.; Guvendi, M. Value of plasma neutrophil gelatinase-associated lipocalin (NGAL) in distinguishing between acute kidney injury (AKI) and chronic kidney disease (CKD). Turk. J. Emerg. Med. 2017, 17, 85–88. [Google Scholar] [CrossRef]
- Gharishvandi, F.; Kazerouni, F.; Ghanei, E.; Rahimipour, A.; Nasiri, M. Comparative assessment of neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C as early biomarkers for early detection of renal failure in patients with hypertension. Iran Biomed. J. 2015, 19, 76–81. [Google Scholar] [CrossRef]
- Bolignano, D.; Lacquaniti, A.; Coppolino, G.; Donato, V.; Campo, S.; Fazio, M.R.; Nicocia, G.; Buemi, M. Neutrophil gelatinaseassociated lipocalin (NGAL) and progression of chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 337–344. [Google Scholar] [CrossRef]
- Giasson, J.; Li, G.H.; Chen, Y. Neutrophil gelatinase-associated lipocalin (NGAL) as a new biomarker for non-acute kidney injury (AKI) diseases. Inflamm. Allergy Drug Targets 2011, 10, 272–282. [Google Scholar] [CrossRef]
- Malyszko, J.; Bachorzewska-Gajewska, H.; Sitniewska, E.; Malyszko, J.S.; Poniatowski, B.; Dobrzycki, S. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in non-diabetic patients with stage 2-4 chronic kidney disease. Ren. Fail. 2008, 30, 625–628. [Google Scholar] [CrossRef]
- Kaufeld, J.K.; Gwinner, W.; Scheffner, I.; Haller, H.G.; Schiffer, M. Urinary NGAL Ratio Is Not a Sensitive Biomarker for Monitoring Acute Tubular Injury in Kidney Transplant Patients: NGAL and ATI in Renal Transplant Patients. J. Transplant. 2012, 2012, 563404. [Google Scholar] [CrossRef]
- Zhou, L.T.; Lv, L.L.; Pan, M.M.; Cao, Y.H.; Liu, H.; Feng, Y.; Ni, H.F.; Liu, B.C. Are Urinary Tubular Injury Markers Useful in Chronic Kidney Disease? A Systematic Review and Meta Analysis. PLoS ONE 2016, 11, e0167334. [Google Scholar] [CrossRef]
- Smith, E.R.; Lee, D.; Cai, M.M.; Tomlinson, L.A.; Ford, M.L.; McMahon, L.P.; Holt, S.G. Urinary neutrophil gelatinase-associated lipocalin may aid prediction of renal decline in patients with non-proteinuric Stages 3 and 4 chronic kidney disease (CKD). Nephrol. Dial. Transplant. 2013, 28, 1569–1579. [Google Scholar] [CrossRef]
- Cai, L.; Rubin, J.; Han, W.; Venge, P.; Xu, S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin. J. Am. Soc. Nephrol. 2010, 5, 2229–2235. [Google Scholar] [CrossRef]
- Khan, Z.; Pandey, M. Role of kidney biomarkers of chronic kidney disease: An update. Saudi J. Biol. Sci. 2014, 21, 294–299. [Google Scholar] [CrossRef]
- Ntrinias, T.; Papasotiriou, M.; Balta, L.; Kalavrizioti, D.; Vamvakas, S.; Papachristou, E.; Goumenos, D.S. Biomarkers in Progressive Chronic Kidney Disease. Still a Long Way to Go. Prilozi 2019, 40, 27–39. [Google Scholar] [CrossRef]
- Viau, A.; El Karoui, K.; Laouari, D.; Burtin, M.; Nguyen, C.; Mori, K.; Pillebout, E.; Berger, T.; Mak, T.W.; Knebelmann, B.; et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J. Clin. Investig. 2010, 120, 4065–4076. [Google Scholar] [CrossRef]
- Xiang, D.; Wang, X.; Liu, P.; Pan, Y.; Zhang, Q.; Chi, X.; Jing, Y.; Duan, X.; Wei, Q.; Wang, J.; et al. Increased NGAL level associated with iron store in chronic kidney disease with anemia. Clin. Exp. Med. 2018, 18, 563–568. [Google Scholar] [CrossRef]
- Kim, I.Y.; Kim, J.H.; Lee, D.W.; Lee, S.B.; Rhee, H.; Song, S.H.; Seong, E.Y.; Kwak, I.S. Plasma neutrophil gelatinase-associated lipocalin is associated with iron status in anemic patients with pre-dialysis chronic kidney disease. Clin. Exp. Nephrol. 2018, 22, 28–34. [Google Scholar] [CrossRef]
- Bolignano, D.; Coppolino, G.; Romeo, A.; De Paola, L.; Buemi, A.; Lacquaniti, A.; Nicocia, G.; Lombardi, L.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) reflects iron status in haemodialysis patients. Nephrol. Dial. Transplant. 2009, 24, 3398–3403. [Google Scholar] [CrossRef]
