Abstract
The increase in life expectancy without a decrease in the years lived without disability leads to the rise of the population aged over 65 years prone to polypharmacy. The novel antidiabetic drugs can improve this global therapeutic and health problem in patients with diabetes mellitus (DM). We aimed to establish the efficacy (A1c hemoglobin reduction) and safety of the newest antidiabetic drugs (considered so due to their novelty in medical practice use), specifically DPP-4i, SGLT-2i, GLP-1 Ra, and tirzepatide. The present meta-analysis followed the protocol registered at Prospero with the CRD42022330442 registration number. The reduction in HbA1c in the DPP4-i class for tenegliptin was 95% CI −0.54 [−1.1, 0.01], p = 0.06; in the SGLT2-iclass for ipragliflozin 95% CI −0.2 [−0.87, 0.47], p = 0.55; and for tofogliflozin 95% CI 3.13 [−12.02, 18.28], p = 0.69, while for tirzepatide it was 0.15, 95% CI [−0.50, 0.80] (p = 0.65). The guidelines for treatment in type 2 DM are provided from cardiovascular outcome trials that report mainly major adverse cardiovascular events and data about efficacy. The newest antidiabetic non-insulinic drugs are reported to be efficient in lowering HbA1c, but this effect depends between classes, molecules, or patients’ age. The newest antidiabetic drugs are proven to be efficient molecules in terms of HbA1c decrease, weight reduction, and safety, but more studies are needed in order to characterize exactly their efficacy and safety profiles.
1. Introduction
The World Health Organization (WHO) reports an increase in life expectancy secondary to decreased mortality rates, but with no decrease in years lived without disability [1,2].
A concomitant rise in the population aged over 65 years, along with the non-transmittable chronic disease epidemics, including type 2 diabetes mellitus (T2DM), cardiovascular diseases (CVD), cancers, and chronic pulmonary diseases, describe a population group mainly affected by an association of various chronic diseases that require multiple medications in their therapeutic management [3,4,5]. Polypharmacy is a generally positive trend, despite its variable definition. It is accepted as being the administration of more than five drugs [6,7,8,9,10,11] and comes with negative effects, such as renal or hepatic function impairment [12,13], living disability, frailty [10,14], hemorrhagic and thrombo-embolic risk, malnutrition [15], long-term care placement or hospitalization [10,16,17], decreased quality of life, or mortality [5,6,7,10,18,19,20]; that came in opposition to the benefits of the antidiabetic, cardioprotective molecules that are proven efficient, safe, and non-inferior to the previous antidiabetic drugs. However, the main challenge is represented by the requirement to establish a proper and predictable treatment [21] and simultaneously avoid poor adherence in self-administering multiple drugs [22], so emphasis should be addressed on the need for a strict and periodic assessment of the risk-benefit balance when continuing or introducing a new therapeutic drug and promoting the personalized medicine [23,24,25].
T2DM is part of the cardio-renal and metabolic syndrome. It is not characterized only by a hyperglycemic status but also by the concomitant complications that appear, both microvascular complications, respectively, retinopathy, neuropathy, or nephropathy; and macrovascular complications, respectively, CVD with its components like stroke, myocardial infarction (MI), coronary artery disease, and peripheral arterial disease [26,27]. Despite the associated comorbidities, other risk factors for polypharmacy in patients with T2DM are represented by older age, that when associated with cognitive impairment may transform polypharmacy into a genuine burden; medication side effects or direct-to-consumer advertisements that can favor the patients to start administering drugs for erectile dysfunction or restless leg syndrome, that are not primarily needed [27]. Therefore, it is important to have comprehensive management to minimize its possible negative consequences [27].
In T2DM, in acute conditions, such as acute MI, stroke, or congestive heart failure, insulin is the main indicated treatment; otherwise, the recommended therapeutic approach includes antidiabetic non-insulin drugs. The novel antidiabetic non-insulin drugs are represented by dipeptidyl peptidase-4 inhibitor (DPP-4i), sodium glucose-2 transporter inhibitors (SGLT-2i), glucagon-like peptide one receptor agonists (GLP-1 Ra), and a dual glucose-dependent insulinotropic polypeptide–GLP-1 receptor agonist that is represented by tirzepatide. Their benefits are secondary to their pleiotropic effects, which exceed only an improvement in the glycemic control, reflected by an improved level of A1C hemoglobin or blood glucose, respectively, due to a reduction in the patient’s body weight, improvement of the lipidic profile, blood pressure (BP) both systolic and diastolic, along with pleiotropic effects, such as a decreased marker of inflammation [28,29,30]. Because T2DM is included in the cardio-renal metabolic syndrome, it is also important to emphasize the benefits of the novel antidiabetic non-insulin drugs from the large cardiovascular outcome trials (CVOT); specifically, a reduction in the major adverse cardiovascular events (MACE), including acute MI, stroke, CV mortality, all-cause mortality, and CV safety [28,29,30].
Aim of the study: The primary endpoint of the study is to establish if there are any differences in the efficacy profile (A1c hemoglobin reduction) for the newest antidiabetic drugs, respectively, DPP-4i, SGLT-2i, GLP-1 Ra, and tirzepatide. The secondary endpoint is to establish differences in the safety (frequency of adverse reaction (AR), such as hypoglycemia–mild (54–70 mg/dL) or severe (<36 mg/dL), and the frequency of class-specific AR profiles for the newest antidiabetic non-insulin drugs—DPP-4i, SGLT-2i, GLP-1 Ra, and tirzepatide.
2. Methods
We developed an easily reproducible protocol for our study following the recommendations of preferred reporting items for systematic reviews and meta-analyses (PRISMA) for the systematic review protocol checklist [31]. Furthermore, we used the population, intervention, comparison, outcome, and study design (PICOS) strategy to guide our study rationale and make a clear, systematic literature search that answers the question: “Is the use of the newest antidiabetic drugs efficient and safe for patients?”.
The studies will be included after searching in two databases, MEDLINE (using PubMed) and Web of Science, using the Boolean operators “AND” and “OR”, and the search strategy: “(efficacy OR safety) AND (novel antidiabetic drugs)”. The inclusion and exclusion criteria are as follows:
- -
- Inclusion criteria: only experimental articles, both clinical trials and randomized controlled trials, published in full-text version in the last 10 years, that include human population over 18 years of age with T2DM, which are prescribed at least one class of novel non-insulin drugs, respectively, DPP-4i, SGLT-2i, GLP-1 Ra and tirzepatide;
- -
- Exclusion criteria: abstracts, short communications, reviews, letters to editors, commentaries, or studies published in a language other than English, published more than 10 years ago, and studies on cell cultures or mammals.
