Effects of Obesity and Calorie Restriction on Cancer Development
Abstract
1. Introduction
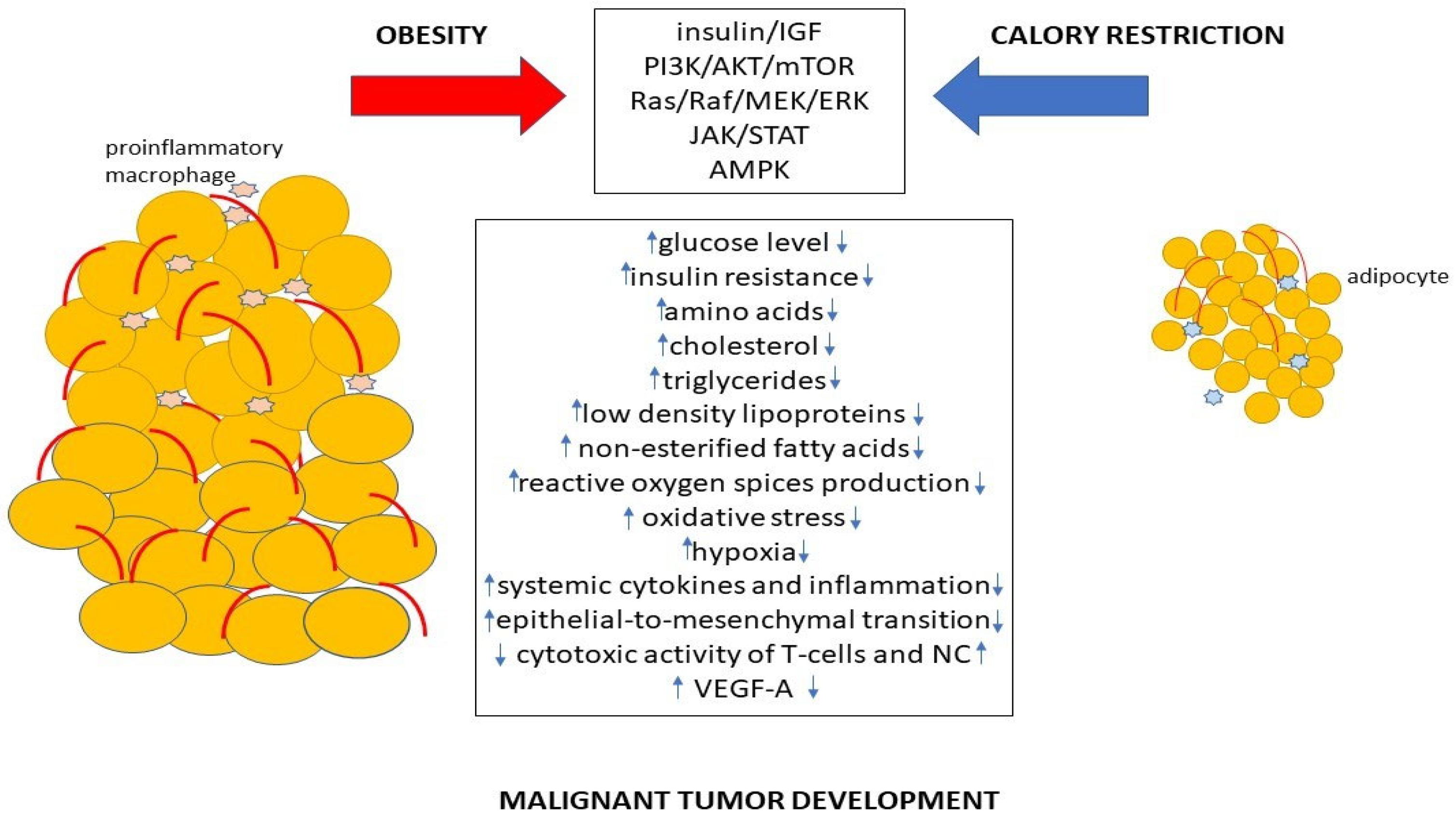
2. Metabolic Alterations Caused by Obesity Leading to Cancer Development
2.1. Carbohydrate Metabolism and Warburg Effect
2.2. Lipid Metabolism
2.3. Hyperinsulinemia, Insulin Resistance and Abnormalities of IGF Axis
2.4. Alterations to Sex Hormone Levels
2.5. Chronic Inflammation
2.6. Adiponectin and Leptin Levels
2.7. Hypoxia
3. Mechanisms Associated with Calorie Restriction Influence
3.1. Alterations to Lipid, Carbohydrate and Protein Metabolism
3.2. MicroRNAs
3.3. Oxidative Stress and Inflammation
3.4. Adipokines and Hormones
3.5. Cell Processes
4. Calorie Restriction as a Treatment Strategy
4.1. Type II Diabetes and Cardiovascular Diseases
4.2. Non-Alcoholic Fatty Liver
4.3. Chronic Kidney Disease
4.4. Metabolic Syndrome
5. Calorie Restriction under Malignant Tumors
5.1. Effects of Calorie Restriction in Laboratory Models with Malignant Tumors
5.2. Effects of Calorie Restriction in Patients with Malignant Tumors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Moliterni, E.; Paolino, G.; Veronese, N.; Bottoni, U.; Corsetti, P.; Cardone, M.; Didona, D.; Lopez, T.; Calvieri, S. Prognostic correlation between vitamin D serological levels, Body Mass Index and clinical-pathological features in melanoma patients. G. Ital. Dermatol. Venereol. 2018, 153, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Lahmann, P.H.; Hughes, M.C.B.; Williams, G.M.; Green, A.C. A prospective study of measured body size and height and risk of keratinocyte cancers and melanoma. Cancer Epidemiol. 2016, 40, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Zhang, M.; Di Martino, J.S.; Bowman, R.L.; Campbell, N.R.; Baksh, S.C.; Simon-Vermot, T.; Kim, I.S.; Haldeman, P.; Mondal, C.; Yong-Gonzales, V.; et al. Adipocyte-Derived Lipids Mediate Melanoma Progression via FATP Proteins. Cancer Discov. 2018, 8, 1006–1025. [Google Scholar] [CrossRef]
- Komatsu, T.; Park, S.; Hayashi, H.; Mori, R.; Yamaza, H.; Shimokawa, I. Mechanisms of Calorie Restriction: A Review of Genes Required for the Life-Extending and Tumor-Inhibiting Effects of Calorie Restriction. Nutrients 2019, 11, 3068. [Google Scholar] [CrossRef]
- Sharmin, F.; Junaed, M.T. Association of Obesity and Serum Gamma Glutamyl Transferase with Impaired Fasting Glucose in Adults at a Tertiary Level Hospital of Bangladesh. Mymensingh. Med. J. 2022, 31, 614–621. [Google Scholar]
- Nagarajan, S.R.; Cross, E.; Sanna, F.; Hodson, L. Dysregulation of hepatic metabolism with obesity: Factors influencing glucose and lipid metabolism. Proc. Nutr. Soc. 2022, 81, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, S.-B.; Lee, S.E.; Kim, J.E.; Kim, J.T.; Kang, Y.E.; Kang, S.G.; Yi, H.-S.; Ko, Y.B.; Lee, K.H.; et al. Expression of LONP1 Is High in Visceral Adipose Tissue in Obesity, and Is Associated with Glucose and Lipid Metabolism. Endocrinol. Metab. 2021, 36, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelstein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.M.; Calvisi, D.F.; Simile, M.M.; Feo, C.F.; Feo, F. The Warburg Effect 97 Years after Its Discovery. Cancers 2020, 12, 2819. [Google Scholar] [CrossRef]
- Krause, N.; Wegner, A. Fructose Metabolism in Cancer. Cells 2020, 9, 2635. [Google Scholar] [CrossRef]
- Carreño, D.V.; Corro, N.B.; Cerda-Infante, J.F.; Echeverría, C.E.; Asencio-Barría, C.A.; Torres-Estay, V.A.; Mayorga-Weber, G.A.; Rojas, P.A.; Véliz, L.P.; Cisternas, P.A.; et al. Dietary Fructose Promotes Prostate Cancer Growth. Cancer Res. 2021, 81, 2824–2832. [Google Scholar] [CrossRef] [PubMed]
- Bu, P.; Chen, K.-Y.; Xiang, K.; Johnson, C.; Crown, S.B.; Rakhilin, N.; Ai, Y.; Wang, L.; Xi, R.; Astapova, I.; et al. Aldolase B-Mediated Fructose Metabolism Drives Metabolic Reprogramming of Colon Cancer Liver Metastasis. Cell Metab. 2018, 27, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2021, 218, e20201606. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, N.; Xu, B.; Chu, Y.; Li, X.; Su, S.; Chen, D.; Li, W.; Shi, Y.; Gao, X.; et al. Fatty acid-induced CD36 expression via O-GlcNAcylation drives gastric cancer metastasis. Theranostics 2019, 9, 5359–5373. [Google Scholar] [CrossRef]
- Gallagher, E.J.; Zelenko, Z.; Neel, B.A.; Antoniou, I.M.; Rajan, L.; Kase, N.; LeRoith, D. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene 2017, 36, 6462–6471. [Google Scholar] [CrossRef]