- Tomasz, G.; Ewa, W.; Jolanta, M. Biomarkers of iron metabolism in chronic kidney disease. Int. Urol. Nephrol. 2021, 53, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, E.K.; Kapitsinou, P.; Pergola, P.E.; Kovesdy, C.P.; Jalal, D.I. Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment. J. Am. Soc. Nephrol. 2020, 31, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Seibert, F.S.; Sitz, M.; Passfall, J.; Haesner, M.; Laschinski, P.; Buhl, M.; Bauer, F.; Rohn, B.; Babel, N.; Westhoff, T.H. Urinary calprotectin, NGAL, and KIM-1 in the differentiation of primarily inflammatory vs. non-inflammatory stable chronic kidney diseases. Ren. Fail. 2021, 43, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; He, Y.; Li, K.; Yang, J.; Li, X.; Lu, R.; Gao, W. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin. Immunol. 2007, 123, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Shin, N.; Shin, M.J.; Yang, B.Y.; Kim, I.Y.; Song, S.H.; Lee, D.W.; Lee, S.B.; Kwak, I.S.; Seong, E.Y. High serum and urine neutrophil gelatinase-associated lipocalin levels are independent predictors of renal progression in patients with immunoglobulin A nephropathy. Korean J. Intern. Med. 2015, 30, 354–361. [Google Scholar] [CrossRef]
- Fang, Y.G.; Chen, N.N.; Cheng, Y.B.; Sun, S.J.; Li, H.X.; Sun, F.; Xiang, Y. Urinary neutrophil gelatinase-associated lipocalin for diagnosis and estimating activity in lupus nephritis: A meta-analysis. Lupus 2015, 24, 1529–1539. [Google Scholar] [CrossRef]
- Palazzo, L.; Lindblom, J.; Mohan, C.; Parodis, I. Current Insights on Biomarkers in Lupus Nephritis: A Systematic Review of the Literature. J. Clin. Med. 2022, 11, 5759. [Google Scholar] [CrossRef]
- Lindblom, J.; Mohan, C.; Parodis, I. Diagnostic, predictive and prognostic biomarkers in systemic lupus erythematosus: Current insights. Curr. Opin. Rheumatol. 2022, 34, 139–149. [Google Scholar] [CrossRef]
- Rubinstein, T.; Pitashny, M.; Putterman, C. The novel role of neutrophil gelatinase-B associated lipocalin (NGAL)/Lipocalin-2 as a biomarker for lupus nephritis. Autoimmun. Rev. 2008, 7, 229–234. [Google Scholar] [CrossRef]
- Bolignano, D.; Coppolino, G.; Campo, S.; Aloisi, C.; Nicocia, G.; Frisina, N.; Buemi, M. Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am. J. Nephrol. 2007, 27, 373–378. [Google Scholar] [CrossRef]
- Vareesangthip, K.; Vareesangthip, K.; Limwongse, C.; Reesukumal, K. Role of Urinary Neutrophil Gelatinase-Associated Lipocalin for Predicting the Severity of Renal Functions in Patients with Autosomal-Dominant Polycystic Kidney Disease. Transplant. Proc. 2017, 49, 950–954. [Google Scholar] [CrossRef]
- Meijer, E.; Boertien, W.E.; Nauta, F.L.; Bakker, S.J.; van Oeveren, W.; Rook, M.; van der Jagt, E.J.; van Goor, H.; Peters, D.J.; Navis, G.; et al. Association of urinary biomarkers with disease severity in patients with autosomal dominant polycystic kidney disease: A cross-sectional analysis. Am. J. Kidney Dis. 2010, 56, 883–895. [Google Scholar] [CrossRef]
- Tsingos, M.; Merlini, L.; Solcà, M.; Goischke, A.; Wilhelm-Bals, A.; Parvex, P. Early Urinary Biomarkers in Pediatric Autosomal Dominant Polycystic Kidney Disease (ADPKD): No Evidence in the Interest of Urinary Neutrophil Gelatinase-Associated Lipocalin (uNGAL). Front. Pediatr. 2019, 7, 88. [Google Scholar] [CrossRef]
- Chuang, H.Y.; Jeng, W.Y.; Wang, E.; Jiang, S.T.; Hsu, C.M.; Hsieh-Li, H.M.; Chiou, Y.Y. Secreted Neutrophil Gelatinase-Associated Lipocalin Shows Stronger Ability to Inhibit Cyst Enlargement of ADPKD Cells Compared with Nonsecreted Form. Cells 2022, 11, 483. [Google Scholar] [CrossRef]
- Malyszko, J.; Malyszko, J.S.; Koc-Zorawska, E.; Kozminski, P.; Mysliwiec, M. Neutrophil gelatinase-associated lipocalin in dialyzed patients is related to residual renal function, type of renal replacement therapy and inflammation. Kidney Blood Press. Res. 2009, 32, 464–469. [Google Scholar] [CrossRef]
- Imamaki, H.; Ishii, A.; Yokoi, H.; Kasahara, M.; Kuwabara, T.; Mori, K.P.; Kato, Y.; Kuwahara, T.; Satoh, M.; Nakatani, K.; et al. Low Serum Neutrophil Gelatinase-associated Lipocalin Level as a Marker of Malnutrition in Maintenance Hemodialysis Patients. PLoS ONE 2015, 10, e0132539. [Google Scholar] [CrossRef]
- Yazdani, M.; Merrikhi, A.; Beni, Z.N.; Baradaran, A.; Soleimani, N.; Musazade, H. Association between neutrophil geletinase-associated lipocalin and iron deficiency anemia in children on chronic dialysis. J. Res. Med. Sci. 2014, 19, 624–628. [Google Scholar]
- Aghsaeifard, Z.; Alizadeh, R.; Bagheri, N. Association between neutrophil gelatinase-associated lipocalin (NGAL) and iron profile in chronic renal disease. Arch. Physiol. Biochem. 2022, 128, 703–707. [Google Scholar] [CrossRef]
