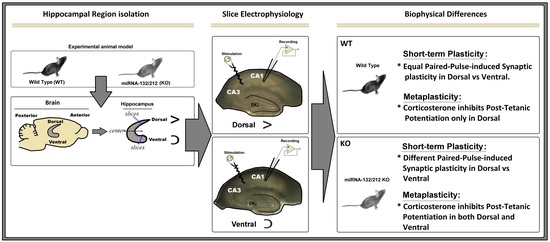
miRNA-132/212 Deficiency Disrupts Selective Corticosterone Modulation of Dorsal vs. Ventral Hippocampal Metaplasticity
Abstract
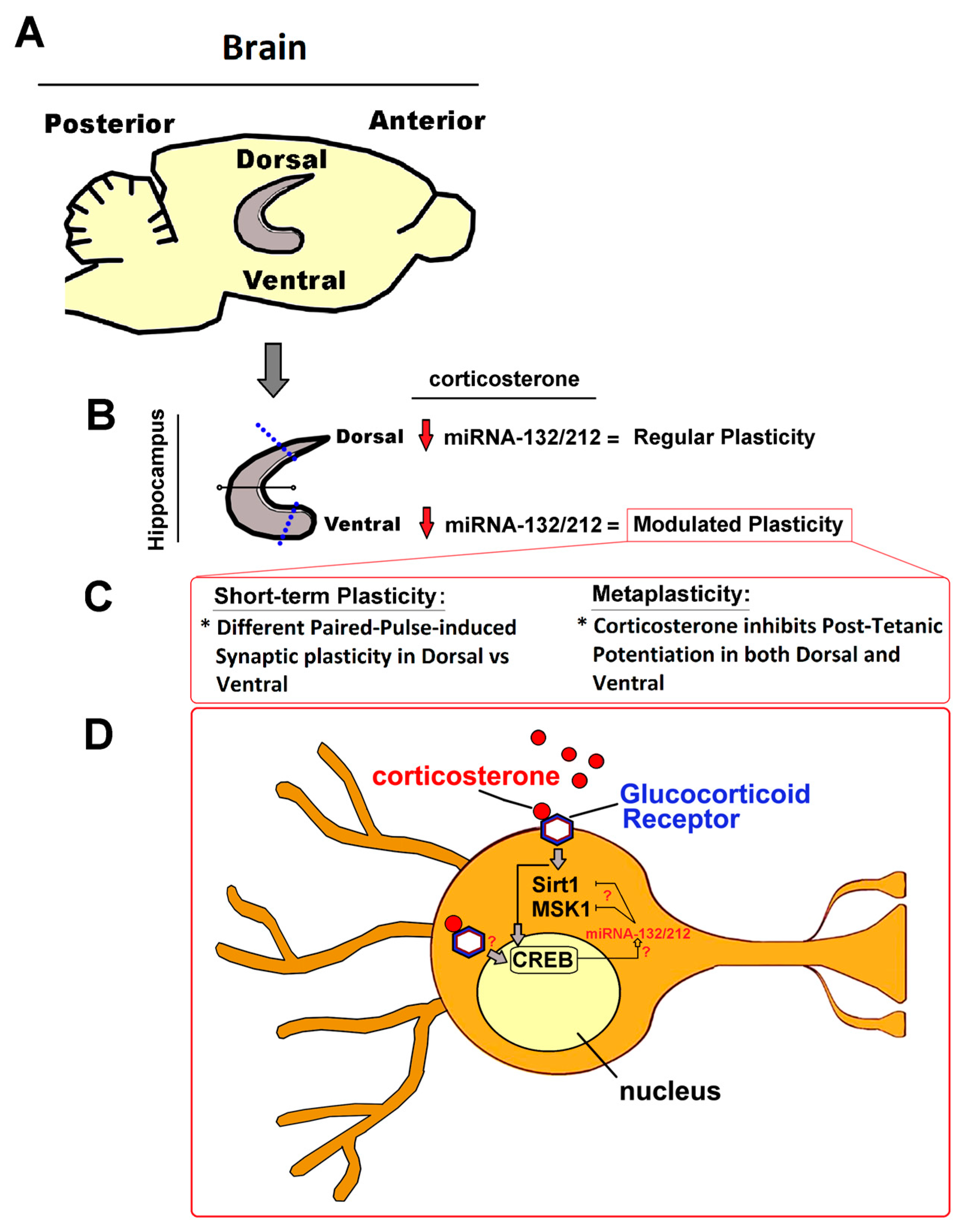
1. Introduction
2. Results
2.1. Comparable Basal Synaptic Transmission in Dorsal and Ventral Hippocampi of WT and miR–132/212−/− Mice
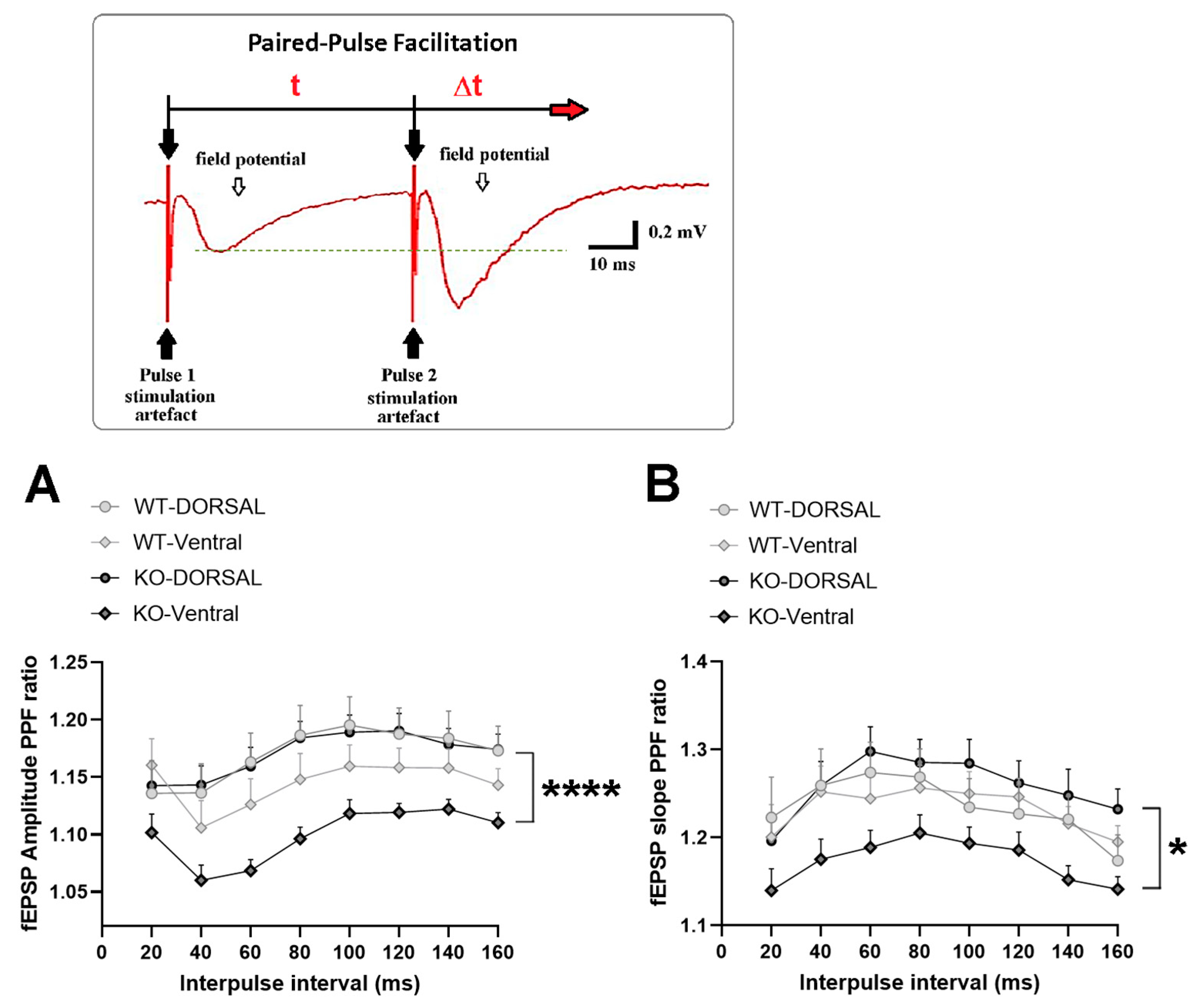
2.2. miRNA-132/212 Gene-Depletion Differentially Affects Short-Term Plasticity in Ventral and Dorsal Hippocampus
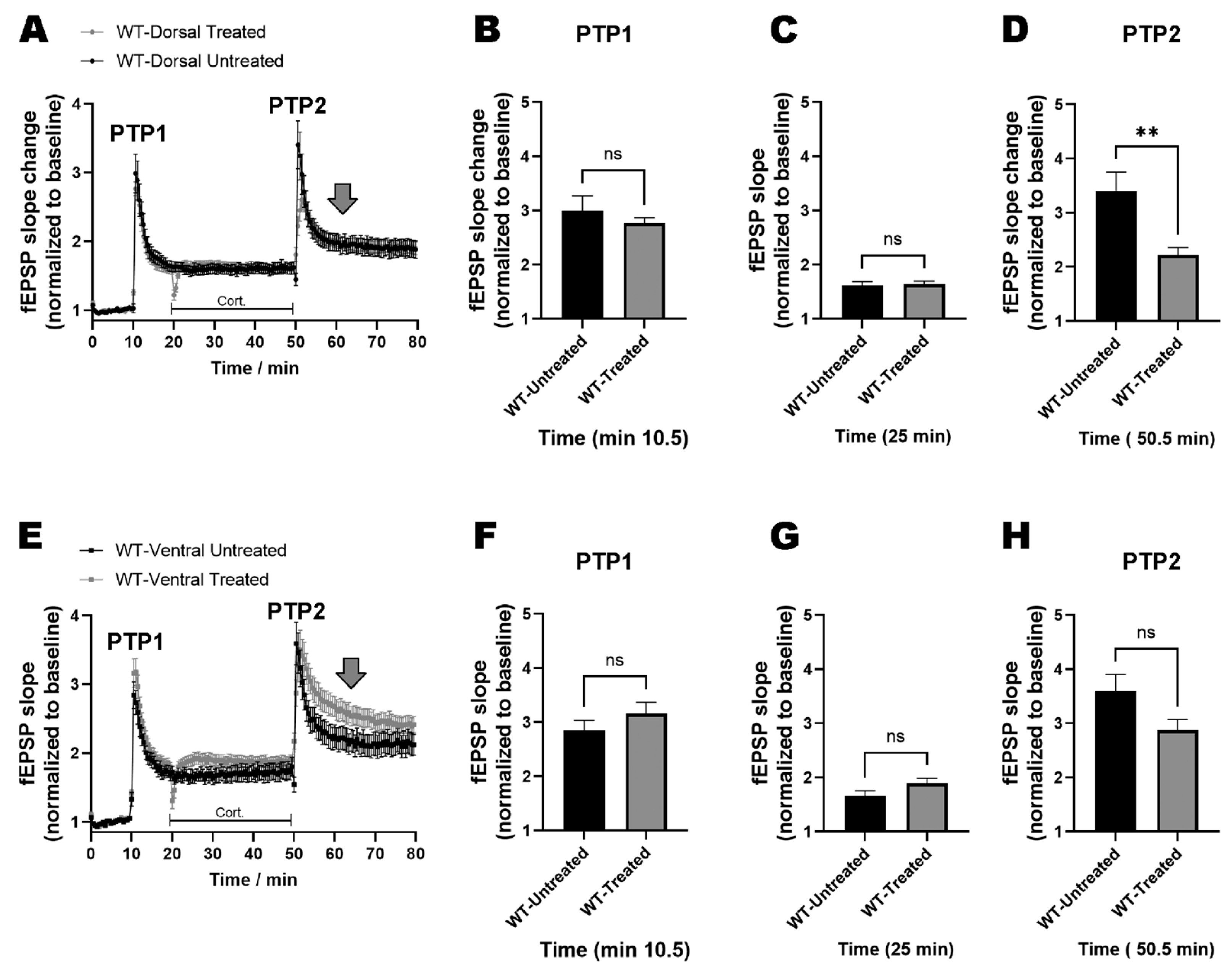
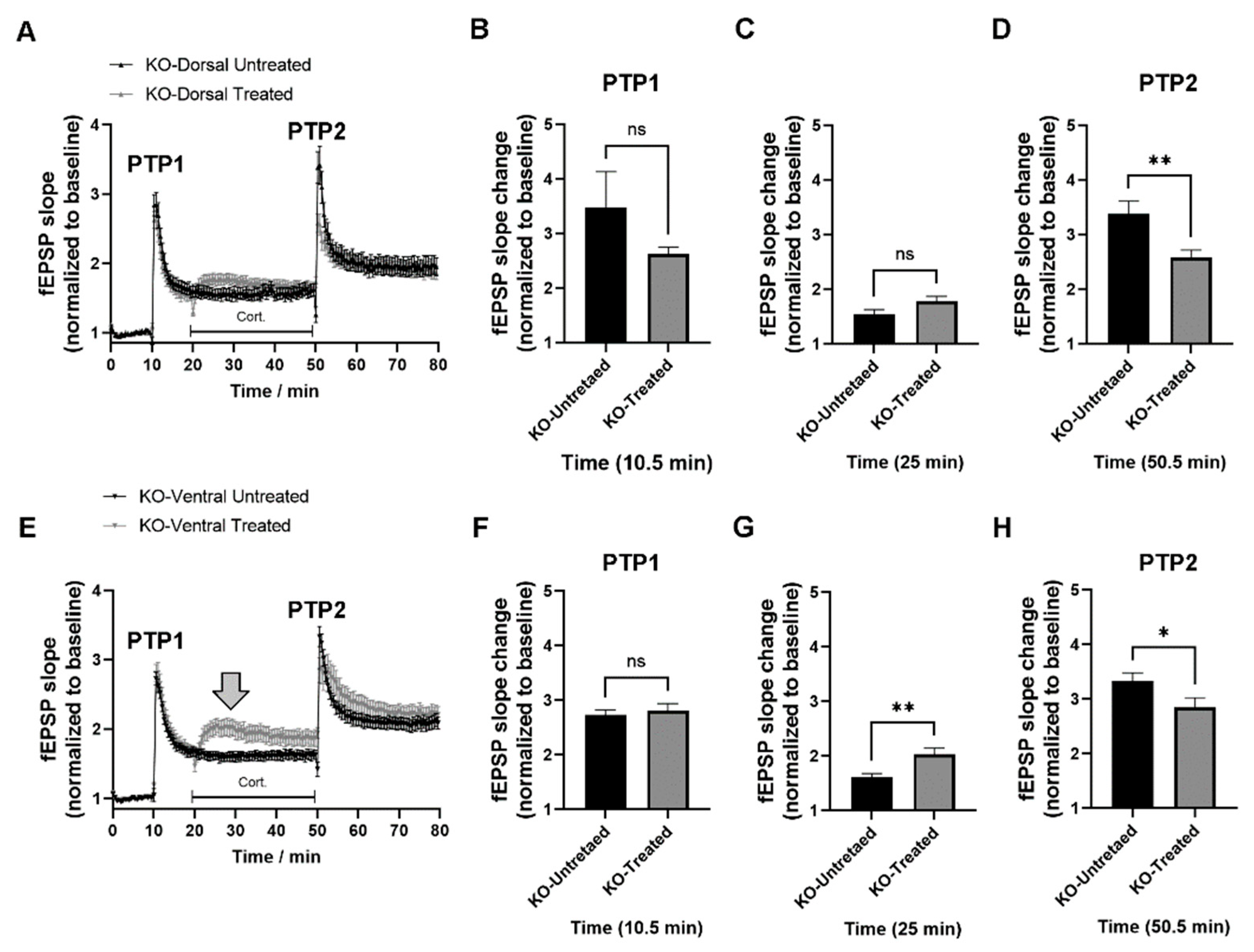
2.3. miRNAs-132/212 Regulate the Region-Specific Effects of Corticosterone on Metaplasticity in the Mouse Hippocampus
2.4. miRNA–132/212 Gene Deletion Disrupts the Region-Specific Effect of Corticosterone on Hippocampal Metaplasticity
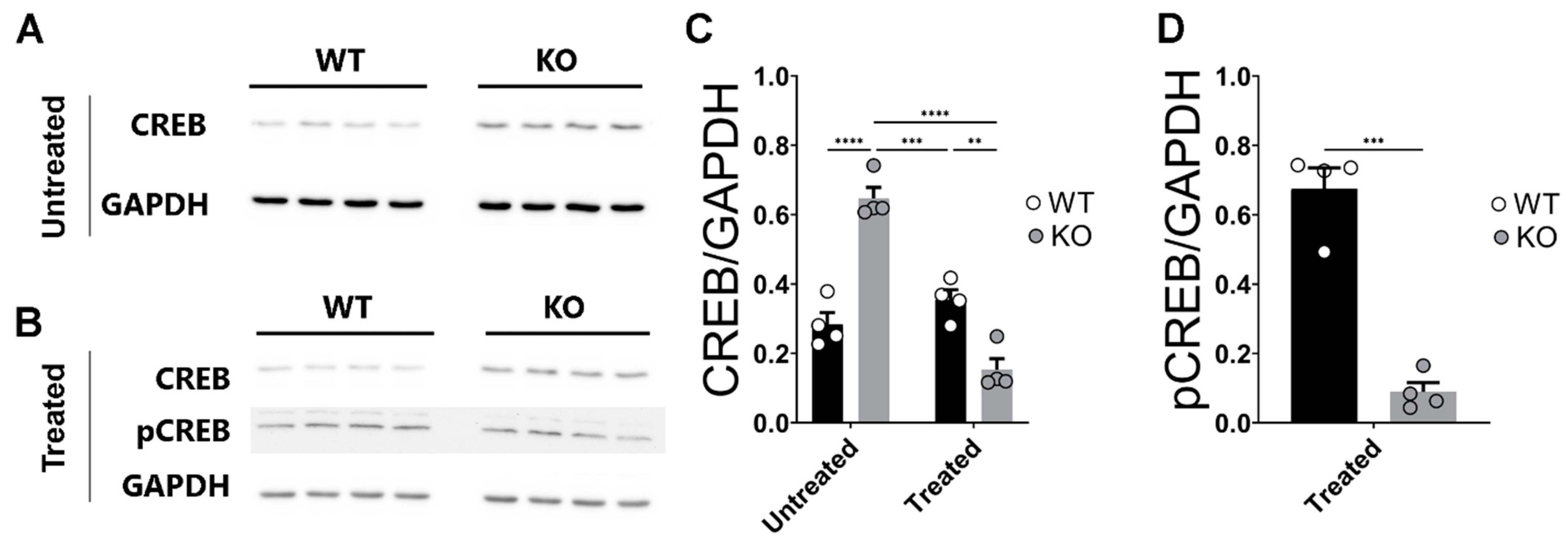
2.5. Deletion of miR–132/212−/− Prevents Enhanced Expression of CREB in the Hippocampi of Corticosterone Treated Slices
2.6. Sirt1 Protein Levels Are Enhanced and Insensitive to Corticosterone Stimulation in the miR–132/212−/− Mice Hippocampi
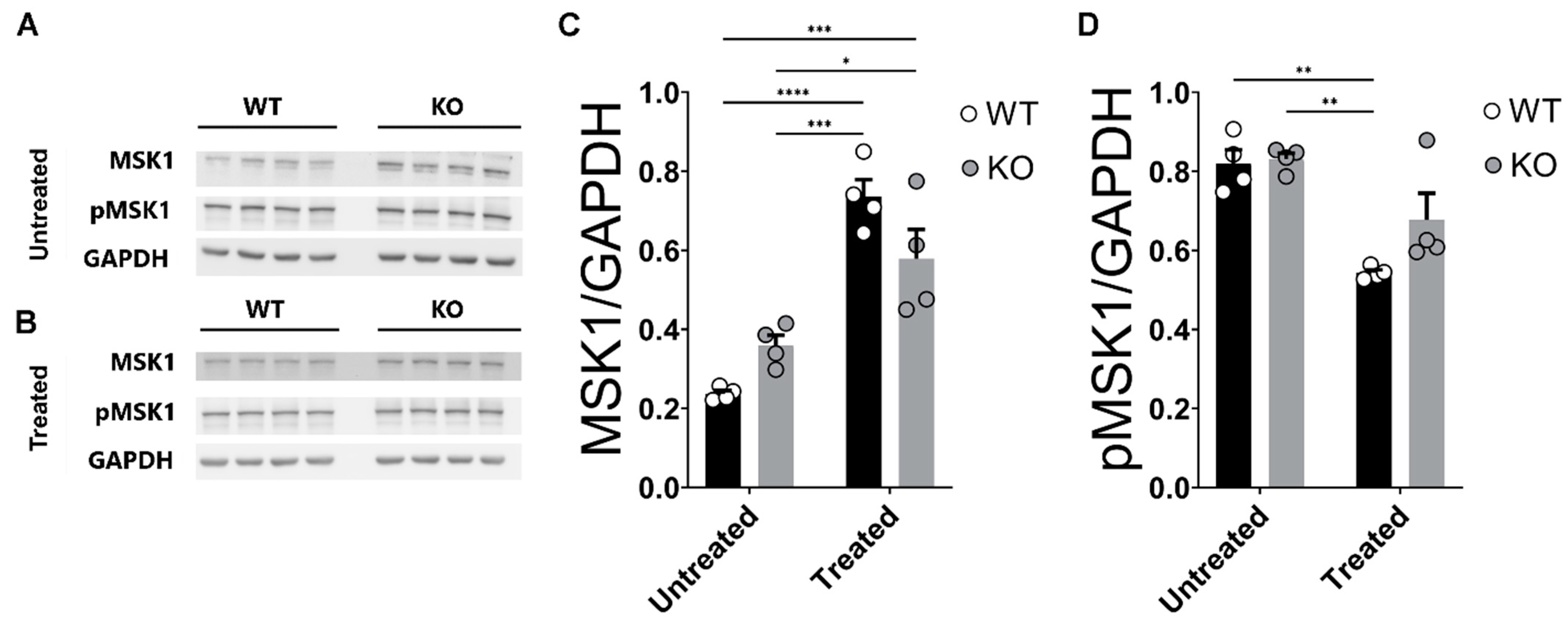
2.7. Corticosterone Effects on MSK1 Levels in WT and miR–132/212−/− Mice Hippocampi
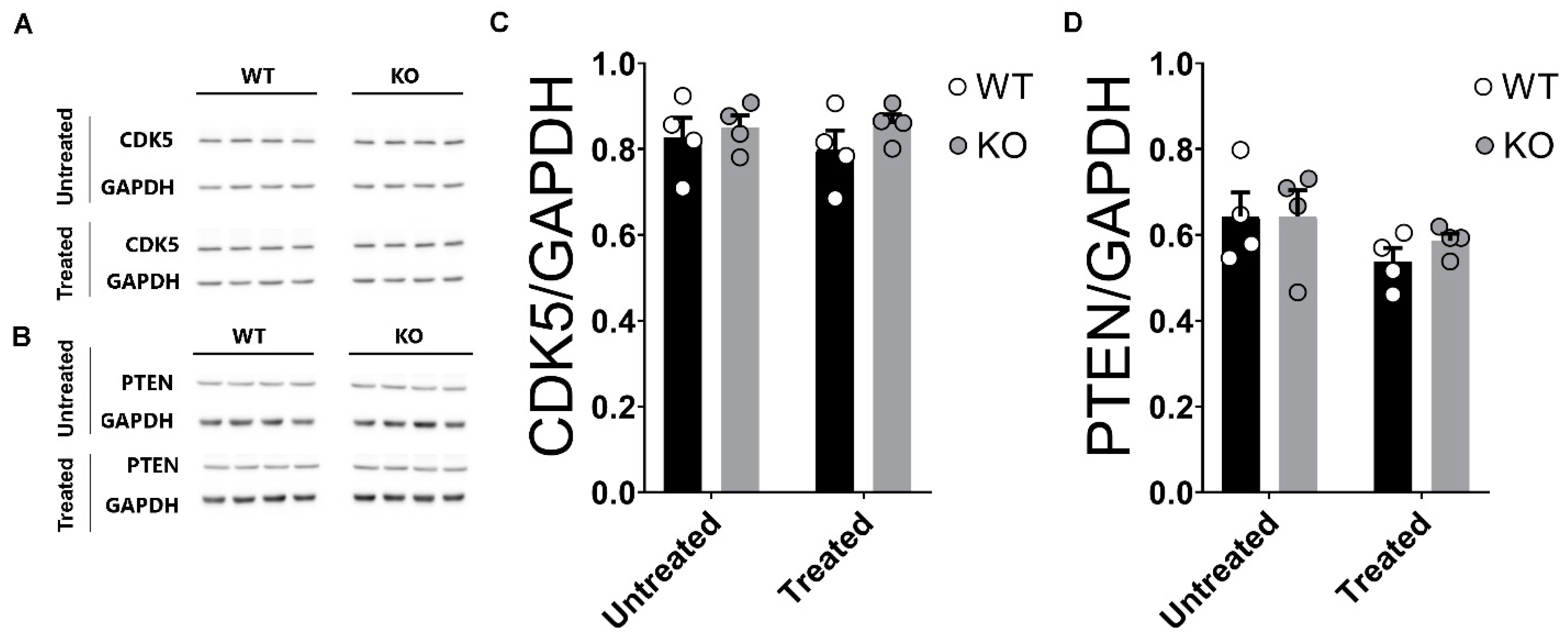
2.8. Unaltered Levels of CDK5 and PTEN in the Hippocampi of miRNA-132/212−/− Mice
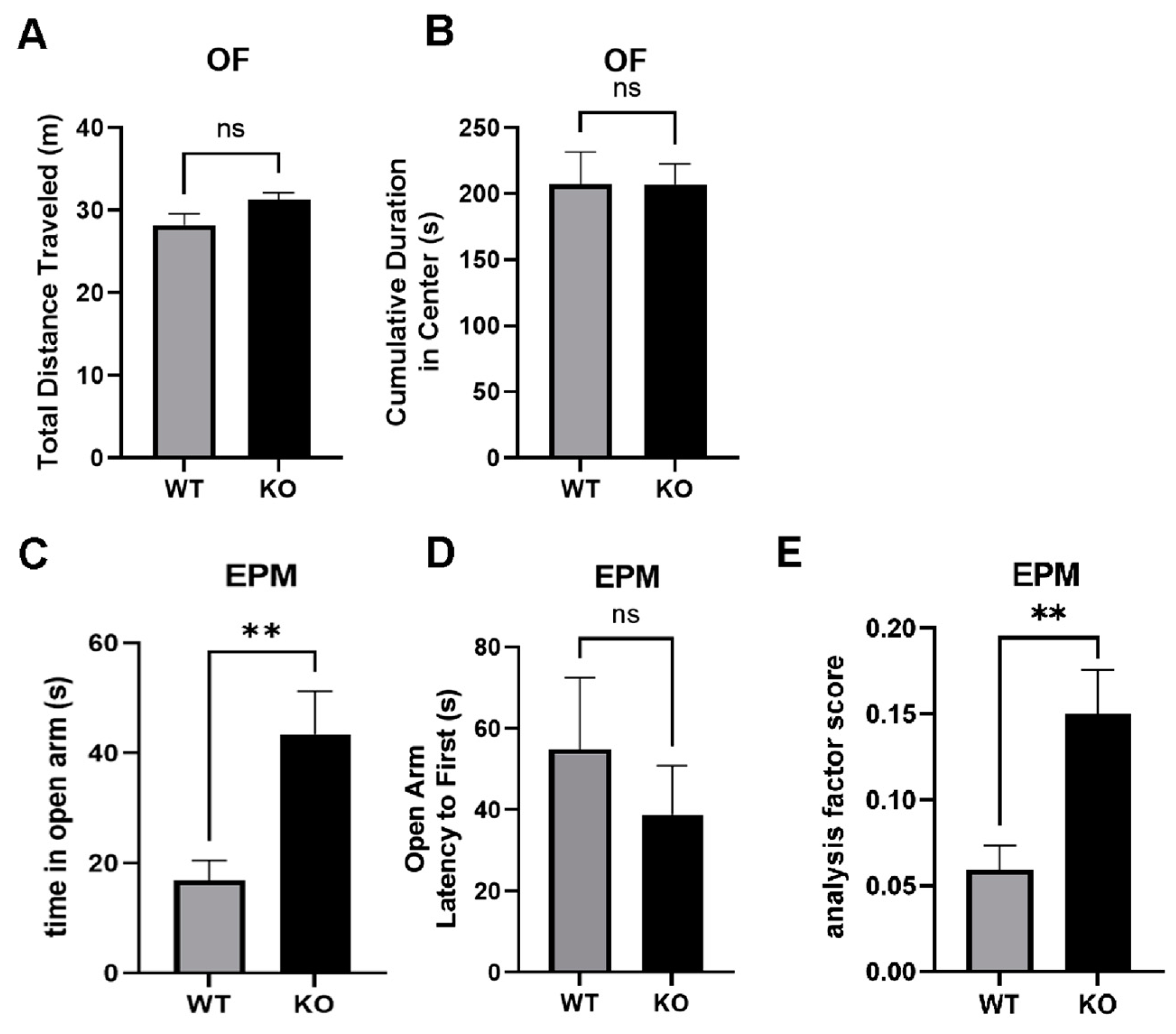
2.9. Reduced Anxiety-Like Behavior in miRNA-132/212−/− Mice
3. Discussion
3.1. miRNAs 132/212 in the Brain Neuronal Function
3.2. Proteins Associated with Mood and Steroid Hormone Signaling
4. Materials and Methods
4.1. Animals
4.2. Animal Grouping and Work Plan
4.3. Slice Electrophysiology
4.4. Western Blotting
4.5. Behavioral Testing
4.6. Open Field Test
4.7. Elevated Plus Maze
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Thau, L.; Gandhi, J.; Sharma, S. Physiology, Cortisol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Payne, J.D.; Jackson, E.D.; Hoscheidt, S.; Ryan, L.; Jacobs, W.J.; Nadel, L. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learn. Mem. (Cold Spring Harb. N. Y.) 2007, 14, 861–868. [Google Scholar] [CrossRef]
- Roozendaal, B.; Okuda, S.; Van der Zee, E.A.; McGaugh, J.L. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci. USA 2006, 103, 6741–6746. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; Maheu, F.; Tu, M.; Fiocco, A.; Schramek, T.E. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007, 65, 209–237. [Google Scholar] [CrossRef]
- Hayley, S.; Poulter, M.O.; Merali, Z.; Anisman, H. The pathogenesis of clinical depression: Stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience 2005, 135, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid regulation of inflammation and its functional correlates: From HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef]
- Munck, A.; Guyre, P.M.; Holbrook, N.J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984, 5, 25–44. [Google Scholar] [CrossRef]
- Aguilera, G. Regulation of pituitary ACTH secretion during chronic stress. Front. Neuroendocr. 1994, 15, 321–350. [Google Scholar] [CrossRef]
- Varghese, F.P.; Brown, E.S. The Hypothalamic-Pituitary-Adrenal Axis in Major Depressive Disorder: A Brief Primer for Primary Care Physicians. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 151–155. [Google Scholar] [CrossRef]
- Owens, M.J.; Nemeroff, C.B. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: Laboratory and clinical studies. Ciba Found. Symp. 1993, 172, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Young, E.A.; Haskett, R.F.; Murphy-Weinberg, V.; Watson, S.J.; Akil, H. Loss of glucocorticoid fast feedback in depression. Arch. Gen. Psychiatry 1991, 48, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Lipov, E.; Kelzenberg, B.; Rothfeld, C.; Abdi, S. Modulation of NGF by cortisol and the Stellate Ganglion Block—Is this the missing link between memory consolidation and PTSD? Med. Hypotheses 2012, 79, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Sagmeister, M.S.; Harper, L.; Hardy, R.S. Cortisol excess in chronic kidney disease—A review of changes and impact on mortality. Front. Endocrinol. 2022, 13, 1075809. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.S.; Rush, A.J.; McEwen, B.S. Hippocampal remodeling and damage by corticosteroids: Implications for mood disorders. Neuropsychopharmacology 1999, 21, 474–484. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Res. 1997, 24, 1–27. [Google Scholar] [CrossRef]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Brinks, V.; van der Mark, M.; de Kloet, R.; Oitzl, M. Emotion and cognition in high and low stress sensitive mouse strains: A combined neuroendocrine and behavioral study in BALB/c and C57BL/6J mice. Front. Behav. Neurosci. 2007, 1, 8. [Google Scholar] [CrossRef]
- Eadie, B.D.; Zhang, W.N.; Boehme, F.; Gil-Mohapel, J.; Kainer, L.; Simpson, J.M.; Christie, B.R. Fmr1 knockout mice show reduced anxiety and alterations in neurogenesis that are specific to the ventral dentate gyrus. Neurobiol. Dis. 2009, 36, 361–373. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, X.; Xie, G.; Zhang, T. Chronic corticosterone exposure impairs emotional regulation and cognitive function through disturbing neural oscillations in mice. Behav. Brain Res. 2022, 434, 114030. [Google Scholar] [CrossRef]
