1. Introduction
The incidence of kidney cancer was ranked 16th among the 36 major cancers globally in 2020, with 431,288 new cases and 179,368 deaths [
1]. There were 75,800 new cancer cases in China in 2016, including 48,000 male and 27,800 female cases [
2]. The reasons for the higher incidence in developed countries are unknown; however, it may be linked to genomic, occupational, and other environmental exposures, such as smoking [
3]. Kidney cancer poses a serious threat to human health.
Kidney renal clear cell carcinoma (KIRC) is the most common subtype of renal cell carcinoma (RCC), accounting for approximately 70% of cases. They are adenocarcinomas derived from renal tubular epithelial cells and are relatively rich in glycogen, phosphoesters, neutral fats, and other substances. Histologically, they are surrounded by a clear cytoplasm, nested cell clusters, and dense endothelial networks [
3]. Although surgical resection is the primary treatment for KIRC, there is still a recurrence rate of approximately 30% and the possibility of distant metastasis. Additionally, the 5-year survival rate after metastasis is low, and conventional chemotherapy and radiotherapy are ineffective in treating all RCC subtypes. Therefore, new treatments, especially molecular targeted therapy, are the main focus of KIRC research [
4,
5,
6,
7].
Trophinin-associated protein (TROAP) is a cytoplasmic protein consisting of 778 amino acid residues rich in proline, and contains three domains homologous to src. The human
TROAP gene encodes the TROAP protein localized on chromosome 12q13.12 [
8,
9,
10]. TROAP is related to bystin and trophinin, which are involved in the initial adhesion process between blastocysts and uterine epithelial cells, and may form complexes involved in embryo implantation [
