Effects of Tail Pinch on BDNF and trkB Expression in the Hippocampus of Roman Low- (RLA) and High-Avoidance (RHA) Rats
Abstract
1. Introduction
2. Results
2.1. Behavioral Measures during TP
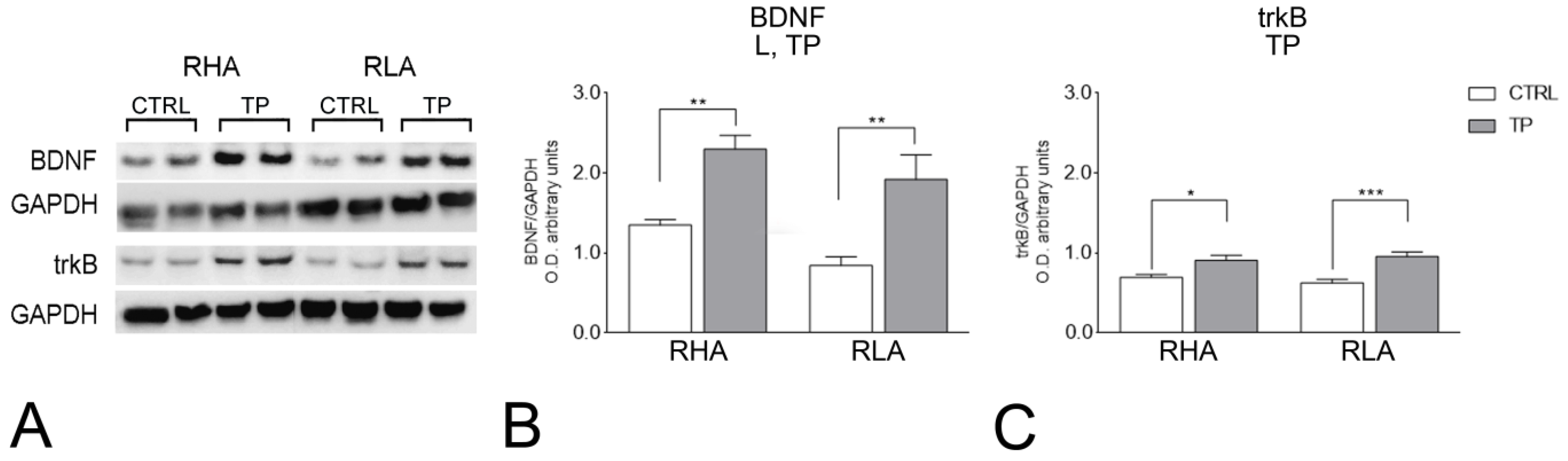