- Scoville, W.B.; Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 1957, 20, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Scoville, W.B.; Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Milner, B. The medial temporal-lobe amnesic syndrome. Psychiatr. Clin. N. Am. 2005, 28, 599–611, 609. [Google Scholar] [CrossRef]
- Milner, B.; Klein, D. Loss of recent memory after bilateral hippocampal lesions: Memory and memories-looking back and looking forward. J. Neurol. Neurosurg. Psychiatry 2016, 87, 230. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, I.; Furini, C.R.; Myskiw, J.C. Fear Memory. Physiol. Rev. 2016, 96, 695–750. [Google Scholar] [CrossRef]
- Patel, P.D.; Katz, M.; Karssen, A.M.; Lyons, D.M. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology 2008, 33, 360–367. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc. Natl. Acad. Sci. USA 1984, 81, 6174–6177. [Google Scholar] [CrossRef]
- Dahmen, B.; Puetz, V.B.; Scharke, W.; von Polier, G.G.; Herpertz-Dahlmann, B.; Konrad, K. Effects of Early-Life Adversity on Hippocampal Structures and Associated HPA Axis Functions. Dev. Neurosci. 2018, 40, 13–22. [Google Scholar] [CrossRef]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- Clow, A.; Thorn, L.; Evans, P.; Hucklebridge, F. The awakening cortisol response: Methodological issues and significance. Stress 2004, 7, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Frodl, T.; O’Keane, V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol. Dis. 2013, 52, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Alfarez, D.N.; Wiegert, O.; Joels, M.; Krugers, H.J. Corticosterone and stress reduce synaptic potentiation in mouse hippocampal slices with mild stimulation. Neuroscience 2002, 115, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.; Carlier, E.; Talmi, M.; Soumireu-Mourat, B. Corticosterone effects on long-term potentiation in mouse hippocampal slices. Neuroendocrinology 1994, 60, 36–41. [Google Scholar] [CrossRef]
- Abraham, W.C.; Bear, M.F. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996, 19, 126–130. [Google Scholar] [CrossRef]
- Caliskan, G.; Stork, O. Hippocampal network oscillations as mediators of behavioural metaplasticity: Insights from emotional learning. Neurobiol. Learn. Mem. 2018, 154, 37–53. [Google Scholar] [CrossRef]
- Holland, L.L.; Wagner, J.J. Primed facilitation of homosynaptic long-term depression and depotentiation in rat hippocampus. J. Neurosci. 1998, 18, 887–894. [Google Scholar] [CrossRef]
- Ambros, V. microRNAs: Tiny regulators with great potential. Cell. 2001, 107, 823–826. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Edbauer, D.; Neilson, J.R.; Foster, K.A.; Wang, C.F.; Seeburg, D.P.; Batterton, M.N.; Tada, T.; Dolan, B.M.; Sharp, P.A.; Sheng, M. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 2010, 65, 373–384. [Google Scholar] [CrossRef]
- Oliver, R.J.; Mandyam, C.D. Regulation of Adult Neurogenesis by Non-coding RNAs: Implications for Substance Use Disorders. Front. Neurosci. 2018, 12, 849. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, T.; Benes, H.; Awad, A.; Bormann, D.; Monje, F.J. Nicotine abolishes memory-related synaptic strengthening and promotes synaptic depression in the neurogenic dentate gyrus of miR-132/212 knockout mice. Addict. Biol. 2020, e12905. [Google Scholar] [CrossRef]
- Baby, N.; Alagappan, N.; Dheen, S.T.; Sajikumar, S. MicroRNA-134-5p inhibition rescues long-term plasticity and synaptic tagging/capture in an Abeta(1-42)-induced model of Alzheimer’s disease. Aging Cell. 2020, 19, e13046. [Google Scholar] [CrossRef] [PubMed]
- Berentsen, B.; Patil, S.; Ronnestad, K.; Goff, K.M.; Pajak, M.; Simpson, T.I.; Wibrand, K.; Bramham, C.R. MicroRNA-34a Acutely Regulates Synaptic Efficacy in the Adult Dentate Gyrus In Vivo. Mol. Neurobiol. 2020, 57, 1432–1445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.P.; Liu, X.L.; Chen, J.J.; Cheng, K.; Bai, S.J.; Zheng, P.; Zhou, C.J.; Wang, W.; Wang, H.Y.; Zhong, L.M.; et al. Circulating microRNA 134 sheds light on the diagnosis of major depressive disorder. Transl. Psychiatry 2020, 10, 95. [Google Scholar] [CrossRef]
- Liu, D.Y.; Zhang, L. MicroRNA-132 promotes neurons cell apoptosis and activates Tau phosphorylation by targeting GTDC-1 in Alzheimer’s disease. Eur. Rev. Med. Pharm. Sci. 2019, 23, 8523–8532. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, P.H. A New Discovery of MicroRNA-455-3p in Alzheimer’s Disease. J. Alzheimers Dis. 2019, 72, S117–S130. [Google Scholar] [CrossRef]
- Herrera-Espejo, S.; Santos-Zorrozua, B.; Alvarez-Gonzalez, P.; Lopez-Lopez, E.; Garcia-Orad, A. A Systematic Review of MicroRNA Expression as Biomarker of Late-Onset Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 8376–8391. [Google Scholar] [CrossRef]
- Salta, E.; De Strooper, B. microRNA-132: A key noncoding RNA operating in the cellular phase of Alzheimer’s disease. FASEB J. 2017, 31, 424–433. [Google Scholar] [CrossRef]
- Hernandez-Rapp, J.; Rainone, S.; Goupil, C.; Dorval, V.; Smith, P.Y.; Saint-Pierre, M.; Vallee, M.; Planel, E.; Droit, A.; Calon, F.; et al. microRNA-132/212 deficiency enhances Abeta production and senile plaque deposition in Alzheimer’s disease triple transgenic mice. Sci. Rep. 2016, 6, 30953. [Google Scholar] [CrossRef]