- Jia, X.Y.; Wei, K.; Chen, J.; Xi, L.H.; Kong, X.L.; Wei, Y.; Wang, L.; Wang, Z.S.; Liu, Y.P.; Liang, L.M.; et al. Association of plasma neutrophil gelatinase-associated lipocalin with parameters of CKD-MBD in maintenance hemodialysis patients. J. Bone Miner. Metab. 2021, 39, 1058–1065. [Google Scholar] [CrossRef]
- Yigit, I.P.; Ulu, R.; Gozel, N.; Taskapan, H.; Ilhan, N.; Dogukan, A. Neutrophil gelatinase-associated lipocalin reflects the severity of anemia without iron deficiency and secondary hyperparathyroidism in hemodialysis patients. North Clin. Istanb. 2017, 4, 36–42. [Google Scholar] [CrossRef]
- Virzì, G.M.; Mattiotti, M.; Manani, S.M.; Gnappi, M.; Tantillo, I.; Corradi, V.; De Cal, M.; Giuliani, A.; Carta, M.; Giavarina, D.; et al. Peritoneal NGAL: A reliable biomarker for PD-peritonitis monitoring. J. Nephrol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Scalzotto, E.; Giavarina, D.; Rodighiero, M.P.; Crepaldi, C.; Day, S.; Ronco, C. The Role of NGAL in Peritoneal Dialysis Effluent in Early Diagnosis of Peritonitis: Case-Control Study in Peritoneal Dialysis Patients. Perit. Dial. Int. 2015, 35, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, A.; Chirico, V.; Mondello, S.; Buemi, A.; Lupica, R.; Fazio, M.R.; Buemi, M.; Aloisi, C. Neutrophil gelatinase-associated lipocalin in peritoneal dialysis reflects status of peritoneum. J. Nephrol. 2013, 26, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Park, K.T.; Lee, J.W.; Cho, E.; Jo, S.K.; Cho, W.Y.; Kim, H.K. Urine neutrophil gelatinase-associated lipocalin predicts graft outcome up to 1 year after kidney transplantation. Transplant. Proc. 2013, 45, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Choi, H.M.; Seo, M.Y.; Lee, J.Y.; Kim, K.; Jun, H.; Jung, C.W.; Park, K.T.; Kim, M.G.; Jo, S.K.; et al. Urine liver-type fatty acid-binding protein predicts graft outcome up to 2 years after kidney transplantation. Transplant. Proc. 2014, 46, 376–380. [Google Scholar] [CrossRef]
- Hollmen, M.E.; Kyllönen, L.E.; Inkinen, K.A.; Lalla, M.L.; Salmela, K.T. Urine neutrophil gelatinase-associated lipocalin is a marker of graft recovery after kidney transplantation. Kidney Int. 2011, 79, 89–98. [Google Scholar] [CrossRef]
- Field, M.; Lowe, D.; Cobbold, M.; Higgins, R.; Briggs, D.; Inston, N.; Ready, A.R. The use of NGAL and IP-10 in the prediction of early acute rejection in highly sensitized patients following HLA-incompatible renal transplantation. Transpl. Int. 2014, 27, 362–370. [Google Scholar] [CrossRef]
- Ramirez-Sandoval, J.C.; Herrington, W.; Morales-Buenrostro, L.E. Neutrophil gelatinase-associated lipocalin in kidney transplantation: A review. Transplant. Rev. 2015, 29, 139–144. [Google Scholar] [CrossRef]
- Kim, S.C.; Page, E.K.; Knechtle, S.J. Urine proteomics in kidney transplantation. Transplant. Rev. 2014, 28, 15–20. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin: A promising biomarker for human acute kidney injury. Biomark. Med. 2010, 4, 265–280. [Google Scholar] [CrossRef]
- Ramirez-Sandoval, J.C.; Barrera-Chimal, J.; Simancas, P.E.; Correa-Rotter, R.; Bobadilla, N.A.; Morales-Buenrostro, L.E. Tubular urinary biomarkers do not identify aetiology of acute kidney injury in kidney transplant recipients. Nephrology 2014, 19, 352–358. [Google Scholar] [CrossRef]
- Cruz, D.N.; Gaiao, S.; Maisel, A.; Ronco, C.; Devarajan, P. Neutrophil gelatinase-associated lipocalin as a biomarker of cardiovascular disease: A systematic review. Clin. Chem. Lab. Med. 2012, 50, 1533–1545. [Google Scholar] [CrossRef]
- Sivalingam, Z.; Larsen, S.B.; Grove, E.L.; Hvas, A.M.; Kristensen, S.D.; Magnusson, N.E. Neutrophil gelatinase-associated lipocalin as a risk marker in cardiovascular disease. Clin. Chem. Lab. Med. 2017, 56, 5–18. [Google Scholar] [CrossRef]
- Yang, H.H.; Wang, X.; Li, S.; Liu, Y.; Akbar, R.; Fan, G.C. Lipocalin family proteins and their diverse roles in cardiovascular disease. Pharmacol. Ther. 2023, 244, 108385. [Google Scholar] [CrossRef]
- Helánová, K.; Pařenica, J.; Dlouhý, V.; Pávková Goldbergová, M.; Cermáková, Z.; Gottwaldová, J.; Spinar, J. The importance of NGAL and cystatin C biomarkers in cardiovascular diseases. Vnitr. Lek. 2012, 58, 286–290. [Google Scholar]