2.2. Western Blot
2.2.1. BDNF Protein Levels
2.2.2. trkB Protein Levels
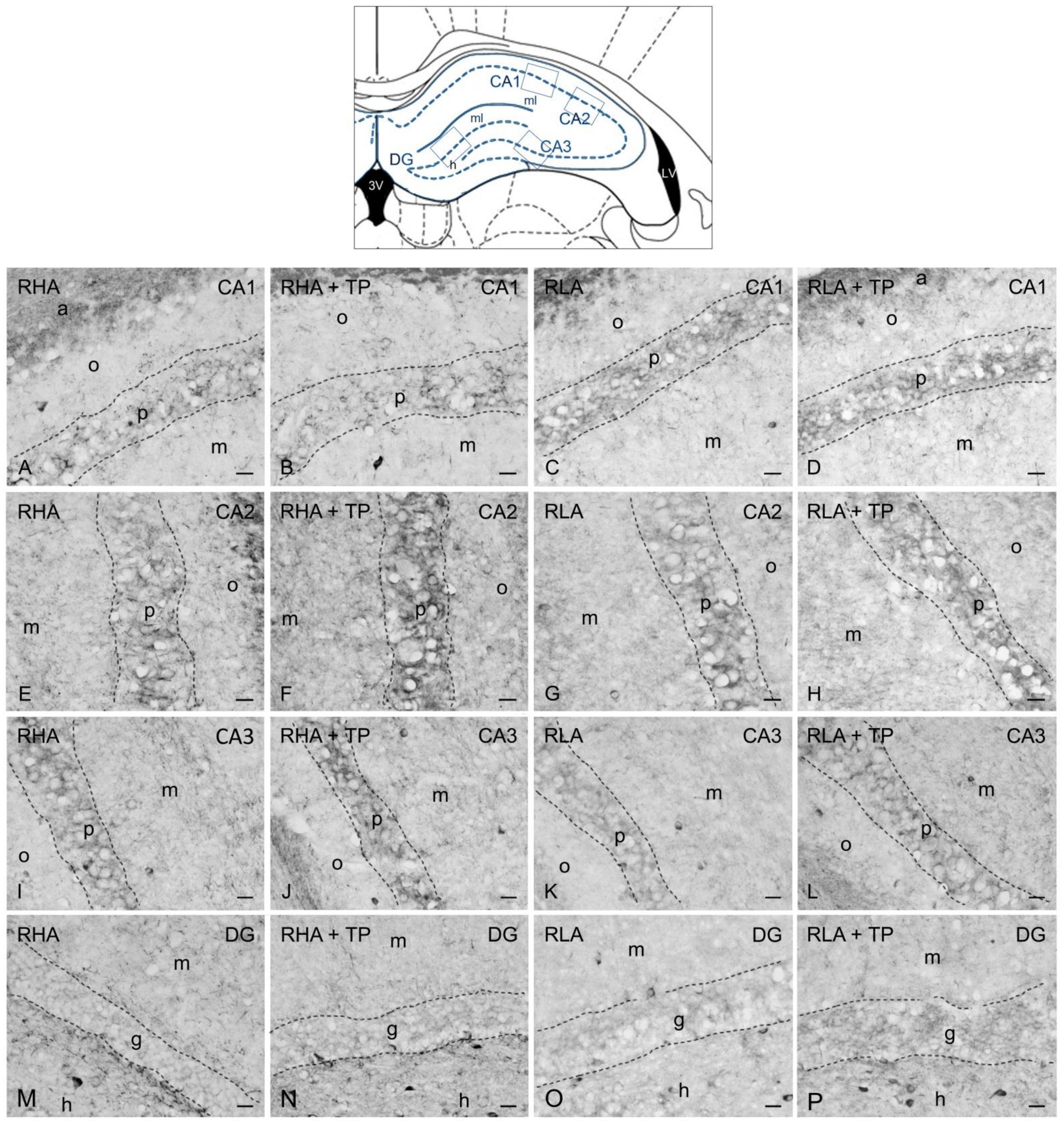