- Salta, E.; Sierksma, A.; Vanden Eynden, E.; De Strooper, B. miR-132 loss de-represses ITPKB and aggravates amyloid and TAU pathology in Alzheimer’s brain. EMBO Mol. Med. 2016, 8, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Aten, S.; Hansen, K.F.; Hoyt, K.R.; Obrietan, K. The miR-132/212 locus: A complex regulator of neuronal plasticity, gene expression and cognition. RNA Dis. 2016, 3. [Google Scholar]
- Hansen, K.F.; Sakamoto, K.; Aten, S.; Snider, K.H.; Loeser, J.; Hesse, A.M.; Page, C.E.; Pelz, C.; Arthur, J.S.; Impey, S.; et al. Targeted deletion of miR-132/-212 impairs memory and alters the hippocampal transcriptome. Learn. Mem. 2016, 23, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Viveros, L.; Chiang, C.K.; Ong, J.L.K.; Hegazi, S.; Cheng, A.H.; Bouchard-Cannon, P.; Fana, M.; Lowden, C.; Zhang, P.; Bothorel, B.; et al. miR-132/212 Modulates Seasonal Adaptation and Dendritic Morphology of the Central Circadian Clock. Cell. Rep. 2017, 19, 505–520. [Google Scholar] [CrossRef]
- Remenyi, J.; Hunter, C.J.; Cole, C.; Ando, H.; Impey, S.; Monk, C.E.; Martin, K.J.; Barton, G.J.; Hutvagner, G.; Arthur, J.S. Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem. J. 2010, 428, 281–291. [Google Scholar] [CrossRef]
- Remenyi, J.; van den Bosch, M.W.; Palygin, O.; Mistry, R.B.; McKenzie, C.; Macdonald, A.; Hutvagner, G.; Arthur, J.S.; Frenguelli, B.G.; Pankratov, Y. miR-132/212 knockout mice reveal roles for these miRNAs in regulating cortical synaptic transmission and plasticity. PLoS ONE 2013, 8, e62509. [Google Scholar] [CrossRef]
- Wanet, A.; Tacheny, A.; Arnould, T.; Renard, P. miR-212/132 expression and functions: Within and beyond the neuronal compartment. Nucleic Acids Res. 2012, 40, 4742–4753. [Google Scholar] [CrossRef]
- Tognini, P.; Pizzorusso, T. MicroRNA212/132 family: Molecular transducer of neuronal function and plasticity. Int. J. Biochem. Cell. Biol. 2012, 44, 6–10. [Google Scholar] [CrossRef]
- Bormann, D.; Stojanovic, T.; Cicvaric, A.; Schuld, G.J.; Cabatic, M.; Ankersmit, H.J.; Monje, F.J. miRNA-132/212 Gene-Deletion Aggravates the Effect of Oxygen-Glucose Deprivation on Synaptic Functions in the Female Mouse Hippocampus. Cells 2021, 10, 1709. [Google Scholar] [CrossRef]
- Stojanovic, T.; Velarde Gamez, D.; Schuld, G.J.; Bormann, D.; Cabatic, M.; Uhrin, P.; Lubec, G.; Monje, F.J. Age-Dependent and Pathway-Specific Bimodal Action of Nicotine on Synaptic Plasticity in the Hippocampus of Mice Lacking the miR-132/212 Genes. Cells 2022, 11, 261. [Google Scholar] [CrossRef]
- Ronovsky, M.; Zambon, A.; Cicvaric, A.; Boehm, V.; Hoesel, B.; Moser, B.A.; Yang, J.; Schmid, J.A.; Haubensak, W.E.; Monje, F.J.; et al. A role for miR-132 in learned safety. Sci. Rep. 2019, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Krugers, H.J.; Joels, M. Beta-adrenergic facilitation of synaptic plasticity in the rat basolateral amygdala in vitro is gradually reversed by corticosterone. Learn. Mem. (Cold Spring Harb. N. Y.) 2009, 16, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kouhnavardi, S.; Ecevitoglu, A.; Dragacevic, V.; Sanna, F.; Arias-Sandoval, E.; Kalaba, P.; Kirchhofer, M.; Lubec, J.; Niello, M.; Holy, M.; et al. A Novel and Selective Dopamine Transporter Inhibitor, (S)-MK-26, Promotes Hippocampal Synaptic Plasticity and Restores Effort-Related Motivational Dysfunctions. Biomolecules 2022, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Cicvaric, A.; Sachernegg, H.M.; Stojanovic, T.; Symmank, D.; Smani, T.; Moeslinger, T.; Uhrin, P.; Monje, F.J. Podoplanin Gene Disruption in Mice Promotes in vivo Neural Progenitor Cells Proliferation, Selectively Impairs Dentate Gyrus Synaptic Depression and Induces Anxiety-Like Behaviors. Front. Cell. Neurosci. 2019, 13, 561. [Google Scholar] [CrossRef] [PubMed]
- Cicvaric, A.; Yang, J.; Bulat, T.; Zambon, A.; Dominguez-Rodriguez, M.; Kuhn, R.; Sadowicz, M.G.; Siwert, A.; Egea, J.; Pollak, D.D.; et al. Enhanced synaptic plasticity and spatial memory in female but not male FLRT2-haplodeficient mice. Sci. Rep. 2018, 8, 3703. [Google Scholar] [CrossRef] [PubMed]
- Cicvaric, A.; Bulat, T.; Bormann, D.; Yang, J.; Auer, B.; Milenkovic, I.; Cabatic, M.; Milicevic, R.; Monje, F.J. Sustained consumption of cocoa-based dark chocolate enhances seizure-like events in the mouse hippocampus. Food Funct. 2018, 9, 1532–1544. [Google Scholar] [CrossRef]
- Cicvaric, A.; Yang, J.; Krieger, S.; Khan, D.; Kim, E.J.; Dominguez-Rodriguez, M.; Cabatic, M.; Molz, B.; Acevedo Aguilar, J.P.; Milicevic, R.; et al. The brain-tumor related protein podoplanin regulates synaptic plasticity and hippocampus-dependent learning and memory. Ann. Med. 2016, 1–17. [Google Scholar] [CrossRef]
- Kim, E.J.; Monje, F.J.; Li, L.; Hoger, H.; Pollak, D.D.; Lubec, G. Alzheimer’s disease risk factor lymphocyte-specific protein tyrosine kinase regulates long-term synaptic strengthening, spatial learning and memory. Cell. Mol. Life Sci. 2013, 70, 743–759. [Google Scholar] [CrossRef]
- Monje, F.J.; Kim, E.J.; Pollak, D.D.; Cabatic, M.; Li, L.; Baston, A.; Lubec, G. Focal adhesion kinase regulates neuronal growth, synaptic plasticity and hippocampus-dependent spatial learning and memory. Neuro-Signals 2012, 20, 1–14. [Google Scholar] [CrossRef]
