The Role of Oxytocin in Early-Life-Stress-Related Neuropsychiatric Disorders
Abstract
1. Introduction
2. The Properties of Oxytocin
3. Oxytocin-Involved Social Behavior
3.1. Positive Social Interactions
3.2. Negative Social Interactions
3.3. Sexual Dimorphism of Oxytocinin Social Behavior
4. Effects of ELS on the Oxytocin System and Central Nervous System
4.1. Effects of ELS on the Oxytocin System
4.2. Abnormalities in Oxytocin System Altered by ELS Correlated with Glial Cells
5. Potential Therapy Strategies of Oxytocin in ELS-Related Neuropsychiatric Disorders
5.1. Autism Spectrum Disorder
5.2. Schizophrenia
5.3. Social Anxiety Disorders
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Early-life stress (ELS) |
| Oxytocin (OXT) |
| Oxytocin receptor (OXTR) |
| Magnocellular neurons (magnOT) |
| Parvocellular neurons (parvOT) |
| Autism spectrum disorder (ASD) |
| Social anxiety disorder (SAD) |
| Post-traumatic stress disorder (PTSD) |
| Bipolar disorder (BD) |
| Amygdala (AMY) |
| Medial prefrontal cortex (mPFC) |
| Anterior cingulate cortex (ACC) |
| Maternal separation (MS) |
| Maternal deprivation (MD) |
| Limited bedding and nesting (LBN) |
| Low level of maternal care (LG) |
| Hypothalamic–pituitary–adrenal (HPA) |
| Corticosterone (CORT) |
| Paraventricular nucleus (PVN) |
| Supraoptic nucleus (SON) |
| Hippocampus (HIP) |
| Lateral portion of the central amygdala (CeL) |
| Medial part of the central amygdala (CeM) |
| Central amygdala nucleus (CeA) |
| Accessory nuclei (AC) |
| Anterior olfactory nucleus (AON) |
| Medial amygdaloid (MeA) |
| Medial preoptic area (MPOA) |
| Nucleus accumbens (Nac) |
| Lateral hypothalamic area (LHA) |
| Corticotropin-releasing hormone (CRH) |
| Spinal cord (SC) |
References
- Schiavone, S.; Jaquet, V.; Trabace, L.; Krause, K.H. Severe life stress and oxidative stress in the brain: From animal models to human pathology. Antioxid. Redox Signal. 2013, 18, 1475–1490. [Google Scholar] [CrossRef]
- Selye, H. A syndrome produced by diverse nocuous agents. 1936. J. Neuropsychiatry Clin. Neurosci. 1998, 10, 230–231. [Google Scholar] [CrossRef]
- Agorastos, A.; Pervanidou, P.; Chrousos, G.P.; Baker, D.G. Developmental Trajectories of Early Life Stress and Trauma: A Narrative Review on Neurobiological Aspects Beyond Stress System Dysregulation. Front. Psychiatry 2019, 10, 118. [Google Scholar] [CrossRef]
- Makris, G.; Eleftheriades, A.; Pervanidou, P. Early Life Stress, Hormones, and Neurodevelopmental Disorders. Horm. Res. Paediatr. 2022, 96, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Juruena, M.F.; Eror, F.; Cleare, A.J.; Young, A.H. The Role of Early Life Stress in HPA Axis and Anxiety. Adv. Exp. Med. Biol. 2020, 1191, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.O.; Crawford, E.; Del Castillo, D. Childhood emotional maltreatment and later psychological distress among college students: The mediating role of maladaptive schemas. Child. Abus. Negl. 2009, 33, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Baracz, S.J.; Everett, N.A.; Cornish, J.L. The impact of early life stress on the central oxytocin system and susceptibility for drug addiction: Applicability of oxytocin as a pharmacotherapy. Neurosci. Biobehav. Rev. 2020, 110, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Colleluori, G.; Galli, C.; Severi, I.; Perugini, J.; Giordano, A. Early Life Stress, Brain Development, and Obesity Risk: Is Oxytocin the Missing Link? Cells 2022, 11, 623. [Google Scholar] [CrossRef]
- Love, T.M. The impact of oxytocin on stress: The role of sex. Curr. Opin. Behav. Sci. 2018, 23, 136–142. [Google Scholar] [CrossRef]
- Lang, R.E.; Heil, J.W.; Ganten, D.; Hermann, K.; Unger, T.; Rascher, W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology 1983, 37, 314–316. [Google Scholar] [CrossRef]
- Nylander, I.; Roman, E. Neuropeptides as mediators of the early-life impact on the brain; implications for alcohol use disorders. Front. Mol. Neurosci. 2012, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Leng, G. The Heart of the Brain: The Hypothalamus and Its Hormones; The MIT Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Carter, C.S. The Role of Oxytocin and Vasopressin in Attachment. Psychodyn. Psychiatry 2017, 45, 499–517. [Google Scholar] [CrossRef]
- García-Cáceres, C.; Balland, E.; Prevot, V.; Luquet, S.; Woods, S.C.; Koch, M.; Horvath, T.L.; Yi, C.-X.; Chowen, J.A.; Verkhratsky, A.; et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci. 2019, 22, 7–14. [Google Scholar] [CrossRef]
- George, J.M. Immunoreactive vasopressin and oxytocin: Concentration in individual human hypothalamic nuclei. Science 1978, 200, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, B.; Truedsson, M.; Djerf, P.; Sundler, F. Oxytocin is expressed throughout the human gastrointestinal tract. Regul. Pept. 2006, 135, 7–11. [Google Scholar] [CrossRef]
- Althammer, F.; Grinevich, V. Diversity of oxytocin neurons: Beyond magno- and parvocellular cell types? J. Neuroendocrinol. 2017, 30, e12549. [Google Scholar] [CrossRef]
- McCormack, S.E.; Blevins, J.E.; Lawson, E.A. Metabolic Effects of Oxytocin. Endocr. Rev. 2020, 41, 121–145. [Google Scholar] [CrossRef]
- Swanson, L.W.; Sawchenko, P.E.; Wiegand, S.J.; Price, J.L. Separate neurons in the paraventricular nucleus project to the median eminence and to the medulla or spinal cord. Brain Res. 1980, 198, 190–195. [Google Scholar] [CrossRef]
- Tang, Y.; Benusiglio, D.; Lefevre, A.; Hilfiger, L.; Althammer, F.; Bludau, A.; Hagiwara, D.; Baudon, A.; Darbon, P.; Schimmer, J.; et al. Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nat. Neurosci. 2020, 23, 1125–1137. [Google Scholar] [CrossRef]
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Cetin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 2012, 73, 553–566. [Google Scholar] [CrossRef]
- Baldi, E.; Costa, A.; Rani, B.; Passani, M.B.; Blandina, P.; Romano, A.; Provensi, G. Oxytocin and Fear Memory Extinction: Possible Implications for the Therapy of Fear Disorders? Int. J. Mol. Sci. 2021, 22, 10000. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, M.J.; Russell, J.T.; Gainer, H. Synthesis, transport, and release of posterior pituitary hormones. Science 1980, 207, 373–378. [Google Scholar] [CrossRef]