Furthermore, the duplicates will be eliminated, and two reviewers will analyze the articles for their relevance, as seen in the flow diagram in Figure 1. To improve the search, each list of selected titles will be shared, and a second screening of the previously selected titles will take place. Finally, a third researcher will be designated to arbitrate any problems that could appear in the selection process.
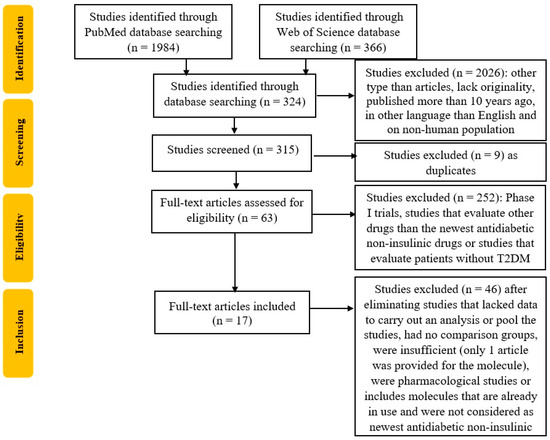
Figure 1.
Flowchart of the study selection process according to PRISMA guidelines.
For data synthesis, we exclude studies with less than two of the same molecules, with insufficient or irrelevant data, or lacking originality. A random effect meta-analysis will combine risk and hazard ratios for individual studies.
Trial registration number: A protocol was submitted to Prospero-International prospective register of systematic reviews to obtain a registration number for our study, respectively, CRD42022330442.
3. Results and Discussions
For DPP-4i, the molecule with a sufficient number of studies [32,33,34,35,36], respectively five, was tenegliptin. The studies’ characteristics (study type, including population and its repartition in control and experimental groups, follow-up duration, mean age, mean HbA1c and mean glycemia, number of hypoglycemic events, and class-specific AR) are shown in Table 1.

Table 1.
Summarized characteristics of studies that included DPP4-i tenegliptin and its results of interest: drug name, patient’s background characteristics, study type, total population, experimental population, control population, HbA1c (hemoglobin A1c), the number of hypoglycemic events, and class-specific adverse reactions.
The results showed that teneligliptin is a promising antidiabetic drug in reducing blood sugar levels with statistical significance for HbA1c (95% CI −0.65 [−1.10, 0.01], p = 0.06), as seen in Figure 2.
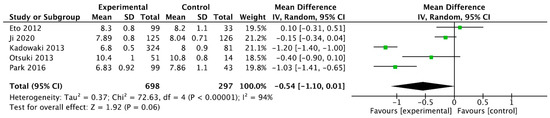
Figure 2.
Forrest plot graphic representation of HbA1c for tenegliptin [32,33,34,35,36]. Green square represents the mean value for each study. Black square represents the mean value of the studies.
For iSGLT-2, the molecules studied enough (five studies) were ipragliflozin [37,38,39,40,41] and tofogliflozin (two studies) [42,43], and the studies’ characteristics (study type, including population and its repartition in control and experimental groups, follow-up duration, mean age, mean HbA1c and mean glycemia, number of hypoglycemic events, and class-specific AR) are shown in Table 2.

Table 2.
Summarized characteristics of studies that included SGLT2-ipragliflozin and tofogliflozin and their results of interest: drug name, patient’s background characteristics, study type, total population, experimental population, control population, HbA1c (hemoglobin A1c), and the number of hypoglycemic events and class-specific adverse reactions.
The results for ipragliflozin and tofogliflozin had no statistical significance, as seen in Figure 3A,B, respectively.
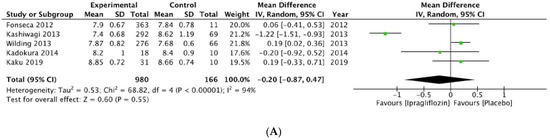
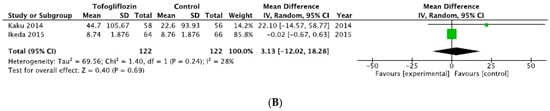
Figure 3.
Forrest plot graphic representation of HbA1c for (A) ipragliflozin [37,38,39,40,41] and (B) tofogliflozin [42,43]. Green square represents the mean value for each study. Black square represents the mean value of the studies.
For dual glucose-dependent insulinotropic polypeptide–GLP-1 receptor agonist represented by tirzepatide [44,45,46,47,48], the studies’ characteristics (study type, including population and its repartition in control and experimental groups, follow-up duration, mean age, mean HbA1c and mean glycemia, number of hypoglycemic events and class-specific AR) are shown in Table 3.

Table 3.
Summarized characteristics of studies that included tirzepatide and their results of interest: drug name, patients’ background characteristics, study type, total population, experimental population, control population, HbA1c (hemoglobin A1c), and the number of hypoglycemic events and class-specific adverse reactions.
For tirzepatide, the 95% CI was 0.15 [−0.50, 0.80] (p = 0.65), as seen in Figure 4.
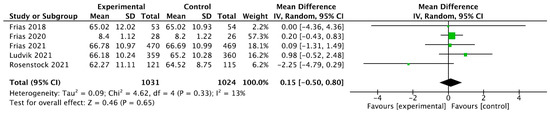
Figure 4.
Forrest plot graphic representation of HbA1c for tirzepatide [44,45,46,47,48]. Green square represents the mean value for each study. Black square represents the mean value of the studies.
Data about each molecule’s weight reduction are shown in Table 4.

Table 4.
Weight reduction reported data for the molecules of interest.
Our study provides comprehensive data about the efficacy of both novel and most recently developed non-insulin antidiabetic drugs. Our results confirm that these molecules are safe regarding glycemic control reflected by HbA1c and as shown by the limited AE or hypoglycemic events. The utility of this systematic review and meta-analysis is emphasized because the majority of data about this type of molecules are the results of large CVOTs, which are also the basis of the national and international guidelines for the treatment of T2DM [49], but in this type of studies, the primary outcomes are centered predominantly on MACE, such as CV death [48,49,50,51,52], nonfatal MI [49,50], nonfatal stroke [49,50], hospitalization, or urgent visits for heart failure (HF) [51,52,53] or sustained ≥50% estimated glomerular filtration rate (eGFR) decline and end-stage kidney disease [54,55]. CVOTs also report data about efficacy, such as A1c hemoglobin [56,57,58], body weight loss [59,60,61], and systolic BP [57,58], and about safety eGFR rates [55] and the incidence of hypoglycemic episodes [58,62]. The benefit of the present study is the synthesis of these scattered results as a mean effect.