- Maggio, N.; Segal, M. Striking variations in corticosteroid modulation of long-term potentiation along the septotemporal axis of the hippocampus. J. Neurosci. 2007, 27, 5757–5765. [Google Scholar] [CrossRef]
- Maggio, N.; Segal, M. Unique regulation of long term potentiation in the rat ventral hippocampus. Hippocampus 2007, 17, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Nagy, V.; Hollstein, R.; Pai, T.P.; Herde, M.K.; Buphamalai, P.; Moeseneder, P.; Lenartowicz, E.; Kavirayani, A.; Korenke, G.C.; Kozieradzki, I.; et al. HACE1 deficiency leads to structural and functional neurodevelopmental defects. Neurol. Genet. 2019, 5, e330. [Google Scholar] [CrossRef] [PubMed]
- Fell, C.W.; Hagelkruys, A.; Cicvaric, A.; Horrer, M.; Liu, L.; Li, J.S.S.; Stadlmann, J.; Polyansky, A.A.; Mereiter, S.; Tejada, M.A.; et al. FIBCD1 is an endocytic GAG receptor associated with a novel neurodevelopmental disorder. EMBO Mol. Med. 2022, 14, e15829. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.A.; Malenka, R.C. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann. N. Y. Acad. Sci. 1999, 868, 515–525. [Google Scholar] [CrossRef]
- Schulz, P.E.; Cook, E.P.; Johnston, D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J. Neurosci. 1994, 14, 5325–5337. [Google Scholar] [CrossRef]
- Bortolotto, Z.A.; Collingridge, G.L. A role for protein kinase C in a form of metaplasticity that regulates the induction of long-term potentiation at CA1 synapses of the adult rat hippocampus. Eur. J. Neurosci. 2000, 12, 4055–4062. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Common molecular and cellular substrates of addiction and memory. Neurobiol. Learn. Mem. 2002, 78, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.F.; Karelina, K.; Sakamoto, K.; Wayman, G.A.; Impey, S.; Obrietan, K. miRNA-132: A dynamic regulator of cognitive capacity. Brain Struct. Funct. 2013, 218, 817–831. [Google Scholar] [CrossRef]
- Fisher, M.L.; LeMalefant, R.M.; Zhou, L.; Huang, G.; Turner, J.R. Distinct Roles of CREB Within the Ventral and Dorsal Hippocampus in Mediating Nicotine Withdrawal Phenotypes. Neuropsychopharmacology 2017, 42, 1599–1609. [Google Scholar] [CrossRef]
- Barco, A.; Patterson, S.; Alarcon, J.M.; Gromova, P.; Mata-Roig, M.; Morozov, A.; Kandel, E.R. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron 2005, 48, 123–137. [Google Scholar] [CrossRef]
- Magill, S.T.; Cambronne, X.A.; Luikart, B.W.; Lioy, D.T.; Leighton, B.H.; Westbrook, G.L.; Mandel, G.; Goodman, R.H. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 20382–20387. [Google Scholar] [CrossRef] [PubMed]
- Haghparast, A.; Taslimi, Z.; Ramin, M.; Azizi, P.; Khodagholi, F.; Hassanpour-Ezatti, M. Changes in phosphorylation of CREB, ERK, and c-fos induction in rat ventral tegmental area, hippocampus and prefrontal cortex after conditioned place preference induced by chemical stimulation of lateral hypothalamus. Behav. Brain Res. 2011, 220, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Hadar, A.; Milanesi, E.; Walczak, M.; Puzianowska-Kuznicka, M.; Kuznicki, J.; Squassina, A.; Niola, P.; Chillotti, C.; Attems, J.; Gozes, I.; et al. SIRT1, miR-132 and miR-212 link human longevity to Alzheimer’s Disease. Sci. Rep. 2018, 8, 8465. [Google Scholar] [CrossRef] [PubMed]
- Herskovits, A.Z.; Guarente, L. SIRT1 in neurodevelopment and brain senescence. Neuron 2014, 81, 471–483. [Google Scholar] [CrossRef]
- Luikart, B.W.; Bensen, A.L.; Washburn, E.K.; Perederiy, J.V.; Su, K.G.; Li, Y.; Kernie, S.G.; Parada, L.F.; Westbrook, G.L. miR-132 mediates the integration of newborn neurons into the adult dentate gyrus. PLoS ONE 2011, 6, e19077. [Google Scholar] [CrossRef]
- Jiang, Y.; Botchway, B.O.A.; Hu, Z.; Fang, M. Overexpression of SIRT1 Inhibits Corticosterone-Induced Autophagy. Neuroscience 2019, 411, 11–22. [Google Scholar] [CrossRef]
- Aten, S.; Page, C.E.; Kalidindi, A.; Wheaton, K.L.; Niraula, A.; Godbout, J.P.; Hoyt, K.R.; Obrietan, K. Data highlighting the expression of two miR-132/212 target genes-Sirt1 and Pten-after chronic stress. Data Brief. 2018, 21, 2323–2329. [Google Scholar] [CrossRef]
- Arthur, J.S. MSK activation and physiological roles. Front. Biosci. 2008, 13, 5866–5879. [Google Scholar] [CrossRef]
- Hauge, C.; Frodin, M. RSK and MSK in MAP kinase signalling. J. Cell. Sci. 2006, 119, 3021–3023. [Google Scholar] [CrossRef]
- Chandramohan, Y.; Droste, S.K.; Arthur, J.S.; Reul, J.M. The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. Eur. J. Neurosci. 2008, 27, 2701–2713. [Google Scholar] [CrossRef]
- Chwang, W.B.; Arthur, J.S.; Schumacher, A.; Sweatt, J.D. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J. Neurosci. 2007, 27, 12732–12742. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mecinas, M.; Trollope, A.F.; Collins, A.; Morfett, H.; Hesketh, S.A.; Kersante, F.; Reul, J.M. Long-lasting behavioral responses to stress involve a direct interaction of glucocorticoid receptors with ERK1/2-MSK1-Elk-1 signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 13806–13811. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Puelles, C.; Calleja-Felipe, M.; Ouro, A.; Bougamra, G.; Arroyo, A.; Diez, I.; Erramuzpe, A.; Cortes, J.; Martinez-Hernandez, J.; Lujan, R.; et al. PTEN Activity Defines an Axis for Plasticity at Cortico-Amygdala Synapses and Influences Social Behavior. Cereb. Cortex 2020, 30, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Mei, F.; Hu, J.; Zhu, M.; Qi, H.; Chen, X.; Li, R.; McNutt, M.A.; Yin, Y. PTENalpha Modulates CaMKII Signaling and Controls Contextual Fear Memory and Spatial Learning. Cell. Rep. 2017, 19, 2627–2641. [Google Scholar] [CrossRef] [PubMed]