- Katagiri, M.; Takahashi, M.; Doi, K.; Myojo, M.; Kiyosue, A.; Ando, J.; Hirata, Y.; Komuro, I. Serum neutrophil gelatinase-associated lipocalin concentration reflects severity of coronary artery disease in patients without heart failure and chronic kidney disease. Heart Vessels 2016, 31, 1595–1602. [Google Scholar] [CrossRef]
- Daniels, L.B.; Barrett-Connor, E.; Clopton, P.; Laughlin, G.A.; Ix, J.H.; Maisel, A.S. Plasma neutrophil gelatinase-associated lipocalin is independently associated with cardiovascular disease and mortality in community-dwelling older adults: The Rancho Bernardo Study. J. Am. Coll. Cardiol. 2012, 59, 1101–1109. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Van der Wal, A.C.; Becker, A.E.; van der Loos, C.M.; Das, P.K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994, 89, 36–44. [Google Scholar] [CrossRef]
- Oberoi, R.; Bogalle, E.P.; Matthes, L.A.; Schuett, H.; Koch, A.K.; Grote, K.; Schieffer, B.; Schuett, J.; Luchtefeld, M. Lipocalin (LCN) 2 Mediates Pro-Atherosclerotic Processes and Is Elevated in Patients with Coronary Artery Disease. PLoS ONE 2015, 10, e0137924. [Google Scholar] [CrossRef]
- Hemdahl, A.L.; Gabrielsen, A.; Zhu, C.; Eriksson, P.; Hedin, U.; Kastrup, J.; Thorén, P.; Hansson, G.K. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, A.; Houard, X.; Philippe, M.; Ollivier, V.; Sebbag, U.; Meilhac, O.; Michel, J.B. Involvement of intraplaque hemorrhage in atherothrombosis evolution via neutrophil protease enrichment. J. Leukoc. Biol. 2007, 82, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Te Boekhorst, B.C.; Bovens, S.M.; Hellings, W.E.; van der Kraak, P.H.; van de Kolk, K.W.; Vink, A.; Moll, F.L.; van Oosterhout, M.F.; de Vries, J.P.; Doevendans, P.A.; et al. Molecular MRI of murine atherosclerotic plaque targeting NGAL: A protein associated with unstable human plaque characteristics. Cardiovasc. Res. 2011, 89, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, W.; Stojkovic, S.; Kaider, A.; Kozakowski, N.; Domenig, C.M.; Burghuber, C.; Nanobachvili, J.; Huber, K.; Klinger, M.; Neumayer, C.; et al. NGAL and MMP-9/NGAL as biomarkers of plaque vulnerability and targets of statins in patients with carotid atherosclerosis. Clin. Chem. Lab. Med. 2017, 56, 147–156. [Google Scholar] [CrossRef]
- Kafkas, N.; Demponeras, C.; Zoubouloglou, F.; Spanou, L.; Babalis, D.; Makris, K. Serum levels of gelatinase associated lipocalin as indicator of the inflammatory status in coronary artery disease. Int. J. Inflam. 2012, 2012, 189797. [Google Scholar] [CrossRef]
- Zografos, T.; Haliassos, A.; Korovesis, S.; Giazitzoglou, E.; Voridis, E.; Katritsis, D. Association of neutrophil gelatinase-associated lipocalin with the severity of coronary artery disease. Am. J. Cardiol. 2009, 104, 917–920. [Google Scholar] [CrossRef]
- Soylu, K.; Aksan, G.; Nar, G.; Özdemir, M.; Gülel, O.; İnci, S.; Aksakal, A.; Soylu, A.İ.; Yılmaz, Ö. Serum neutrophil gelatinase-associated lipocalin levels are correlated with the complexity and the severity of atherosclerosis in acute coronary syndrome. Anatol. J. Cardiol. 2015, 15, 450–455. [Google Scholar] [CrossRef]
- Sahinarslan, A.; Kocaman, S.A.; Bas, D.; Akyel, A.; Ercin, U.; Zengin, O.; Timurkaynak, T. Plasma neutrophil gelatinase-associated lipocalin levels in acute myocardial infarction and stable coronary artery disease. Coron. Artery Dis. 2011, 22, 333–338. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Peng, Y.; Gao, H.; Wang, Y.; Zhao, J.; Pan, C. Plasma neutrophil gelatinase-associated lipocalin levels are associated with the presence and severity of coronary heart disease. PLoS ONE 2019, 14, e0220841. [Google Scholar] [CrossRef]
- Paulsson, J.; Dadfar, E.; Held, C.; Jacobson, S.H.; Lundahl, J. Activation of peripheral and in vivo transmigrated neutrophils in patients with stable coronary artery disease. Atherosclerosis 2007, 192, 328–334. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, Y.; Wang, S.; Chen, P.; Pei, Y.; Zhang, J.; Zhang, R.; Niu, G.; Gu, F.; Li, X. Clinical significance of neutrophil gelatinase-associated lipocalin and sdLDL-C for coronary artery disease in patients with type 2 diabetes mellitus aged ≥65 years. Cardiovasc. Diabetol. 2022, 21, 252. [Google Scholar] [CrossRef]