11]. Multiple studies have shown that TROAP expression in breast and colorectal cancer, ovarian adenocarcinoma, hepatocellular carcinoma, gastric cancer and other tumors enhances malignancy and promotes tumor development [
10,
12,
13,
14,
15]. Additionally, data have shown that elevated
TROAP expression positively correlates with the clinical severity of hepatocellular carcinoma, indicating a poor overall and disease-free survival [
10]. TROAP can affect tumor progression through various pathways; for example, it can promote disease progression through the Wnt3/survival protein signaling pathway in prostate cancer [
16]. In addition, Han Liu et al. found that TROAP regulated cell division cycle 20 (CDC20) and influenced spindle microtubules (ASPM) to promote malignant tumor development during the S and G2 phases of the cell cycle [
17]. Lastly,
TROAP can be used as the target of some microRNAs to inhibit the proliferative activity of cancer cells [
13]. For instance, in KIRC, miR-532-3p reduced cancer cell viability, migration, and invasion by targeting
TROAP [
18]. However, the downstream regulatory mechanism of TROAP in KIRC has not been elucidated.
In this study, we defined the expression of TROAP in KIRC cells, and explored the role and downstream mechanism of TROAP in KIRC cells.
3. Discussion
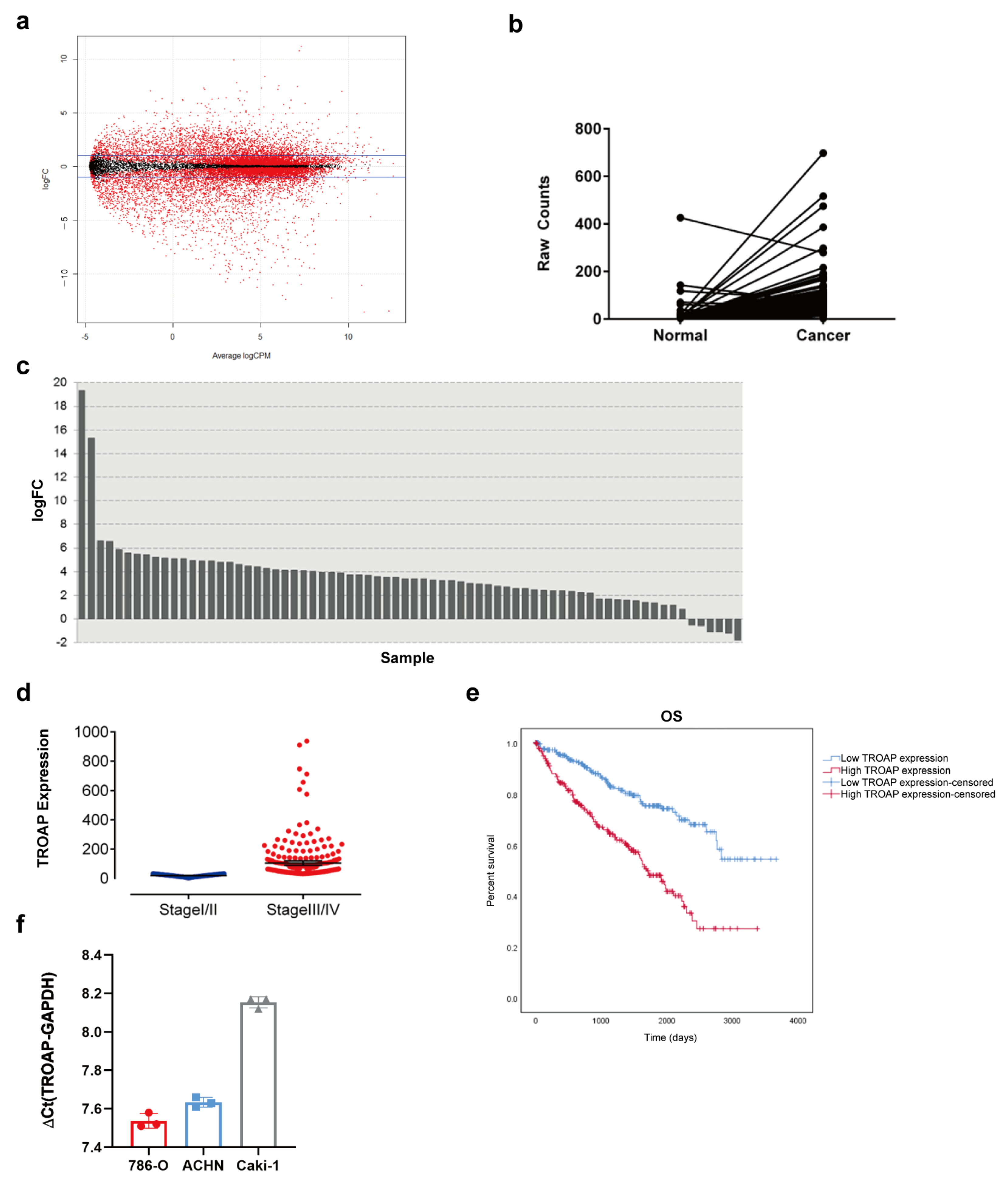
In this study, through bioinformatics analysis and the TCGA database, we screened out a significantly different