- Sperow, M.; Berry, R.B.; Bayazitov, I.T.; Zhu, G.; Baker, S.J.; Zakharenko, S.S. Phosphatase and tensin homologue (PTEN) regulates synaptic plasticity independently of its effect on neuronal morphology and migration. J. Physiol. 2012, 590, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Santos, A.C.; Farfel, J.M.; Grinberg, L.T.; Ferretti, R.E.; Campos, A.H.; Cunha, I.W.; Begnami, M.D.; Rocha, R.M.; Carraro, D.M.; et al. Repair of oxidative DNA damage, cell-cycle regulation and neuronal death may influence the clinical manifestation of Alzheimer’s disease. PLoS ONE 2014, 9, e99897. [Google Scholar] [CrossRef]
- Quan, Q.; Qian, Y.; Li, X.; Li, M. CDK5 Participates in Amyloid-beta Production by Regulating PPARgamma Phosphorylation in Primary Rat Hippocampal Neurons. J. Alzheimers Dis. 2019, 71, 443–460. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Y.; Liang, R.; Zhang, Y. Inhibition of cyclin-dependent kinase 5 activity alleviates diabetes-related cognitive deficits. FASEB J. 2019, 33, 14506–14515. [Google Scholar] [CrossRef]
- Brossaud, J.; Roumes, H.; Helbling, J.C.; Moisan, M.P.; Pallet, V.; Ferreira, G.; Biyong, E.F.; Redonnet, A.; Corcuff, J.B. Retinoic acid increases glucocorticoid receptor phosphorylation via cyclin-dependent kinase 5. Mol. Cell. Neurosci. 2017, 82, 96–104. [Google Scholar] [CrossRef]
- Jin, X.; Sasamoto, K.; Nagai, J.; Yamazaki, Y.; Saito, K.; Goshima, Y.; Inoue, T.; Ohshima, T. Phosphorylation of CRMP2 by Cdk5 Regulates Dendritic Spine Development of Cortical Neuron in the Mouse Hippocampus. Neural Plast. 2016, 2016, 6790743. [Google Scholar] [CrossRef]
- Tomizawa, K.; Ohta, J.; Matsushita, M.; Moriwaki, A.; Li, S.T.; Takei, K.; Matsui, H. Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity. J. Neurosci. 2002, 22, 2590–2597. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Ando, K.; Takeda, S.; Satoh, Y.; Seki, T.; Itohara, S.; Greengard, P.; Kirino, Y.; Nairn, A.C.; Suzuki, T. Neuron-specific phosphorylation of Alzheimer’s beta-amyloid precursor protein by cyclin-dependent kinase 5. J. Neurochem. 2000, 75, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Agasse, F.; Mendez-David, I.; Christaller, W.; Carpentier, R.; Braz, B.Y.; David, D.J.; Saudou, F.; Humbert, S. Chronic Corticosterone Elevation Suppresses Adult Hippocampal Neurogenesis by Hyperphosphorylating Huntingtin. Cell. Rep. 2020, 32, 107865. [Google Scholar] [CrossRef]
- Mitic, M.; Simic, I.; Djordjevic, J.; Radojcic, M.B.; Adzic, M. Gender-specific effects of fluoxetine on hippocampal glucocorticoid receptor phosphorylation and behavior in chronically stressed rats. Neuropharmacology 2013, 70, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Adzic, M.; Djordjevic, J.; Djordjevic, A.; Niciforovic, A.; Demonacos, C.; Radojcic, M.; Krstic-Demonacos, M. Acute or chronic stress induce cell compartment-specific phosphorylation of glucocorticoid receptor and alter its transcriptional activity in Wistar rat brain. J. Endocrinol. 2009, 202, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, L.; Zhang, Y.; Du, J.; Hu, J.; Bao, H.; Xing, Y.; Si, Y. Phosphatase and Tensin Homolog Deleted on Chromosome Ten Knockdown Attenuates Cognitive Deficits by Inhibiting Neuroinflammation in a Mouse Model of Perioperative Neurocognitive Disorder. Neuroscience 2021, 468, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.E.; Lee, H.J.; Chae, C.W.; Cho, J.H.; Jung, Y.H.; Kim, J.S.; Kim, S.Y.; Lim, J.R.; Han, H.J. BNIP3L/NIX-mediated mitophagy protects against glucocorticoid-induced synapse defects. Nat. Commun. 2021, 12, 487. [Google Scholar] [CrossRef]
- Chen, D.; Lan, G.; Li, R.; Mei, Y.; Shui, X.; Gu, X.; Wang, L.; Zhang, T.; Gan, C.L.; Xia, Y.; et al. Melatonin ameliorates tau-related pathology via the miR-504-3p and CDK5 axis in Alzheimer’s disease. Transl. Neurodegener. 2022, 11, 27. [Google Scholar] [CrossRef]
- Inouye, M.O.; Colameo, D.; Ammann, I.; Winterer, J.; Schratt, G. miR-329- and miR-495-mediated Prr7 down-regulation is required for homeostatic synaptic depression in rat hippocampal neurons. Life Sci. Alliance 2022, 5. [Google Scholar] [CrossRef]
- Li, Y.; Fan, C.; Wang, L.; Lan, T.; Gao, R.; Wang, W.; Yu, S.Y. MicroRNA-26a-3p rescues depression-like behaviors in male rats via preventing hippocampal neuronal anomalies. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Aten, S.; Page, C.E.; Kalidindi, A.; Wheaton, K.; Niraula, A.; Godbout, J.P.; Hoyt, K.R.; Obrietan, K. miR-132/212 is induced by stress and its dysregulation triggers anxiety-related behavior. Neuropharmacology 2019, 144, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Travis, S.G.; Coupland, N.J.; Hegadoren, K.; Silverstone, P.H.; Huang, Y.; Carter, R.; Fujiwara, E.; Seres, P.; Malykhin, N.V. Effects of cortisol on hippocampal subfields volumes and memory performance in healthy control subjects and patients with major depressive disorder. J. Affect. Disord. 2016, 201, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M., Jr.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Borras, J.; Vuilleumier, P. Amygdala function in emotion, cognition, and behavior. Handb. Clin. Neurol. 2022, 187, 359–380. [Google Scholar] [CrossRef]