- Landgraf, R.; Neumann, I.D. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocr. 2004, 25, 150–176. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D.; Landgraf, R. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012, 35, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Charlet, A.; Grinevich, V. Oxytocin Mobilizes Midbrain Dopamine toward Sociality. Neuron 2017, 95, 235–237. [Google Scholar] [CrossRef]
- Sjoholm, I. Oxytocinase and its possible significance in the degradation of oxytocin during pregnancy. FEBS Lett. 1969, 4, 135–139. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Dumais, K.M.; Veenema, A.H. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front. Neuroendocr. 2016, 40, 1–23. [Google Scholar] [CrossRef]
- Stoop, R. Neuromodulation by oxytocin and vasopressin. Neuron 2012, 76, 142–159. [Google Scholar] [CrossRef]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef]
- Baudon, A.; Clauss Creusot, E.; Althammer, F.; Schaaf, C.P.; Charlet, A. Emerging role of astrocytes in oxytocin-mediated control of neural circuits and brain functions. Prog. Neurobiol. 2022, 217, 102328. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.W.; Neuner, S.; Polepalli, J.S.; Beier, K.T.; Wright, M.; Walsh, J.J.; Lewis, E.M.; Luo, L.; Deisseroth, K.; Dolen, G.; et al. Gating of social reward by oxytocin in the ventral tegmental area. Science 2017, 357, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Oettl, L.L.; Ravi, N.; Schneider, M.; Scheller, M.F.; Schneider, P.; Mitre, M.; da Silva Gouveia, M.; Froemke, R.C.; Chao, M.V.; Young, W.S.; et al. Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron 2016, 90, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Raam, T.; McAvoy, K.M.; Besnard, A.; Veenema, A.H.; Sahay, A. Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat. Commun. 2017, 8, 2001. [Google Scholar] [CrossRef]
- Knoop, M.; Possovre, M.-L.; Jacquens, A.; Charlet, A.; Baud, O.; Darbon, P. The Role of Oxytocin in Abnormal Brain Development: Effect on Glial Cells and Neuroinflammation. Cells 2022, 11, 3899. [Google Scholar] [CrossRef]
- Song, Z.; Albers, H.E. Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Front. Neuroendocr. 2018, 51, 14–24. [Google Scholar] [CrossRef]
- Abramova, O.; Zorkina, Y.; Ushakova, V.; Zubkov, E.; Morozova, A.; Chekhonin, V. The role of oxytocin and vasopressin dysfunction in cognitive impairment and mental disorders. Neuropeptides 2020, 83, 102079. [Google Scholar] [CrossRef]
- Wahis, J.; Baudon, A.; Althammer, F.; Kerspern, D.; Goyon, S.; Hagiwara, D.; Lefevre, A.; Barteczko, L.; Boury-Jamot, B.; Bellanger, B.; et al. Astrocytes mediate the effect of oxytocin in the central amygdala on neuronal activity and affective states in rodents. Nat. Neurosci. 2021, 24, 529–541. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, S.; Bai, X.; Gao, Y.; Liu, G.; Wang, X.; Liu, D.; Li, T.; Hao, A.; Wang, Z. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J. Neuroinflammation 2016, 13, 77. [Google Scholar] [CrossRef]
- Szeto, A.; Sun-Suslow, N.; Mendez, A.J.; Hernandez, R.I.; Wagner, K.V.; McCabe, P.M. Regulation of the macrophage oxytocin receptor in response to inflammation. Am. J. Physiology. Endocrinol. Metab. 2017, 312, E183–E189. [Google Scholar] [CrossRef]
- Inoue, T.; Yamakage, H.; Tanaka, M.; Kusakabe, T.; Shimatsu, A.; Satoh-Asahara, N. Oxytocin Suppresses Inflammatory Responses Induced by Lipopolysaccharide through Inhibition of the eIF-2-ATF4 Pathway in Mouse Microglia. Cells 2019, 8, 527. [Google Scholar] [CrossRef] [PubMed]
- Guttenplan, K.A.; Weigel, M.K.; Adler, D.I.; Couthouis, J.; Liddelow, S.A.; Gitler, A.D.; Barres, B.A. Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model. Nat. Commun. 2020, 11, 3753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Marsh, S.E.; Walker, A.J.; Kamath, T.; Dissing-Olesen, L.; Hammond, T.R.; de Soysa, T.Y.; Young, A.M.H.; Murphy, S.; Abdulraouf, A.; Nadaf, N.; et al. Dissection of artifactual and confounding glial signatures by single-cell sequencing of mouse and human brain. Nat. Neurosci. 2022, 25, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, Z.; Zhou, L.; Darmanis, S.; Neff, N.F.; Okamoto, J.; Gulati, G.; Bennett, M.L.; Sun, L.O.; Clarke, L.E.; et al. Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 2019, 101, 207–223.e210. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.F.; Pusapati, S.; Khenhrani, R.R.; Farooqi, M.S.; Sarwar, S.; Alnasarat, A.; Mathur, N.; Metz, C.N.; LeRoith, D.; Tracey, K.J.; et al. Oxytocin and Related Peptide Hormones: Candidate Anti-Inflammatory Therapy in Early Stages of Sepsis. Front. Immunol. 2022, 13, 864007. [Google Scholar] [CrossRef]
- Tang, Y.; Shi, Y.; Gao, Y.; Xu, X.; Han, T.; Li, J.; Liu, C. Oxytocin system alleviates intestinal inflammation by regulating macrophages polarization in experimental colitis. Clin. Sci. (Lond. Engl. 1979) 2019, 133, 1977–1992. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, B.; Cheon, I.S.; Goplen, N.P.; Jiang, L.; Zhang, R.; Peebles, R.S.; Mack, M.; Kaplan, M.H.; Limper, A.H.; et al. PPAR-γ in Macrophages Limits Pulmonary Inflammation and Promotes Host Recovery following Respiratory Viral Infection. J. Virol. 2019, 93, e00030-19. [Google Scholar] [CrossRef]
- Eckertova, M.; Ondrejcakova, M.; Krskova, K.; Zorad, S.; Jezova, D. Subchronic treatment of rats with oxytocin results in improved adipocyte differentiation and increased gene expression of factors involved in adipogenesis. Br. J. Pharmacol. 2011, 162, 452–463. [Google Scholar] [CrossRef]
- Atasoy, D.; Betley, J.N.; Su, H.H.; Sternson, S.M. Deconstruction of a neural circuit for hunger. Nature 2012, 488, 172–177. [Google Scholar] [CrossRef]
- Li, X.H.; Matsuura, T.; Xue, M.; Chen, Q.Y.; Liu, R.H.; Lu, J.S.; Shi, W.; Fan, K.; Zhou, Z.; Miao, Z.; et al. Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Rep. 2021, 36, 109411. [Google Scholar] [CrossRef]
- Liu, Y.; Li, A.; Bair-Marshall, C.; Xu, H.; Jee, H.J.; Zhu, E.; Sun, M.; Zhang, Q.; Lefevre, A.; Chen, Z.S.; et al. Oxytocin promotes prefrontal population activity via the PVN-PFC pathway to regulate pain. Neuron 2023, 111, 1795–1811.e7. [Google Scholar] [CrossRef] [PubMed]