TROAP gene, which had a higher expression level in KIRC cancer tissues than in adjacent tissues. Through test analysis, we found that the high expression of TROAP was related to the T and pathological stages of KIRC. The upregulation of TROAP may be one of the factors leading to a poor prognosis in patients with KIRC. Similarly, TROAP is upregulated in other malignant tumors, such as liver, ovarian, prostate, and colon cancers [
10,
12,
13,
19]. The high expressions of
TROAP could promote the proliferation and metastasis of cancer cells, leading to a poor prognosis [
14]. Therefore, we performed qPCR on KIRC cell lines (786-O, ACHN, and CAKI-1) and found that
TROAP mRNA was highly expressed in these cell lines. This proves that
TROAP expression was upregulated in KIRC. Subsequently, we used lentivirus to interfere with
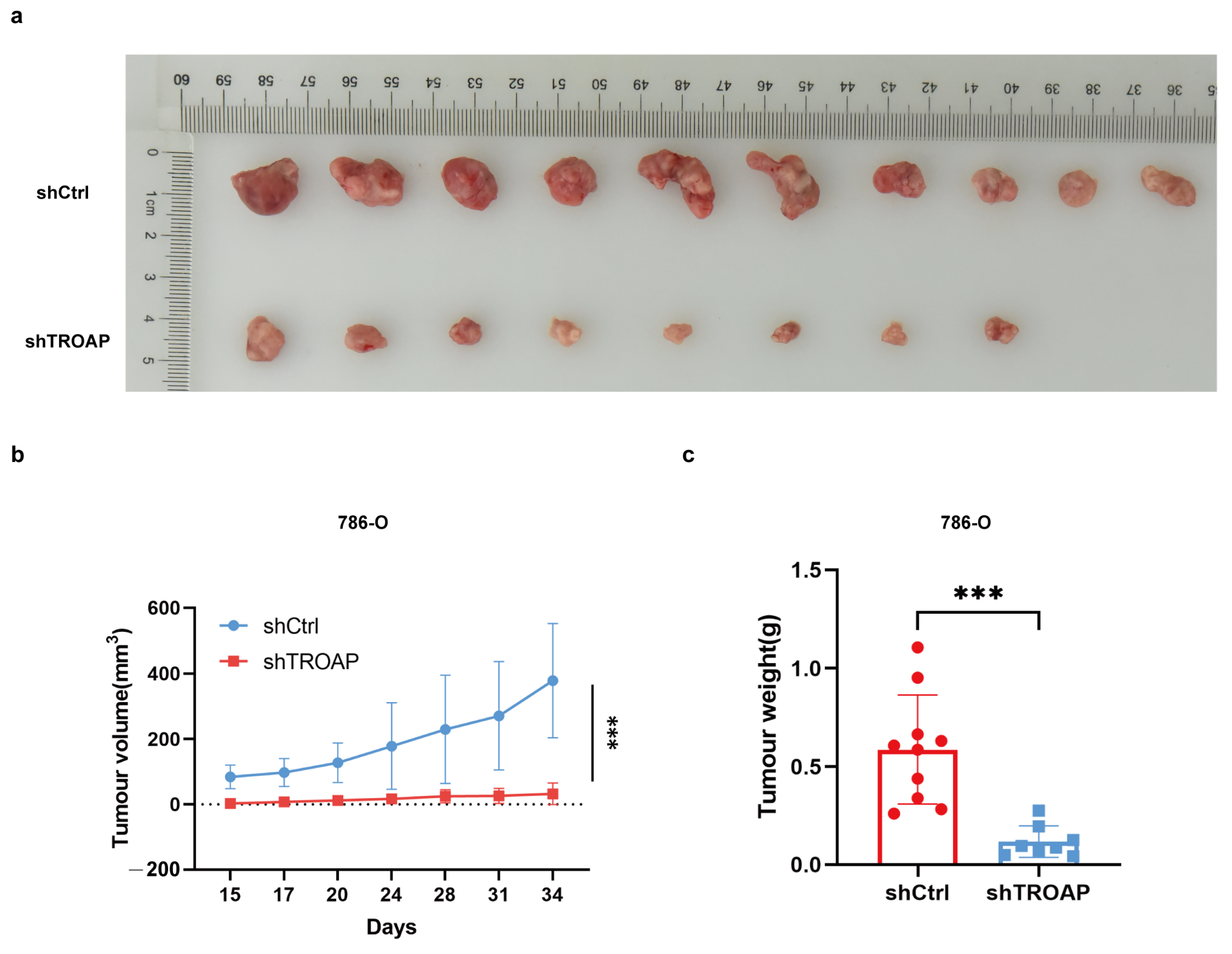
TROAP expression in 786-O and ACHN cells. We observed that TROAP promoted KIRC cell proliferation, migration, and invasion, and affected the cell cycle and apoptosis. We then considered whether interfering with TROAP could affect tumor growth in vivo. Xenograft tumor experiments in nude mice confirmed our suspicion that tumor growth was slowed in the
TROAP knockdown group. These experiments demonstrated that TROAP is an oncogene that could aggravate the malignancy of cancer cells.
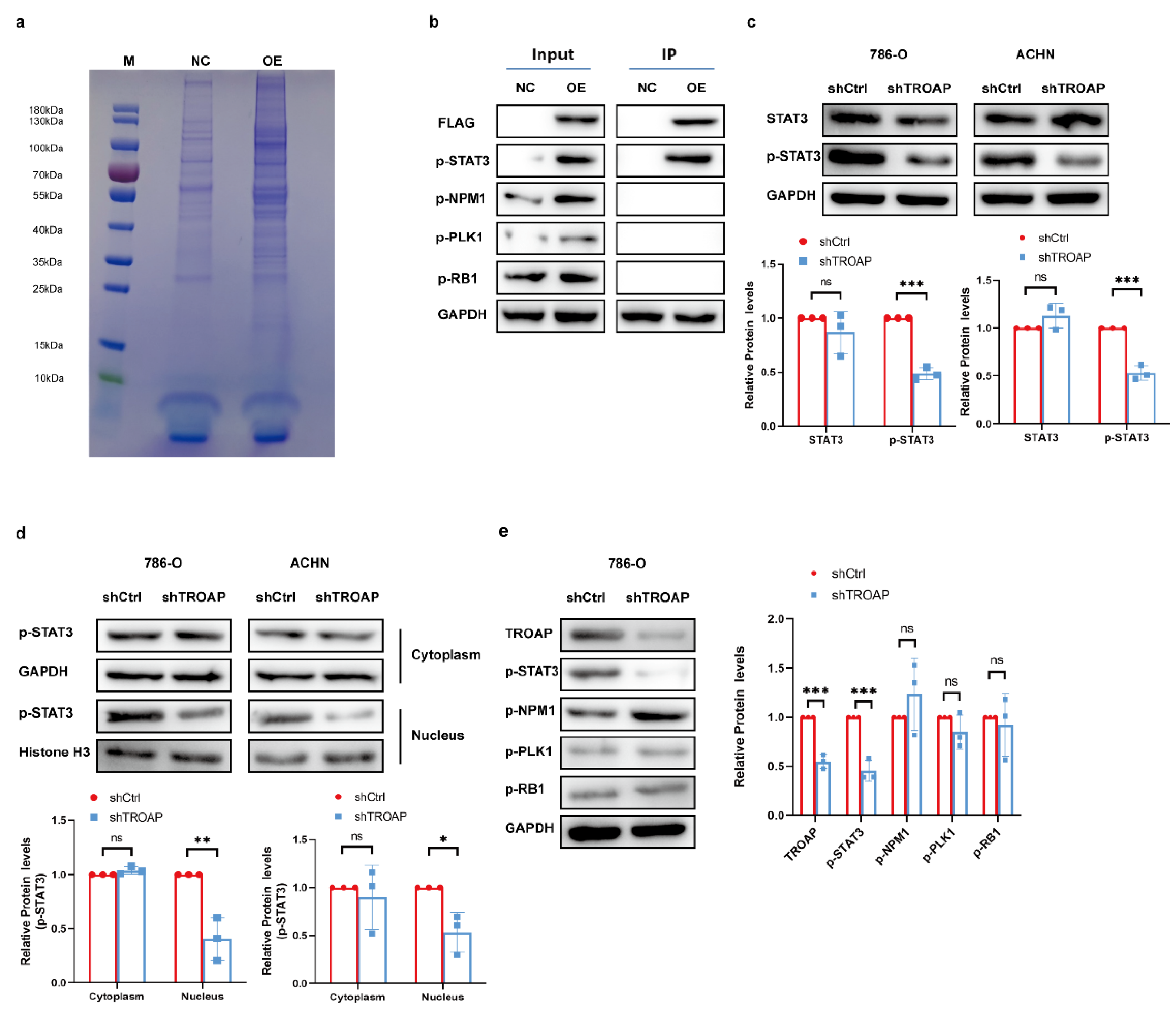
Additionally, we explored the specific regulatory mechanism by which TROAP exerts its oncogenic effects in KIRC. Mir-532-3p, upstream of
TROAP, reportedly attenuates KIRC cell viability, migration, and invasion [
18]. However, its mechanism of action was not explored; we performed CO-IP experiments to achieve this. After mass spectrometry analysis, screening, and Western blot experiments, we found that TROAP can combine with STAT3. TROAP could directly bind to the activated p-STAT3 protein; the p-STAT3 protein levels were notably elevated by
