Evaluation of the Antiviral Efficacy of Subcutaneous Nafamostat Formulated with Glycyrrhizic Acid against SARS-CoV-2 in a Murine Model
Abstract
1. Introduction
2. Results
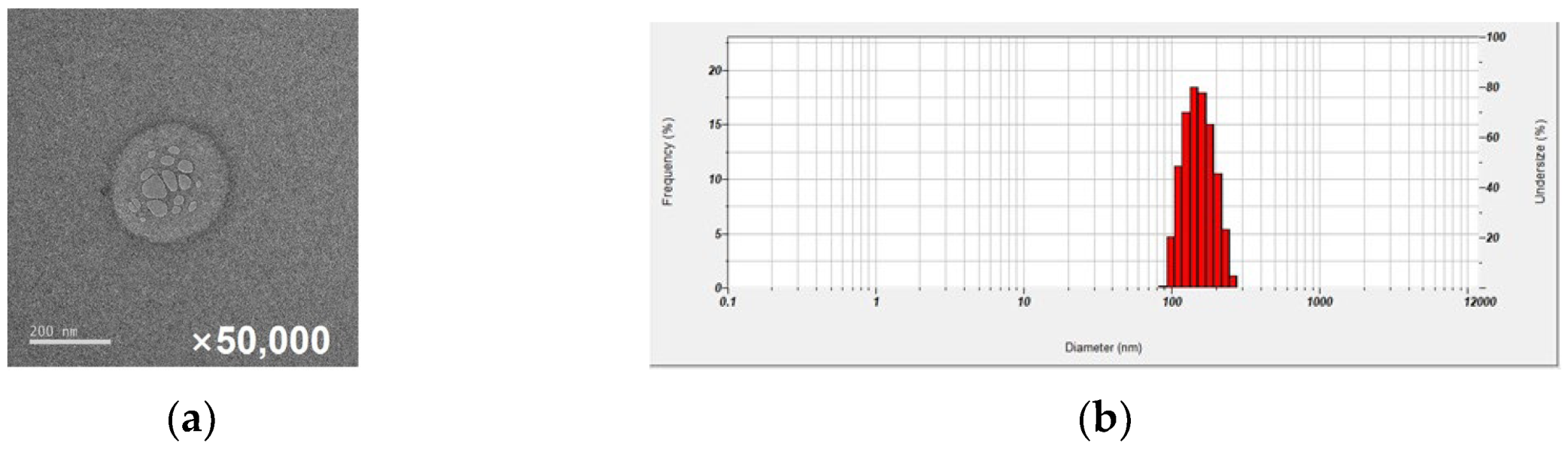
2.1. Nafamostat Formulation with Glycyrrhizic Acid: Characterization of NM-Loaded Micelle NPs
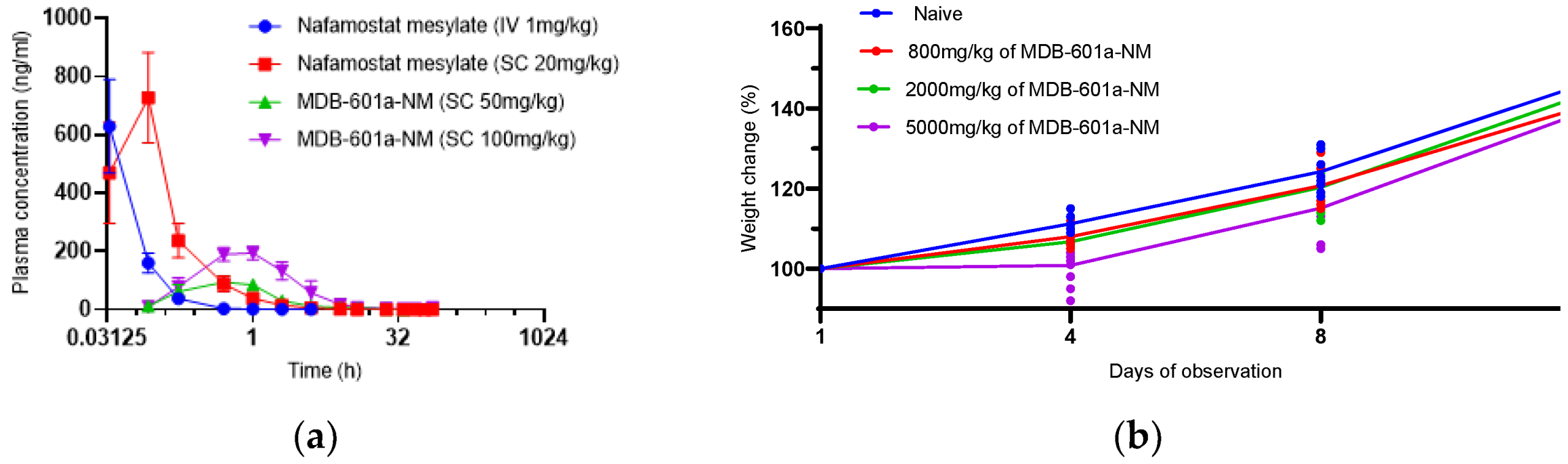
2.2. Pharmacokinetics and Single-Dose Toxicity Test of MDB-601a-NM in Sprague-Dawley Rats
2.3. Evaluation of Single-Dose Toxicity of MDB-601a-NM in Sprague-Dawley Rats
2.4. In Vivo Protective Efficacy of MDB-601a-NM against SARS-CoV-2 Infection
2.5. Assessment of MDB-601a-NM Therapy in Inhibiting Viral Replication in SARS-CoV-2 Infected Tissue
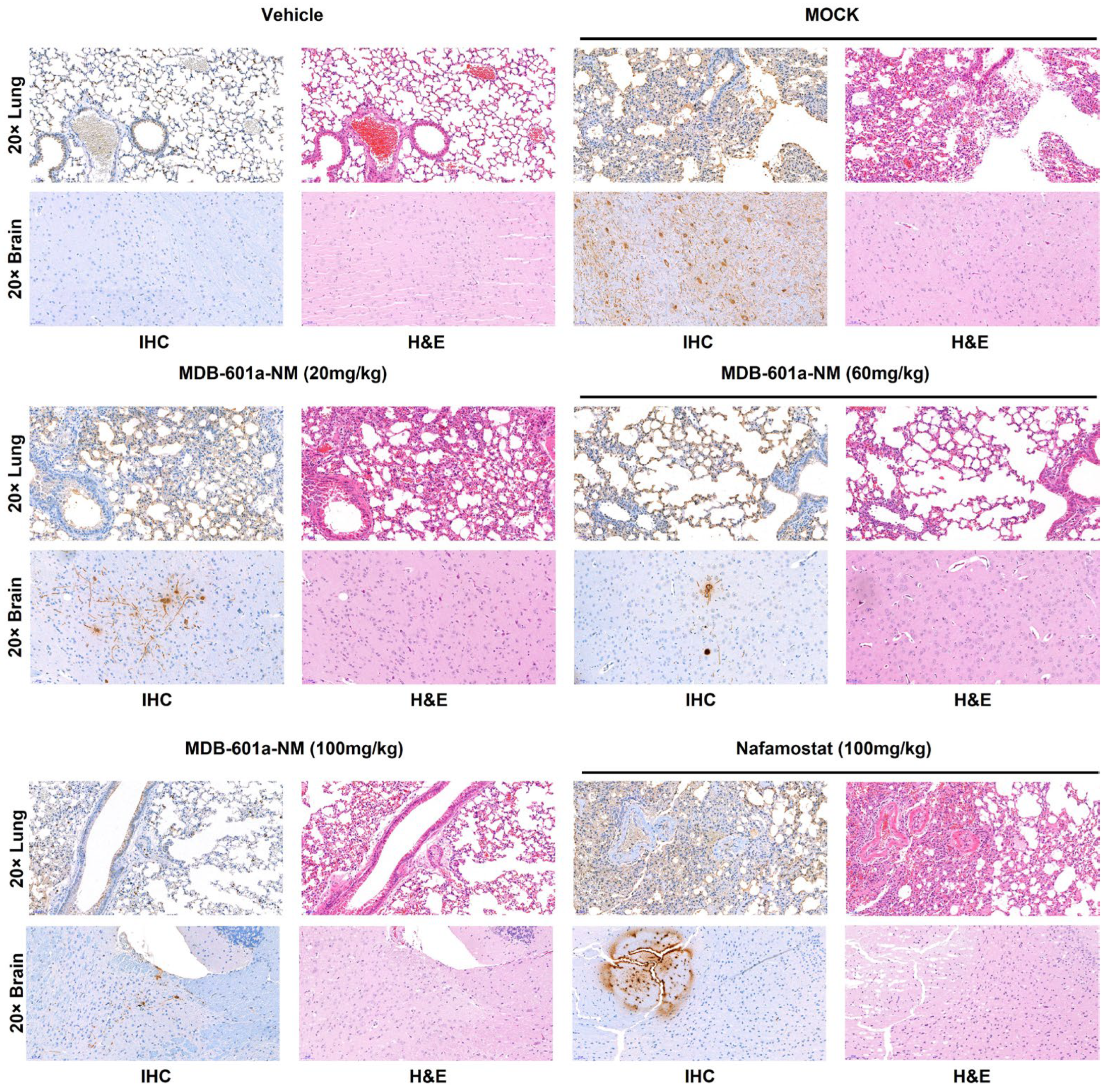
2.6. Comparison of Histopathological Features in Mice Treated with MDB-601a-NM Therapy and Infected with SARS-CoV-2
3. Discussion
4. Materials and Methods
4.1. Preparation of Nafamostat-Loaded Micelles
4.2. Characterization of Nafamostat-Loaded Micelles
4.3. Cell Culture and Virus
4.4. Pharmacokinetics and Single-Dose Toxicity of MDB-601a-NM
4.5. In Vivo Efficacy Evaluation in K18-ACE2 Transgenic Mice
4.6. Viral Titration
4.7. Histology and Immunohistochemistry
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV). 2020. Available online: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) (accessed on 30 January 2020).
- Jangra, S.; Ye, C.; Rathnasinghe, R.; Stadlbauer, D.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Chernet, R.L. SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe 2021, 2, e283–e284. [Google Scholar] [PubMed]