- Vasan, R.S.; Pan, S.; Xanthakis, V.; Beiser, A.; Larson, M.G.; Seshadri, S.; Mitchell, G.F. Arterial Stiffness and Long-Term Risk of Health Outcomes: The Framingham Heart Study. Hypertension 2022, 79, 1045–1056. [Google Scholar] [CrossRef]
- Soylu, K.; Nar, G.; Aksan, G.; Gedikli, Ö.; İnci, S.; Yuksel, S.; Nar, R.; İdil Soylu, A.; Gulel, O.; Şahin, M. Serum neutrophil gelatinase-associated lipocalin levels and aortic stiffness in noncritical coronary artery disease. Cardiorenal Med. 2014, 4, 147–154. [Google Scholar] [CrossRef]
- Cheng, L.; Xing, H.; Mao, X.; Li, L.; Li, X.; Li, Q. Lipocalin-2 promotes m1 macrophages polarization in a mouse cardiac ischaemia-reperfusion injury model. Scand. J. Immunol. 2015, 81, 31–38. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Buonafine, M.; Boukhalfa, I.; Ibarrola, J.; Fernández-Celis, A.; Kolkhof, P.; Rossignol, P.; Girerd, N.; Mulder, P.; López-Andrés, N.; et al. Aldosterone Target NGAL (Neutrophil Gelatinase-Associated Lipocalin) Is Involved in Cardiac Remodeling After Myocardial Infarction Through NFκB Pathway. Hypertension 2017, 70, 1148–1156. [Google Scholar] [CrossRef]
- Freitas, I.A.; Lima, N.A.; Silva, G.B.D., Jr.; Castro, R.L., Jr.; Patel, P.; Lima, C.C.V.; Lino, D.O.D.C. Novel biomarkers in the prognosis of patients with atherosclerotic coronary artery disease. Rev. Port. Cardiol. 2020, 39, 667–672. [Google Scholar] [CrossRef]
- Avci, A.; Ozturk, B.; Demir, K.; Akyürek, F.; Altunkeser, B.B. The Prognostic Utility of Plasma NGAL Levels in ST Segment Elevation in Myocardial Infarction Patients. Adv. Prev. Med. 2020, 2020, 4637043. [Google Scholar] [CrossRef]
- Yang, B.; Fan, P.; Xu, A.; Lam, K.S.; Berger, T.; Mak, T.W.; Tse, H.F.; Yue, J.W.; Song, E.; Vanhoutte, P.M.; et al. Improved functional recovery to I/R injury in hearts from lipocalin-2 deficiency mice: Restoration of mitochondrial function and phospholipids remodeling. Am. J. Transl. Res. 2012, 4, 60–71. [Google Scholar]
- Karetnikova, V.; Osokina, A.; Gruzdeva, O.; Uchasova, E.; Zykov, M.; Kalaeva, V.; Kashtalap, V.; Shafranskaya, K.; Barbarash, O. Serum neutrophil gelatinase-associated lipocalin the estimation of hospital prognosis in patients with ST-elevated myocardial infarction. PLoS ONE 2017, 12, e0180816. [Google Scholar] [CrossRef]
- Fan, Y.; Zou, C. Prognostic value of neutrophil gelatinase-associated lipocalin in patients with acute ST-segment elevation myocardial infarction: A meta-analysis. Eur. J. Prev. Cardiol. 2019, 26, 444–446. [Google Scholar] [CrossRef]
- Lindberg, S.; Pedersen, S.H.; Mogelvang, R.; Jensen, J.S.; Flyvbjerg, A.; Galatius, S.; Magnusson, N.E. Prognostic utility of neutrophil gelatinase-associated lipocalin in predicting mortality and cardiovascular events in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J. Am. Coll. Cardiol. 2012, 60, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Helanova, K.; Littnerova, S.; Kubena, P.; Ganovska, E.; Pavlusova, M.; Kubkova, L.; Jarkovsky, J.; Pavkova Goldbergova, M.; Lipkova, J.; Gottwaldova, J.; et al. Prognostic impact of neutrophil gelatinase-associated lipocalin and B-type natriuretic in patients with ST-elevation myocardial infarction treated by primary PCI: A prospective observational cohort study. BMJ Open 2015, 5, e006872. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Beltrami, M.; Pellegrini, M.; Nuti, R. Natriuretic peptides and NGAL in heart failure: Does a link exist? Clin. Chim. Acta 2012, 413, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; Simic, D.; Radovanovic, S.; Simic, T. Novel Biomarkers of Heart Failure. Adv. Clin. Chem. 2017, 79, 93–152. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, S.; Chen, Z.; Adhikari, B.K.; Zhang, Y.; Zhang, J.; Sun, J.; Wang, Y. Cardiac biomarkers of heart failure in chronic kidney disease. Clin. Chim. Acta 2020, 510, 298–310. [Google Scholar] [CrossRef]
- Yndestad, A.; Landrø, L.; Ueland, T.; Dahl, C.P.; Flo, T.H.; Vinge, L.E.; Espevik, T.; Frøland, S.S.; Husberg, C.; Christensen, G.; et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur. Heart J. 2009, 30, 1229–1236. [Google Scholar] [CrossRef]
- Bolignano, D.; Basile, G.; Parisi, P.; Coppolino, G.; Nicocia, G.; Buemi, M. Increased plasma neutrophil gelatinase-associated lipocalin levels predict mortality in elderly patients with chronic heart failure. Rejuvenation Res. 2009, 12, 7–14. [Google Scholar] [CrossRef]