The limitation of the present study is the low number of included studies because there are molecules where only two studies met the inclusion criteria and, also, the scarce data about the molecules from each of the GIP-GLP1 and GIP-GLP-1-Glucagon that are not included.
3.1. DPP-4i
For the DPP-4i class, which is more known for its high tolerability, the metabolic control is reported to have a reduction in HbA1c of 0.6–0.8% [63]. There are also studies that reported that HbA1c reduction to be of only 0.3% in case of a longer follow-up period both for alogliptin and sitagliptin [64]. TECOS trial is a good example because in the first 4 months, sitagliptin obtained the largest decrease, and at 3 years, the benefit was only 0.3% [65], identical to the SAVOR-TIMI study [66] and similar to the CARMELINA trial where the reduction for linagliptin on HbA1c was only 0.36% [67]. At any rate, despite the modest decrease of HbA1c and the slight weight loss, compared to GLP-1 Ra, DPP-4i have few side effects, with a negligible risk of hypoglycemia [63].
From the AR point of view, a review by Gomez-Peralta et al. [63] reported that the incidence of hypoglycemic events is lower when comparing sitagliptin with a placebo or an insulin-increasing regimen; similar to vildagliptin as compared to a placebo, when adding a DPP-4i to an insufficient insulin regimen [63]. Moreover, it seems that even in the case of patients with T2DM and severe chronic kidney disease, the treatment with DPP-4i is safe regarding hypoglycemic events [63]. The data for the elderly population also show a safe profile for linagliptin compared to metformin, to sulfonylurea, to a basal insulin regimen, or to insulin treatment in general, while for vildagliptin, the evidence compares to insulin treatment [63]. Another important group that seems to benefit from DPP-4i, alone or when added to insulin regimens, are the hospitalized patients, where there is no increase in the hypoglycemic events but with better metabolic control [63].
3.2. SGLT-2i
In the case of SGLT-2i, in the DAPA-HF study, there is a reported reduction of HbA1c in the case of dapagliflozin of 0.21% [68], while other studies report a greater reduction of 0.89% [69], or even 2.66% but in those cases when the baseline HbA1c from the beginning of the study was higher [70]. In EMPEROR-Reduced (empagliflozin outcome trial in patients with chronic heart failure with reduced ejection fraction), empagliflozin is reported to reduce HbA1c, but without a specific value, so its range of efficacy on A1c hemoglobin is reported by other studies with the values that range between 0.11–0.82%, and similar to dapagliflozin a greater effect is encountered in the case of higher levels of A1c hemoglobin at the beginning of treatment [70,71].
Other efficacy elements are reported in the EMPAREG-OUTCOME trial, where empagliflozin reduces BP with 2.46 mmHg for systolic and 1.46 mmHg for diastolic, without raising the ventricular rhythm [70]. On the other hand, they are reported to be associated with a small increase in LDL-cholesterol and HDL-cholesterol levels and a small decrease in triglyceride and small dense LDL-cholesterol levels [70]. Moreover, empagliflozin reduced CV mortality in the EMPAREG-OUTCOME trial [72]. Empagliflozin is neutral on lipid metabolism and has a slower rate of decline in eGFR [51,73].
As AR, when talking about hypoglycemic events, empagliflozin, dapagliflozin, or canagliflozin does not increase their incidence [70], but it is important to emphasize that their use in association with representants of sulfonylurea favors the increase in hypoglycemic events incidence [70]. On the other hand, when talking about a class-specific AR, the urinary tract infections (UTIs) in large CVOTs or RCTs, the data are conflicting, from a decrease in incidence to no significant difference, and eventually to an increase in UTI incidence [70]. When taking each molecule, for dapagliflozin, the DECLARE-TIMI trial and real-life studies reported an increase in the incidence of UTIs, similar to empagliflozin in the EMPAREG-OUTCOME trial [70,74]. Another important class-specific AR is represented by genital infections, which are reported to have increased and even to favor the treatment discontinuation in the DECLARE-TIMI study, for dapagliflozin, in the EMPAREG-OTCOME trial for empagliflozin, and in the CANVAS trial for canagliflozin [70,74]. The cited factors reported to favor the occurrence of infections in the genital sphere are represented by the female gender and by the history of similar infections previously [70,74].
3.3. GLP-1 Ra
For the representants of the GLP-1 Ra class, semaglutide data are provided in the Pioneer trials. The reported HbA1c reduction ranged between 0.6% and 1.1% as compared to the placebo in the Pioneer 1 trial [75], of 1.3% as compared to the 0.9% reduction for empagliflozin [76], with the highest reported reduction of 1.7% in the Pioneer 9 trial [61]. The weight reduction ranged between 0.2 and 2.6 kg [75] compared to the placebo, or of 4.7 kg versus 3.8 kg in the case of empagliflozin [76]. The ARs reported no hypoglycemic events, including severe episodes, for oral semaglutide in the PIONEER 9 trial; one hypoglycemic event, without severe episodes, for oral semaglutide in the PIONEER 1 trial, and fewer hypoglycemic events than in the empagliflozin group, including one severe episode, for both oral semaglutide and empagliflozin, in the PIONEER 2 trial [61,75,76]. Moreover, when talking about the class-specific gastrointestinal (GI) AR, the most reported ARs were mainly constipation, along with a decrease of appetite, nausea, diarrhea, and abdominal discomfort for oral semaglutide in the PIONEER 9 trial; nausea and diarrhea, along with vomiting and decreased appetite for semaglutide in the PIONEER 1 trial; while nonserious nausea, of mild to moderate severity, but transient, along with diarrhea, vomiting, and a decreased appetite for semaglutide in the PIONEER 2 trial [61,75,76].
Discontinuation due to a drug-related AR was encountered in 10.7% of patients treated with oral semaglutide in the PIONEER 2 trial, for 7.4% of patients treated with oral semaglutide in the PIONEER 1 trial, and for 0.2% of patients treated with oral semaglutide in the PIONEER 9 trial [61,75,76].
Other trials that evaluate semaglutide are represented by Sustain trials, which reported a decrease of HbA1c of 1.55% (95% CI −1.74, −1.36), a body weight (BW) reduction of 3.73 kg (95% CI −4.54, −2.91) [77]. When talking about AR, there were no episodes of either hypoglycemia or severe hypoglycemia reported; and the class-specific GI AR encountered are nausea, vomiting, constipation, and diarrhea for semaglutide in sustain trials [77]. Regarding treatment discontinuation due to AR related to the treatment, it took place in only 3% of patients [77].