- Marlin, B.J.; Froemke, R.C. Oxytocin modulation of neural circuits for social behavior. Dev. Neurobiol. 2017, 77, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Borland, J.M.; Grantham, K.N.; Aiani, L.M.; Frantz, K.J.; Albers, H.E. Role of oxytocin in the ventral tegmental area in social reinforcement. Psychoneuroendocrinology 2018, 95, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Christoffel, D.J.; Malenka, R.C. Neural circuits regulating prosocial behaviors. Neuropsychopharmacology 2022, 48, 79–89. [Google Scholar] [CrossRef]
- Xiao, L.; Priest, M.F.; Nasenbeny, J.; Lu, T.; Kozorovitskiy, Y. Biased Oxytocinergic Modulation of Midbrain Dopamine Systems. Neuron 2017, 95, 368–384.e365. [Google Scholar] [CrossRef]
- Song, Z.; Borland, J.M.; Larkin, T.E.; O’Malley, M.; Albers, H.E. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology 2016, 74, 164–172. [Google Scholar] [CrossRef]
- Nardou, R.; Lewis, E.M.; Rothhaas, R.; Xu, R.; Yang, A.; Boyden, E.; Dölen, G. Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature 2019, 569, 116–120. [Google Scholar] [CrossRef]
- Hurlemann, R.; Scheele, D. Dissecting the Role of Oxytocin in the Formation and Loss of Social Relationships. Biol. Psychiatry 2016, 79, 185–193. [Google Scholar] [CrossRef]
- Barraza, J.A.; Zak, P.J. Empathy toward strangers triggers oxytocin release and subsequent generosity. Ann. N. Y. Acad. Sci. 2009, 1167, 182–189. [Google Scholar] [CrossRef]
- Walum, H.; Young, L.J. The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci. 2018, 19, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Perkeybile, A.M.; Bales, K.L. Intergenerational transmission of sociality: The role of parents in shaping social behavior in monogamous and non-monogamous species. J. Exp. Biol. 2017, 220, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R.; Shapiro, L.E. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. USA 1992, 89, 5981–5985. [Google Scholar] [CrossRef] [PubMed]
- Bethlehem, R.A.I.; Lombardo, M.V.; Lai, M.C.; Auyeung, B.; Crockford, S.K.; Deakin, J.; Soubramanian, S.; Sule, A.; Kundu, P.; Voon, V.; et al. Intranasal oxytocin enhances intrinsic corticostriatal functional connectivity in women. Transl. Psychiatry 2017, 7, e1099. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.M.; Young, L.J. Comparative Perspectives on Oxytocin and Vasopressin Receptor Research in Rodents and Primates: Translational Implications. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, A.; Lee, J.; Sato, N. Oxytocin in the anterior cingulate cortex is involved in helping behaviour. Behav. Brain Res. 2020, 393, 112790. [Google Scholar] [CrossRef]
- Burkett, J.P.; Andari, E.; Johnson, Z.V.; Curry, D.C.; de Waal, F.B.; Young, L.J. Oxytocin-dependent consolation behavior in rodents. Science 2016, 351, 375–378. [Google Scholar] [CrossRef]
- Crick, F.H. Thinking about the brain. Sci. Am. 1979, 241, 219–232. [Google Scholar] [CrossRef]
- Crockford, C.; Wittig, R.M.; Langergraber, K.; Ziegler, T.E.; Zuberbuhler, K.; Deschner, T. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc. Biol. Sci. 2013, 280, 20122765. [Google Scholar] [CrossRef]
- Wittig, R.M.; Crockford, C.; Deschner, T.; Langergraber, K.E.; Ziegler, T.E.; Zuberbuhler, K. Food sharing is linked to urinary oxytocin levels and bonding in related and unrelated wild chimpanzees. Proc. Biol. Sci. 2014, 281, 20133096. [Google Scholar] [CrossRef]
- Samuni, L.; Preis, A.; Mundry, R.; Deschner, T.; Crockford, C.; Wittig, R.M. Oxytocin reactivity during intergroup conflict in wild chimpanzees. Proc. Natl. Acad. Sci. USA 2017, 114, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.A.; Prange, A.J., Jr. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl. Acad. Sci. USA 1979, 76, 6661–6665. [Google Scholar] [CrossRef] [PubMed]
- Froemke, R.C.; Jones, B.J. Development of auditory cortical synaptic receptive fields. Neurosci. Biobehav. Rev. 2011, 35, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Carcea, I.; Caraballo, N.L.; Marlin, B.J.; Ooyama, R.; Riceberg, J.S.; Mendoza Navarro, J.M.; Opendak, M.; Diaz, V.E.; Schuster, L.; Alvarado Torres, M.I.; et al. Oxytocin neurons enable social transmission of maternal behaviour. Nature 2021, 596, 553–557. [Google Scholar] [CrossRef]
- Inada, K.; Hagihara, M.; Tsujimoto, K.; Abe, T.; Konno, A.; Hirai, H.; Kiyonari, H.; Miyamichi, K. Plasticity of neural connections underlying oxytocin-mediated parental behaviors of male mice. Neuron 2022, 110, 2009–2023.e2005. [Google Scholar] [CrossRef]
- Yuan, W.; He, Z.; Hou, W.; Wang, L.; Li, L.; Zhang, J.; Yang, Y.; Jia, R.; Qiao, H.; Tai, F. Role of oxytocin in the medial preoptic area (MPOA) in the modulation of paternal behavior in mandarin voles. Horm. Behav. 2019, 110, 46–55. [Google Scholar] [CrossRef]
- Barchi-Ferreira, A.M.; Osorio, F.L. Associations between oxytocin and empathy in humans: A systematic literature review. Psychoneuroendocrinology 2021, 129, 105268. [Google Scholar] [CrossRef]
- Akinrinade, I.; Kareklas, K.; Teles, M.C.; Reis, T.K.; Gliksberg, M.; Petri, G.; Levkowitz, G.; Oliveira, R.F. Evolutionarily conserved role of oxytocin in social fear contagion in zebrafish. Science 2023, 379, 1232–1237. [Google Scholar] [CrossRef]
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673–676. [Google Scholar] [CrossRef]
- Menon, R.; Grund, T.; Zoicas, I.; Althammer, F.; Fiedler, D.; Biermeier, V.; Bosch, O.J.; Hiraoka, Y.; Nishimori, K.; Eliava, M.; et al. Oxytocin Signaling in the Lateral Septum Prevents Social Fear during Lactation. Curr. Biol. 2018, 28, 1066–1078.e6. [Google Scholar] [CrossRef]
- Huber, D.; Veinante, P.; Stoop, R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 2005, 308, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, I.; Young, E.A. Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinology 1997, 22, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Latt, H.M.; Koga, Y.; Nishiki, T.; Matsui, H. Oxytocin and Stress: Neural Mechanisms, Stress-Related Disorders, and Therapeutic Approaches. Neuroscience 2019, 417, 1–10. [Google Scholar] [CrossRef]
- Owen, S.F.; Tuncdemir, S.N.; Bader, P.L.; Tirko, N.N.; Fishell, G.; Tsien, R.W. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature 2013, 500, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Froemke, R.C.; Young, L.J. Oxytocin, Neural Plasticity, and Social Behavior. Annu. Rev. Neurosci. 2021, 44, 359–381. [Google Scholar] [CrossRef] [PubMed]
