Dynamic Patterns of Mammalian Mitochondrial DNA Replication Uncovered Using SSiNGLe-5′ES
Abstract
1. Introduction
2. Results
2.1. Establishment and Validation of an NGS-Based Strategy for the Detection of Mitochondrial DNA Replication Origins
2.2. Nucleotide-Level Analysis of the Initiation Sites within the Annotated Human Origins
2.3. Dynamic Pattern of the Initiation Sites Is a Common Feature of Mammalian Mitochondria
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Biological Material and DNA Isolation
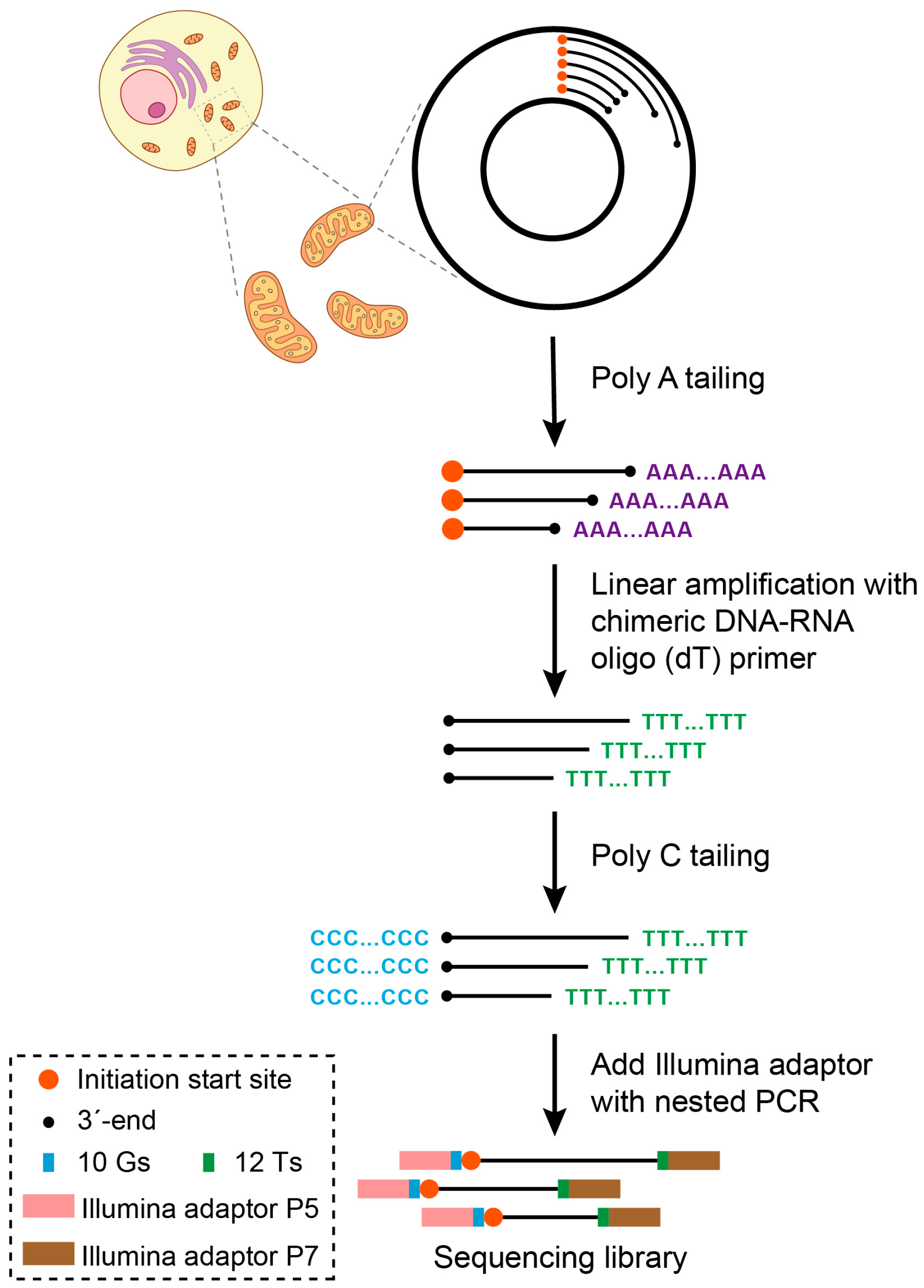
4.3. SSiNGLe-5′ES: Wet Lab
4.4. SSiNGLe-5′ES: Bioinformatics
4.5. In Silico OL Structure Analysis
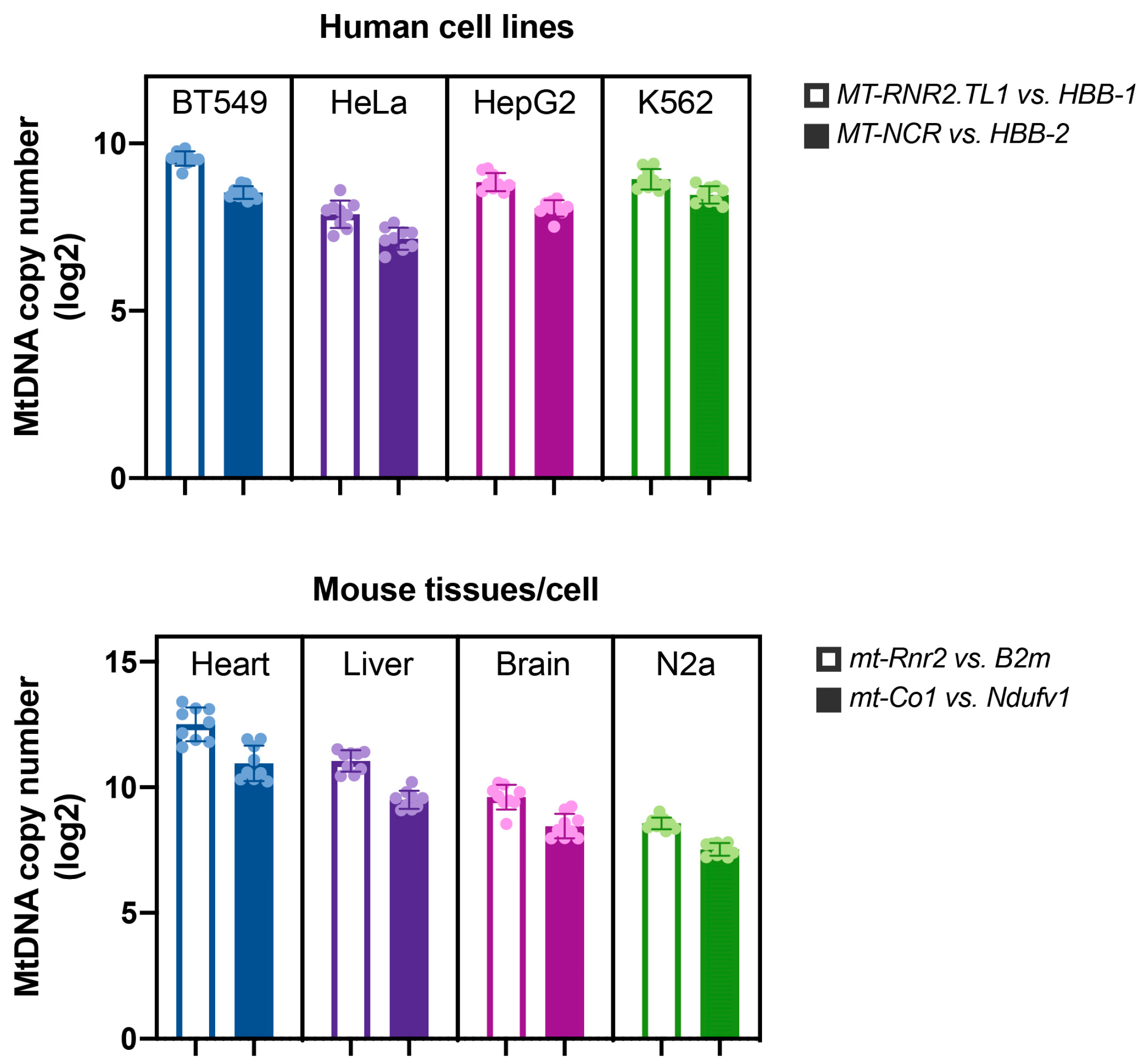
4.6. Mitochondrial DNA Copy Number Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roger, A.J.; Muñoz-Gómez, S.A.; Kamikawa, R. The Origin and Diversification of Mitochondria. Curr. Biol. 2017, 27, R1177–R1192. [Google Scholar] [CrossRef] [PubMed]
- Copeland, W.C. Defects of Mitochondrial DNA Replication. J. Child Neurol. 2014, 29, 1216–1224. [Google Scholar] [CrossRef]
- Nikkanen, J.; Forsström, S.; Euro, L.; Paetau, I.; Kohnz, R.A.; Wang, L.; Chilov, D.; Viinamäki, J.; Roivainen, A.; Marjamäki, P.; et al. Mitochondrial DNA Replication Defects Disturb Cellular DNTP Pools and Remodel One-Carbon Metabolism. Cell Metab. 2016, 23, 635–648. [Google Scholar] [CrossRef]
- Stumpf, J.D.; Copeland, W.C. Mitochondrial DNA Replication and Disease: Insights from DNA Polymerase γ Mutations. Cell. Mol. Life Sci. 2011, 68, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Almannai, M.; El-Hattab, A.W.; Scaglia, F. Mitochondrial DNA Replication: Clinical Syndromes. Essays Biochem. 2018, 62, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sampath, H. Mitochondrial DNA Integrity: Role in Health and Disease. Cells 2019, 8, 100. [Google Scholar] [CrossRef]
- Robberson, D.L.; Kasamatsu, H.; Vinograd, J. Replication of Mitochondrial DNA. Circular Replicative Intermediates in Mouse L Cells. Proc. Natl. Acad. Sci. USA 1972, 69, 737–741. [Google Scholar] [CrossRef]
- Clayton, D.A. Replication of Animal Mitochondrial DNA. Cell 1982, 28, 693–705. [Google Scholar] [CrossRef]
- Holt, I.J.; Lorimer, H.E.; Jacobs, H.T. Coupled Leading- and Lagging-Strand Synthesis of Mammalian Mitochondrial DNA. Cell 2000, 100, 515–524. [Google Scholar] [CrossRef]