TROAP overexpression and reduced with
TROAP-knockdown.
TROAP-knockdown remarkably decreased the nuclear enrichment of p-STAT3 protein. In subcutaneous models, p-STAT3 levels were found to be significantly lower in
TROAP-knockout tumors compared to control tumors, which is consistent with in vitro findings. STAT3 overexpression prevented inhibition of KIRC proliferation and metastasis after
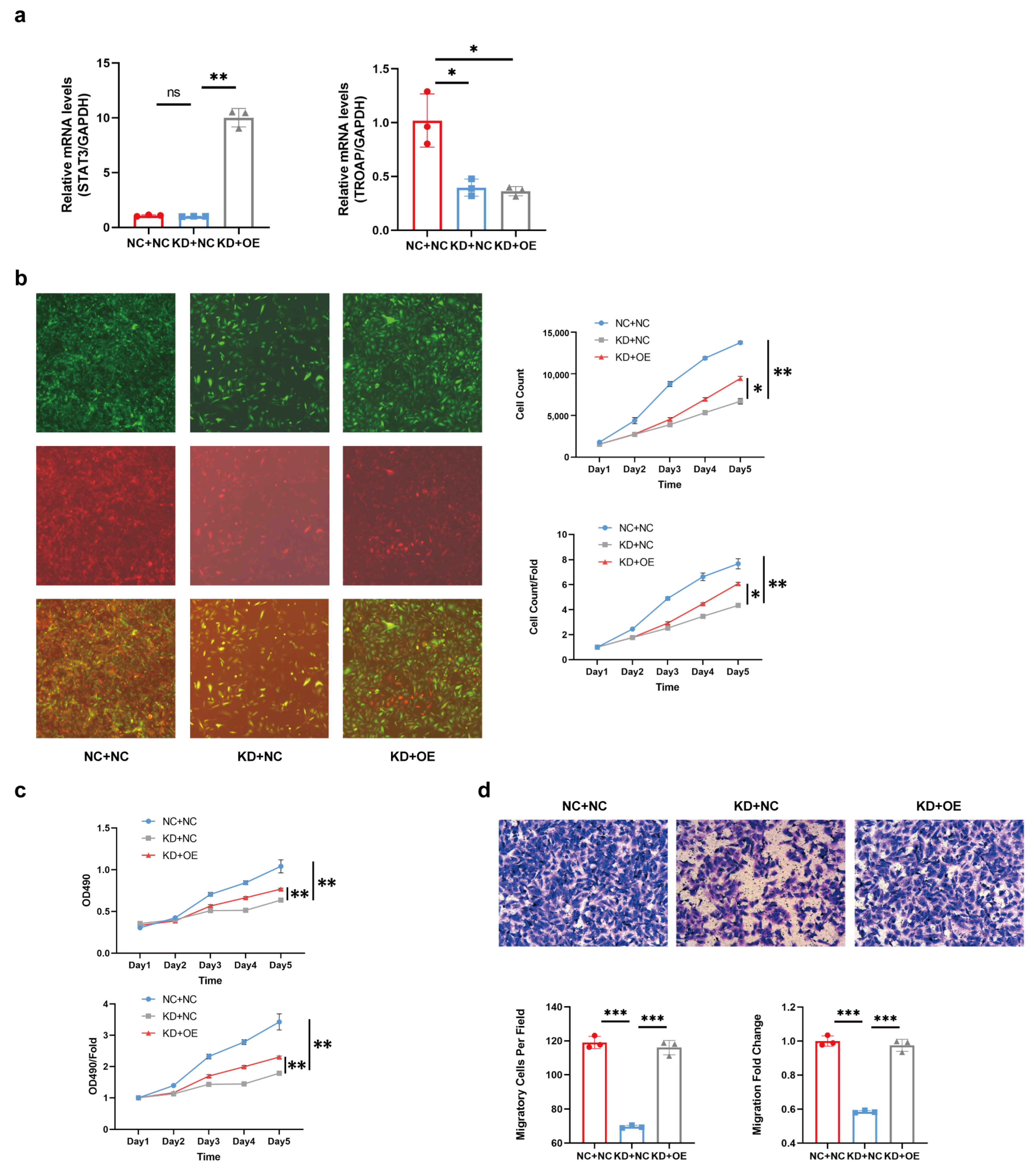
TROAP interference. The combined effects of TROAP and STAT3 may contribute to the carcinogenic effect.
STAT protein family is a family of cytoplasmic transcription factors, including STAT3 [
20]. STAT3 consists of 770 amino acids and contains 6 functionally conserved domains. As a cytokine receptor, STAT3 is tightly regulated by negative modulators and is inactive when unstimulated. Upon stimulation (primarily through phosphorylation induced by upstream ligands), STAT3 dimerizes and translocates to the nucleus, where it binds to DNA and becomes functional [
21]. As a member of the cytokine receptor family, STAT3 plays an important role in the proliferation and metastasis of various cancers. For example, STAT3 participates in the regulation of SOX4 to promote tumor progression in hepatic cell carcinoma [
22]. The IL6/STAT3 pathway hijacked estrogen receptor alpha enhancers to promote cancer cell proliferation in breast cancer [
23]. Similarly, we found that in KIRC, STAT3 promoted tumor proliferation, invasion, and metastasis, and played a prooncogenic role in KIRC.
TROAP is reportedly involved in the initial adhesion process between blastocysts and uterine epithelial cells. Additionally, it is associated with the microscopic cytoskeleton and mitosis and could affect the cell cycle [