- Eliava, M.; Melchior, M.; Knobloch-Bollmann, H.S.; Wahis, J.; da Silva Gouveia, M.; Tang, Y.; Ciobanu, A.C.; Triana Del Rio, R.; Roth, L.C.; Althammer, F.; et al. A New Population of Parvocellular Oxytocin Neurons Controlling Magnocellular Neuron Activity and Inflammatory Pain Processing. Neuron 2016, 89, 1291–1304. [Google Scholar] [CrossRef] [PubMed]
- de la Mora, M.P.; Pérez-Carrera, D.; Crespo-Ramírez, M.; Tarakanov, A.; Fuxe, K.; Borroto-Escuela, D.O. Signaling in dopamine D2 receptor-oxytocin receptor heterocomplexes and its relevance for the anxiolytic effects of dopamine and oxytocin interactions in the amygdala of the rat. Biochim. Biophys. Acta 2016, 1862, 2075–2085. [Google Scholar] [CrossRef]
- Marsh, N.; Marsh, A.A.; Lee, M.R.; Hurlemann, R. Oxytocin and the Neurobiology of Prosocial Behavior. Neuroscientist 2021, 27, 604–619. [Google Scholar] [CrossRef]
- Eckstein, M.; Becker, B.; Scheele, D.; Scholz, C.; Preckel, K.; Schlaepfer, T.E.; Grinevich, V.; Kendrick, K.M.; Maier, W.; Hurlemann, R. Oxytocin facilitates the extinction of conditioned fear in humans. Biol. Psychiatry 2015, 78, 194–202. [Google Scholar] [CrossRef]
- Liu, N.; Hadj-Bouziane, F.; Jones, K.B.; Turchi, J.N.; Averbeck, B.B.; Ungerleider, L.G. Oxytocin modulates fMRI responses to facial expression in macaques. Proc. Natl. Acad. Sci. USA 2015, 112, E3123–E3130. [Google Scholar] [CrossRef]
- Ferretti, V.; Maltese, F.; Contarini, G.; Nigro, M.; Bonavia, A.; Huang, H.; Gigliucci, V.; Morelli, G.; Scheggia, D.; Managò, F.; et al. Oxytocin Signaling in the Central Amygdala Modulates Emotion Discrimination in Mice. Curr. Biol. 2019, 29, 1938–1953.e6. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.T.; Althammer, F.; Silva da Gouveia, M.; Goyon, S.; Eliava, M.; Lefevre, A.; Kerspern, D.; Schimmer, J.; Raftogianni, A.; Wahis, J.; et al. A Fear Memory Engram and Its Plasticity in the Hypothalamic Oxytocin System. Neuron 2019, 103, 133–146.e8. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.E.M.; Lukas, M.; Wolf, H.N.; Durante, E.; Lorenz, A.; Mayer, A.L.; Bludau, A.; Bosch, O.J.; Grinevich, V.; Egger, V.; et al. Oxytocin and vasopressin within the ventral and dorsal lateral septum modulate aggression in female rats. Nat. Commun. 2021, 12, 2900. [Google Scholar] [CrossRef] [PubMed]
- Duque-Wilckens, N.; Torres, L.Y.; Yokoyama, S.; Minie, V.A.; Tran, A.M.; Petkova, S.P.; Hao, R.; Ramos-Maciel, S.; Rios, R.A.; Jackson, K.; et al. Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc. Natl. Acad. Sci. USA 2020, 117, 26406–26413. [Google Scholar] [CrossRef]
- Haussler, H.U.; Jirikowski, G.F.; Caldwell, J.D. Sex differences among oxytocin-immunoreactive neuronal systems in the mouse hypothalamus. J. Chem. Neuroanat. 1990, 3, 271–276. [Google Scholar]
- Qiao, X.; Yan, Y.; Wu, R.; Tai, F.; Hao, P.; Cao, Y.; Wang, J. Sociality and oxytocin and vasopressin in the brain of male and female dominant and subordinate mandarin voles. J. Comp. Physiol. A 2014, 200, 149–159. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Pan, Y.; Wang, Z.; Zhang, Z. Species differences in the immunoreactive expression of oxytocin, vasopressin, tyrosine hydroxylase and estrogen receptor alpha in the brain of Mongolian gerbils (Meriones unguiculatus) and Chinese striped hamsters (Cricetulus barabensis). PLoS ONE 2013, 8, e65807. [Google Scholar] [CrossRef]
- Xu, L.; Pan, Y.; Young, K.A.; Wang, Z.; Zhang, Z. Oxytocin and vasopressin immunoreactive staining in the brains of Brandt’s voles (Lasiopodomys brandtii) and greater long-tailed hamsters (Tscherskia triton). Neuroscience 2010, 169, 1235–1247. [Google Scholar] [CrossRef]
- Nakajima, M.; Gorlich, A.; Heintz, N. Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell 2014, 159, 295–305. [Google Scholar] [CrossRef]
- Li, K.; Nakajima, M.; Ibanez-Tallon, I.; Heintz, N. A Cortical Circuit for Sexually Dimorphic Oxytocin-Dependent Anxiety Behaviors. Cell 2016, 167, 60–72.e11. [Google Scholar] [CrossRef]
- Reh, R.K.; Dias, B.G.; Nelson, C.A., 3rd; Kaufer, D.; Werker, J.F.; Kolb, B.; Levine, J.D.; Hensch, T.K. Critical period regulation across multiple timescales. Proc. Natl. Acad. Sci. USA 2020, 117, 23242–23251. [Google Scholar] [CrossRef] [PubMed]
- Molet, J.; Maras, P.M.; Avishai-Eliner, S.; Baram, T.Z. Naturalistic rodent models of chronic early-life stress. Dev. Psychobiol. 2014, 56, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Birnie, M.T.; Kooiker, C.L.; Short, A.K.; Bolton, J.L.; Chen, Y.; Baram, T.Z. Plasticity of the Reward Circuitry After Early-Life Adversity: Mechanisms and Significance. Biol. Psychiatry 2020, 87, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Baram, T.Z. Toward Understanding How Early-Life Stress Reprograms Cognitive and Emotional Brain Networks. Neuropsychopharmacology 2016, 41, 197–206. [Google Scholar] [CrossRef]
- Biagini, G.; Pich, E.M.; Carani, C.; Marrama, P.; Agnati, L.F. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int. J. Dev. Neurosci. 1998, 16, 187–197. [Google Scholar] [CrossRef]
- Huot, R.L.; Plotsky, P.M.; Lenox, R.H.; McNamara, R.K. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002, 950, 52–63. [Google Scholar] [CrossRef]
- Derks, N.A.; Krugers, H.J.; Hoogenraad, C.C.; Joels, M.; Sarabdjitsingh, R.A. Effects of Early Life Stress on Synaptic Plasticity in the Developing Hippocampus of Male and Female Rats. PLoS ONE 2016, 11, e0164551. [Google Scholar] [CrossRef]
- Baram, T.Z.; Davis, E.P.; Obenaus, A.; Sandman, C.A.; Small, S.L.; Solodkin, A.; Stern, H. Fragmentation and unpredictability of early-life experience in mental disorders. Am. J. Psychiatry 2012, 169, 907–915. [Google Scholar] [CrossRef]
- Juif, P.E.; Salio, C.; Zell, V.; Melchior, M.; Lacaud, A.; Petit-Demouliere, N.; Ferrini, F.; Darbon, P.; Hanesch, U.; Anton, F.; et al. Peripheral and central alterations affecting spinal nociceptive processing and pain at adulthood in rats exposed to neonatal maternal deprivation. Eur. J. Neurosci. 2016, 44, 1952–1962. [Google Scholar] [CrossRef]