Exenatide was evaluated in duration trials and was reported to provide a reduction of HbA1c of 1.9% in weekly administration and a 1.5% reduction in case of daily administration in the Duration-1 trial [78]; a similar reduction in HbA1c of 1.5% in the weekly formulation was reported in the DURATION-2 trial [79]. On BW, both daily and weekly exenatide provide a reduction that ranges between 3.6 kg and 3.7 kg in Duration-1 [78], while in the DURATION-2 trial, there was only a 2.3 kg reduction in BW [79]. From the AR, no episodes of severe hypoglycemia, but a few episodes of minor hypoglycemia, especially in patients also treated with sulfonylurea, were encountered for exenatide in the DURATION-1 trial. In contrast, no episodes of severe hypoglycemia, but with 1% episode of minor hypoglycemia, were encountered for exenatide in the DURATION-2 trial [78,79]. The class-specific GI AR reported were nausea, vomiting, and diarrhea for exenatide in the DURATION-1 trial; and nausea, diarrhea, vomiting, and constipation for exenatide in the DURATION-2 trial [78,79]. It is important to emphasize that the GI AR were less likely to appear in the weekly presentation [78,79]. Discontinuation of treatment due to ARs was encountered for exenatide once a week in 6.1% of patients and, respectively, for exenatide twice a day in 4.8% of patients in the DURATION-1 trial, while 6.25% of patients discontinued the treatment due to related AR for exenatide once weekly in DURATION-2 trial [78,79].
Dulaglutide was evaluated in the AWARD trials, and for the metabolic control and AR for a follow-up of 26 weeks in the case of the AWARD-1 trial and 52 weeks in the case of the AWARD-2 trial. It was reported to provide a reduction of fasting plasma glucose of −43 ± 2 mg/dL and of −1.36 ± 0.08% for HbA1c in AWARD-1 trial [80] and of −27 ± 3 mg/dL for fasting plasma glucose level and of −1.08 ± 0.06% for HbA1c in the AWARD-2 trial [81]. The efficacy of BW reduction was −1.30 ± 0.29 kg for dulaglutide in the AWARD-1 trial [80] and −1.87 ± 0.24 kg for dulaglutide in the AWARD-2 trial [81]. From the AR perspective, hypoglycemic events were 10.4% for exenatide in the AWARD-1 trial, less than in the exenatide comparator, without episodes of severe hypoglycemia [80], while a rate of 55.3% hypoglycemic events as compared to 69.1% in the insulin glargine comparator group, with similar rated of severe hypoglycemic events (2 events in each group) for dulaglutide in the AWARD-2 trial [81]. The reported class-specific GI AR were nausea, diarrhea, and vomiting and were transient and mild to moderate in intensity for dulaglutide in the AWARD-1 trial [80]. Mild to moderate diarrhea and nausea were also reported in the AWARD-2 trial [81]. When discussing discontinuing the treatment due to developed AR, there were no significant differences between dulaglutide and comparators in the AWARD-1 and AWARD-2 trials [80,81].
3.4. Tirzepatide
The SURPASS clinical trials are the dedicated studies for tirzepatide that showed benefits on HbA1c reduction that vary from 1.24% to 2.58%, while the benefits on reduction of BW ranged from 5.4 kg to 11.7 kg [82]. ARs were specific to the GLP-1 Ra class and the GI AR—vomiting, nausea, constipation, and diarrhea, but with reduced CV events. It is important to emphasize that the discontinuation rate due to AR was low, at only 8.5%, and was not significantly higher when compared to the semaglutide comparator [82].
3.5. Future Perspectives
There are reported data that the innovation in the field of antidiabetic non-insulinic drugs is ongoing, and agents such as LY3437943, a novel triple agonist peptide at the glucagon receptor (GCGR), glucose-dependent insulinotropic polypeptide receptor (GIPR), and GLP-1R proved in phase 1 studies are safe and tolerable with similar benefits regarding weight and glycemic profiles to other incretins [83,84]; amylin analog represented by cagrilinitide that, without lifestyle intervention, reduced BW and improved glycemic control in healthy subjects [85]; cagrisema, a fixed combination of semaglutide and cagrilintide, that in phase 2 studies provided a higher HbA1c and BW reduction compared to both its components [86], while cotadutide bamadutide and SAR425899, dual GLP-1 and glucagon receptor agonists, reduced HbA1c along with BW in patients with T2DM and who are overweight or obese [87,88]. Other innovative treatments are represented by AMG-133, an antibody blocking the activation of glucose-dependent insulinotropic polypeptide receptor (GIPR) to which are conjugated GLP-1R peptide agonists that proved to have significant weight benefits in phase 1 studies on patients with obesity but without T2DM [89]. Also in development are molecules, such as a novel GLP-1R agonist, consisting of a DPP-IV-resistant GLP-1 fragment fused to the light chains of a humanized GLP-1R antibody by a peptide linker that acts like a structural highly specific antibody but with the properties of a GLP-1 agonist. It has proven efficient in normalizing glycemic fluctuations, improving β-cell function, and reducing BW in mice with T2DM [90,91]. Danuglipron, an oral GLP-1 Ra, also proved efficient and safe in treating mice with T2DM [92]. It is important to emphasize the importance of the association of nutritional intervention because there are reported data stating that intensive weight management, per se, can lead to important weight reduction and even to T2DM remission [93,94,95].
3.6. Strengths and Limitations
The major strength of our meta-analysis is that it showed that the newest antidiabetic non-insulinic drugs, respectively DPP-4i, SGLT-2i, GLP-1 Ra, and tirzepatide are proven to be efficient molecules in terms of HbA1c, weight reduction, and safety, such as hypoglycemia and a class-specific AR.
The primary limitation of our research is the predefined search, which limited the included studies and, respectively, the number of the molecules for each of the classes (e.g., only tenegliptin for DPP-4i, only ipragliflozin and tofogliflozin for SGLT-2i or not all the commercially available GLP-1 RA molecules) or the type populations that were available to be analyzed (e.g., AR comparison between elderly versus the rest of the population). Another limitation is the high rate of excluded full-text articles due to a high percentage of identified articles of Phase I trials, studies that evaluate drugs other than the newest antidiabetic non-insulinic drugs, or studies that evaluate patients without T2DM. Finally, another limitation is that drug dose, time, or disease severity were not evaluated.
4. Conclusions
There are few studies, including CVOTs, that report extensive data about the efficacy and safety of the novel non-insulin antidiabetic drugs, but they prove to be efficient molecules in terms of HbA1c and body weight reduction and offer these results in safe conditions, with low rates of hypoglycemic events, including severe ones; with low rates of specific AR and with low rates of discontinuation due to AR secondary to administered treatment. Moreover, there is hope for even better due to the innovative molecules still being developed. Nevertheless, there is a need for more studies of these novel non-insulin antidiabetic drugs, along with a need for translating the results into real-life settings to verify their favorable effects at the patient’s bedside.