8,
24]. Some pathways involved in TROAP have been identified in prostate, liver, and colorectal cancers [
13,
16,
25]. We found that STAT3 could act as a TROAP-interacting protein and that TROAP could promote KIRC proliferation, migration, and metastasis with the help of STAT3. In 786-O cells, STAT3 overexpression restored the decreased cell proliferation and metastasis caused by
TROAP-knockdown, indicating that TROAP can bind to STAT3 to regulate the degree of KIRC malignancy. In our research, we investigated the specific binding mode of TROAP and STAT3, and verified whether the binding of TROAP and STAT3 affects the degree of STAT3 phosphorylation. However, we did not confirm the correlation between the TROAP and JAK/STAT3 pathways in KIRC, which was the most studied pathway of them [
26,
27,
28]. Furthermore, in this study, although we referred to and analyzed a large number of clinical samples of KIRC in public databases, and selected TROAP as the research target based on this, we did not collect clinical samples of KIRC patients for testing in subsequent studies, which may need to be gradually improved in future studies. In addition, we did not conduct caudal vein transfer experiments in nude mice to verify whether TROAP had the ability to promote KIRC distant transfer in vivo, which needs to be supplemented in our subsequent studies. In future, we will explore the effects of TROAP on the nuclear translocation of STAT3 and its binding to DNA. Further research on TROAP and STAT3 can provide a direction for selecting early diagnostic markers for KIRC and may bring new ideas for molecular targeted therapy development.