- Bowmaker, M.; Yang, M.Y.; Yasukawa, T.; Reyes, A.; Jacobs, H.T.; Huberman, J.A.; Holt, I.J. Mammalian Mitochondrial DNA Replicates Bidirectionally from an Initiation Zone. J. Biol. Chem. 2003, 278, 50961–50969. [Google Scholar] [CrossRef]
- Yasukawa, T.; Yang, M.-Y.; Jacobs, H.T.; Holt, I.J. A Bidirectional Origin of Replication Maps to the Major Noncoding Region of Human Mitochondrial DNA. Mol. Cell 2005, 18, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A.; Cecconi, C.; Tkachuk, A.N.; Bustamante, C.; Clayton, D.A. Replication of Mitochondrial DNA Occurs by Strand Displacement with Alternative Light-Strand Origins, Not via a Strand-Coupled Mechanism. Genes. Dev. 2005, 19, 2466–2476. [Google Scholar] [CrossRef] [PubMed]
- Holt, I.J.; Reyes, A. Human Mitochondrial DNA Replication. Cold Spring Harb. Perspect. Biol. 2012, 4, a012971. [Google Scholar] [CrossRef]
- Reyes, A.; Kazak, L.; Wood, S.R.; Yasukawa, T.; Jacobs, H.T.; Holt, I.J. Mitochondrial DNA Replication Proceeds via a “bootlace” Mechanism Involving the Incorporation of Processed Transcripts. Nucleic Acids Res. 2013, 41, 5837–5850. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, T.; Kang, D. An Overview of Mammalian Mitochondrial DNA Replication Mechanisms. J. Biochem. 2018, 164, 183–193. [Google Scholar] [CrossRef]
- Cupp, J.D.; Nielsen, B.L. Minireview: DNA Replication in Plant Mitochondria. Mitochondrion 2014, 19 Pt B, 231–237. [Google Scholar] [CrossRef]
- Chen, X.J.; Clark-Walker, G.D. Unveiling the Mystery of Mitochondrial DNA Replication in Yeasts. Mitochondrion 2018, 38, 17–22. [Google Scholar] [CrossRef]
- Falkenberg, M. Mitochondrial DNA Replication in Mammalian Cells: Overview of the Pathway. Essays Biochem. 2018, 62, 287–296. [Google Scholar] [CrossRef]
- Mignotte, B.; Barat, M.; Mounolou, J.C. Characterization of a Mitochondrial Protein Binding to Single-Stranded DNA. Nucleic Acids Res. 1985, 13, 1703–1716. [Google Scholar] [CrossRef]
- Miralles Fusté, J.; Shi, Y.; Wanrooij, S.; Zhu, X.; Jemt, E.; Persson, Ö.; Sabouri, N.; Gustafsson, C.M.; Falkenberg, M. In Vivo Occupancy of Mitochondrial Single-Stranded DNA Binding Protein Supports the Strand Displacement Mode of DNA Replication. PLoS Genet. 2014, 10, e1004832. [Google Scholar] [CrossRef]
- Martens, P.A.; Clayton, D.A. Mechanism of Mitochondrial DNA Replication in Mouse L-Cells: Localization and Sequence of the Light-Strand Origin of Replication. J. Mol. Biol. 1979, 135, 327–351. [Google Scholar] [CrossRef] [PubMed]