- Oikonomou, E.; Tsalamandris, S.; Karlis, D.; Siasos, G.; Chrysohoou, C.; Vogiatzi, G.; Dimitropoulos, S.; Charalambous, G.; Kouskouni, E.; Tousoulis, D. The association among biomarkers of renal and heart function in patients with heart failure: The role of NGAL. Biomark. Med. 2018, 12, 1323–1330. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Ruocco, G.; Pellegrini, M.; De Gori, C.; Del Castillo, G.; Franci, B.; Nuti, R.; Ronco, C. Comparison of Neutrophil Gelatinase-Associated Lipocalin Versus B-Type Natriuretic Peptide and Cystatin C to Predict Early Acute Kidney Injury and Outcome in Patients with Acute Heart Failure. Am. J. Cardiol. 2015, 116, 104–111. [Google Scholar] [CrossRef]
- Maisel, A.S.; Mueller, C.; Fitzgerald, R.; Brikhan, R.; Hiestand, B.C.; Iqbal, N.; Clopton, P.; van Veldhuisen, D.J. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: The NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur. J. Heart Fail. 2011, 13, 846–851. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Alvelos, M.; Pimentel, R.; Pinho, E.; Gomes, A.; Lourenço, P.; Teles, M.J.; Almeida, P.; Guimarães, J.T.; Bettencourt, P. Neutrophil gelatinase-associated lipocalin in the diagnosis of type 1 cardio-renal syndrome in the general ward. Clin. J. Am. Soc. Nephrol. 2011, 6, 476–481. [Google Scholar] [CrossRef]
- Nasonova, S.N.; Zhirov, I.V.; Ledyakhova, M.V.; Sharf, T.V.; Bosykh, E.G.; Masenko, V.P.; Tereshchenko, S.N. Early diagnosis of acute renal injury in patients with acute decompensation of chronic heart failure. Ter. Arkh. 2019, 91, 67–73. [Google Scholar] [CrossRef]
- Dankova, M.; Minarikova, Z.; Danko, J.; Gergel, J.; Pontuch, P.; Goncalvesova, E. Novel biomarkers for prediction of acute kidney injury in acute heart failure. Bratisl. Lek. Listy 2020, 121, 321–324. [Google Scholar] [CrossRef]
- Thai, H.P.; Bui, B.H.; Anh, T.H.; Van, M.H. Value of Plasma NGAL and Creatinine on First Day of Admission in the Diagnosis of Cardiorenal Syndrome Type 1. Cardiol. Res. Pract. 2020, 2020, 2789410. [Google Scholar] [CrossRef]
- Gembillo, G.; Visconti, L.; Giusti, M.A.; Siligato, R.; Gallo, A.; Santoro, D.; Mattina, A. Cardiorenal Syndrome: New Pathways and Novel Biomarkers. Biomolecules 2021, 11, 1581. [Google Scholar] [CrossRef]
- Song, X.; Cai, D.; Zhang, B. Clinical values of serum NGAL combined with NT-proBNP in the early prognosis of type 1 cardiorenal syndrome. Am. J. Transl. Res. 2021, 13, 3363–3368. [Google Scholar]
- Angelini, A.; Castellani, C.; Virzì, G.M.; Fedrigo, M.; Thiene, G.; Valente, M.; Ronco, C.; Vescovo, G. The Role of Congestion in Cardiorenal Syndrome Type 2: New Pathophysiological Insights into an Experimental Model of Heart Failure. Cardiorenal Med. 2015, 6, 61–72. [Google Scholar] [CrossRef]
- Sonmez, O.; Ertem, F.U.; Vatankulu, M.A.; Erdogan, E.; Tasal, A.; Kucukbuzcu, S.; Goktekin, O. Novel fibro-inflammation markers in assessing left atrial remodeling in non-valvular atrial fibrillation. Med. Sci. Monit. 2014, 20, 463–470. [Google Scholar] [CrossRef]
- Mlodawska, E.; Tomaszuk-Kazberuk, A.; Lopatowska, P.; Waszkiewicz, E.; Bachorzewska-Gajewska, H.; Malyszko, J.; Michniewicz, E.; Dobrzycki, S.; Musial, W.J. Matrix Metalloproteinase Neutrophil Gelatinase-Associated Lipocalin Complex Predicts Atrial Fibrillation Recurrence after Electrical Cardioversion in Obese Patients. Cardiorenal Med. 2016, 7, 11–20. [Google Scholar] [CrossRef]
- Malyszko, J.; Bachorzewska-Gajewska, H.; Malyszko, J.S.; Pawlak, K.; Dobrzycki, S. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in hypertensive and normotensive patients with coronary artery disease. Nephrology 2008, 13, 153–156. [Google Scholar] [CrossRef]
- Araos, P.; Amador, C.A. Neutrophil gelatinase-associated lipocalin as an immunomodulator in endocrine hypertension. Front. Endocrinol. 2022, 13, 1006790. [Google Scholar] [CrossRef] [PubMed]