- Feldt, M.; Menard, J.; Rosendahl, A.H.; Lettiero, B.; Bendahl, P.-O.; Belting, M.; Borgquist, S. The effect of statin treatment on intratumoral cholesterol levels and LDL receptor expression: A window-of-opportunity breast cancer trial. Cancer Metab. 2020, 8, 25. [Google Scholar] [CrossRef]
- Raftopulos, N.L.; Washaya, T.C.; Niederprüm, A.; Egert, A.; Hakeem-Sanni, M.F.; Varney, B.; Aishah, A.; Georgieva, M.L.; Olsson, E.; dos Santos, D.Z.; et al. Prostate cancer cell proliferation is influenced by LDL-cholesterol availability and cholesteryl ester turnover. Cancer Metab. 2022, 10, 1. [Google Scholar] [CrossRef]
- Dos Santos, C.R.; Fonseca, I.; Dias, S.; De Almeida, J.C.M. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Cancer 2014, 14, 132. [Google Scholar] [CrossRef]
- Cornish, A.J.; Law, P.J.; Timofeeva, M.; Palin, K.; Farrington, S.M.; Palles, C.; Jenkins, M.A.; Casey, G.; Brenner, H.; Chang-Claude, J.; et al. Modifiable pathways for colorectal cancer: A mendelian randomisation analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 55–62. [Google Scholar] [CrossRef]
- Hohneck, A.L.; Rosenkaimer, S.; Hofheinz, R.-D.; Akin, I.; Borggrefe, M.; Gerhards, S. Blood Cholesterol and Outcome of Patients with Cancer under Regular Cardiological Surveillance. Curr. Oncol. 2021, 28, 863–872. [Google Scholar] [CrossRef]
- Penson, P.; Long, D.L.; Howard, G.; Howard, V.J.; Jones, S.R.; Martin, S.S.; Mikhailidis, D.P.; Muntner, P.; Rizzo, M.; Rader, D.J.; et al. Associations between cardiovascular disease, cancer, and very low high-density lipoprotein cholesterol in the REasons for Geographical and Racial Differences in Stroke (REGARDS) study. Cardiovasc. Res. 2019, 115, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Attané, C.; Milhas, D.; Dirat, B.; Dauvillier, S.; Guerard, A.; Gilhodes, J.; Lazar, I.; Alet, N.; Laurent, V.; et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. J. Clin. Investig. 2017, 2, e87489. [Google Scholar] [CrossRef]
- Madak-Erdogan, Z.; Band, S.; Zhao, Y.C.; Smith, B.P.; Kulkoyluoglu-Cotul, E.; Zuo, Q.; Casiano, A.S.; Wrobel, K.; Rossi, G.; Smith, R.L.; et al. Free Fatty Acids Rewire Cancer Metabolism in Obesity-Associated Breast Cancer via Estrogen Receptor and mTOR Signaling. Cancer Res. 2019, 79, 2494–2510. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Cheng, X.; Hu, Y.; Song, R.; Li, G. Insulin-like growth factor 1 and metabolic parameters are associated with nonalcoholic fatty liver disease in obese children and adolescents. Acta Paediatr. 2017, 106, 298–303. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; He, T.; Yang, W.; Wang, L.; Zhang, L.; Guo, M. Epigenetic silencing of IGFBPL1 promotes esophageal cancer growth by activating PI3K-AKT signaling. Clin. Epigenetics 2020, 12, 22. [Google Scholar] [CrossRef]
- Aleem, E.; Nehrbass, D.; Klimek, F.; Mayer, D.; Bannasch, P. Upregulation of the Insulin Receptor and Type I Insulin-Like Growth Factor Receptor Are Early Events in Hepatocarcinogenesis. Toxicol. Pathol. 2011, 39, 524–543. [Google Scholar] [CrossRef] [PubMed]
- Adamek, A.; Kasprzak, A. Insulin-Like Growth Factor (IGF) System in Liver Diseases. Int. J. Mol. Sci. 2018, 19, 1308. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jiang, J.; Zhang, X.; Zhang, M.; Fu, Y. Comprehensive Analysis of IGFBPs as Biomarkers in Gastric Cancer. Front. Oncol. 2021, 11, 723131. [Google Scholar] [CrossRef]
- Nur, S.I.; Ozturk, A.; Kavas, M.; Bulut, I.; Alparslan, S.; Aydogan, E.S.; Atinkaya, B.C.; Kolay, M.; Coskun, A. IGFBP-4: A promising biomarker for lung cancer. J. Med. Biochem. 2021, 40, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Hermani, A.; Shukla, A.; Medunjanin, S.; Werner, H.; Mayer, D. Insulin-like growth factor binding protein-4 and -5 modulate ligand-dependent estrogen receptor-α activation in breast cancer cells in an IGF-independent manner. Cell. Signal. 2013, 25, 1395. [Google Scholar] [CrossRef]
- Kalledsøe, L.; Dragsted, L.O.; Hansen, L.; Kyrø, C.; Grønbæk, H.; Tjønneland, A.; Olsen, A. The insulin-like growth factor family and breast cancer prognosis: A prospective cohort study among postmenopausal women in Denmark. Growth Horm. IGF Res. 2018, 44, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ciulei, G.; Orasan, O.H.; Coste, S.C.; Cozma, A.; Negrean, V.; Procopciuc, L.M. Vitamin D and the insulin-like growth factor system: Implications for colorectal neoplasia. Eur. J. Clin. Investig. 2020, 50, e13265. [Google Scholar] [CrossRef]
- Lazúrová, I.; Jochmanová, I.; Sotak, Š.; Špaková, I.; Mareková, M. Is there a role for the IGF system and epidermal growth factor (EGF) in the pathogenesis of adrenocortical adenomas? A preliminary case-control study. Physiol. Res. 2020, 69, 1085–1094. [Google Scholar] [CrossRef]