- Tada, T.; Zhou, H.; Dcosta, B.M.; Samanovic, M.I.; Chivukula, V.; Herati, R.S.; Hubbard, S.R.; Mulligan, M.J.; Landau, N.R. Increased resistance of SARS-CoV-2 Omicron variant to neutralization by vaccine-elicited and therapeutic antibodies. EBioMedicine 2022, 78, 103944. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [PubMed]
- Chen, X.; Xu, Z.; Zeng, S.; Wang, X.; Liu, W.; Qian, L.; Wei, J.; Yang, X.; Shen, Q.; Gong, Z.; et al. The Molecular Aspect of Antitumor Effects of Protease Inhibitor Nafamostat Mesylate and Its Role in Potential Clinical Applications. Front. Oncol. 2019, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Kang, Y.-J.; Jang, H.M.; Jung, H.-Y.; Cho, J.-H.; Park, S.-H.; Kim, Y.-L.; Kim, C.-D. Nafamostat mesilate as an anticoagulant during continuous renal replacement therapy in patients with high bleeding risk: A randomized clinical trial. Medicine 2015, 94, e2392. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-G.; Zhang, M.; Yu, D.; Shao, J.-P.; Chen, Y.-C.; Liu, X.-Q. A method for quantifying the unstable and highly polar drug nafamostat mesilate in human plasma with optimized solid-phase extraction and ESI-MS detection: More accurate evaluation for pharmacokinetic study. Anal. Bioanal. Chem. 2008, 391, 1063–1071. [Google Scholar] [PubMed]
- Kim, C.-H. Anti–SARS-CoV-2 natural products as potentially therapeutic agents. Front. Pharmacol. 2021, 12, 590509. [Google Scholar] [CrossRef] [PubMed]
- Hoever, G.; Baltina, L.; Michaelis, M.; Kondratenko, R.; Baltina, L.; Tolstikov, G.A.; Doerr, H.W.; Cinatl, J., Jr. Antiviral Activity of Glycyrrhizic Acid Derivatives against SARS−Coronavirus. J. Med. Chem. 2005, 48, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Huang, X.; Lai, Y.; Liu, X.; Jiang, Y.; Zhan, S. Glycyrrhizic acid for COVID-19: Findings of targeting pivotal inflammatory pathways triggered by SARS-CoV-2. Front. Pharmacol. 