- Simic, G.; Tkalcic, M.; Vukic, V.; Mulc, D.; Spanic, E.; Sagud, M.; Olucha-Bordonau, F.E.; Vuksic, M.; P, R.H. Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules 2021, 11, 823. [Google Scholar] [CrossRef]
- Kim, J.J.; Lee, H.J.; Han, J.S.; Packard, M.G. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J. Neurosci. 2001, 21, 5222–5228. [Google Scholar] [CrossRef]
- Segall, L.A.; Milet, A.; Tronche, F.; Amir, S. Brain glucocorticoid receptors are necessary for the rhythmic expression of the clock protein, PERIOD2, in the central extended amygdala in mice. Neurosci. Lett. 2009, 457, 58–60. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Fan, H.; Wang, Z.; Huang, J.; Wen, G.; Wang, X.; Xie, Q.; Qiu, P. Brain-derived neurotrophic factor upregulates synaptic GluA1 in the amygdala to promote depression in response to psychological stress. Biochem. Pharm. 2021, 192, 114740. [Google Scholar] [CrossRef]
- Mackiewicz, K.L.; Sarinopoulos, I.; Cleven, K.L.; Nitschke, J.B. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proc. Natl. Acad. Sci. USA 2006, 103, 14200–14205. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- Floriou-Servou, A.; von Ziegler, L.; Stalder, L.; Sturman, O.; Privitera, M.; Rassi, A.; Cremonesi, A.; Thony, B.; Bohacek, J. Distinct Proteomic, Transcriptomic, and Epigenetic Stress Responses in Dorsal and Ventral Hippocampus. Biol. Psychiatry 2018, 84, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Sato, S.; Tsutsui, K.; Witter, M.P.; Iijima, T. Organization of multisynaptic inputs to the dorsal and ventral dentate gyrus: Retrograde trans-synaptic tracing with rabies virus vector in the rat. PLoS ONE 2013, 8, e78928. [Google Scholar] [CrossRef] [PubMed]
- Steullet, P.; Cabungcal, J.H.; Kulak, A.; Kraftsik, R.; Chen, Y.; Dalton, T.P.; Cuenod, M.; Do, K.Q. Redox dysregulation affects the ventral but not dorsal hippocampus: Impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J. Neurosci. 2010, 30, 2547–2558. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.M.; Ito, H.T.; Moser, E.I.; Moser, M.B. Functional diversity along the transverse axis of hippocampal area CA1. FEBS Lett. 2014, 588, 2470–2476. [Google Scholar] [CrossRef]
- Moser, M.B.; Moser, E.I. Functional differentiation in the hippocampus. Hippocampus 1998, 8, 608–619. [Google Scholar] [CrossRef]
- Strange, B.A.; Witter, M.P.; Lein, E.S.; Moser, E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. 2014, 15, 655–669. [Google Scholar] [CrossRef]
- Torrisi, S.A.; Lavanco, G.; Maurel, O.M.; Gulisano, W.; Laudani, S.; Geraci, F.; Grasso, M.; Barbagallo, C.; Caraci, F.; Bucolo, C.; et al. A novel arousal-based individual screening reveals susceptibility and resilience to PTSD-like phenotypes in mice. Neurobiol. Stress. 2021, 14, 100286. [Google Scholar] [CrossRef]
- Maurel, O.M.; Torrisi, S.A.; Barbagallo, C.; Purrello, M.; Salomone, S.; Drago, F.; Ragusa, M.; Leggio, G.M. Dysregulation of miR-15a-5p, miR-497a-5p and miR-511-5p Is Associated with Modulation of BDNF and FKBP5 in Brain Areas of PTSD-Related Susceptible and Resilient Mice. Int. J. Mol. Sci. 2021, 22, 5157. [Google Scholar] [CrossRef]
- Belovicova, K.; Bogi, E.; Csatlosova, K.; Dubovicky, M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip. Toxicol. 2017, 10, 40–43. [Google Scholar] [CrossRef]
- Hogg, S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol. Biochem. Behav. 1996, 54, 21–30. [Google Scholar] [CrossRef]
- Bourin, M.; Hascoet, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [PubMed]
- Hagenbuch, N.; Feldon, J.; Yee, B.K. Use of the elevated plus-maze test with opaque or transparent walls in the detection of mouse strain differences and the anxiolytic effects of diazepam. Behav. Pharmacol. 2006, 17, 31–41. [Google Scholar] [PubMed]
- Ramos, A. Animal models of anxiety: Do I need multiple tests? Trends Pharmacol. Sci. 2008, 29, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Violle, N.; Balandras, F.; Le Roux, Y.; Desor, D.; Schroeder, H. Variations in illumination, closed wall transparency and/or extramaze space influence both baseline anxiety and response to diazepam in the rat elevated plus-maze. Behav. Brain Res. 2009, 203, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Piasecki, C.C.; Lonstein, J.S. Use of the light-dark box to compare the anxiety-related behavior of virgin and postpartum female rats. Pharmacol. Biochem. Behav. 2011, 100, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Steimer, T. Animal models of anxiety disorders in rats and mice: Some conceptual issues. Dialogues Clin. Neurosci. 2011, 13, 495–506. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Fote, G.M.; Blakeman, S.; Cahuzac, E.L.; Newbold, S.A.; Picciotto, M.R. Multiple Nicotinic Acetylcholine Receptor Subtypes in the Mouse Amygdala Regulate Affective Behaviors and Response to Social Stress. Neuropsychopharmacology 2016, 41, 1579–1587. [Google Scholar] [CrossRef]
- Pidoplichko, V.I.; Prager, E.M.; Aroniadou-Anderjaska, V.; Braga, M.F. alpha7-Containing nicotinic acetylcholine receptors on interneurons of the basolateral amygdala and their role in the regulation of the network excitability. J. Neurophysiol. 2013, 110, 2358–2369. [Google Scholar] [CrossRef]