- Danoff, J.S.; Wroblewski, K.L.; Graves, A.J.; Quinn, G.C.; Perkeybile, A.M.; Kenkel, W.M.; Lillard, T.S.; Parikh, H.I.; Golino, H.F.; Gregory, S.G.; et al. Genetic, epigenetic, and environmental factors controlling oxytocin receptor gene expression. Clin. Epigenetics 2021, 13, 23. [Google Scholar] [CrossRef]
- Francis, D.D.; Young, L.J.; Meaney, M.J.; Insel, T.R. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: Gender differences. J. Neuroendocrinol. 2002, 14, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Loken, L.S.; Wessberg, J.; Morrison, I.; McGlone, F.; Olausson, H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009, 12, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Stewart, R.A.; Demas, G.E.; Alberts, J.R. Maternal contact differentially modulates central and peripheral oxytocin in rat pups during a brief regime of mother-pup interaction that induces a filial huddling preference. J. Neuroendocrinol. 2012, 24, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Hertenstein, M.J.; Verkamp, J.M.; Kerestes, A.M.; Holmes, R.M. The communicative functions of touch in humans, nonhuman primates, and rats: A review and synthesis of the empirical research. Genet. Soc. Gen. Psychol. Monogr. 2006, 132, 5–94. [Google Scholar] [CrossRef] [PubMed]
- Onaka, T.; Takayanagi, Y. The oxytocin system and early-life experience-dependent plastic changes. J. Neuroendocrinol. 2021, 33, e13049. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Lindell, S.G.; Driscoll, C.A.; Zhou, Z.; Yuan, Q.; Schwandt, M.L.; Miller-Crews, I.; Simpson, E.A.; Paukner, A.; Ferrari, P.F.; et al. Early rearing history influences oxytocin receptor epigenetic regulation in rhesus macaques. Proc. Natl. Acad. Sci. USA 2017, 114, 11769–11774. [Google Scholar] [CrossRef]
- Champagne, F.; Diorio, J.; Sharma, S.; Meaney, M.J. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 12736–12741. [Google Scholar] [CrossRef]
- Thornton, J.L.; Everett, N.A.; Webb, P.; Turner, A.J.; Cornish, J.L.; Baracz, S.J. Adolescent oxytocin administration reduces depression-like behaviour induced by early life stress in adult male and female rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 110, 110279. [Google Scholar] [CrossRef]
- Yoshida, M.; Takayanagi, Y.; Inoue, K.; Kimura, T.; Young, L.J.; Onaka, T.; Nishimori, K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 2259–2271. [Google Scholar] [CrossRef]
- Boccia, M.L.; Petrusz, P.; Suzuki, K.; Marson, L.; Pedersen, C.A. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience 2013, 253, 155–164. [Google Scholar] [CrossRef]
- Han, R.T.; Kim, Y.B.; Park, E.H.; Kim, J.Y.; Ryu, C.; Kim, H.Y.; Lee, J.; Pahk, K.; Shanyu, C.; Kim, H.; et al. Long-Term Isolation Elicits Depression and Anxiety-Related Behaviors by Reducing Oxytocin-Induced GABAergic Transmission in Central Amygdala. Front. Mol. Neurosci. 2018, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, T.; Anselmo-Franci, J.A.; Li, P.; Callahan, M.F.; Morris, M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998, 781, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Standley, J. Music therapy research in the NICU: An updated meta-analysis. Neonatal Netw. NN 2012, 31, 311–316. [Google Scholar] [CrossRef]
- Vittner, D.; Butler, S.; Smith, K.; Makris, N.; Brownell, E.; Samra, H.; McGrath, J. Parent Engagement Correlates With Parent and Preterm Infant Oxytocin Release During Skin-to-Skin Contact. Adv. Neonatal Care Off. J. Natl. Assoc. Neonatal Nurses 2019, 19, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Vittner, D.; McGrath, J.; Robinson, J.; Lawhon, G.; Cusson, R.; Eisenfeld, L.; Walsh, S.; Young, E.; Cong, X. Increase in Oxytocin from Skin-to-Skin Contact Enhances Development of Parent-Infant Relationship. Biol. Res. Nurs. 2018, 20, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Palazzi, A.; Meschini, R.; Piccinini, C.A. NICU music therapy effects on maternal mental health and preterm infant’s emotional arousal. Infant Ment. Health J. 2021, 42, 672–689. [Google Scholar] [CrossRef]
- Filippa, M.; Monaci, M.G.; Spagnuolo, C.; Serravalle, P.; Daniele, R.; Grandjean, D. Maternal speech decreases pain scores and increases oxytocin levels in preterm infants during painful procedures. Sci. Rep. 2021, 11, 17301. [Google Scholar] [CrossRef]
- Havranek, T.; Lestanova, Z.; Mravec, B.; Strbak, V.; Bakos, J.; Bacova, Z. Oxytocin Modulates Expression of Neuron and Glial Markers in the Rat Hippocampus. Folia Biol. 2017, 63, 91–97. [Google Scholar]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Bazargani, N.; Attwell, D. Astrocyte calcium signaling: The third wave. Nat. Neurosci. 2016, 19, 182–189. [Google Scholar] [CrossRef]
- Roque, A.; Ochoa-Zarzosa, A.; Torner, L. Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain Behav. Immun. 2016, 55, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Banqueri, M.; Mendez, M.; Gomez-Lazaro, E.; Arias, J.L. Early life stress by repeated maternal separation induces long-term neuroinflammatory response in glial cells of male rats. Stress 2019, 22, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- Perea, G.; Gomez, R.; Mederos, S.; Covelo, A.; Ballesteros, J.J.; Schlosser, L.; Hernandez-Vivanco, A.; Martin-Fernandez, M.; Quintana, R.; Rayan, A.; et al. Activity-dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte-neuron networks. eLife 2016, 5, e20362. [Google Scholar] [CrossRef]
- Ta, T.T.; Dikmen, H.O.; Schilling, S.; Chausse, B.; Lewen, A.; Hollnagel, J.O.; Kann, O. Priming of microglia with IFN-gamma slows neuronal gamma oscillations in situ. Proc. Natl. Acad. Sci. USA 2019, 116, 4637–4642. [Google Scholar] [CrossRef]
- Hasan, M.; Kanna, M.S.; Jun, W.; Ramkrishnan, A.S.; Iqbal, Z.; Lee, Y.; Li, Y. Schema-like learning and memory consolidation acting through myelination. FASEB J. 2019, 33, 11758–11775. [Google Scholar] [CrossRef]
- Alaerts, K.; Bernaerts, S.; Prinsen, J.; Dillen, C.; Steyaert, J.; Wenderoth, N. Oxytocin induces long-lasting adaptations within amygdala circuitry in autism: A treatment-mechanism study with randomized placebo-controlled design. Neuropsychopharmacology 2020, 45, 1141–1149. [Google Scholar] [CrossRef]
- Sardinha, V.M.; Guerra-Gomes, S.; Caetano, I.; Tavares, G.; Martins, M.; Reis, J.S.; Correia, J.S.; Teixeira-Castro, A.; Pinto, L.; Sousa, N.; et al. Astrocytic signaling supports hippocampal-prefrontal theta synchronization and cognitive function. Glia 2017, 65, 1944–1960. [Google Scholar] [CrossRef]