- Giovannucci, E. Insulin, Insulin-Like Growth Factors and Colon Cancer: A Review of the Evidence. J. Nutr. 2001, 131, 3109S–3120S. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T.; Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; et al. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, W.; Ma, J.; Dai, W.; Liu, L.; Guo, S.; Chen, J.; Wang, H.; Yang, Y.; Yi, X.; et al. Aberrant SIRT6 expression contributes to melanoma growth: Role of the autophagy paradox and IGF-AKT signaling. Autophagy 2018, 14, 518–533. [Google Scholar] [CrossRef]
- Liu, X.; Mi, J.; Qin, H.; Li, Z.; Chai, J.; Li, M.; Wu, J.; Xu, J. E2F1/IGF-1R Loop Contributes to BRAF Inhibitor Resistance in Melanoma. J. Investig. Dermatol. 2020, 140, 1295.e1–1299.e1. [Google Scholar] [CrossRef] [PubMed]
- Molehin, D.; Rasha, F.; Rahman, R.L.; Pruitt, K. Regulation of aromatase in cancer. Mol. Cell. Biochem. 2021, 476, 2449–2464. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, N.M.; Arthur, R.; Manson, J.E.; Chlebowski, R.T.; Kroenke, C.H.; Peterson, L.; Cheng, T.D.; Feliciano, E.C.; Lane, D.; Luo, J.; et al. Association of Body Fat and Risk of Breast Cancer in Postmenopausal Women With Normal Body Mass Index: A Secondary Analysis of a Randomized Clinical Trial and Observational Study. JAMA Oncol. 2019, 5, 155–163. [Google Scholar] [CrossRef]
- Arthur, R.S.; Xue, X.; Kamensky, V.; Chlebowski, R.T.; Simon, M.; Luo, J.; Shadyab, A.H.; Neuhouser, M.L.; Banack, H.; Ho, G.Y.; et al. The association between DXA-derived body fat measures and breast cancer risk among postmenopausal women in the Women’s Health Initiative. Cancer Med. 2020, 9, 1581–1599. [Google Scholar] [CrossRef] [PubMed]
- Pabona, J.M.P.; Burnett, A.F.; Brown, D.M.; Quick, C.M.; Simmen, F.A.; Montales, M.T.E.; Liu, S.J.; Rose, T.; Alhallak, I.; Siegel, E.R.; et al. Metformin Promotes Anti-tumor Biomarkers in Human Endometrial Cancer Cells. Reprod. Sci. 2020, 27, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Farris, M.; McNeil, J.; Friedenreich, C. Obesity and Endometrial Cancer. In Obesity and Cancer; Recent Results in Cancer Research; Pischon, T., Nimptsch, K., Eds.; Springer: Cham, Switzerland, 2016; Volume 208, pp. 107–136. [Google Scholar] [CrossRef]
- Kaaks, R.; Lukanova, A.; Kurzer, M.S. Obesity, endogenous hormones, and endometrial cancer risk: A synthetic review. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1531–1543. [Google Scholar]
- Gonzalo-Encabo, P.; Valadés, D.; García-Honduvilla, N.; Blanco, A.D.C.; Friedenreich, C.M.; Pérez-López, A. Exercise type and fat mass loss regulate breast cancer-related sex hormones in obese and overweight postmenopausal women. Eur. J. Appl. Physiol. 2020, 120, 1277–1287. [Google Scholar] [CrossRef]
- DeJongh, J.; Ahsman, M.; Snelder, N. A population K-PD model analysis of long-term testosterone inhibition in prostate cancer patients undergoing intermittent androgen deprivation therapy. J. Pharmacokinet. Pharmacodyn. 2021, 48, 465–477. [Google Scholar] [CrossRef]
- Porcaro, A.B.; Cerrato, C.; Tafuri, A.; Bianchi, A.; Gallina, S.; Orlando, R.; Amigoni, N.; Rizzetto, R.; Gozzo, A.; Migliorini, F.; et al. Low endogenous testosterone levels are associated with the extend of lymphnodal invasion at radical prostatectomy and extended pelvic lymph node dissection. Int. Urol. Nephrol. 2021, 53, 2027–2039. [Google Scholar] [CrossRef]
- Porcaro, A.B.; Tafuri, A.; Panunzio, A.; Mazzucato, G.; Cerrato, C.; Gallina, S.; Bianchi, A.; Rizzetto, R.; Amigoni, N.; Serafin, E.; et al. Endogenous testosterone density is an independent predictor of pelvic lymph node invasion in high-risk prostate cancer: Results in 201 consecutive patients treated with radical prostatectomy and extended pelvic lymph node dissection. Int. Urol. Nephrol. 2022, 54, 541–550. [Google Scholar] [CrossRef]
- Lainez, N.M.; Coss, D. Obesity, Neuroinflammation, and Reproductive Function. Endocrinology 2019, 160, 2719–2736. [Google Scholar] [CrossRef]
- Pasquali, R.; Oriolo, C. Obesity and Androgens in Women. Front. Horm. Res. 2019, 53, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Maček, P.; Molinari, N.; Sobočan, M.; Knez, J. What Role do Androgens Play in Endometrial Cancer? J. Pers. Med. 2023, 13, 341. [Google Scholar] [CrossRef] [PubMed]
- Reyes-García, J.; Montaño, L.M.; Carbajal-García, A.; Wang, Y.-X. Sex Hormones and Lung Inflammation. Adv. Exp. Med. Biol. 2021, 1304, 259–321. [Google Scholar] [CrossRef]