- Laviolette, S.R. Molecular and neuronal mechanisms underlying the effects of adolescent nicotine exposure on anxiety and mood disorders. Neuropharmacology 2021, 184, 108411. [Google Scholar] [CrossRef]
- Zhang, M.; Bian, Z. Alzheimer’s Disease and microRNA-132: A Widespread Pathological Factor and Potential Therapeutic Target. Front. Neurosci. 2021, 15, 687973. [Google Scholar] [CrossRef]
- Alkadhi, K.A.; Alzoubi, K.H.; Srivareerat, M.; Tran, T.T. Chronic psychosocial stress exacerbates impairment of synaptic plasticity in beta-amyloid rat model of Alzheimer’s disease: Prevention by nicotine. Curr. Alzheimer Res. 2011, 8, 718–731. [Google Scholar] [CrossRef]
- Alkadhi, K.A.; Srivareerat, M.; Tran, T.T. Intensification of long-term memory deficit by chronic stress and prevention by nicotine in a rat model of Alzheimer’s disease. Mol. Cell. Neurosci. 2010, 45, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, L.; Tornese, P.; Sala, N.; Lee, F.S.; Popoli, M.; Ieraci, A. Acute stress induces an aberrant increase of presynaptic release of glutamate and cellular activation in the hippocampus of BDNF(Val/Met) mice. J. Cell. Physiol. 2022, 237, 3834–3844. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Rouse, J.; Zhang, A.; Cariati, S.; Cohen, P.; Comb, M.J. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 1996, 15, 4629–4642. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rapp, J.; Smith, P.Y.; Filali, M.; Goupil, C.; Planel, E.; Magill, S.T.; Goodman, R.H.; Hebert, S.S. Memory formation and retention are affected in adult miR-132/212 knockout mice. Behav. Brain Res. 2015, 287, 15–26. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, H.R.; Lee, C.Y.; Hyun, S.A.; Ko, M.Y.; Lee, B.S.; Hwang, D.Y.; Ka, M. 2-Phenylethylamine (PEA) Ameliorates Corticosterone-Induced Depression-Like Phenotype via the BDNF/TrkB/CREB Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 9103. [Google Scholar] [CrossRef]
- Lu, Y.; Sareddy, G.R.; Wang, J.; Wang, R.; Li, Y.; Dong, Y.; Zhang, Q.; Liu, J.; O’Connor, J.C.; Xu, J.; et al. Neuron-Derived Estrogen Regulates Synaptic Plasticity and Memory. J. Neurosci. 2019, 39, 2792–2809. [Google Scholar] [CrossRef]
- Okun, E.; Griffioen, K.; Barak, B.; Roberts, N.J.; Castro, K.; Pita, M.A.; Cheng, A.; Mughal, M.R.; Wan, R.; Ashery, U.; et al. Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 15625–15630. [Google Scholar] [CrossRef]
- Hansen, K.F.; Sakamoto, K.; Wayman, G.A.; Impey, S.; Obrietan, K. Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLoS ONE 2010, 5, e15497. [Google Scholar] [CrossRef]
- Lambert, T.J.; Storm, D.R.; Sullivan, J.M. MicroRNA132 modulates short-term synaptic plasticity but not basal release probability in hippocampal neurons. PLoS ONE 2010, 5, e15182. [Google Scholar] [CrossRef]
- Silva, A.J.; Kogan, J.H.; Frankland, P.W.; Kida, S. CREB and memory. Annu. Rev. Neurosci. 1998, 21, 127–148. [Google Scholar] [CrossRef]
- Meyer, H.C.; Odriozola, P.; Cohodes, E.M.; Mandell, J.D.; Li, A.; Yang, R.; Hall, B.S.; Haberman, J.T.; Zacharek, S.J.; Liston, C.; et al. Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans. Proc. Natl. Acad. Sci. USA 2019, 116, 26970–26979. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.A.; Kutlu, M.G.; Gould, T.J. Nicotine disrupts safety learning by enhancing fear associated with a safety cue via the dorsal hippocampus. J. Psychopharmacol. 2017, 31, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol. Learn. Mem. 2002, 78, 578–595. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, E.; Verissimo, C.S.; Mariman, R.; Kamphorst, J.T.; Barbosa, J.S.; Zweers, T.; Champagne, D.L.; Schouten, T.; Meijer, O.C.; de Kloet, E.R.; et al. MicroRNA 18 and 124a down-regulate the glucocorticoid receptor: Implications for glucocorticoid responsiveness in the brain. Endocrinology 2009, 150, 2220–2228. [Google Scholar] [CrossRef]
- Hillerer, K.M.; Slattery, D.A.; Pletzer, B. Neurobiological mechanisms underlying sex-related differences in stress-related disorders: Effects of neuroactive steroids on the hippocampus. Front. Neuroendocr. 2019, 55, 100796. [Google Scholar] [CrossRef]
- Gurvich, C.; Thomas, N.; Kulkarni, J. Sex differences in cognition and aging and the influence of sex hormones. Handb. Clin. Neurol. 2020, 175, 103–115. [Google Scholar] [CrossRef]
- Hajali, V.; Andersen, M.L.; Negah, S.S.; Sheibani, V. Sex differences in sleep and sleep loss-induced cognitive deficits: The influence of gonadal hormones. Horm. Behav. 2019, 108, 50–61. [Google Scholar] [CrossRef]
- Lambert, K.G.; Kinsley, C.H. Sex differences and gonadal hormones influence susceptibility to the activity-stress paradigm. Physiol. Behav. 1993, 53, 1085–1090. [Google Scholar] [CrossRef]
- McEwen, B.S.; Milner, T.A. Understanding the broad influence of sex hormones and sex differences in the brain. J. Neurosci. Res. 2017, 95, 24–39. [Google Scholar] [CrossRef]