- Araos, P.; Prado, C.; Lozano, M.; Figueroa, S.; Espinoza, A.; Berger, T.; Mak, T.W.; Jaisser, F.; Pacheco, R.; Michea, L.; et al. Dendritic cells are crucial for cardiovascular remodeling and modulate neutrophil gelatinase-associated lipocalin expression upon mineralocorticoid receptor activation. J. Hypertens. 2019, 37, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Tarjus, A.; Martínez-Martínez, E.; Amador, C.; Latouche, C.; El Moghrabi, S.; Berger, T.; Mak, T.W.; Fay, R.; Farman, N.; Rossignol, P.; et al. Neutrophil Gelatinase-Associated Lipocalin, a Novel Mineralocorticoid Biotarget, Mediates Vascular Profibrotic Effects of Mineralocorticoids. Hypertension 2015, 66, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.Y.M.; Trinh, K.; Li, J.; Hahn, S.H.; Endre, Z.H.; Rogers, N.M.; Alexander, S.I. Biomarkers in Cardiorenal Syndrome and Potential Insights into Novel Therapeutics. Front. Cardiovasc. Med. 2022, 9, 868658. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, D.D.; Feng, Y.M.; Huang, Z.Q.; Xie, Y.B.; Zhou, J.; Li, J. Relationship between morning peak phenomenon and early renal injury NGAL in H-type hypertension. Blood Press. 2022, 31, 200–206. [Google Scholar] [CrossRef]
- Aksan, G.; İnci, S.; Nar, G.; Siğirci, S.; Gedikli, Ö.; Soylu, K.; Nar, R.; Yüksel, S.; Şahin, M. Serum neutrophıl gelatınase-assocıated lıpocalın levels in patients with non-dipper hypertension. Clin. Investig. Med. 2015, 38, 53–62. [Google Scholar] [CrossRef]
- Albert, C.; Haase, M.; Albert, A.; Ernst, M.; Kropf, S.; Bellomo, R.; Westphal, S.; Braun-Dullaeus, R.C.; Haase-Fielitz, A.; Elitok, S. Predictive Value of Plasma NGAL:Hepcidin-25 for Major Adverse Kidney Events after Cardiac Surgery with Cardiopulmonary Bypass: A Pilot Study. Ann. Lab. Med. 2021, 41, 357–365. [Google Scholar] [CrossRef]
- Ghonemy, T.A.; Amro, G.M. Plasma neutrophil gelatinase-associated lipocalin (NGAL) and plasma cystatin C (CysC) as biomarker of acute kidney injury after cardiac surgery. Saudi J. Kidney Dis. Transpl. 2014, 25, 582–588. [Google Scholar] [CrossRef]
- Yuan, S.M. Acute Kidney Injury after Cardiac Surgery: Risk Factors and Novel Biomarkers. Braz. J. Cardiovasc. Surg. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Zhou, F.; Luo, Q.; Wang, L.; Han, L. Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: A meta-analysis. Eur. J. Cardiothorac. Surg. 2016, 49, 746–755. [Google Scholar] [CrossRef]
- Karaolanis, G.; Moris, D.; Palla, V.V.; Karanikola, E.; Bakoyiannis, C.; Georgopoulos, S. Neutrophil Gelatinase Associated Lipocalin (NGAL) as a Biomarker. Does It Apply in Abdominal Aortic Aneurysms? A Review of Literature. Indian J. Surg. 2015, 77, 1313–1317. [Google Scholar] [CrossRef]
- Tarín, C.; Fernandez-Garcia, C.E.; Burillo, E.; Pastor-Vargas, C.; Llamas-Granda, P.; Castejón, B.; Ramos-Mozo, P.; Torres-Fonseca, M.M.; Berger, T.; Mak, T.W.; et al. Lipocalin-2 deficiency or blockade protects against aortic abdominal aneurysm development in mice. Cardiovasc. Res. 2016, 111, 262–273. [Google Scholar] [CrossRef]
- Ramos-Mozo, P.; Madrigal-Matute, J.; Vega de Ceniga, M.; Blanco-Colio, L.M.; Meilhac, O.; Feldman, L.; Michel, J.B.; Clancy, P.; Golledge, J.; Norman, P.E.; et al. Increased plasma levels of NGAL, a marker of neutrophil activation, in patients with abdominal aortic aneurysm. Atherosclerosis 2012, 220, 552–556. [Google Scholar] [CrossRef]
- Aqrawi, L.A.; Galtung, H.K.; Vestad, B.; Øvstebø, R.; Thiede, B.; Rusthen, S.; Young, A.; Guerreiro, E.M.; Utheim, T.P.; Chen, X.; et al. Identification of potential saliva and tear biomarkers in primary Sjögren’s syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res. Ther. 2017, 19, 14. [Google Scholar] [CrossRef]
- Aqrawi, L.A.; Jensen, J.L.; Fromreide, S.; Galtung, H.K.; Skarstein, K. Expression of NGAL-specific cells and mRNA levels correlate with inflammation in the salivary gland, and its overexpression in the saliva, of patients with primary Sjögren’s syndrome. Autoimmunity 2020, 53, 333–343. [Google Scholar] [CrossRef]
- Metallinou, D.; Lykeridou, K.; Karampas, G.; Liosis, G.T.; Skevaki, C.; Rizou, M.; Papassotiriou, I.; Rizos, D. Postpartum human breast milk levels of neutrophil gelatinase-associated lipocalin (NGAL) and matrix metalloproteinase-9 (MMP-9)/NGAL complex in normal and pregnancies complicated with insulin-dependent gestational diabetes mellitus. A prospective pilot case-control study. J. Obstet. Gynaecol. 2020, 40, 461–467. [Google Scholar] [CrossRef]