2021, 12, 631206. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.; Han, H.-S.; Jeong, J.; Park, E.-M.; Shim, K.-S. A Novel Computational Approach for the Discovery of Drug Delivery System Candidates for COVID-19. Int. J. Mol. Sci. 2021, 22, 2815. [Google Scholar] [PubMed]
- Ngo, D.L.; Yamamoto, N.; Tran, V.A.; Nguyen, N.G.; Phan, D.; Lumbanraja, F.R.; Kubo, M.; Satou, K. Application of word embedding to drug repositioning. J. Biomed. Sci. Eng. 2016, 9, 7–16. [Google Scholar] [CrossRef]
- Maibaum, L.; Dinner, A.R.; Chandler, D. Micelle formation and the hydrophobic effect. J. Phys. Chem. B 2004, 108, 6778–6781. [Google Scholar] [CrossRef]

| Gender | Dose (mg/kg) | No. of Animals | Clinical Sign | Days of Observation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||||
| Male | Naïve | 5 | NOA a | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 800 | 5 | NOA | 5 | 5 | 5 | 4 | 1 | 2 | 2 | 4 | 4 | ||||||
| Swelling b | 1 | 5 | 5 | 5 | 5 | 5 | 4 | 3 | 3 | 1 | 1 | ||||||
| 2000 | 5 | NOA | 5 | 5 | 2 | ||||||||||||
| Swelling | 3 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |||||
| 5000 | 5 | NOA | 5 | 5 | |||||||||||||
| Swelling | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |||||
| Female | Naïve | 5 | NOA | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 800 | 5 | NOA | 5 | 5 | 5 | 3 | 1 | 1 | 2 | 2 | |||||||
| Swelling | 2 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 3 | 3 | ||||||
| 2000 | 5 | NOA | 5 | 5 | 3 | ||||||||||||