- Panaro, M.A.; Benameur, T.; Porro, C. Hypothalamic Neuropeptide Brain Protection: Focus on Oxytocin. J. Clin. Med. 2020, 9, 1534. [Google Scholar] [CrossRef]
- Baskerville, T.A.; Douglas, A.J. Dopamine and oxytocin interactions underlying behaviors: Potential contributions to behavioral disorders. CNS Neurosci. Ther. 2010, 16, e92–e123. [Google Scholar] [CrossRef] [PubMed]
- Purba, J.S.; Hofman, M.A.; Swaab, D.F. Decreased number of oxytocin-immunoreactive neurons in the paraventricular nucleus of the hypothalamus in Parkinson’s disease. Neurology 1994, 44, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Liang, M.; Munesue, S.; Deguchi, K.; Harashima, A.; Furuhara, K.; Yuhi, T.; Zhong, J.; Akther, S.; Goto, H.; et al. Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Commun. Biol. 2019, 2, 76. [Google Scholar] [CrossRef] [PubMed]
- Ino, D.; Tanaka, Y.; Hibino, H.; Nishiyama, M. A fluorescent sensor for real-time measurement of extracellular oxytocin dynamics in the brain. Nat. Methods 2022, 19, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Wang, H.; Wang, P.; Geng, L.; Mei, L.; Osakada, T.; Wang, L.; Tang, Y.; Kania, A.; Grinevich, V.; et al. A genetically encoded sensor measures temporal oxytocin release from different neuronal compartments. Nat. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, H.; Wang, P.; Cui, W.; Xu, K.; Chen, D.; Hu, M.; Li, Z.; Geng, X.; Wei, S. Oxytocin and serotonin in the modulation of neural function: Neurobiological underpinnings of autism-related behavior. Front. Neurosci. 2022, 16, 919890. [Google Scholar] [CrossRef]
- Zhang, R.; Jia, M.X.; Zhang, J.S.; Xu, X.J.; Shou, X.J.; Zhang, X.T.; Li, L.; Li, N.; Han, S.P.; Han, J.S. Transcutaneous electrical acupoint stimulation in children with autism and its impact on plasma levels of arginine-vasopressin and oxytocin: A prospective single-blinded controlled study. Res. Dev. Disabil. 2012, 33, 1136–1146. [Google Scholar] [CrossRef]
- Moerkerke, M.; Peeters, M.; de Vries, L.; Daniels, N.; Steyaert, J.; Alaerts, K.; Boets, B. Endogenous Oxytocin Levels in Autism-A Meta-Analysis. Brain Sci. 2021, 11, 1545. [Google Scholar] [CrossRef]
- Parker, K.J.; Oztan, O.; Libove, R.A.; Sumiyoshi, R.D.; Jackson, L.P.; Karhson, D.S.; Summers, J.E.; Hinman, K.E.; Motonaga, K.S.; Phillips, J.M.; et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc. Natl. Acad. Sci. USA 2017, 114, 8119–8124. [Google Scholar] [CrossRef]
- Guastella, A.J.; Einfeld, S.L.; Gray, K.M.; Rinehart, N.J.; Tonge, B.J.; Lambert, T.J.; Hickie, I.B. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol. Psychiatry 2010, 67, 692–694. [Google Scholar] [CrossRef]
- Hollander, E.; Bartz, J.; Chaplin, W.; Phillips, A.; Sumner, J.; Soorya, L.; Anagnostou, E.; Wasserman, S. Oxytocin increases retention of social cognition in autism. Biol. Psychiatry 2007, 61, 498–503. [Google Scholar] [CrossRef]
- Sikich, L.; Kolevzon, A.; King, B.; McDougle, C.; Sanders, K.; Kim, S.-J.; Spanos, M.; Chandrasekhar, T.; Trelles, P.; Rockhill, C.; et al. Intranasal Oxytocin in Children and Adolescents with Autism Spectrum Disorder. N. Engl. J. Med. 2021, 385, 1462–1473. [Google Scholar] [CrossRef]
- Andari, E.; Duhamel, J.R.; Zalla, T.; Herbrecht, E.; Leboyer, M.; Sirigu, A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc. Natl. Acad. Sci. USA 2010, 107, 4389–4394. [Google Scholar] [CrossRef]
- Yatawara, C.J.; Einfeld, S.L.; Hickie, I.B.; Davenport, T.A.; Guastella, A.J. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Mol. Psychiatry 2016, 21, 1225–1231. [Google Scholar] [CrossRef]
- Anagnostou, E.; Soorya, L.; Chaplin, W.; Bartz, J.; Halpern, D.; Wasserman, S.; Wang, A.T.; Pepa, L.; Tanel, N.; Kushki, A.; et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: A randomized controlled trial. Mol. Autism 2012, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Averbeck, B.B.; Bobin, T.; Evans, S.; Shergill, S.S. Emotion recognition and oxytocin in patients with schizophrenia. Psychol. Med. 2012, 42, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Feifel, D.; Macdonald, K.; Nguyen, A.; Cobb, P.; Warlan, H.; Galangue, B.; Minassian, A.; Becker, O.; Cooper, J.; Perry, W.; et al. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol. Psychiatry 2010, 68, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Scantamburlo, G.; Hansenne, M.; Geenen, V.; Legros, J.J.; Ansseau, M. Additional intranasal oxytocin to escitalopram improves depressive symptoms in resistant depression: An open trial. Eur. Psychiatry 2015, 30, 65–68. [Google Scholar] [CrossRef]
- Donadon, M.F.; Martin-Santos, R.; Osório, F.L. Oxytocin effects on the cognition of women with postpartum depression: A randomized, placebo-controlled clinical trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110098. [Google Scholar] [CrossRef]
- Bertsch, K.; Gamer, M.; Schmidt, B.; Schmidinger, I.; Walther, S.; Kästel, T.; Schnell, K.; Büchel, C.; Domes, G.; Herpertz, S.C. Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. Am. J. Psychiatry 2013, 170, 1169–1177. [Google Scholar] [CrossRef]
- Bartz, J.; Simeon, D.; Hamilton, H.; Kim, S.; Crystal, S.; Braun, A.; Vicens, V.; Hollander, E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc. Cogn. Affect. Neurosci. 2011, 6, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Troisi, A. Ethological research in clinical psychiatry: The study of nonverbal behavior during interviews. Neurosci. Biobehav. Rev. 1999, 23, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, I.; Poudel, G.; Kordsachia, C.; Wu, Q.; Thomson, H.; Georgiou-Karistianis, N.; Stout, J.C. Oxytocin selectively modulates brain processing of disgust in Huntington’s disease gene carriers. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 81, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, I.; Phan, K.L.; Wood, A.; Angstadt, M.; Chua, P.; Heinrichs, M.; Stout, J.C.; Nathan, P.J. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 2010, 35, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Guastella, A.J.; Howard, A.L.; Dadds, M.R.; Mitchell, P.; Carson, D.S. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology 2009, 34, 917–923. [Google Scholar] [CrossRef]