Author Contributions
Conceptualization T.S., L.-I.S. and I.-C.B.; methodology C.S., A.J., S.B. and M.B.; software A.P.S., validation M.R., T.S. and A.A.R., investigation L.-I.S., I.-C.B. and C.S.; resources A.J. and M.B.; data curation S.B., T.S. and A.P.S.; writing—original draft preparation T.S., L.-I.S., I.-C.B. and A.P.S.; writing—review and editing E.M., M.R., S.B. and C.S.; visualization A.P.S. and M.R.; supervision M.B. and A.A.R.; project administration T.S. and A.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
Conflicts of Interest
Stefan Busnatu is an Honorary Advisor of the Romania Minister of Health, currently a nucleus member within the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology (EAPC)/European Society of Cardiology and a member within EAPC Young Community Core Group 2022–2024. He has given lectures, received honoraria and research support, and participated in conferences, advisory boards, and clinical trials sponsored by pharmaceutical and biotechnology companies including AstraZeneca, Pfizer, Novartis, Medtronic, BTL, Phillips, Sonoscape and Janssen. Andrej Janez has served as a consultant and is on speakers’ bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk, Abbott, Sanofi, and Medtronic. Emir Muzurović has given lectures and participated in conferences and advisory boards sponsored by pharmaceutical companies, including Novo Nordisk, Boehringer Ingelheim, AstraZeneca, Medtronic, Merck Sharp & Dohme, Novartis, Sanofi, and Servier. The rest of the authors declare no conflict of interest. Manfredi Rizzo has given lectures, received honoraria and research support, and participated in conferences, advisory boards, and clinical trials sponsored by many pharmaceutical companies, including Amgen, Astra Zeneca, Boehringer Ingelheim, Kowa, Eli Lilly, Meda, Mylan, Merck Sharp & Dohme, Novo Nordisk, Novartis, Roche Diagnostics, Sanofi and Servier. Anca Pantea Stoian is currently the President of the Romanian National Diabetes Committee. She has given lectures, received honoraria and research support, and participated in conferences, advisory boards, and clinical trials sponsored by pharmaceutical companies, including AstraZeneca, Amgen, Boehringer Ingelheim, Medtronic, Eli Lilly, Merck, Novo Nordisk, MSD, Medochemie, Novartis, Roche Diagnostics, and Sanofi. Ali A. Rizvi, Maciej Banach, Cristian Serafinceanu, Teodor Salmen, Liviu-Ionut Serbanoiu, and Ioana-Cristina Bica has no relevant conflicts of interest to disclose.
References
- World Health Organization. Global Health Estimates: Life Expectancy and Healthy Life Expectancy. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy#:~:text=Globally%2C%20life%20expectancy%20has%20increased,reduced%20years%20lived%20with%20disability (accessed on 2 May 2022).
- Brown, G.C. Living too long: The current focus of medical research on increasing the quantity, rather than the quality, of life is damaging our health and harming the economy. EMBO Rep. 2015, 16, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yang, Y.; Schwebel, D.C.; Liu, Z.; Li, L.; Cheng, P.; Ning, P.; Hu, G. Population ageing and mortality during 1990–2017: A global decomposition analysis. PLoS Med. 2020, 17, e1003138. [Google Scholar] [CrossRef] [PubMed]
- Salive, M.E. Multimorbidity in older adults. Epidemiol. Rev. 2013, 35, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Chiang-Hanisko, L.; Tan, J.Y.; Chiang, L.C. Polypharmacy issues in older adults. Hu Li Za Zhi 2014, 61, 97–104. [Google Scholar]
- Czarkowski, W.M.; Bisch, S.; Mleczko, K.; Dziadkiewicz, P.; Kmita, D.A.; Wójcik, M.; Janecki, M. Polypragmasy as a therapeutic problem among palliative and geriatric patients. Med. Paliatywna W Park. 2021, 13, 24. [Google Scholar] [CrossRef]
- Davis, J.W.; Chung, R.; Juarez, D.T. Prevalence of comorbid conditions with aging among patients with diabetes and cardiovascular disease. Hawaii J. Med. Public Health 2011, 70, 209–213. [Google Scholar]
- Mair, A.; Wilson, M.; Dreischulte, T. Addressing the Challenge of Polypharmacy. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 661–681. [Google Scholar] [CrossRef]
- Sarkar, S. Geriatric polypharmacy: A growing epidemic. How to prevent it? Br. J. Med. Med. Res. 2017, 21, 1–11. [Google Scholar] [CrossRef]
- Okpechi, I.G.; Tinwala, M.M.; Muneer, S.; Zaidi, D.; Ye, F.; Hamonic, L.N.; Khan, M.; Sultana, N.; Brimble, S.; Grill, A.; et al. Prevalence of polypharmacy and associated adverse health outcomes in adult patients with chronic kidney disease: Protocol for a systematic review and meta-analysis. Syst. Rev. 2021, 10, 198. [Google Scholar] [CrossRef]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef]
- Pedraza, L.; Laosa, O.; Rodríguez-Mañas, L.; Gutiérrez-Romero, D.F.; Frías, J.; Carnicero, J.A.; Ramírez, E. Drug Induced Liver Injury in Geriatric Patients Detected by a Two-Hospital Prospective Pharmacovigilance Program: A Comprehensive Analysis Using the RousselUclaf Causality Assessment Method. Front. Pharmacol. 2021, 11, 2060. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, J.I.; Shikata, T.; Ito, S.; Kimura, T.; Takamoto, K.; Manabe, E.; Asakura, M.; Ishihara, M.; Tsujino, T. Polypharmacy is associated with accelerated deterioration of renal function in cardiovascular outpatients. Cardiol. Res. 2020, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Boudreau, R.M.; Donohue, J.M.; Zgibor, J.C.; Marcum, Z.A.; Costacou, T.; Newman, A.B.; Waters, T.M.; Strotmeyer, E.S. Persistent polypharmacy and fall injury risk: The Health, Aging and Body Composition Study. BMC Geriatr. 2021, 21, 710. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Itoh, T.; Yabe, A.; Imai, S.; Nakamura, Y.; Mizokami, Y.; Okouchi, Y.; Ikeshita, A.; Kominato, H. Polypharmacy is associated with malnutrition and activities of daily living disability among daycare facility users: A cross-sectional study. Medicine 2021, 100, e27073. [Google Scholar] [CrossRef]