- Yang, X.D.; Jiang, S.; Wang, G.; Zhang, R.; Zhang, J.; Zhu, J.S. Link of obesity and gastrointestinal cancer: Crossroad of inflammation and oxidative stress. J. Biol. Regul. Homeost. Agents 2015, 29, 755–760. [Google Scholar] [PubMed]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White adipose tissue dysfunction in obesity and aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Tulotta, C.; Ottewell, P. The role of IL-1B in breast cancer bone metastasis. Endocr. Relat. Cancer 2018, 25, R421–R434. [Google Scholar] [CrossRef]
- Manore, S.G.; Doheny, D.L.; Wong, G.L.; Lo, H.-W. IL-6/JAK/STAT3 Signaling in Breast Cancer Metastasis: Biology and Treatment. Front. Oncol. 2022, 12, 866014. [Google Scholar] [CrossRef]
- Malhab, L.J.B.; Abdel-Rahman, W.M. Obesity and inflammation: Colorectal cancer engines. Curr. Mol. Pharmacol. 2022, 15, 620–646. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and obesity: Role and clinical implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Malvi, P.; Chaube, B.; Singh, S.V.; Mohammad, N.; Vijayakumar, M.V.; Singh, S.; Chouhan, S.; Bhat, M.K. Elevated circulatory levels of leptin and resistin impair therapeutic efficacy of dacarbazine in melanoma under obese state. Cancer Metab. 2018, 6, 2. [Google Scholar] [CrossRef]
- Schmidt, S.; Monk, J.M.; Robinson, L.E.; Mourtzakis, M. The integrative role of leptin, oestrogen and the insulin family in obesity-associated breast cancer: Potential effects of exercise. Obes. Rev. 2015, 16, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-C. The role of visfatin in cancer proliferation, angiogenesis, metastasis, drug resistance and clinical prognosis. Cancer Manag. Res. 2019, 11, 3481–3491. [Google Scholar] [CrossRef]
- Venkateshaiah, S.U.; Khan, S.; Ling, W.; Bam, R.; Li, X.; van Rhee, F.; Usmani, S.; Barlogie, B.; Epstein, J.; Yaccoby, S. NAMPT/PBEF1 enzymatic activity is indispensable for myeloma cell growth and osteoclast activity. Exp. Hematol. 2013, 41, 547–557. [Google Scholar] [CrossRef]
- Reizes, O.; Berger, N.A. (Eds.) Adipocytokines, Energy Balance, and Cancer; Springer International Publishing: Cham, Switzerland, 2017; Energy Balance and Cancer 12; ISBN 978-3-319-41677-9. [Google Scholar]
- García-Miranda, A.; Garcia-Hernandez, A.; Castañeda-Saucedo, E.; Navarro-Tito, N.; Maycotte, P. Adipokines as Regulators of Autophagy in Obesity-Linked Cancer. Cells 2022, 11, 3230. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A. Metabolic pathways in obesity-related breast cancer. Nat. Rev. Endocrinol. 2021, 17, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Liu, S.; Klionsky, D.J.; Lip, G.Y.H.; Tuomilehto, J.; Kavalakatt, S.; Pereira, D.M.; Samali, A.; Ren, J. ER stress in obesity pathogenesis and management. Trends Pharmacol. Sci. 2022, 43, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Redman, L.M. Impact of calorie restriction on energy metabolism in humans. Exp. Gerontol. 2020, 133, 110875. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Das, S.K.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; Ravussin, E.; et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): Exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 673–683. [Google Scholar] [CrossRef]
- Park, C.Y.; Park, S.; Kim, M.S.; Kim, H.-K.; Han, S.N. Effects of mild calorie restriction on lipid metabolism and inflammation in liver and adipose tissue. Biochem. Biophys. Res. Commun. 2017, 490, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Pons, V.; Riera, J.; Capó, X.; Martorell, M.; Sureda, A.; Tur, J.A.; Drobnic, F.; Pons, A. Calorie restriction regime enhances physical performance of trained athletes. J. Int. Soc. Sports Nutr. 2018, 15, 12. [Google Scholar] [CrossRef]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-obese Humans. Cell Metab. 2019, 30, 462–476. [Google Scholar] [CrossRef]
- Giardina, S.; Hernández-Alonso, P.; Salas-Salvadó, J.; Rabassa-Soler, A.; Bulló, M. Modulation of Human Subcutaneous Adipose Tissue MicroRNA Profile Associated with Changes in Adiposity-Related Parameters. Mol. Nutr. Food Res. 2018, 62, 1700594. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, G.; Filardi, T.; Sabato, C.; Vacca, A.; Migliaccio, S.; Morano, S.; Ferretti, E. Tissue and circulating microRNAs as biomarkers of response to obesity treatment strategies. J. Endocrinol. Investig. 2021, 44, 1159–1174. [Google Scholar] [CrossRef] [PubMed]