- Alvares, G.A.; Chen, N.T.; Balleine, B.W.; Hickie, I.B.; Guastella, A.J. Oxytocin selectively moderates negative cognitive appraisals in high trait anxious males. Psychoneuroendocrinology 2012, 37, 2022–2031. [Google Scholar] [CrossRef]
- Koch, S.B.J.; van Zuiden, M.; Nawijn, L.; Frijling, J.L.; Veltman, D.J.; Olff, M. Intranasal Oxytocin Administration Dampens Amygdala Reactivity towards Emotional Faces in Male and Female PTSD Patients. Neuropsychopharmacology 2016, 41, 1495–1504. [Google Scholar] [CrossRef]
- El-Ansary, A.; Al-Ayadhi, L. Neuroinflammation in autism spectrum disorders. J. Neuroinflammation 2012, 9, 265. [Google Scholar] [CrossRef]
- Lee, J.H.; Espinera, A.R.; Chen, D.; Choi, K.E.; Caslin, A.Y.; Won, S.; Pecoraro, V.; Xu, G.Y.; Wei, L.; Yu, S.P. Neonatal inflammatory pain and systemic inflammatory responses as possible environmental factors in the development of autism spectrum disorder of juvenile rats. J. Neuroinflammation 2016, 13, 109. [Google Scholar] [CrossRef]
- Iannucci, A.; Caneparo, V.; Raviola, S.; Debernardi, I.; Colangelo, D.; Miggiano, R.; Griffante, G.; Landolfo, S.; Gariglio, M.; De Andrea, M. Toll-like receptor 4-mediated inflammation triggered by extracellular IFI16 is enhanced by lipopolysaccharide binding. PLoS Pathog. 2020, 16, e1008811. [Google Scholar] [CrossRef]
- Alabdali, A.; Al-Ayadhi, L.; El-Ansary, A. Association of social and cognitive impairment and biomarkers in autism spectrum disorders. J. Neuroinflammation 2014, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, S.; Liu, X.; Zheng, Y.; Li, L.; Meng, S. Oxytocin improves animal behaviors and ameliorates oxidative stress and inflammation in autistic mice. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 107, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Friuli, M.; Eramo, B.; Valenza, M.; Scuderi, C.; Provensi, G.; Romano, A. Targeting the Oxytocinergic System: A Possible Pharmacological Strategy for the Treatment of Inflammation Occurring in Different Chronic Diseases. Int. J. Mol. Sci. 2021, 22, 10250. [Google Scholar] [CrossRef]
- Meziane, H.; Schaller, F.; Bauer, S.; Villard, C.; Matarazzo, V.; Riet, F.; Guillon, G.; Lafitte, D.; Desarmenien, M.G.; Tauber, M.; et al. An Early Postnatal Oxytocin Treatment Prevents Social and Learning Deficits in Adult Mice Deficient for Magel2, a Gene Involved in Prader-Willi Syndrome and Autism. Biol. Psychiatry 2015, 78, 85–94. [Google Scholar] [CrossRef]
- Schaller, F.; Watrin, F.; Sturny, R.; Massacrier, A.; Szepetowski, P.; Muscatelli, F. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum. Mol. Genet. 2010, 19, 4895–4905. [Google Scholar] [CrossRef] [PubMed]
- Moy, S.S.; Teng, B.L.; Nikolova, V.D.; Riddick, N.V.; Simpson, C.D.; Van Deusen, A.; Janzen, W.P.; Sassano, M.F.; Pedersen, C.A.; Jarstfer, M.B. Prosocial effects of an oxytocin metabolite, but not synthetic oxytocin receptor agonists, in a mouse model of autism. Neuropharmacology 2019, 144, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, D.H.; Levitt, P. Autism spectrum disorders: Developmental disconnection syndromes. Curr. Opin. Neurobiol. 2007, 17, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Duan, X.; Mantini, D.; Chen, H. Alteration of functional connectivity in autism spectrum disorder: Effect of age and anatomical distance. Sci. Rep. 2016, 6, 26527. [Google Scholar] [CrossRef]
- Choe, K.Y.; Bethlehem, R.A.I.; Safrin, M.; Dong, H.; Salman, E.; Li, Y.; Grinevich, V.; Golshani, P.; DeNardo, L.A.; Penagarikano, O.; et al. Oxytocin normalizes altered circuit connectivity for social rescue of the Cntnap2 knockout mouse. Neuron 2022, 110, 795–808.e6. [Google Scholar] [CrossRef]
- Goh, K.K.; Chen, C.Y.; Wu, T.H.; Chen, C.H.; Lu, M.L. Crosstalk between Schizophrenia and Metabolic Syndrome: The Role of Oxytocinergic Dysfunction. Int. J. Mol. Sci. 2022, 23, 7092. [Google Scholar] [CrossRef]
- Winship, I.R.; Dursun, S.M.; Baker, G.B.; Balista, P.A.; Kandratavicius, L.; Maia-de-Oliveira, J.P.; Hallak, J.; Howland, J.G. An Overview of Animal Models Related to Schizophrenia. Can. J. Psychiatry 2019, 64, 5–17. [Google Scholar] [CrossRef]
- Montag, C.; Brockmann, E.M.; Bayerl, M.; Rujescu, D.; Müller, D.J.; Gallinat, J. Oxytocin and oxytocin receptor gene polymorphisms and risk for schizophrenia: A case-control study. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry 2013, 14, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Feifel, D.; Macdonald, K.; Cobb, P.; Minassian, A. Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophr. Res. 2012, 139, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.K.; Chen, C.H.; Lane, H.Y. Oxytocin in Schizophrenia: Pathophysiology and Implications for Future Treatment. Int. J. Mol. Sci. 2021, 22, 2146. [Google Scholar] [CrossRef]
- Young, J.W.; Geyer, M.A. Developing treatments for cognitive deficits in schizophrenia: The challenge of translation. J. Psychopharmacol. 2015, 29, 178–196. [Google Scholar] [CrossRef]
- Millan, M.J.; Bales, K.L. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: The CNTRICS initiative. Neurosci. Biobehav. Rev. 2013, 37, 2166–2180. [Google Scholar] [CrossRef]
- Ferguson, J.N.; Young, L.J.; Hearn, E.F.; Matzuk, M.M.; Insel, T.R.; Winslow, J.T. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000, 25, 284–288. [Google Scholar] [CrossRef]
- Ferguson, J.N.; Aldag, J.M.; Insel, T.R.; Young, L.J. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 8278–8285. [Google Scholar] [CrossRef]
- Crawley, J.N.; Chen, T.; Puri, A.; Washburn, R.; Sullivan, T.L.; Hill, J.M.; Young, N.B.; Nadler, J.J.; Moy, S.S.; Young, L.J.; et al. Social approach behaviors in oxytocin knockout mice: Comparison of two independent lines tested in different laboratory environments. Neuropeptides 2007, 41, 145–163. [Google Scholar] [CrossRef]