- Resnick, B.; Galik, E.; Boltz, M.; Holmes, S.; Fix, S.; Vigne, E.; Zhu, S.; Lewis, R. Polypharmacy in Assisted Living and Impact on Clinical Outcomes. Consult. Pharm. 2018, 33, 321–330. [Google Scholar] [CrossRef]
- Fukuba, N.; Nishida, M.; Hayashi, M.; Furukawa, N.; Ishitobi, H.; Nagaoka, M.; Takahashi, Y.; Fukuhara, H.; Yuki, M.; Komazawa, Y.; et al. The Relationship between Polypharmacy and Hospital-stay Duration: A Retrospective Study. Cureus 2020, 12, e7267. [Google Scholar] [CrossRef]
- Schenker, Y.; Park, S.Y.; Jeong, K.; Pruskowski, J.; Kavalieratos, D.; Resick, J.; Abernethy, A.; Kutner, J.S. Associations between Polypharmacy, Symptom Burden, and Quality of Life in Patients with Advanced, Life-Limiting Illness. J. Gen. Inter. Med. 2019, 34, 559–566. [Google Scholar] [CrossRef]
- Bosch-Lenders, D.; Jansen, J.; Stoffers, H.E.J.H.; Winkens, B.; Aretz, K.; Twellaar, M.; Schols, J.M.G.A.; van der Kuy, P.-H.M.; Knottnerus, J.A.; van den Akker, M. The Effect of a Comprehensive, Interdisciplinary Medication Review on Quality of Life and Medication Use in Community-Dwelling Older People with Polypharmacy. J. Clin. Med. 2021, 10, 600. [Google Scholar] [CrossRef]
- Kurczewska-Michalak, M.; Lewek, P.; Jankowska-Polańska, B.; Giardini, A.; Granata, N.; Maffoni, M.; Costa, E.; Midão, L.; Kardas, P. Polypharmacy Management in the Older Adults: A Scoping Review of Available Interventions. Front. Pharmacol. 2021, 12, 734045. [Google Scholar] [CrossRef]
- Payne, R.A. The epidemiology of polypharmacy. Clin. Med. 2016, 16, 465–469. [Google Scholar] [CrossRef]
- Franchi, C.; Ludergnani, M.; Merlino, L.; Nobili, A.; Fortino, I.; Leoni, O.; Ardoino, I. Multiple Medication Adherence and Related Outcomes in Community-Dwelling Older People on Chronic Polypharmacy: A Retrospective Cohort Study on Administrative Claims Data. Int. J. Environ. Res. Public Health 2022, 19, 5692. [Google Scholar] [CrossRef] [PubMed]
- Munger, M.A. Polypharmacy and combination therapy in the management of hypertension in elderly patients with comorbid diabetes mellitus. Drugs Aging 2010, 27, 871–883. [Google Scholar] [CrossRef]
- Ermakov, D.; Fomina, E.; Kartashova, O. Specific features of rational pharmacotherapy in elderly patients. Eur. J. Hosp. Pharm. 2021. [Google Scholar] [CrossRef]
- Shakib, S. Problems of polypharmacy. Aust. Fam. Physician 2002, 31, 125–128. [Google Scholar] [PubMed]
- Dobrică, E.C.; Găman, M.A.; Cozma, M.A.; Bratu, O.G.; Pantea Stoian, A.; Diaconu, C.C. Polypharmacy in Type 2 Diabetes Mellitus: Insights from an Internal Medicine Department. Medicina 2019, 55, 436. [Google Scholar] [CrossRef] [PubMed]
- Peron, E.P.; Ogbonna, K.C.; Donohoe, K.L. Antidiabetic medications and polypharmacy. Clin. Geriatr. Med. 2015, 31, 17–27. [Google Scholar] [CrossRef]
- Siasos, G.; Bletsa, E.; Stampouloglou, P.K.; Paschou, S.A.; Oikonomou, E.; Tsigkou, V.; Antonopoulos, A.S.; Vavuranakis, M.; Tousoulis, D. Novel Antidiabetic Agents: Cardiovascular and Safety Outcomes. Curr. Pharm. Des. 2020, 26, 5911–5932. [Google Scholar] [CrossRef]
- Zhuang, X.D.; He, X.; Yang, D.Y.; Guo, Y.; He, J.G.; Xiao, H.P.; Liao, X.X. Comparative cardiovascular outcomes in the era of novel antidiabetic agents: A comprehensive network meta-analysis of 166,371 participants from 170 randomized controlled trials. Cardiovasc. Diabetol. 2018, 17, 79. [Google Scholar] [CrossRef]
- Mazin, I.; Chernomordik, F.; Fefer, P.; Matetzky, S.; Beigel, R. The Impact of Novel Antidiabetic Medications on CV Outcomes: A New Therapeutic Horizon for Diabetic and Non-Diabetic Cardiac Patients. J. Clin. Med. 2022, 11, 1904. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Otsuki, H.; Kosaka, T.; Nakamura, K.; Shimomura, F.; Kuwahara, Y.; Tsukamoto, T. Safety and efficacy of teneligliptin: A novel DPP-4 inhibitor for hemodialysis patients with type 2 diabetes. Int. Urol. Nephrol. 2014, 46, 427–432. [Google Scholar] [CrossRef]
- Kadowaki, T.; Kondo, K. Efficacy, safety and dose-response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2013, 15, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Eto, T.; Inoue, S.; Kadowaki, T. Effects of once-daily teneligliptin on 24-h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: A 4-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 2012, 14, 1040–1046. [Google Scholar] [CrossRef]
- Hong, S.; Park, C.Y.; Han, K.A.; Chung, C.H.; Ku, B.J.; Jang, H.C.; Ahn, C.W.; Lee, M.K.; Moon, M.K.; Son, H.S.; et al. Efficacy and safety of teneligliptin, a novel dipeptidyl peptidase-4 inhibitor, in Korean patients with type 2 diabetes mellitus: A 24-week multicentre, randomized, double-blind, placebo-controlled phase III trial. Diabetes Obes. Metab. 2016, 18, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Ma, J.; Lu, W.; Liu, J.; Zeng, J.; Yang, J.; Li, W.; Zhang, X.; Xiao, X.; Takayanagi, G.; et al. Phase III, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of teneligliptin monotherapy in Chinese patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. J. Diabetes Investig. 2021, 12, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.; Ferrannini, E.; Fonseca, V.A.; Wilpshaar, W.; Dhanjal, P.; Houzer, A. Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: A dose-finding study. Diabetes Obes. Metab. 2013, 15, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.A.; Ferrannini, E.; Wilding, J.P.; Wilpshaar, W.; Dhanjal, P.; Ball, G.; Klasen, S. Active- and placebo-controlled dose-finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J. Diabetes Complicat. 2013, 27, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Kadokura, T.; Akiyama, N.; Kashiwagi, A.; Utsuno, A.; Kazuta, K.; Yoshida, S.; Nagase, I.; Smulders, R.; Kageyama, S. Pharmacokinetic and pharmacodynamic study of ipragliflozin in Japanese patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Diabetes Res. Clin. Pract. 2014, 106, 50–56. [Google Scholar] [CrossRef]