- Wanrooij, S.; Miralles Fusté, J.; Stewart, J.B.; Wanrooij, P.H.; Samuelsson, T.; Larsson, N.-G.; Gustafsson, C.M.; Falkenberg, M. In Vivo Mutagenesis Reveals That OriL Is Essential for Mitochondrial DNA Replication. EMBO Rep. 2012, 13, 1130–1137. [Google Scholar] [CrossRef]
- Yasukawa, T.; Reyes, A.; Cluett, T.J.; Yang, M.-Y.; Bowmaker, M.; Jacobs, H.T.; Holt, I.J. Replication of Vertebrate Mitochondrial DNA Entails Transient Ribonucleotide Incorporation throughout the Lagging Strand. EMBO J. 2006, 25, 5358–5371. [Google Scholar] [CrossRef] [PubMed]
- Berglund, A.-K.; Navarrete, C.; Engqvist, M.K.M.; Hoberg, E.; Szilagyi, Z.; Taylor, R.W.; Gustafsson, C.M.; Falkenberg, M.; Clausen, A.R. Nucleotide Pools Dictate the Identity and Frequency of Ribonucleotide Incorporation in Mitochondrial DNA. PLoS Genet. 2017, 13, e1006628. [Google Scholar] [CrossRef]
- Robberson, D.L.; Clayton, D.A. Replication of Mitochondrial DNA in Mouse L Cells and Their Thymidine Kinase—Derivatives: Displacement Replication on a Covalently-Closed Circular Template. Proc. Natl. Acad. Sci. USA 1972, 69, 3810–3814. [Google Scholar] [CrossRef] [PubMed]
- Koike, K.; Wolstenholme, D.R. Evidence for Discontinuous Replication of Circular Mitochondrial DNA Molecules from Novikoff Rat Ascites Hepatoma Cells. J. Cell Biol. 1974, 61, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Pikó, L.; Matsumoto, L. Complex Forms and Replicative Intermediates of Mitochondrial DNA in Tissues from Adult and Senescent Mice. Nucleic Acids Res. 1977, 4, 1301–1314. [Google Scholar] [CrossRef]
- Kang, D.; Miyako, K.; Kai, Y.; Irie, T.; Takeshige, K. In Vivo Determination of Replication Origins of Human Mitochondrial DNA by Ligation-Mediated Polymerase Chain Reaction. J. Biol. Chem. 1997, 272, 15275–15279. [Google Scholar] [CrossRef]
- Crews, S.; Ojala, D.; Posakony, J.; Nishiguchi, J.; Attardi, G. Nucleotide Sequence of a Region of Human Mitochondrial DNA Containing the Precisely Identified Origin of Replication. Nature 1979, 277, 192–198. [Google Scholar] [CrossRef]
- Wong, T.W.; Clayton, D.A. In Vitro Replication of Human Mitochondrial DNA: Accurate Initiation at the Origin of Light-Strand Synthesis. Cell 1985, 42, 951–958. [Google Scholar] [CrossRef]