- Brady, E.M.; Gulsin, G.S.; Mirkes, E.M.; Parke, K.; Kanagala, P.; Ng, L.L.; Graham-Brown, M.P.M.; Athithan, L.; Henson, J.; Redman, E.; et al. Fibro-inflammatory recovery and type 2 diabetes remission following a low calorie diet but not exercise training: A secondary analysis of the DIASTOLIC randomised controlled trial. Diabet. Med. 2022, 39, e14884. [Google Scholar] [CrossRef] [PubMed]
- Imbert, A.; Vialaneix, N.; Marquis, J.; Vion, J.; Charpagne, A.; Metairon, S.; Laurens, C.; Moro, C.; Boulet, N.; Walter, O.; et al. Network Analyses Reveal Negative Link Between Changes in Adipose Tissue GDF15 and BMI During Dietary-induced Weight Loss. J. Clin. Endocrinol. Metab. 2022, 107, e130–e142. [Google Scholar] [CrossRef] [PubMed]
- Il’yasova, D.; Fontana, L.; Bhapkar, M.; Pieper, C.F.; Spasojevic, I.; Redman, L.M.; Das, S.K.; Huffman, K.M.; Kraus, W.E.; the CALERIE Study Investigators. Effects of 2 years of caloric restriction on oxidative status assessed by urinary F2-isoprostanes: The CALERIE 2 randomized clinical trial. Aging Cell 2018, 17, e12719. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Robinson-Cohen, C.; Ellis, C.; Headley, S.A.E.; Tuttle, K.; Wood, R.J.; Evans, E.E.; Milch, C.M.; Moody, K.A.; Germain, M.; et al. Metabolic Effects of Diet and Exercise in Patients with Moderate to Severe CKD: A Randomized Clinical Trial. J. Am. Soc. Nephrol. 2017, 29, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Ming, Y.; Wu, M.; Jing, J.; Xu, S.; Li, H.; Zhu, Y. Effects of Caloric Restriction and Rope-Skipping Exercise on Cardiometabolic Health: A Pilot Randomized Controlled Trial in Young Adults. Nutrients 2021, 13, 3222. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, F.; de Bruin, R.W.F.; Van Steeg, H.; Beekhof, P.; Wackers, P.; Hesselink, D.A.; Hoeijmakers, J.H.J.; Dollé, M.E.T.; IJzermans, J.N.M. Protein and calorie restriction may improve outcomes in living kidney donors and kidney transplant recipients. Aging 2020, 12, 12441–12467. [Google Scholar] [CrossRef] [PubMed]
- Kvitne, K.E.; Krogstad, V.; Wegler, C.; Johnson, L.K.; Kringen, M.K.; Hovd, M.H.; Hertel, J.K.; Heijer, M.; Sandbu, R.; Skovlund, E.; et al. Short- and long-term effects of body weight, calorie restriction and gastric bypass on CYP1A2, CYP2C19 and CYP2C9 activity. Br. J. Clin. Pharmacol. 2022, 88, 4121–4133. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, M.; Malesza, I.J.; Bajerska, J.; Chmurzyńska, A.; Muzsik, A.; Bermagambetova, S.; Mądry, E.; Walkowiak, J.; Lisowska, A. Energy-restricted Central-European diet stimulates liver microsomal function in obese postmenopausal women-a randomized nutritional trial with a comparison to energy-restricted Mediterranean diet. Eur. Rev. Med. Pharm. Sci. 2020, 24, 11165–11171. [Google Scholar] [CrossRef]
- Hołowko, J.; Michalczyk, M.M.; Zając, A.; Czerwińska-Rogowska, M.; Ryterska, K.; Banaszczak, M.; Jakubczyk, K.; Stachowska, E. Six Weeks of Calorie Restriction Improves Body Composition and Lipid Profile in Obese and Overweight Former Athletes. Nutrients 2019, 11, 1461. [Google Scholar] [CrossRef] [PubMed]
- De Luis, D.A.; Primo, D.; Izaola, O.; Hoyos, E.G.; Gomez, J.J.L.; Ortola, A.; Aller, R. Role of the variant in adiponectin gene rs266729 on weight loss and cardiovascular risk factors after a hypocaloric diet with the Mediterranean pattern. Nutrition 2019, 60, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Klein, S.; Holloszy, J.O.; Premachandra, B.N. Effect of Long-Term Calorie Restriction with Adequate Protein and Micronutrients on Thyroid Hormones. J. Clin. Endocrinol. Metab. 2006, 91, 3232–3235. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Friedmann, A.J.; Holloszy, J.O.; Fontana, L. Long-term effects of calorie restriction on serum sex-hormone concentrations in men. Aging Cell 2010, 9, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Razny, U.; Goralska, J.; Calder, P.C.; Gruca, A.; Childs, C.E.; Kapusta, M.; Slowinska-Solnica, K.; Dembinska-Kiec, A.; Solnica, B.; Malczewska-Malec, M. The Effect of Caloric Restriction with and without n-3 PUFA Supplementation on Bone Turnover Markers in Blood of Subjects with Abdominal Obesity: A Randomized Placebo-Controlled Trial. Nutrients 2021, 13, 3096. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-A.; Blache, C.A.; Bajana, S.; Hasan, N.; Kamal, M.; Morita, Y.; Gupta, V.; Tsolmon, B.; Suh, K.S.; Gorenstein, D.G.; et al. The effect of soluble E-selectin on tumor progression and metastasis. BMC Cancer 2016, 16, 331. [Google Scholar] [CrossRef]