- Kong, F.; Zhen, Z.; Li, J.; Huang, L.; Wang, X.; Song, Y.; Liu, J. Sex-related neuroanatomical basis of emotion regulation ability. PLoS ONE 2014, 9, e97071. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.; Wainwright, S.R.; Galea, L.A. Sex hormones and adult hippocampal neurogenesis: Regulation, implications, and potential mechanisms. Front. Neuroendocr. 2016, 41, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Koss, W.A.; Frick, K.M. Sex differences in hippocampal function. J. Neurosci. Res. 2017, 95, 539–562. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E.; MacLusky, N.J. Sex differences in hippocampal area CA3 pyramidal cells. J. Neurosci. Res. 2017, 95, 563–575. [Google Scholar] [CrossRef]
- Morgan, C.P.; Bale, T.L. Sex differences in microRNA-mRNA networks: Examination of novel epigenetic programming mechanisms in the sexually dimorphic neonatal hypothalamus. Biol. Sex. Differ. 2017, 8, 27. [Google Scholar] [CrossRef]
- Murphy, S.J.; Lusardi, T.A.; Phillips, J.I.; Saugstad, J.A. Sex differences in microRNA expression during development in rat cortex. Neurochem. Int. 2014, 77, 24–32. [Google Scholar] [CrossRef]
- Sheinerman, K.; Tsivinsky, V.; Mathur, A.; Kessler, D.; Shaz, B.; Umansky, S. Age- and sex-dependent changes in levels of circulating brain-enriched microRNAs during normal aging. Aging (Albany N. Y.) 2018, 10, 3017–3041. [Google Scholar] [CrossRef]
- Jirkof, P.; Bratcher, N.; Medina, L.; Strasburg, D.; Ebert, P.; Gaskill, B.N. The effect of group size, age and handling frequency on inter-male aggression in CD 1 mice. Sci. Rep. 2020, 10, 2253. [Google Scholar] [CrossRef]
- Svenson, K.L.; Paigen, B. Recommended housing densities for research mice: Filling the gap in data-driven alternatives. FASEB J. 2019, 33, 3097–3111. [Google Scholar] [CrossRef]
- Nguyen, P.V.; Abel, T.; Kandel, E.R. Requirement of a critical period of transcription for induction of a late phase of LTP. Sci. (N. Y.) 1994, 265, 1104–1107. [Google Scholar] [CrossRef]
- Nguyen, P.V.; Kandel, E.R. Brief theta-burst stimulation induces a transcription-dependent late phase of LTP requiring cAMP in area CA1 of the mouse hippocampus. Learn. Mem. (Cold Spring Harb. N. Y.) 1997, 4, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Ladron de Guevara-Miranda, D.; Millon, C.; Rosell-Valle, C.; Perez-Fernandez, M.; Missiroli, M.; Serrano, A.; Pavon, F.J.; Rodriguez de Fonseca, F.; Martinez-Losa, M.; Alvarez-Dolado, M.; et al. Long-lasting memory deficits in mice withdrawn from cocaine are concomitant with neuroadaptations in hippocampal basal activity, GABAergic interneurons and adult neurogenesis. Dis. Model. Mech. 2017, 10, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Manas-Padilla, M.C.; Avila-Gamiz, F.; Gil-Rodriguez, S.; Ladron de Guevara-Miranda, D.; Rodriguez de Fonseca, F.; Santin, L.J.; Castilla-Ortega, E. Persistent changes in exploration and hyperactivity coexist with cognitive impairment in mice withdrawn from chronic cocaine. Physiol. Behav. 2021, 240, 113542. [Google Scholar] [CrossRef]
- Bailey, K.R.; Crawley, J.N. Anxiety-Related Behaviors in Mice. In Methods of Behavior Analysis in Neuroscience, 2nd ed.; Buccafusco, J.J., Ed.; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Manas-Padilla, M.C.; Gil-Rodriguez, S.; Sampedro-Piquero, P.; Avila-Gamiz, F.; Rodriguez de Fonseca, F.; Santin, L.J.; Castilla-Ortega, E. Remote memory of drug experiences coexists with cognitive decline and abnormal adult neurogenesis in an animal model of cocaine-altered cognition. Addict. Biol. 2021, 26, e12886. [Google Scholar] [CrossRef]
- Pinheiro, S.H.; Zangrossi, H., Jr.; Del-Ben, C.M.; Graeff, F.G. Elevated mazes as animal models of anxiety: Effects of serotonergic agents. Acad. Bras. Cienc. 2007, 79, 71–85. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Shen, H.; Shen, Z.; Wu, L.; Mo, F.; Li, M. Physiological stress-induced corticosterone increases heme uptake via KLF4-HCP1 signaling pathway in hippocampus neurons. Sci. Rep. 2017, 7, 5745. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouhnavardi, S.; Cabatic, M.; Mañas-Padilla, M.C.; Malabanan, M.-A.; Smani, T.; Cicvaric, A.; Muñoz Aranzalez, E.A.; Koenig, X.; Urban, E.; Lubec, G.; et al. miRNA-132/212 Deficiency Disrupts Selective Corticosterone Modulation of Dorsal vs. Ventral Hippocampal Metaplasticity. Int. J. Mol. Sci. 2023, 24, 9565. https://doi.org/10.3390/ijms24119565
Kouhnavardi S, Cabatic M, Mañas-Padilla MC, Malabanan M-A, Smani T, Cicvaric A, Muñoz Aranzalez EA, Koenig X, Urban E, Lubec G, et al. miRNA-132/212 Deficiency Disrupts Selective Corticosterone Modulation of Dorsal vs. Ventral Hippocampal Metaplasticity. International Journal of Molecular Sciences. 2023; 24(11):9565. https://doi.org/10.3390/ijms24119565
Chicago/Turabian StyleKouhnavardi, Shima, Maureen Cabatic, M. Carmen Mañas-Padilla, Marife-Astrid Malabanan, Tarik Smani, Ana Cicvaric, Edison Alejandro Muñoz Aranzalez, Xaver Koenig, Ernst Urban, Gert Lubec, and et al. 2023. "miRNA-132/212 Deficiency Disrupts Selective Corticosterone Modulation of Dorsal vs. Ventral Hippocampal Metaplasticity" International Journal of Molecular Sciences 24, no. 11: 9565. https://doi.org/10.3390/ijms24119565
APA StyleKouhnavardi, S., Cabatic, M., Mañas-Padilla, M. C., Malabanan, M.-A., Smani, T., Cicvaric, A., Muñoz Aranzalez, E. A., Koenig, X., Urban, E., Lubec, G., Castilla-Ortega, E., & Monje, F. J. (2023). miRNA-132/212 Deficiency Disrupts Selective Corticosterone Modulation of Dorsal vs. Ventral Hippocampal Metaplasticity. International Journal of Molecular Sciences, 24(11), 9565. https://doi.org/10.3390/ijms24119565