Overall, we concluded that TROAP was highly expressed in KIRC and promoted cancer cell proliferation, invasion, and metastasis. Additionally, the TROAP and STAT3 interaction affected KIRC proliferation and metastasis (
Figure 6). However, a more specific mechanism needs to be explored.
4. Materials and Methods
4.1. Bioinformatics Analysis
TROAP expression was downloaded from the Cancer Genome Atlas (TCGA) online database (
http://tcga-data.nci.nih.gov/tcga/) which were accessed on 25 June 2020. First, we selected keywords related to renal clear cell carcinoma on the data download page and downloaded the RNAseq files in batches. Based on the barcode information of the samples, the original data files of the 72 paired samples were split from the sample list (
Supplementary Table S1). Second, for each gene symbol, we selected the transcript with the highest expression for analysis. If the number of raw reads of all samples corresponding to that symbol was <50, the data for that symbol was considered unavailable. The trimmed mean of the M-value (TMM) method was used for data normalization. Furthermore, we performed primary quality control by observing the biological coefficient of variation (BCV). A negative binomial general linear model was used for the statistical analyses of multiple paired samples to calculate the
p-values with a filtering criterion of <0.05. Simultaneously, the fold-change (FC) value of the sample was calculated using log2 (Cancer/Normal) and the filtering standard as ≥1 and ≤−1. We used the Mann–Whitney U test to analyze the significance of the differences in
TROAP gene expression levels at different levels of different clinical data. In this study, we used Spearman’s test to analyze the correlation between the
TROAP expression level in cancer tissues and clinical data. We preliminarily explored the potential role of these clinical data in the occurrence and development of renal clear cell carcinoma. Lastly, the Kaplan–Meier method (log-rank test statistic) was used to test whether the effect of the
TROAP gene expression level on survival time was significant. All TCGA data were calculated and processed using the R software (
http://www.r-project.org) and SPSS 17.0.
4.2. Quantitative Real-Time PCR
RNA extraction from 786-O, ACHN, and Caki-1 cell lines was performed using the TRIzol kit (Pufei, Shanghai, China). RNA was then reverse-transcribed into cDNA using the M-MLV kit (Promega, Madison, MI, USA), according to the manufacturer’s instructions. The primer sequences used for GAPDH, TROAP, and STAT3 are listed in
Supplementary Table S2. SYBR Master Mix (Takara, San Jose, CA, USA) was used for PCR on an ABI 7500 PCR system (ABI, Co. Ltd., Lees Summit, MO, USA). The qRT-PCR protocol included initiation at 95 °C for 15 s, followed by 45 cycles at 95 °C for 5 s and 60 °C for 30 s. The relative mRNA expression (TROAP/GAPDH or STAT3/GAPDH) was determined using the 2
−∆∆Ct method. All analyses were conducted in triplicate.
4.3. Cell Culture
The human KIRC cell lines 786-O, ACHN, and Caki-1 were purchased from the cell bank of the Chinese Academy of Science (Shanghai, China). The cells were cultured in Dulbecco modified Eagle’s medium (DMEM; Corning, NY, USA), supplemented with 10% fetal bovine serum (Ausbian, Adelaide, Australia). The incubator conditions were controlled at 37 °C and 5% CO2.
4.4. Lentivirus-Mediated Small Hairpin RNA Construction and Determination of Efficiency
ShTROAP was designed based on the
TROAP (NM_005480, Infection
https://www.ncbi.nlm.nih.gov/nuccore/NM_005480) target sequence (CCCCCGGCAAGCCACGAAGGATC), and a non-silencing shRNA sequence (TTCTCCGAACGTGTCACGT) was used as a negative control. After designing the RNA interference target, a single-stranded DNA oligo containing an interference sequence was synthesized and annealed to produce double-stranded DNA. Subsequently, the digested lentiviral vector was directly connected to the restriction sites at both ends. The ligation product was transferred into the prepared Escherichia coli competent cells, and the positive recombinant was identified via PCR and sent for sequencing verification. Subsequently, the sequencing results were compared with the correct clones for plasmid extraction. Additionally, the
TROAP and
STAT3 overexpression lentiviruses, LV-TROAP and LV-STAT3, were constructed, and their sequences are listed in the table (
Supplementary Table S3). The transfection efficiency was >80% based on green-fluorescent protein fluorescence.