- Cunha, G.M.; Guzman, G.; De Mello, L.L.C.; Trein, B.; Spina, L.; Bussade, I.; Prata, J.M.; Sajoux, I.; Countinho, W. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients With Obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef]
- Martens, C.R.; Denman, B.A.; Mazzo, M.R.; Armstrong, M.L.; Reisdorph, N.; McQueen, M.B.; Chonchol, M.; Seals, D.R. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 2018, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Corrigendum: Calorie restriction for long-term remission of type 2 diabetes. Clin. Med. 2019, 19, 192. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Cortinovis, M.; Trillini, M.; Parvanova, A.; Abbate, M.; Satriano, C.; Salvetti, F.; Bossi, A.C.; Trevisan, R.; Perna, A.; et al. Long-term kidney and systemic effects of calorie restriction in overweight or obese type 2 diabetic patients (C.Re.S.O. 2 randomized controlled trial). Diabetes Res. Clin. Pract. 2022, 185, 109804. [Google Scholar] [CrossRef]
- Tragni, E.; Vigna, L.; Ruscica, M.; Macchi, C.; Casula, M.; Santelia, A.; Catapano, A.L.; Magni, P. Reduction of Cardio-Metabolic Risk and Body Weight through a Multiphasic Very-Low Calorie Ketogenic Diet Program in Women with Overweight/Obesity: A Study in a Real-World Setting. Nutrients 2021, 13, 1804. [Google Scholar] [CrossRef]
- Oliveira, C.; Silveira, E.A.; Rosa, L.; Santos, A.; Rodrigues, A.P.; Mendonça, C.; Silva, L.; Gentil, P.; Rebelo, A.C. Risk Factors Associated with Cardiac Autonomic Modulation in Obese Individuals. J. Obes. 2020, 2020, 7185249. [Google Scholar] [CrossRef]
- Gonçalinho, G.H.F.; Roggerio, A.; Goes, M.F.d.S.; Avakian, S.D.; Leal, D.P.; Strunz, C.M.C.; Mansur, A.P. Comparison of Resveratrol Supplementation and Energy Restriction Effects on Sympathetic Nervous System Activity and Vascular Reactivity: A Randomized Clinical Trial. Molecules 2021, 26, 3168. [Google Scholar] [CrossRef] [PubMed]
- Heiston, E.M.; Gilbertson, N.M.; Eichner, N.Z.M.; Malin, S.K. A Low-Calorie Diet with or without Exercise Reduces Postprandial Aortic Waveform in Females with Obesity. Med. Sci. Sports Exerc. 2021, 53, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Ristic-Medic, D.; Kovacic, M.; Takic, M.; Arsic, A.; Petrovic, S.; Paunovic, M.; Jovicic, M.; Vucic, V. Calorie-Restricted Mediterranean and Low-Fat Diets Affect Fatty Acid Status in Individuals with Nonalcoholic Fatty Liver Disease. Nutrients 2020, 13, 15. [Google Scholar] [CrossRef]
- Dorling, J.L.; Ravussin, E.; Redman, L.M.; Bhapkar, M.; Huffman, K.M.; Racette, S.B.; Das, S.K.; Apolzan, J.W.; Kraus, W.E.; Höchsmann, C.; et al. Effect of 2 years of calorie restriction on liver biomarkers: Results from the CALERIE phase 2 randomized controlled trial. Eur. J. Nutr. 2021, 60, 1633–1643. [Google Scholar] [CrossRef]
- Bruci, A.; Tuccinardi, D.; Tozzi, R.; Balena, A.; Santucci, S.; Frontani, R.; Mariani, S.; Basciani, S.; Spera, G.; Gnessi, L.; et al. Very Low-Calorie Ketogenic Diet: A Safe and Effective Tool for Weight Loss in Patients with Obesity and Mild Kidney Failure. Nutrients 2020, 12, 333. [Google Scholar] [CrossRef]
- Lapik, I.A.; Gapparova, K.M.; Chehonina, Y. Efficiency estimation of diet therapy with protein component modification in patients with obesity and purine metabolism disorder. Vopr. Pitan. 2019, 88, 80–87. [Google Scholar] [PubMed]
- Sanllorente, A.; Soria-Florido, M.T.; Castañer, O.; Lassale, C.; Salas-Salvadó, J.; Martínez-González, M.Á.; Subirana, I.; Ros, E.; Corella, D.; Estruch, R.; et al. A lifestyle intervention with an energy-restricted Mediterranean diet and physical activity enhances HDL function: A substudy of the PREDIMED-Plus randomized controlled trial. Am. J. Clin. Nutr. 2021, 114, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Vizthum, D.; Henry-Barron, B.; Schweitzer, A.; Cassard, S.D.; Kossoff, E.; Hartman, A.L.; Kapogiannis, D.; Sullivan, P.; Baer, D.J.; et al. Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 23, 33–39. [Google Scholar] [CrossRef]
- Mukherjee, P.; Augur, Z.M.; Li, M.; Hill, C.; Greenwood, B.; Domin, M.A.; Kondakci, G.; Narain, N.R.; Kiebish, M.A.; Bronson, R.T.; et al. Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma. Commun. Biol. 2019, 2, 200. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh-Gohari, S.; Feichtinger, R.G.; Vidali, S.; Locker, F.; Rutherford, T.; O’donnel, M.; Stöger-Kleiber, A.; Mayr, J.A.; Sperl, W.; Kofler, B. A ketogenic diet supplemented with medium-chain triglycerides enhances the anti-tumor and anti-angiogenic efficacy of chemotherapy on neuroblastoma xenografts in a CD1-nu mouse model. Oncotarget 2017, 8, 64728–64744. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Rudderow, A.L.; Sossong, A.M.; Lewis, K.N.; Younts, C.; Pearson, K.J.; Bernier, M.; de Cabo, R. Perinatal diet influences health and survival in a mouse model of leukemia. Geroscience 2020, 42, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Morioka, T.; Yamazaki, S.; Yanagihara, H.; Sunaoshi, M.; Kaminishi, M.; Kakinuma, S. Calorie Restriction Suppresses the Progression of Radiation-Induced Intestinal Tumours in C3B6F1 ApcMin/+ Mice. Anticancer Res. 2021, 41, 1365–1375. [Google Scholar] [CrossRef]