| Swelling | 2 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |||||
| 5000 | 5 | NOA | 5 | 5 | 1 | ||||||||||||
| Swelling | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.H.; Lee, W.H.; Min, S.C.; Kim, B.K.; Park, O.B.; Chokkakula, S.; Ahn, S.J.; Oh, S.; Park, J.-H.; Jung, J.W.; et al. Evaluation of the Antiviral Efficacy of Subcutaneous Nafamostat Formulated with Glycyrrhizic Acid against SARS-CoV-2 in a Murine Model. Int. J. Mol. Sci. 2023, 24, 9579. https://doi.org/10.3390/ijms24119579
Jeong JH, Lee WH, Min SC, Kim BK, Park OB, Chokkakula S, Ahn SJ, Oh S, Park J-H, Jung JW, et al. Evaluation of the Antiviral Efficacy of Subcutaneous Nafamostat Formulated with Glycyrrhizic Acid against SARS-CoV-2 in a Murine Model. International Journal of Molecular Sciences. 2023; 24(11):9579. https://doi.org/10.3390/ijms24119579
Chicago/Turabian StyleJeong, Ju Hwan, Woong Hee Lee, Seong Cheol Min, Beom Kyu Kim, On Bi Park, Santosh Chokkakula, Seong Ju Ahn, Sol Oh, Ji-Hyun Park, Ji Won Jung, and et al. 2023. "Evaluation of the Antiviral Efficacy of Subcutaneous Nafamostat Formulated with Glycyrrhizic Acid against SARS-CoV-2 in a Murine Model" International Journal of Molecular Sciences 24, no. 11: 9579. https://doi.org/10.3390/ijms24119579
APA StyleJeong, J. H., Lee, W. H., Min, S. C., Kim, B. K., Park, O. B., Chokkakula, S., Ahn, S. J., Oh, S., Park, J.-H., Jung, J. W., Jung, J. M., Kim, E.-G., & Song, M.-S. (2023). Evaluation of the Antiviral Efficacy of Subcutaneous Nafamostat Formulated with Glycyrrhizic Acid against SARS-CoV-2 in a Murine Model. International Journal of Molecular Sciences, 24(11), 9579. https://doi.org/10.3390/ijms24119579