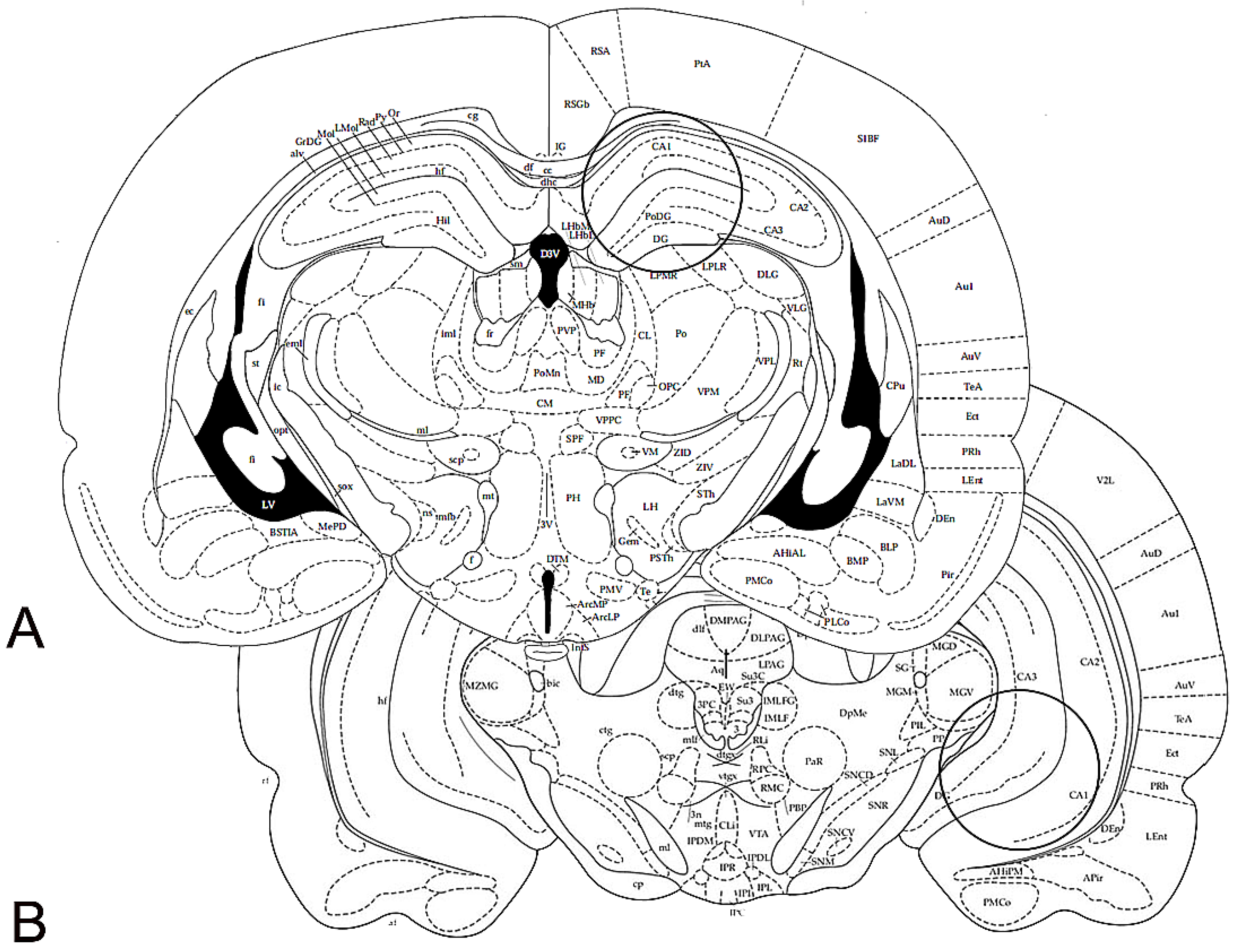
2.3. Immunohistochemistry
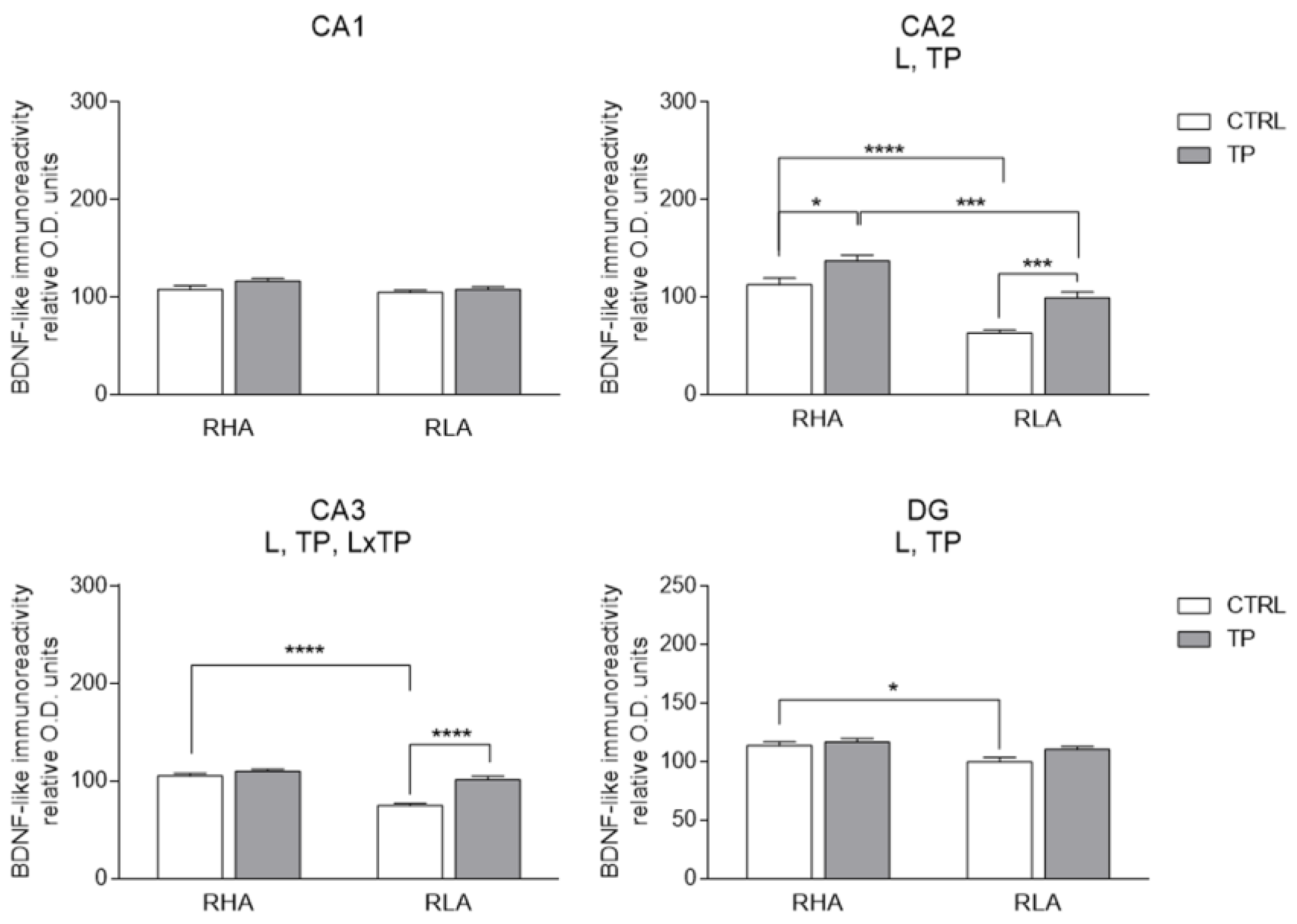