- Bowers, L.W.; Doerstling, S.S.; Shamsunder, M.G.; Lineberger, C.G.; Rossi, E.L.; Montgomery, S.A.; Coleman, M.F.; Gong, W.; Parker, J.S.; Howell, A.; et al. Reversing the Genomic, Epigenetic, and Triple-Negative Breast Cancer–Enhancing Effects of Obesity. Cancer Prev. Res. 2022, 15, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Caiola, E.; Falcetta, F.; Giordano, S.; Marabese, M.; Garassino, M.C.; Broggini, M.; Pastorelli, R.; Brunelli, L. Co-occurring KRAS mutation/LKB1 loss in non-small cell lung cancer cells results in enhanced metabolic activity susceptible to caloric restriction: An in vitro integrated multilevel approach. J. Exp. Clin. Cancer Res. 2018, 37, 302. [Google Scholar] [CrossRef]
- Kim, S.W.; Cha, M.-J.; Lee, S.-K.; Song, B.-W.; Jin, X.; Lee, J.M.; Park, J.H.; Lee, J.D. Curcumin Treatment in Combination with Glucose Restriction Inhibits Intracellular Alkalinization and Tumor Growth in Hepatoma Cells. Int. J. Mol. Sci. 2019, 20, 2375. [Google Scholar] [CrossRef] [PubMed]
- Castejón, M.; Plaza, A.; Martinez-Romero, J.; Fernandez-Marcos, P.J.; Cabo, R.; Diaz-Ruiz, A. Energy Restriction and Colorectal Cancer: A Call for Additional Research. Nutrients 2020, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.; Wenger, K.J.; von Mettenheim, N.; Bojunga, J.; Vetter, M.; Diehl, B.; Franz, K.; Gerlach, R.; Ronellenfitsch, M.W.; Harter, P.; et al. Short-term fasting in glioma patients: Analysis of diet diaries and metabolic parameters of the ERGO2 trial. Eur. J. Nutr. 2022, 61, 477–487. [Google Scholar] [CrossRef]
- Tang, C.-C.; Huang, T.-C.; Tien, F.-M.; Lin, J.-M.; Yeh, Y.-C.; Lee, C.-Y. Safety, Feasibility, and Effects of Short-Term Calorie Reduction during Induction Chemotherapy in Patients with Diffuse Large B-Cell Lymphoma: A Pilot Study. Nutrients 2021, 13, 3268. [Google Scholar] [CrossRef]
- Kirkham, A.A.; King, K.; Joy, A.A.; Pelletier, A.B.; Mackey, J.R.; Young, K.; Zhu, X.; Meza-Junco, J.; Basi, S.K.; Hiller, J.P.; et al. Rationale and design of the Diet Restriction and Exercise-induced Adaptations in Metastatic breast cancer (DREAM) study: A 2-arm, parallel-group, phase II, randomized control trial of a short-term, calorie-restricted, and ketogenic diet plus exercise during intravenous chemotherapy versus usual care. BMC Cancer 2021, 21, 1093. [Google Scholar] [CrossRef]
- Kirkham, A.A.; Paterson, D.I.; Prado, C.M.; Mackey, J.R.; Courneya, K.S.; Pituskin, E.; Thompson, R.B. Rationale and design of the Caloric Restriction and Exercise protection from Anthracycline Toxic Effects (CREATE) study: A 3-arm parallel group phase II randomized controlled trial in early breast cancer. BMC Cancer 2018, 18, 864. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, D.; Zaramella, A.; Fabris, F.; Sánchez-Rodríguez, R.; Nucci, D.; Fassan, M.; Nardi, M.; Benna, C.; Cristofori, C.; Morbin, T.; et al. Insulin/IGF-1 Signaling Is Downregulated in Barrett’s Esophagus Patients Undergoing a Moderate Calorie and Protein Restriction Program: A Randomized 2-Year Trial. Nutrients 2021, 13, 3638. [Google Scholar] [CrossRef]
- De Man, F.M.; van Eerden, R.A.; van Doorn, G.M.; Hoop, E.; Koolen, S.L.W.; Olieman, J.F.; de Bruijn, P.; Veraart, J.N.; van Halteren, H.K.; Sandberg, Y.; et al. Effects of Protein and Calorie Restriction on the Metabolism and Toxicity Profile of Irinotecan in Cancer Patients. Clin. Pharmacol. Ther. 2021, 109, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Le Noci, V.; Sommariva, M.; Bianchi, F.; Triulzi, T.; Tagliabue, E.; Balsari, A.; Sfondrini, L. Local Administration of Caloric Restriction Mimetics to Promote the Immune Control of Lung Metastases. J. Immunol. Res. 2019, 2019, 2015892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, S.; Tan, Q.; Cheng, D.; Dai, Q.; Yang, Z.; Zhang, L.; Li, F.; Zuo, Y.; Dai, W.; et al. Combination therapy with ropivacaine-loaded liposomes and nutrient deprivation for simultaneous cancer therapy and cancer pain relief. Theranostics 2020, 10, 4885–4899. [Google Scholar] [CrossRef]