- Naudé, P.J.W.; Ramakers, I.H.G.B.; Van der Flier, W.M.; Jiskoot, L.C.; Reesink, F.E.; Claassen, J.A.H.R.; Koek, H.L.; Eisel, U.L.M.; De Deyn, P.P. Serum and cerebrospinal fluid Neutrophil gelatinase-associated lipocalin (NGAL) levels as biomarkers for the conversion from mild cognitive impairment to Alzheimer’s disease dementia. Neurobiol. Aging 2021, 107, 1–10. [Google Scholar] [CrossRef]
- Wu, C.Y.; Bawa, K.K.; Ouk, M.; Leung, N.; Yu, D.; Lanctôt, K.L.; Herrmann, N.; Pakosh, M.; Swardfager, W. Neutrophil activation in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis of protein markers in blood and cerebrospinal fluid. Ageing Res. Rev. 2020, 62, 101130. [Google Scholar] [CrossRef]
- Platanaki, C.; Paraskevas, T.; Delastic, A.L.; Michailides, C.; Kantanis, A.; Polychronopoulos, P.; Marangos, M.; Velissaris, D. The role of cerebrospinal fluid levels of neutrophil gelatinase-associated lipocalin (NGAL) and electroencephalography in the assessment of impaired consciousness in the context of infection. Rom. J. Intern. Med. 2023, 61, 112–115. [Google Scholar] [CrossRef]
- Osuka, K.; Watanabe, Y.; Suzuki, C.; Iwami, K.; Miyachi, S. Sequential expression of neutrophil chemoattractants in cerebrospinal fluid after subarachnoid hemorrhage. J. Neuroimmunol. 2021, 357, 577610. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Gallo, J.; Zapletalová, J.; Prošková, J.; Juráňová, J.; Lovečková, Y. Glucose, Lactate, NGAL and Coefficient of Energy Balance in Synovial Fluid in Patients with Hip and Knee Prosthetic Joint Infection. Acta Chir. Orthop. Traumatol. Cech 2022, 89, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, Z.; Li, M.; Li, W.; Fang, X.; Zhang, W. Synovial fluid neutrophil gelatinase-associated lipocalin can be used to accurately diagnose prosthetic joint infection. Int. J. Infect. Dis. 2022, 123, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Vergara, A.; Fernández-Pittol, M.J.; Muñoz-Mahamud, E.; Morata, L.; Bosch, J.; Vila, J.; Soriano, A.; Casals-Pascual, C. Evaluation of Lipocalin-2 as a Biomarker of Periprosthetic Joint Infection. J. Arthroplasty 2019, 34, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, X.; Ni, M.; Fu, J.; Xu, C.; Chai, W.; Chen, J.Y. What is the performance of novel synovial biomarkers for detecting periprosthetic joint infection in the presence of inflammatory joint disease? Bone Joint J. 2021, 103, 32–38. [Google Scholar] [CrossRef]
- Sesen, J.; Driscoll, J.; Moses-Gardner, A.; Orbach, D.B.; Zurakowski, D.; Smith, E.R. Non-invasive Urinary Biomarkers in Moyamoya Disease. Front. Neurol. 2021, 12, 661952. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, M.; Yan, X.; Cao, L.; Barnes, P.J.; Adcock, I.M.; Huang, M.; Yao, X. Increased neutrophil gelatinase-associated lipocalin (NGAL) promotes airway remodelling in chronic obstructive pulmonary disease. Clin. Sci. 2017, 131, 1147–1159. [Google Scholar] [CrossRef]
- Xiao, R.; Chen, R. Neutrophil gelatinase-associated lipocalin as a potential novel biomarker for ventilator-associated lung injury. Mol. Med. Rep. 2017, 15, 3535–3540. [Google Scholar] [CrossRef]
- Wu, K.A.; Wu, C.C.; Liu, Y.C.; Hsueh, P.C.; Chin, C.Y.; Wang, C.L.; Chu, C.M.; Shih, L.J.; Yang, C.Y. Combined serum biomarkers in the noninvasive diagnosis of complicated parapneumonic effusions and empyema. BMC Pulm. Med. 2019, 19, 108. [Google Scholar] [CrossRef]
- Faria, D.K.; Faria, C.S.; Doi, D.; Acencio, M.M.P.; Antonangelo, L. Hybrid panel of biomarkers can be useful in the diagnosis of pleural and peritoneal effusions. Clin. Chim. Acta 2019, 497, 48–53. [Google Scholar] [CrossRef]
- Beghini, J.; Giraldo, P.C.; Linhares, I.M.; Ledger, W.J.; Witkin, S.S. Neutrophil Gelatinase-Associated Lipocalin Concentration in Vaginal Fluid: Relation to Bacterial Vaginosis and Vulvovaginal Candidiasis. Reprod. Sci. 2015, 22, 964–968. [Google Scholar] [CrossRef]
- Beghini, J.; Giraldo, P.C.; Eleutério, J., Jr.; Do Amaral, R.L.; Polpeta, N.C.; Gonçalves, A.K. Vaginal Inflammation: Association between Leukocyte Concentration and Levels of Immune Mediators. Am. J. Reprod. Immunol. 2016, 75, 126–133. [Google Scholar] [CrossRef]
- Vajrychova, M.; Kacerovsky, M.; Tambor, V.; Hornychova, H.; Lenco, J. Microbial invasion and histological chorioamnionitis upregulate neutrophil-gelatinase associated lipocalin in preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 2016, 29, 12–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).