4.5. Cell Proliferation and Colony Formation Assays
The proliferation of 786-O and ACHN cells was detected using Celigo and MTT assays. After reaching the logarithmic growth phase, both the shCtrl and shTROAP cell cultures were trypsinized and resuspended in DMEM. The cells were plated in five 96-well plates, followed by incubation at 37 °C and 5% CO2. After incubation for 1, 2, 3, 4, and 5 days, the cells were incubated with 20 μL of 5 mg/mL MTT for 4 h. Lastly, we measured the optical density at a wavelength of 490 nm. Based on these data, we performed statistical data mapping and constructed cell proliferation curves. The assay was performed in triplicate. In the Celigo assay, cell collection and plate-laying were the same as those in the MTT assay. Celigo was performed 1, 2, 3, 4, and 5 days after plate-laying. The number of cells with green fluorescence in the plates of each scan well was accurately calculated by adjusting the input parameters of the analysis settings. The data were statistically plotted, and the cell proliferation curves were planned for the 5 days.
4.6. Apoptosis Analysis and Cell Cycle Detection
Flow cytometry was used to determine the apoptosis rate and cell cycle. The shCtrl and shTROAP cell cultures were trypsinized and resuspended in DMEM after reaching the logarithmic growth phase. The cells were centrifuged at 1300 rpm for 5 min, the supernatant was discarded, and the cell precipitate was washed with D-Hanks (pH = 7.2–7.4) precooled at 4 °C. The cells were washed with 1 × binding buffer and centrifuged for 3 min. The pellets were resuspended in 200 μL 1 × binding buffer and stained with 10 μL Annexin V-APC for 10–15 min at 24° in the dark. The cells were analyzed using a flow cytometer (Thermo Fisher Scientific, Waltham, MA, USA) within 1 h, to determine the proportion of the cells in each cell cycle phase per group and the apoptosis rate. For the cell cycle assay, the cells were harvested in the same manner as for the apoptosis assay, washed, and precipitated. The harvested cells were centrifuged at 1300 rpm for 5 min and fixed in 75% ethanol precooled at 4 °C for at least 1 h. Next, the cells were centrifuged at 1300 rpm for 5 min to remove the fixant, and the cell precipitate was washed with D-Hanks. According to the number of cells, a certain volume of the cell staining solution (40 × PI mother solution (2 mg/mL): 100 × RNase mother solution (10 mg/mL): 1 × D-Hanks = 25: 10: 1000) (0.6–1 mL) was used, so that the cell passing rate was 300–800 cells/s when loading the machine. The cells were analyzed using a flow cytometer (Thermo Fisher Scientific, USA) within 1 h to determine the proportion of cells in each cell cycle phase per group. The data obtained were analyzed using the ModFit software (version 3.1). All assays were performed in triplicate.
4.7. Western Blot Analysis
Protein expression was analyzed via Western blotting. Briefly, the total protein was extracted using an immunoprecipitation protein lysis buffer (Beyotime Biotechnology, Shanghai, China). Protein concentrations were determined using the bicinchoninic acid assay (BCA) protein determination kit (Beyotime Biotechnology, Shanghai, China). Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Each protein sample (100 mg) was loaded onto 10% SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane (Merckmillipore, Darmstadt, Germany) for 150 min. The primary antibodies were incubated in blocking buffer (TBST solution with 5% skim milk) overnight at 4 °C. The next day, the membranes were washed. However, the secondary antibodies were diluted with a blocking buffer, and the PVDF membranes were incubated for 1.5 h at room temperature and washed. Lastly, X-ray visualization was performed using the Pierce™ ECL Western blotting substrate kit (Thermo, USA). The following primary antibodies were used: anti-GAPDH (Santa Cruz, CA, USA), anti-TROAP (Sigma, Roedermark, Germany), anti-FLAG (Sigma-Aldrich, Germany), anti-STAT3 (CST, Danvers, DE, USA), anti-PLK1 (CST, USA), anti-RB1 (CST, USA), and anti-NPM1 (Abcam, Cambridge, UK). The secondary antibodies used included anti-Rabbit IgG (CST, USA) and anti-mouse IgG (CST, USA).