- Heintz, C.; Doktor, T.K.; Lanjuin, A.; Escoubas, C.; Zhang, Y.; Weir, H.J.; Dutta, S.; Silva-García, C.G.; Bruun, G.H.; Morantte, I.; et al. Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans. Nature 2017, 541, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.; Tung, N.; Casanova-Acebes, M.; Chang, C.; Cantoni, C.; Zhang, D.; Wirtz, T.H.; Naik, S.; Rose, S.A.; Brocker, C.N.; et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell 2019, 178, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.C.; Westermark, A.M.; Zhang, Y.; Yuan, C.; Li, Z.; Lau, A.N.; Sapp, K.M.; Wolpin, B.M.; Heiden, M.G.V. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature 2021, 599, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Martin, C.K.; Newton, R.L.; Apolzan, J.W.; Arnold, C.L.; Davis, T.C.; Price-Haywood, E.G.; Denstel, K.D.; Mire, E.F.; Thethi, T.K.; et al. Weight Loss in Underserved Patients—A Cluster-Randomized Trial. N. Engl. J. Med. 2020, 383, 909–918. [Google Scholar] [CrossRef]
- Di Malta, C.; Siciliano, D.; Calcagni, A.; Monfregola, J.; Punzi, S.; Pastore, N.; Eastes, A.N.; Davis, O.; De Cegli, R.; Zampelli, A.; et al. Transcriptional activation of RagD GTPase controls mTORC1 and promotes cancer growth. Science 2017, 356, 1188–1192. [Google Scholar] [CrossRef]
- Vodnala, S.K.; Eil, R.; Kishton, R.J.; Sukumar, M.; Yamamoto, T.N.; Ha, N.-H.; Lee, P.-H.; Shin, M.; Patel, S.J.; Yu, Z.; et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 2019, 363, eaau0135. [Google Scholar] [CrossRef] [PubMed]
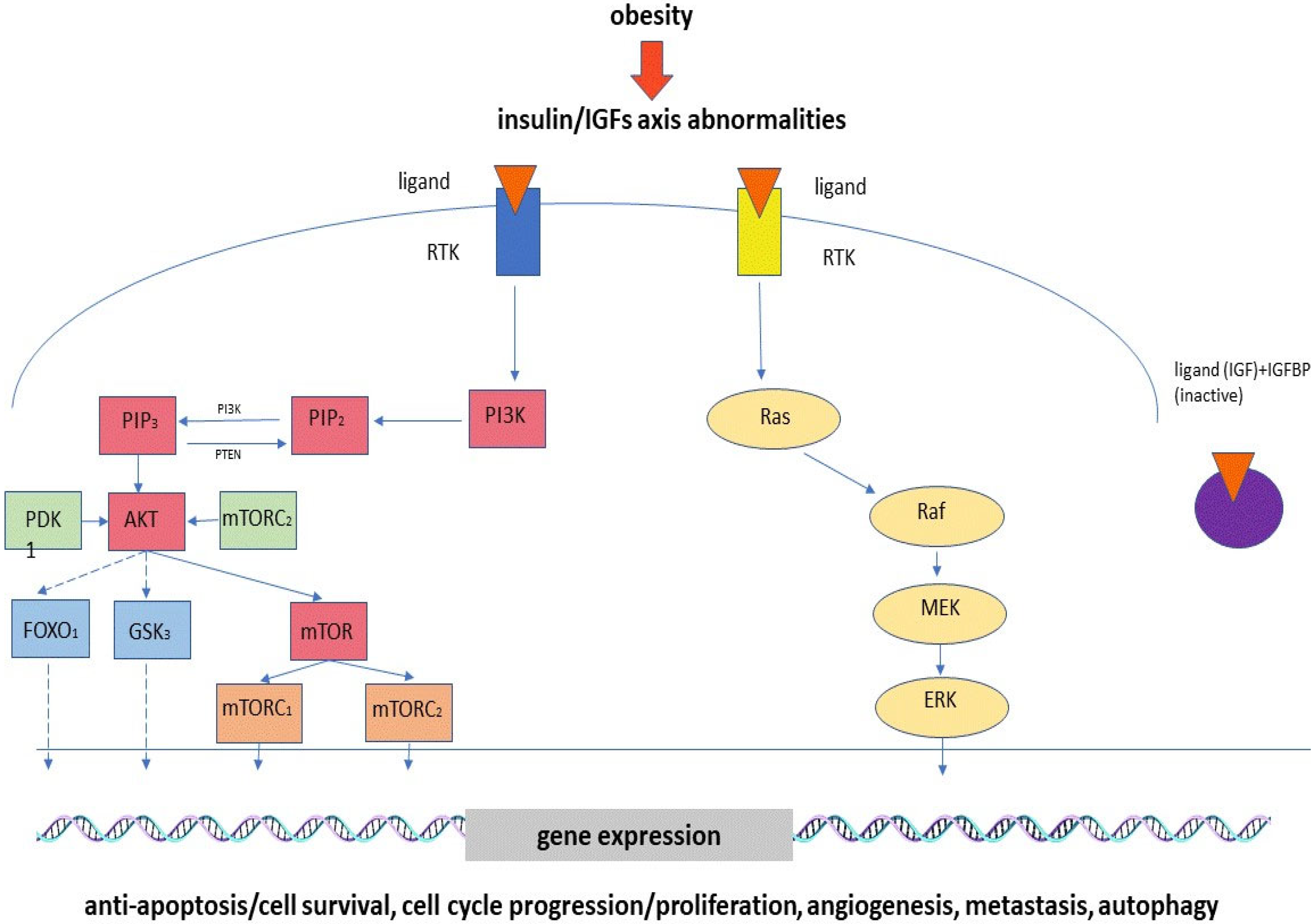
| Types of Cancer | Signaling Pathway | Genes | Up-Regulation or Down-Regulation | Effects | References |
|---|---|---|---|---|---|
| Esophageal cancer | IGF/PI3K/AKT | IGFBPL1 | Down-regulation | Inhibition of esophageal cancer cell clonal formation and proliferation; induction of cell apoptosis and G1/S phase arrest | Liu 2020 [28] |
| Hepatocellular carcinoma | IGF/PI3K/AKT; | IGF1 | Up-regulation | Stimulation of DNA synthesis and cyclin D1 expression, inhibition of proteasome-mediated cathepsin B (CTSB) degradation in hepatocellular carcinoma cells; | Adamek 2018 [30] |
| IGF2 | Up-regulation | Stimulation of neoangiogenesis in hepatocellular carcinoma; | |||
| Adrenocortical adenoma | IGF/JAK-STAT | IGF1 | Up-regulation | Stimulation of adrenocortical adenoma cell growth, differentiation, proliferation and survival | Lazúrová 2020 [36] |
| IGF2 | Up-regulation | Stimulation of adrenocortical adenoma cell growth, differentiation, proliferation and survival | |||
| Colorectal cancer | IGF-1/IGFBP | VEGF | Up-regulation | Stimulation of neoangiogenesis in colorectal cancer | Ciulei 2020 [35] |
| Colon cancer | IGF/PI3K/AKT/mTOR/MAPK | IGFs | Up-regulation | Stimulation of colon cancer cell growth, differentiation, proliferation and survival | Giovannucci 2001 [37] |
| Melanoma | IGF/AKT | IGFs | Up-regulation | Stimulation of autophagy in melanoma cells | Wang 2018 [40] |
| Breast cancer | IL-6/JAK/STAT3 | IL-6 | Up-regulation | Stimulation of breast cancer cell proliferation and invasiveness, suppressing apoptosis | Manore 2022 [60] |
| HIF1α | HIF1α | Up-regulation | Stimulation of breast cancer cell proliferation | Brown 2021 [70] | |
| p53 | p53 | Down-regulation | Blockage of breast cancer cell apoptosis | Brown 2021 [70] | |
| AMPK | AMPK | Up-regulation | Stimulation of breast cancer cell proliferation | Wang 2017 [25] | |
| Endometrium cancer | ER/IGF | IGF1 | Up-regulation | Stimulation of endometrium cell proliferation and inhibition of apoptosis | Shaw 2016 [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergeeva, E.; Ruksha, T.; Fefelova, Y. Effects of Obesity and Calorie Restriction on Cancer Development. Int. J. Mol. Sci. 2023, 24, 9601. https://doi.org/10.3390/ijms24119601
Sergeeva E, Ruksha T, Fefelova Y. Effects of Obesity and Calorie Restriction on Cancer Development. International Journal of Molecular Sciences. 2023; 24(11):9601. https://doi.org/10.3390/ijms24119601
Chicago/Turabian StyleSergeeva, Ekaterina, Tatiana Ruksha, and Yulia Fefelova. 2023. "Effects of Obesity and Calorie Restriction on Cancer Development" International Journal of Molecular Sciences 24, no. 11: 9601. https://doi.org/10.3390/ijms24119601
APA StyleSergeeva, E., Ruksha, T., & Fefelova, Y. (2023). Effects of Obesity and Calorie Restriction on Cancer Development. International Journal of Molecular Sciences, 24(11), 9601. https://doi.org/10.3390/ijms24119601