- Seligmann, H. Mitochondrial TRNAs as Light Strand Replication Origins: Similarity between Anticodon Loops and the Loop of the Light Strand Replication Origin Predicts Initiation of DNA Replication. Biosystems 2010, 99, 85–93. [Google Scholar] [CrossRef]
- Cao, H.; Salazar-García, L.; Gao, F.; Wahlestedt, T.; Wu, C.-L.; Han, X.; Cai, Y.; Xu, D.; Wang, F.; Tang, L.; et al. Novel Approach Reveals Genomic Landscapes of Single-Strand DNA Breaks with Nucleotide Resolution in Human Cells. Nat. Commun. 2019, 10, 5799. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, T.J.; Minczuk, M. In D-Loop: 40 Years of Mitochondrial 7S DNA. Exp. Gerontol. 2014, 56, 175–181. [Google Scholar] [CrossRef]
- Fusté, J.M.; Wanrooij, S.; Jemt, E.; Granycome, C.E.; Cluett, T.J.; Shi, Y.; Atanassova, N.; Holt, I.J.; Gustafsson, C.M.; Falkenberg, M. Mitochondrial RNA Polymerase Is Needed for Activation of the Origin of Light-Strand DNA Replication. Mol. Cell 2010, 37, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.D.; Clayton, D.A. Priming of Human Mitochondrial DNA Replication Occurs at the Light-Strand Promoter. Proc. Natl. Acad. Sci. USA 1985, 82, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Gillum, A.M.; Clayton, D.A. Mechanism of Mitochondrial DNA Replication in Mouse L-Cells: RNA Priming during the Initiation of Heavy-Strand Synthesis. J. Mol. Biol. 1979, 135, 353–368. [Google Scholar] [CrossRef]
- Bibb, M.J.; van Etten, R.A.; Wright, C.T.; Walberg, M.W.; Clayton, D.A. Sequence and Gene Organization of Mouse Mitochondrial DNA. Cell 1981, 26, 167–180. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.-S.; Kroemer, G.; Galluzzi, L. Mitochondrial Metabolism and Cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef]
- Kopinski, P.K.; Singh, L.N.; Zhang, S.; Lott, M.T.; Wallace, D.C. Mitochondrial DNA Variation and Cancer. Nat. Rev. Cancer 2021, 21, 431–445. [Google Scholar] [CrossRef]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The Human Genome Browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef]
- Gruber, A.R.; Lorenz, R.; Bernhart, S.H.; Neuböck, R.; Hofacker, I.L. The Vienna RNA Websuite. Nucleic Acids Res. 2008, 36, W70–W74. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.; Bernhart, S.H.; Höner Zu Siederdissen, C.; Tafer, H.; Flamm, C.; Stadler, P.F.; Hofacker, I.L. ViennaRNA Package 2.0. Algorithms Mol. Biol. 2011, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Jiang, L.; Bhasin, S.; Khan, S.M.; Swerdlow, R.H. DNA Extraction Procedures Meaningfully Influence QPCR-Based MtDNA Copy Number Determination. Mitochondrion 2009, 9, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.N.; Shahni, R.; Rodriguez-de-Ledesma, A.; Laftah, A.; Cunningham, P. Mitochondrial DNA as a Non-Invasive Biomarker: Accurate Quantification Using Real Time Quantitative PCR without Co-Amplification of Pseudogenes and Dilution Bias. Biochem. Biophys. Res. Commun. 2011, 412, 1–7. [Google Scholar] [CrossRef]
- Lee, W.; Johnson, J.; Gough, D.J.; Donoghue, J.; Cagnone, G.L.M.; Vaghjiani, V.; Brown, K.A.; Johns, T.G.; St John, J.C. Mitochondrial DNA Copy Number Is Regulated by DNA Methylation and Demethylation of POLGA in Stem and Cancer Cells and Their Differentiated Progeny. Cell Death Dis. 2015, 6, e1664. [Google Scholar] [CrossRef]
- Malik, A.N.; Czajka, A.; Cunningham, P. Accurate Quantification of Mouse Mitochondrial DNA without Co-Amplification of Nuclear Mitochondrial Insertion Sequences. Mitochondrion 2016, 29, 59–64. [Google Scholar] [CrossRef]
- Graf Einsiedel, H.; Taube, T.; Hartmann, R.; Wellmann, S.; Seifert, G.; Henze, G.; Seeger, K. Deletion Analysis of P16(INKa) and P15(INKb) in Relapsed Childhood Acute Lymphoblastic Leukemia. Blood 2002, 99, 4629–4631. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Luo, L.; Huang, Y.; Lu, M.; Tang, L.; Diao, Y.; Kapranov, P. Dynamic Patterns of Mammalian Mitochondrial DNA Replication Uncovered Using SSiNGLe-5′ES. Int. J. Mol. Sci. 2023, 24, 9711. https://doi.org/10.3390/ijms24119711
Xu D, Luo L, Huang Y, Lu M, Tang L, Diao Y, Kapranov P. Dynamic Patterns of Mammalian Mitochondrial DNA Replication Uncovered Using SSiNGLe-5′ES. International Journal of Molecular Sciences. 2023; 24(11):9711. https://doi.org/10.3390/ijms24119711
Chicago/Turabian StyleXu, Dongyang, Lingcong Luo, Yu Huang, Meng Lu, Lu Tang, Yong Diao, and Philipp Kapranov. 2023. "Dynamic Patterns of Mammalian Mitochondrial DNA Replication Uncovered Using SSiNGLe-5′ES" International Journal of Molecular Sciences 24, no. 11: 9711. https://doi.org/10.3390/ijms24119711
APA StyleXu, D., Luo, L., Huang, Y., Lu, M., Tang, L., Diao, Y., & Kapranov, P. (2023). Dynamic Patterns of Mammalian Mitochondrial DNA Replication Uncovered Using SSiNGLe-5′ES. International Journal of Molecular Sciences, 24(11), 9711. https://doi.org/10.3390/ijms24119711