4.8. Wound-Healing Assay
The ShCtrl and shTROAP cells were cultured in DMEM supplemented with 10% fetal bovine serum. The cells were cultured in a 5% incubator at 37 °C, with three replicates per group, and the culture system at 100 μL/well. The next day, the low-concentration serum medium was changed, and a scratch meter was used to align the lower end of the 96-well plate at the central part; a scratch was formed by nudging upward. The cells were rinsed gently 2–3 times using a serum-free medium, and a low concentration of serum medium (0.5% fetal bovine serum (FBS)) was added. Pictures were taken at 0 h. The cells were cultured in a 37 °C, 5% CO2 incubator and scanned with Celigo at the appropriate time according to the degree of healing. The area of migration was analyzed using Celigo. This assay was performed in triplicate.
4.9. Cell Migration Assay and Invasion Assay
Cell migration was evaluated using a trans-well assay. The Corning transfer and invasion kits (Corning, NY, USA) were used for cell migration and invasion experiments. The invasion kit should be placed in a 37 °C incubator for 2 h, with 500 µL of a serum-free medium added to the upper and lower chambers to rehydrate the Matrigel matrix layer. The 786-O and ACHN cell lines were added to the kit at a density of 8 × 104/well, 30% FBS medium was added to the lower chamber, and the cells were cultured for 24 h. The non-metastatic and non-invasive cells in the chamber were gently removed with a cotton swab. The chamber was placed in 4% paraformaldehyde fixative for 30 min and stained with Giemsa staining fluid (Sigma, Germany). The field of view was randomly selected for each trans-well chamber, and four (100×) and nine photos (200×) were taken. The 200× photos were used for cell counting and data analysis. This assay was performed in triplicate.
4.10. Tumor Formation Assay in a Nude Mouse Model
After trypsin digestion of the shCtrl and shTROAP tumorigenic cells in the logarithmic growth phase, the cell suspension was resuspended in the complete medium cell suspension (0.2 mL) containing 1 × 107 cells injected into the right cutaneous axilla of 4-week-old BALB/c nude mice (Charles River, China). After 15 days, the tumor volumes were measured and collected 2 to 3 times per week for a total of 5 times. On day 33, an overdose of 2% sodium pentobarbital was administered, and death was confirmed via cervical dislocation. Lastly, the tumors were removed with medical scissors and tweezers, arranged on a whiteboard, and photographed for preservation. A ruler was required as a reference to read a specific scale. The animal experiment was reviewed and approved by the Ethics Review Committee of Jiangnan University (JN. No. 20201030c0561225[274]).
4.11. Co-Immunoprecipitation (Co-IP) Assay and Shotgun Mass Spectrometry Analysis
The experiment was performed using 293T cells. Using PCR, the gene sequence encoding the 3 × FLAG tag was fused to the 5’ end of the target gene to generate a 3 × FLAG-target fusion gene. Next, the 3 × FLAG-target fusion gene was inserted into the corresponding lentiviral expression vector to prepare the P3 × FLAG-Target expression plasmid. The empty lentivirus vector and P3 × FLAG-target plasmid were co-transfected into 293T cells with helper plasmids. Lentivirus particles, lenti-control, and lenti-3 × FLAG-target were harvested and prepared. The total cell protein in the supernatant was collected via centrifugation for BCA protein quantification. The protein complexes were purified by CO-IP using FLAG beads (Sigma). CO-IP samples were subjected to SDS-PAGE and Coomassie Brilliant Blue staining. According to each sample lane to be tested on the gel chart, the tape was cut off, and the protein in each sample tape was enzymatically hydrolyzed with trypsin, and the protein was cut into peptide segments. Liquid chromatography–tandem mass spectrometry (LC-MS) was performed for each peptide sample. Each sample’s original quality spectrum file was checked using the PD/MASCOT software (version 2.1) to obtain the protein identification results (
Supplementary Table S4). Gene ontology and KEGG analyses were performed on the list of proteins specifically identified in the overexpression (OE) group. The genes that may interact with the target genes were selected for Western blot verification.
4.12. Statistical Analysis
Data were analyzed using the SPSS software (version 17.0). The Kaplan–Meier method was used to analyze the relationship between TROAP expression and prognosis, and a Cox regression model was used for multivariate analysis. The values are expressed as the mean ± SD. The Student’s t-test was used to analyze the differences between the two groups. Statistical significance was set at p < 0.05. The images were produced using the GraphPad Prism 8 and Illustrator CC 2018 software.