2.3.1. BDNF-like Immunoreactivity
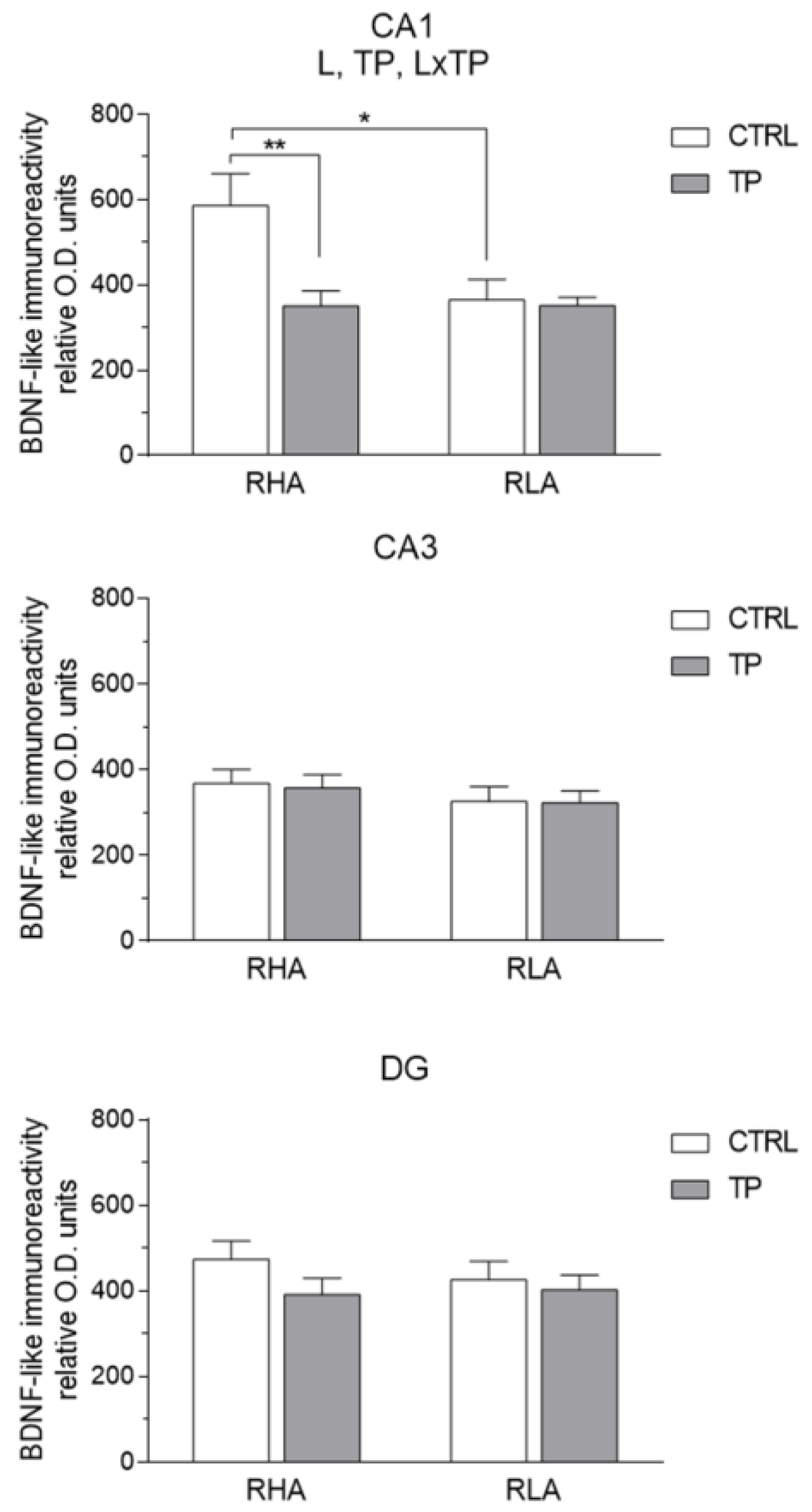
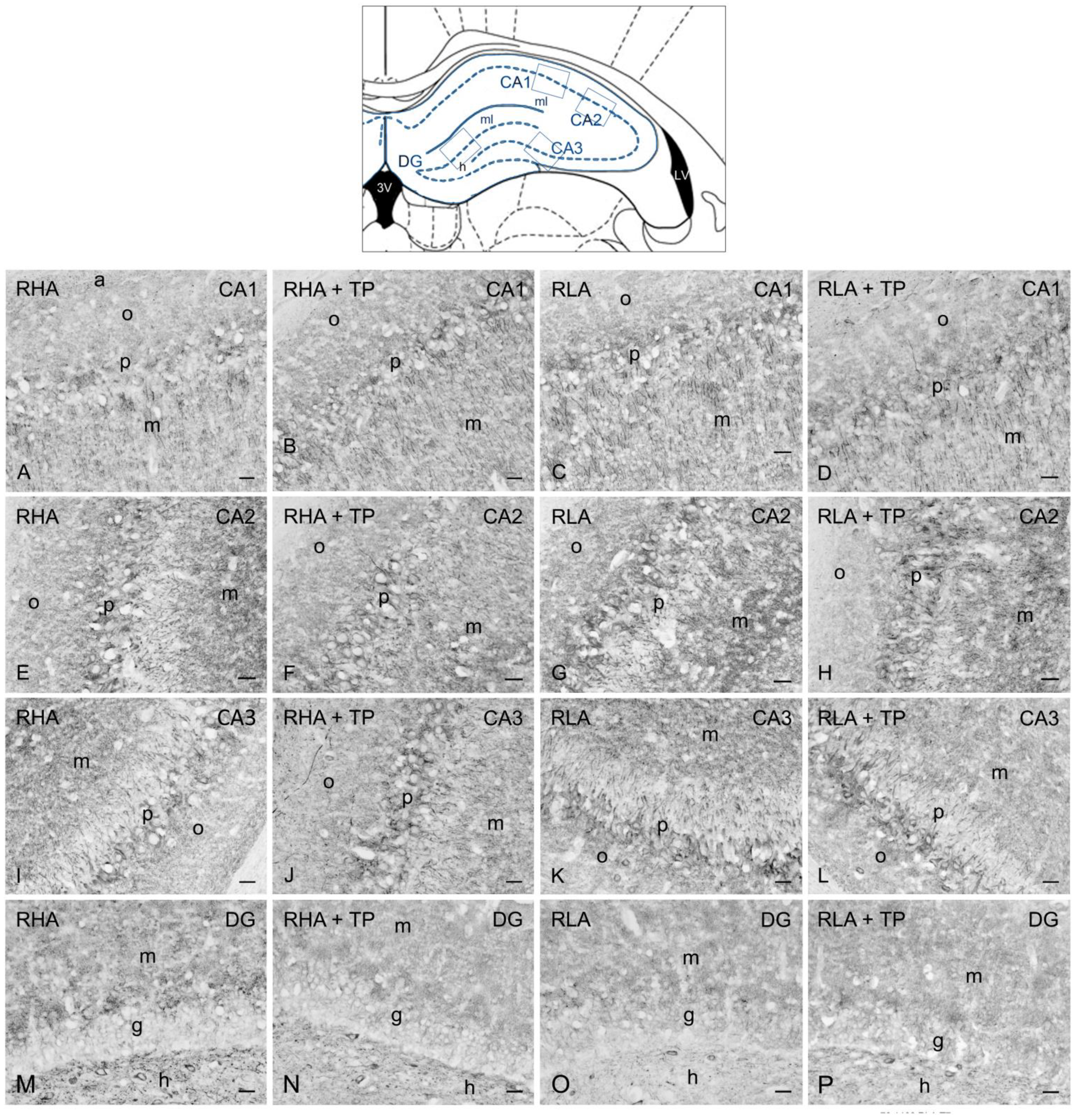