- Popik, P.; van Ree, J.M. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur. Neuropsychopharmacol. 1991, 1, 555–560. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, H.; Yang, X.; Huang, K.; Zhang, X.; Li, C. Decreased Serum Oxytocin and Increased Homocysteine in First-Episode Schizophrenia Patients. Front. Psychiatry 2019, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Jobst, A.; Dehning, S.; Ruf, S.; Notz, T.; Buchheim, A.; Henning-Fast, K.; Meißner, D.; Meyer, S.; Bondy, B.; Müller, N.; et al. Oxytocin and vasopressin levels are decreased in the plasma of male schizophrenia patients. Acta Neuropsychiatr. 2014, 26, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Guzel, D.; Yazici, A.B.; Pek, T.M.; Doganay, S.; Simsek, A.B.S.; Saglam, K.; Turan, C.; Yazici, E. Atrial natriuretic peptide and posterior pituitary neurohormone changes in patients with acute schizophrenia. Neuropsychiatr. Dis. Treat. 2018, 14, 1855–1860. [Google Scholar] [CrossRef]
- Aydın, O.; Lysaker, P.H.; Balıkçı, K.; Ünal-Aydın, P.; Esen-Danacı, A. Associations of oxytocin and vasopressin plasma levels with neurocognitive, social cognitive and meta cognitive function in schizophrenia. Psychiatry Res. 2018, 270, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Aydın, O.; Balıkçı, K.; Taş, C.; Ünal-Aydın, P.; Taneli, F.; Esen-Danacı, A. Assessing the relationship between attachment, parental attitude and plasma oxytocin in schizophrenia patients and their unaffected siblings. Nord. J. Psychiatry 2019, 73, 51–57. [Google Scholar] [CrossRef]
- Strauss, G.P.; Keller, W.R.; Koenig, J.I.; Gold, J.M.; Ossenfort, K.L.; Buchanan, R.W. Plasma oxytocin levels predict olfactory identification and negative symptoms in individuals with schizophrenia. Schizophr. Res. 2015, 162, 57–61. [Google Scholar] [CrossRef]
- Strauss, G.P.; Keller, W.R.; Koenig, J.I.; Sullivan, S.K.; Gold, J.M.; Buchanan, R.W. Endogenous oxytocin levels are associated with the perception of emotion in dynamic body expressions in schizophrenia. Schizophr. Res. 2015, 162, 52–56. [Google Scholar] [CrossRef]
- Kirsch, P.; Esslinger, C.; Chen, Q.; Mier, D.; Lis, S.; Siddhanti, S.; Gruppe, H.; Mattay, V.S.; Gallhofer, B.; Meyer-Lindenberg, A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 11489–11493. [Google Scholar] [CrossRef]
- Cardoso, C.; Kingdon, D.; Ellenbogen, M.A. A meta-analytic review of the impact of intranasal oxytocin administration on cortisol concentrations during laboratory tasks: Moderation by method and mental health. Psychoneuroendocrinology 2014, 49, 161–170. [Google Scholar] [CrossRef]
- Leppanen, J.; Ng, K.W.; Kim, Y.-R.; Tchanturia, K.; Treasure, J. Meta-analytic review of the effects of a single dose of intranasal oxytocin on threat processing in humans. J. Affect. Disord. 2018, 225, 167–179. [Google Scholar] [CrossRef]
- Dodhia, S.; Hosanagar, A.; Fitzgerald, D.A.; Labuschagne, I.; Wood, A.G.; Nathan, P.J.; Phan, K.L. Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology 2014, 39, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Bakermans-Kranenburg, M.J.; van IJzendoorn, M.H. Sniffing around oxytocin: Review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl. Psychiatry 2013, 3, e258. [Google Scholar] [CrossRef] [PubMed]
- Zik, J.B.; Roberts, D.L. The many faces of oxytocin: Implications for psychiatry. Psychiatry Res. 2015, 226, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Olff, M.; Frijling, J.L.; Kubzansky, L.D.; Bradley, B.; Ellenbogen, M.A.; Cardoso, C.; Bartz, J.A.; Yee, J.R.; van Zuiden, M. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 2013, 38, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
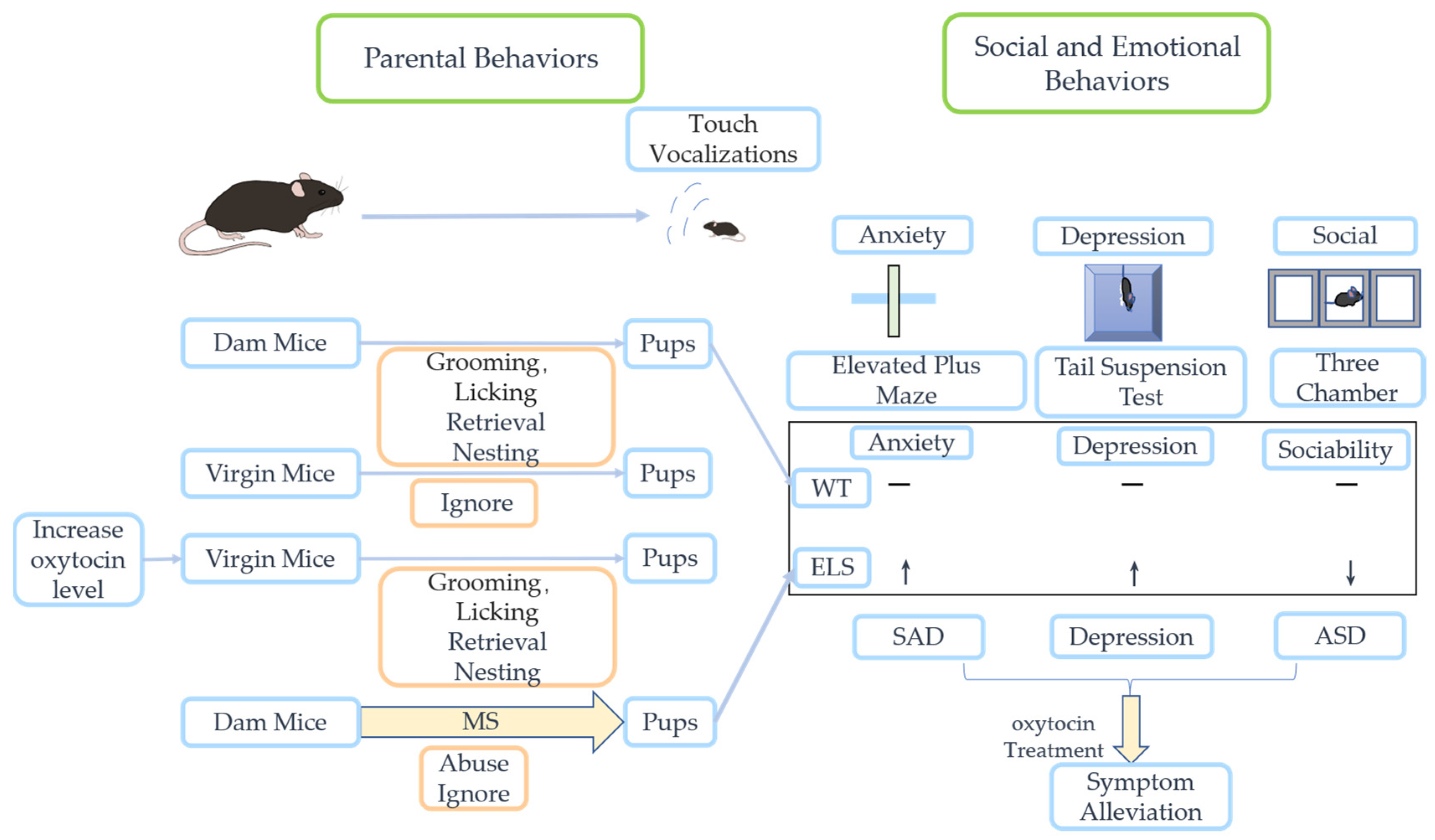
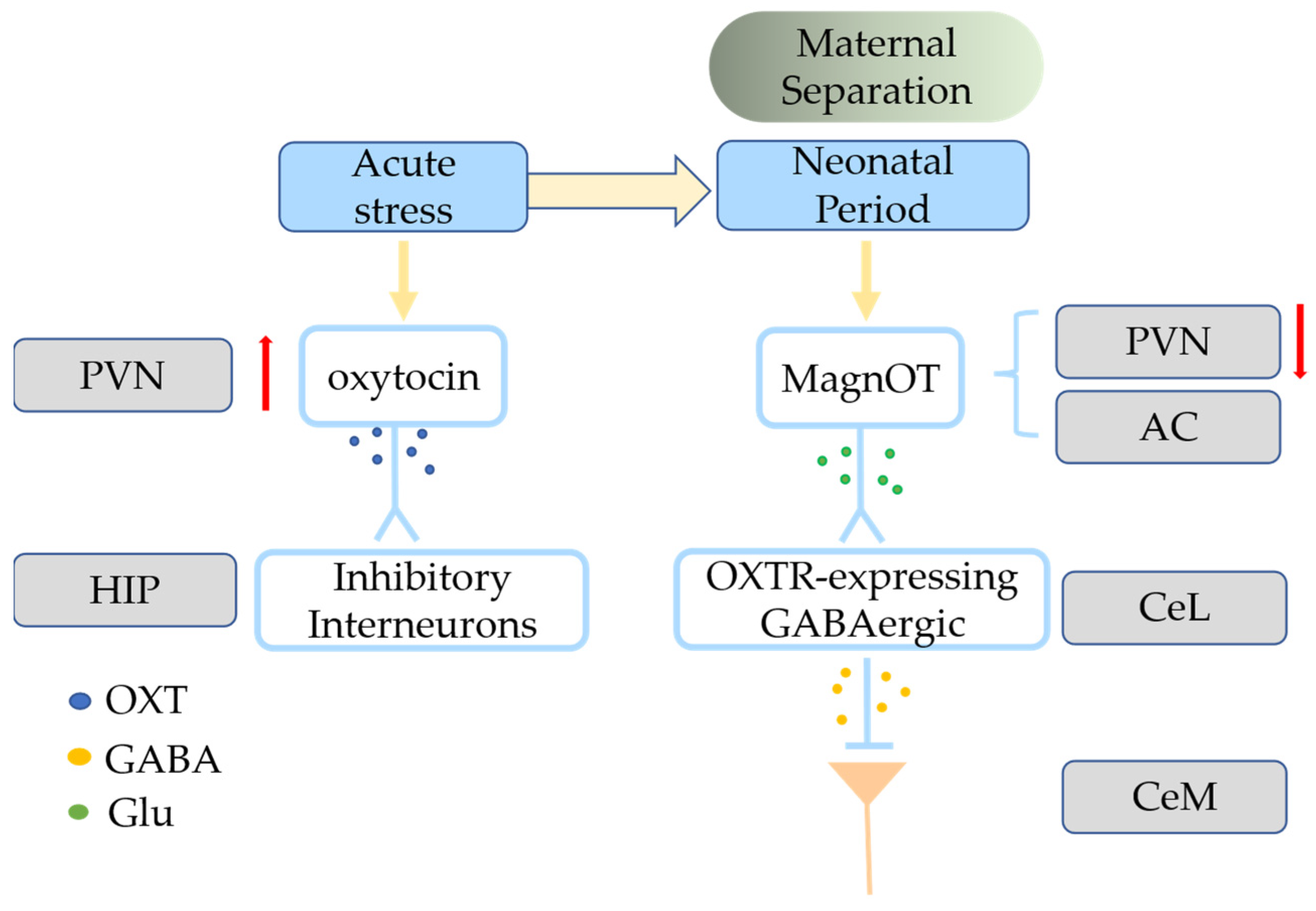
| Diseases | Therapies | The Role of Oxytocin |
|---|---|---|
| Autism Spectrum Disorder, ASD | 1. Intranasal 2. Oxytocin infusion 3. Intranasal 4. Intranasal 5. Oxytocin infusion 6. Intranasal 7. Intranasal | 1. Improving the social skills of children with ASD [150] 2. Single doses of oxytocin administered to individuals with ASD improve the processing of social information [152] 3. Improved emotion recognition [151] 4. Improved social learning 5. Significant reduction in repetitive behaviors [154] 6. Improved social responsiveness of children with ASD [155] 7. No significant changes in the primary outcome measures, but after 6 weeks, results suggested improvements in measures of social cognition [156] |
| Schizophrenia | 1. Intranasal 2. Intranasal | 1. Improvement in global emotion recognition [157] 2. Reduced scores on the positive and negative symptom scale and clinical global impression-improvement scale [158] |
| Depression | 1. Intranasal 2. Intranasal | 1. Reducing depressive symptoms [159] 2. Oxytocin increased response bias for happiness and reduced negative thoughts with postpartum depression [160] |
| Borderline Personality Disorder (BPD) | 1. Intranasal 2. Intranasal | 1. Reduced vigilant attention to social threat cues [161] 2. Decreasing trust and cooperation in BPD patients [162] 3. After treatment, BPD patients showed more affiliative behavior [163] |
| Huntington’s Disease | 1. Intranasal | 1. Oxytocin normalized brain activity to disgusted faces in Huntington’s disease carriers [164] |
| Social Anxiety Disorder (SAD) | 1. Intranasal 2. Intranasal 3. Intranasal | 1. Suppressing the response to fear signals by inhibiting the amygdala [165] 2. Decreases maladaptive cognitions about performance in exposure tasks over time [166] 3. Oxytocin moderates negative self-appraisals after a social stressor for high trait, anxious male participants [167] |
| Post-Traumatic Stress Disorder (PTSD) | 1. Intranasal | 1. Reduced amygdala response to emotional faces in PTSD patients [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Song, D.; Yan, Y.; Quan, Z.; Qing, H. The Role of Oxytocin in Early-Life-Stress-Related Neuropsychiatric Disorders. Int. J. Mol. Sci. 2023, 24, 10430. https://doi.org/10.3390/ijms241310430
Jin Y, Song D, Yan Y, Quan Z, Qing H. The Role of Oxytocin in Early-Life-Stress-Related Neuropsychiatric Disorders. International Journal of Molecular Sciences. 2023; 24(13):10430. https://doi.org/10.3390/ijms241310430
Chicago/Turabian StyleJin, Yue, Da Song, Yan Yan, Zhenzhen Quan, and Hong Qing. 2023. "The Role of Oxytocin in Early-Life-Stress-Related Neuropsychiatric Disorders" International Journal of Molecular Sciences 24, no. 13: 10430. https://doi.org/10.3390/ijms241310430
APA StyleJin, Y., Song, D., Yan, Y., Quan, Z., & Qing, H. (2023). The Role of Oxytocin in Early-Life-Stress-Related Neuropsychiatric Disorders. International Journal of Molecular Sciences, 24(13), 10430. https://doi.org/10.3390/ijms241310430