- Kashiwagi, A.; Kazuta, K.; Yoshida, S.; Nagase, I. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J. Diabetes Investig. 2014, 5, 382–391. [Google Scholar] [CrossRef]
- Kaku, K.; Isaka, H.; Toyoshima, J.; Sakatani, T. Clinical pharmacology study of ipragliflozin in Japanese patients with type 1 diabetes mellitus: A phase 2, randomized, placebo-controlled trial. Diabetes Obes. Metab. 2019, 21, 1445–1454. [Google Scholar] [CrossRef]
- Kaku, K.; Watada, H.; Iwamoto, Y.; Utsunomiya, K.; Terauchi, Y.; Tobe, K.; Tanizawa, Y.; Araki, E.; Ueda, M.; Suganami, H.; et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: A combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc. Diabetol. 2014, 13, 65. [Google Scholar] [PubMed]
- Ikeda, S.; Takano, Y.; Cynshi, O.; Tanaka, R.; Christ, A.D.; Boerlin, V.; Beyer, U.; Beck, A.; Ciorciaro, C.; Meyer, M.; et al. A novel and selective sodium-glucose cotransporter-2 inhibitor, tofogliflozin, improves glycaemic control and lowers body weight in patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2015, 17, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Fernández Landó, L.; Mao, H.; Cui, X.; Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K.; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Giorgino, F.; Jódar, E.; Frias, J.P.; Fernández Landó, L.; Brown, K.; Bray, R.; Rodríguez, Á. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial. Lancet 2021, 398, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef]
- Frias, J.P.; Nauck, M.A.; Van, J.; Benson, C.; Bray, R.; Cui, X.; Milicevic, Z.; Urva, S.; Haupt, A.; Robins, D.A. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes Obes. Metab. 2020, 22, 938–946. [Google Scholar]
- Eckel, R.H.; Farooki, A.; Henry, R.R.; Koch, G.G.; Leiter, L.A. Cardiovascular Outcome Trials in Type 2 Diabetes: What Do They Mean for Clinical Practice? Clin. Diabetes 2019, 37, 316–337. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. NEJM 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. NEJM 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. NEJM 2021, 384, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. NEJM 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.; McMurray, J.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. NEJM 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. NEJM 2020, 383, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyan, N.A.; Koshy, R.M.; Jacob, K.; Pappachan, J.M. Efficacy and cardiovascular safety of DPP-4 inhibitors. Curr. Drug Saf. 2021, 16, 154–164. [Google Scholar] [CrossRef]
- Ali, A.; Bain, S.; Hicks, D.; Jones, P.N.; Patel, D.C.; Evans, M.; Fernando, K.; James, J.; Milne, N.; Viljoen, A.; et al. SGLT2 Inhibitors: Cardiovascular Benefits Beyond HbA1c—Translating Evidence into Practice. Diabetes Ther. 2019, 10, 1595–1622. [Google Scholar] [CrossRef]
- Consoli, A.; Formoso, G.; Baldassarre, M.P.A.; Febo, F. A comparative safety review between GLP-1 receptor agonists and SGLT2 inhibitors for diabetes treatment. Expert Opin. Drug Saf. 2018, 17, 293–302. [Google Scholar] [CrossRef]
- Rodbard, H.W.; Buse, J.B.; Woo, V.; Vilsbøll, T.; Langbakke, I.H.; Kvist, K.; Gough, S.C. Benefits of combination of insulin degludec and liraglutide are independent of baseline glycated haemoglobin level and duration of type 2 diabetes. Diabetes Obes. Metab. 2016, 18, 40–48. [Google Scholar] [CrossRef]
- Ahrén, B.; Masmiquel, L.; Kumar, H.; Sargin, M.; Karsbøl, J.D.; Jacobsen, S.H.; Chow, F. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): A 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017, 5, 341–354. [Google Scholar] [CrossRef]
- Yamada, Y.; Katagiri, H.; Hamamoto, Y.; Deenadayalan, S.; Navarria, A.; Nishijima, K.; Seino, Y.; PIONEER 9 Investigators. Dose-response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): A 52-week, phase 2/3a, randomised, controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 377–391. [Google Scholar] [CrossRef]
- Bloomgarden, Z.T. Implications of the recent CVOTs in type 2 diabetes: The right place for DPP-IV inhibitors today. Diabetes Res. Clin. Pract. 2019, 157, 107906. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Peralta, F.; Abreu, C.; Gomez-Rodriguez, S.; Barranco, R.J.; Umpierrez, G.E. Safety and Efficacy of DPP4 Inhibitor and Basal Insulin in Type 2 Diabetes: An Updated Review and Challenging Clinical Scenarios. Diabetes Ther. 2018, 9, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.; Jandeleit-Dahm, K.A. Dipeptidyl peptidase-4 inhibitors and cardiovascular and renal disease in type 2 diabetes: What have we learned from the CARMELINA trial? Diab. Vasc. Dis. Res. 2019, 16, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of Linagliptin vs. Placebo on Major Cardiovascular Events in Adults with Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019, 321, 69–79. [Google Scholar] [CrossRef]
- Kaplinsky, E. DAPA-HF trial: Dapagliflozin evolves from a glucose-lowering agent to a therapy for heart failure. Drugs Context 2020, 9, 2019-11-3. [Google Scholar] [CrossRef]
- Ferrannini, E.; Ramos, S.J.; Salsali, A.; Tang, W.; List, J.F. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: A randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2010, 33, 2217–2224. [Google Scholar] [CrossRef]
- Tentolouris, A.; Vlachakis, P.; Tzeravini, E.; Eleftheriadou, I.; Tentolouris, N. SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. Int. J. Environ. Res. Public Health 2019, 16, 2965. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Khan, M.S.; Marx, N.; Lam, C.; Schnaidt, S.; Ofstad, A.P.; Brueckmann, M.; Jamal, W.; et al. Effect of Empagliflozin on Cardiovascular and Renal Outcomes in Patients with Heart Failure by Baseline Diabetes Status: Results from the EMPEROR-Reduced Trial. Circulation 2021, 143, 337–349. [Google Scholar] [CrossRef]
- Fitchett, D.; Inzucchi, S.E.; Cannon, C.P.; McGuire, D.K.; Scirica, B.M.; Johansen, O.E.; Sambevski, S.; Kaspers, S.; Pfarr, E.; George, J.T.; et al. Empagliflozin Reduced Mortality and Hospitalization for Heart Failure across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial. Circulation 2019, 139, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, S.; Celik, M.; Vural, A.; Aydin, B. The effect of low and high dose empagliflozin on HbA1c and lipid profile in type 2 diabetes mellitus: A real-world data. North. Clin. Istanb. 2019, 7, 167–173. [Google Scholar] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Rosenstock, J.; Terauchi, Y.; Altuntas, Y.; Lalic, N.M.; Morales Villegas, E.C.; Jeppesen, O.K.; Christiansen, E.; Hertz, C.L.; Haluzík, M.; et al. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison with Placebo in Patients with Type 2 Diabetes. Diabetes Care 2019, 42, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Rodbard, H.W.; Rosenstock, J.; Canani, L.H.; Deerochanawong, C.; Gumprecht, J.; Lindberg, S.Ø.; Lingvay, I.; Søndergaard, A.L.; Treppendahl, M.B.; Montanya, E.; et al. Oral Semaglutide Versus Empagliflozin in Patients with Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care 2019, 42, 2272–2281. [Google Scholar] [CrossRef]
- Sorli, C.; Harashima, S.I.; Tsoukas, G.M.; Unger, J.; Karsbøl, J.D.; Hansen, T.; Bain, S.C. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): A double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 251–260. [Google Scholar] [CrossRef]
- Drucker, D.J.; Buse, J.B.; Taylor, K.; Kendall, D.M.; Trautmann, M.; Zhuang, D.; Porter, L.; DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: A randomised, open-label, non-inferiority study. Lancet 2008, 372, 1240–1250. [Google Scholar] [CrossRef]
- Bergenstal, R.M.; Wysham, C.; Macconell, L.; Malloy, J.; Walsh, B.; Yan, P.; Wilhelm, K.; Malone, J.; Porter, L.E.; DURATION-2 Study Group. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): A randomised trial. Lancet 2010, 376, 431–439. [Google Scholar] [CrossRef]
- Wysham, C.; Blevins, T.; Arakaki, R.; Colon, G.; Garcia, P.; Atisso, C.; Kuhstoss, D.; Lakshmanan, M. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care 2014, 37, 2159–2167. [Google Scholar] [CrossRef]
- Giorgino, F.; Benroubi, M.; Sun, J.H.; Zimmermann, A.G.; Pechtner, V. Efficacy and Safety of Once-Weekly Dulaglutide Versus Insulin Glargine in Patients with Type 2 Diabetes on Metformin and Glimepiride (AWARD-2). Diabetes Care 2015, 38, 2241–2249. [Google Scholar] [CrossRef]
- Nauck, M.A.; D’Alessio, D.A. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc. Diabetol. 2022, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Coskun, T.; Urva, S.; Roell, W.C.; Qu, H.; Loghin, C.; Moyers, J.S.; O’Farrell, L.S.; Briere, D.A.; Sloop, K.W.; Thomas, M.K.; et al. LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: From discovery to clinical proof of concept. Cell Metab. 2022, 34, 1234–1247.e9. [Google Scholar] [CrossRef] [PubMed]
- Urva, S.; Coskun, T.; Loh, M.T.; Du, Y.; Thomas, M.K.; Gurbuz, S.; Haupt, A.; Benson, C.T.; Hernandez-Illas, M.; D’Alessio, D.A.; et al. LY3437943, a novel triple GIP, GLP-1, and glucagon receptor agonist in people with type 2 diabetes: A phase 1b, multicentre, double-blind, placebo-controlled, randomised, multiple-ascending dose trial. Lancet 2022, 400, 1869–1881. [Google Scholar] [CrossRef]
- Enebo, L.B.; Berthelsen, K.K.; Kankam, M.; Lund, M.T.; Rubino, D.M.; Satylganova, A.; Lau, D.C.W. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2·4 mg for weight management: A randomised, controlled, phase 1b trial. Lancet 2021, 397, 1736–1748. [Google Scholar] [CrossRef]
- Sudlow, A.; Pournaras, D.J.; le Roux, C.W. Combining metabolic surgery with medications for type 2 diabetes: Is there a benefit? J. Bariatr. Surg. 2023, 2, 13. [Google Scholar]
- Nahra, R.; Wang, T.; Gadde, K.M.; Oscarsson, J.; Stumvoll, M.; Jermutus, L.; Hirshberg, B.; Ambery, P. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults with Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care 2021, 44, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Visentin, R.; Göbel, B.; Riz, M.; Cobelli, C.; Klabunde, T.; Dalla Man, C. Improved postprandial glucose metabolism in type 2 diabetes by the dual glucagon-like peptide-1/glucagon receptor agonist SAR425899 in comparison with liraglutide. Diabetes Obes. Metab. 2021, 23, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Flatt, P.R.; Conlon, J.M. An update on peptide-based therapies for type 2 diabetes and obesity. Peptides 2023, 161, 170939. [Google Scholar] [CrossRef]
- Li, C.; Yang, M.; Wang, X.; Zhang, H.; Yao, C.; Sun, S.; Liu, Q.; Pan, H.; Liu, S.; Huan, Y.; et al. Glutazumab, a novel long-lasting GLP-1/anti-GLP-1R antibody fusion protein, exerts anti-diabetic effects through targeting dual receptor binding sites. Biochem. Pharmacol. 2018, 150, 46–53. [Google Scholar] [CrossRef]
- Jepsen, M.M.; Christensen, M.B. Emerging glucagon-like peptide 1 receptor agonists for the treatment of obesity. Expert Opin. Emerg. Drugs 2021, 26, 231–243. [Google Scholar] [CrossRef]
- Saxena, A.R.; Gorman, D.N.; Esquejo, R.M.; Bergman, A.; Chidsey, K.; Buckeridge, C.; Griffith, D.A.; Kim, A.M. Danuglipron (PF-06882961) in type 2 diabetes: A randomized, placebo-controlled, multiple ascending-dose phase 1 trial. Nat. Med. 2021, 27, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Sachinidis, A.; Nikolic, D.; Stoian, A.P.; Papanas, N.; Tarar, O.; Rizvi, A.A.; Rizzo, M. Cardiovascular outcomes trials with incretin-based medications: A critical review of data available on GLP-1 receptor agonists and DPP-4 inhibitors. Metabolism 2020, 111, 154343. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.M.; Rizvi, A.A.; Giglio, R.V.; Stoian, A.P.; Ligi, D.; Mannello, F. Impact of Glucose-Lowering Medications on Cardiovascular and Metabolic Risk in Type 2 Diabetes. J. Clin. Med. 2020, 9, 912. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).