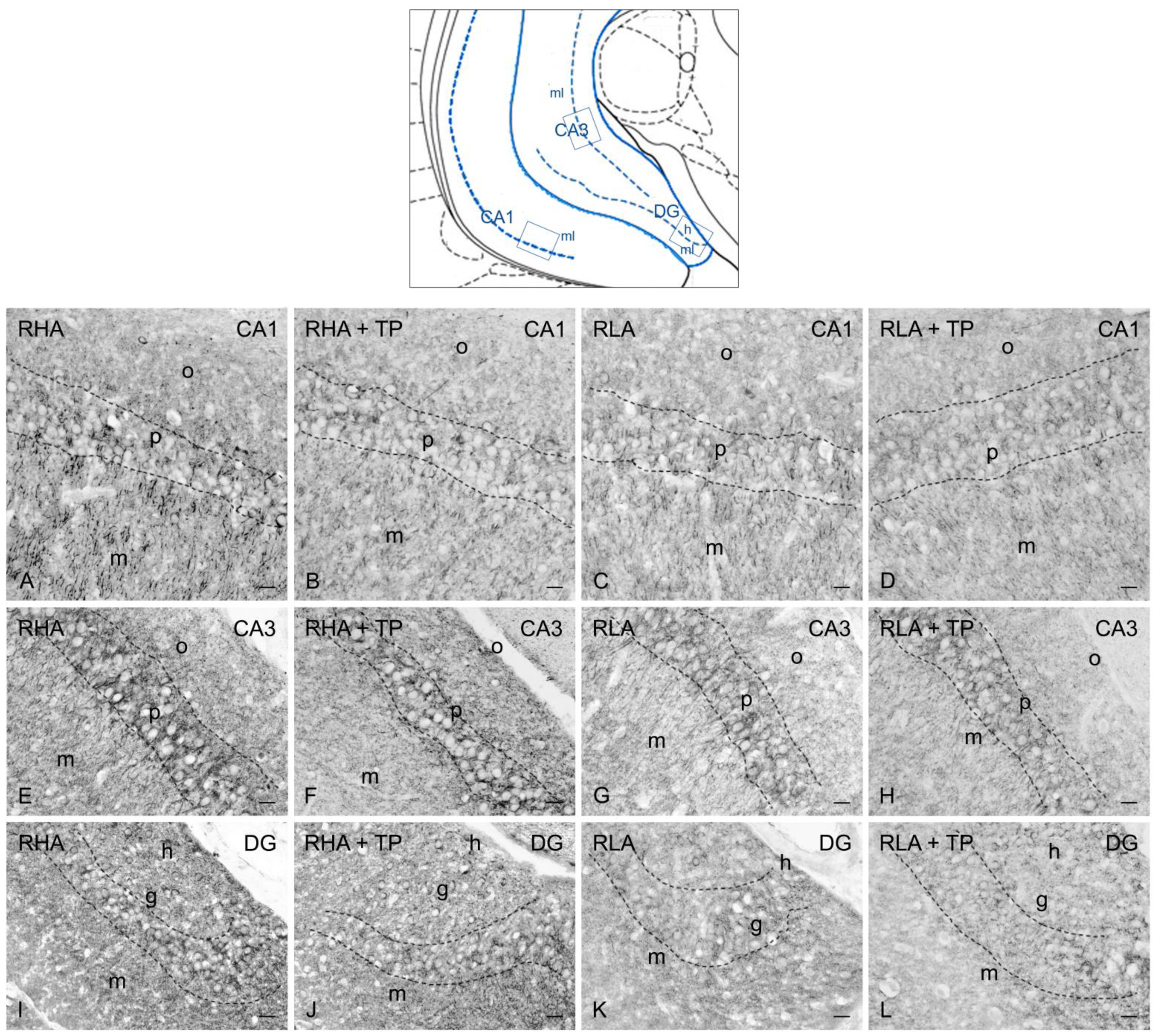
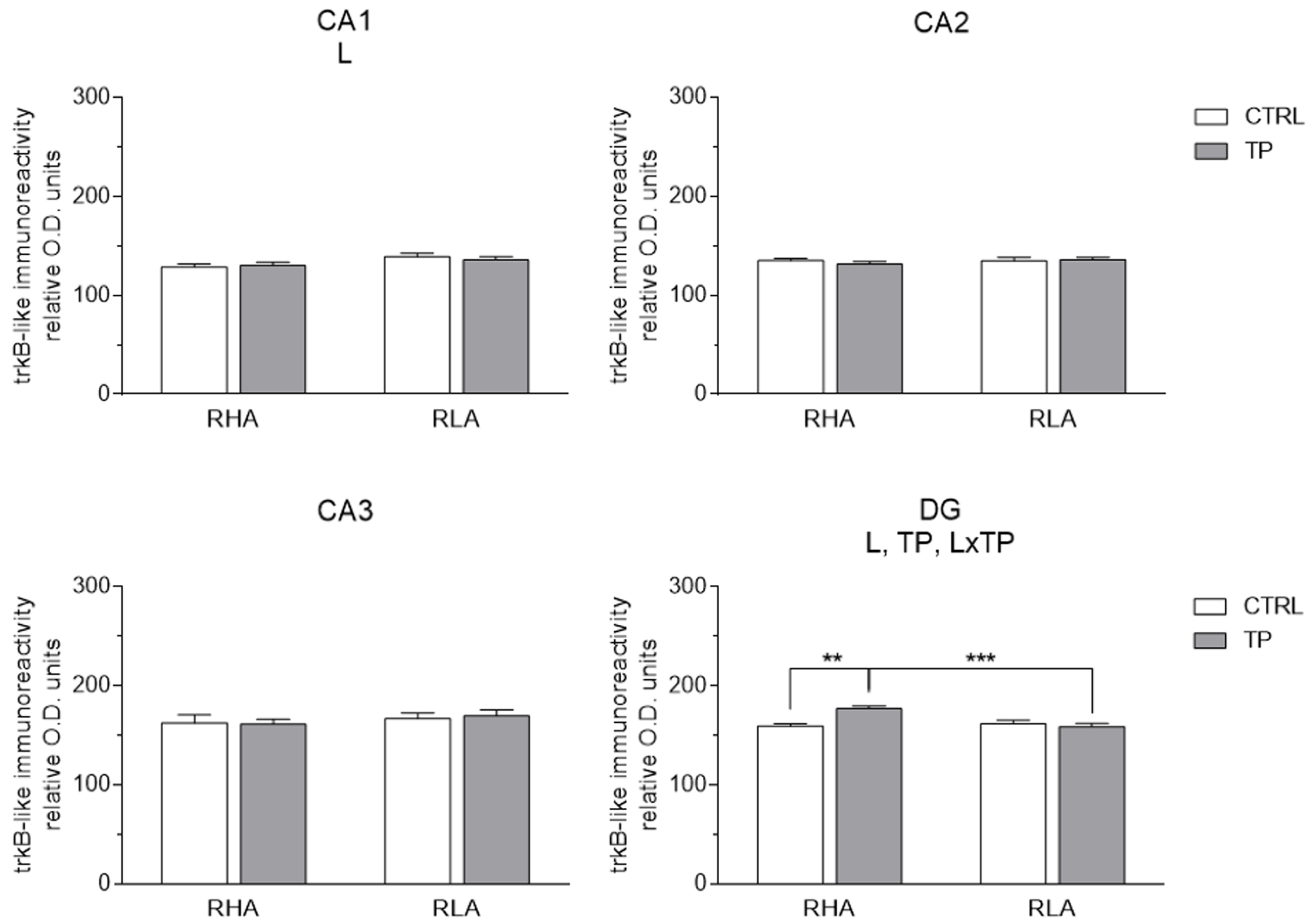
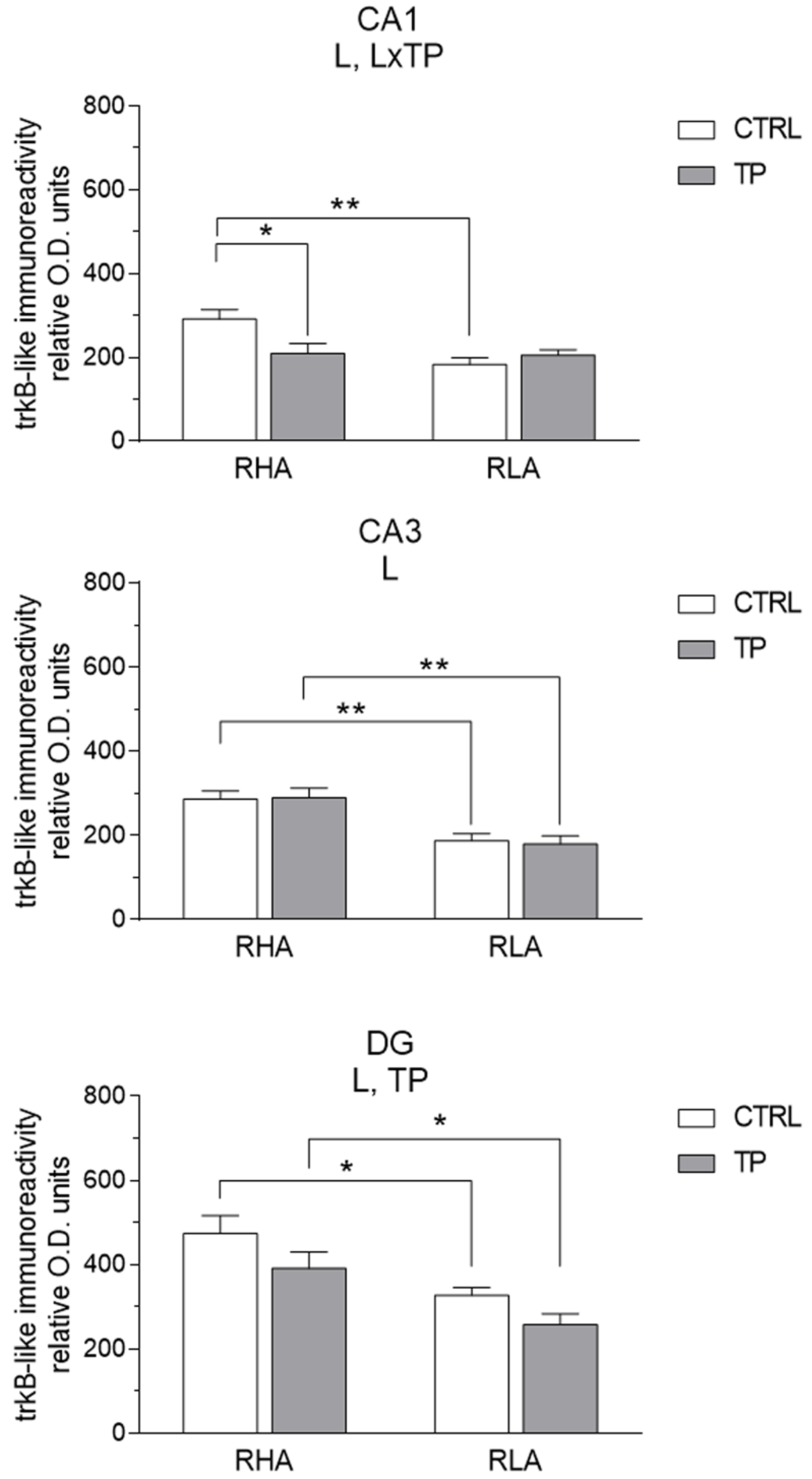
2.3.2. trkB-like Immunoreactivity
3. Discussion
3.1. Effect of Tail Pinch-Induced Acute Stress on the BDNF and trkB Protein Levels in the Dorsal and Ventral Hippocampus
3.2. Effect of Tail Pinch on the Regional and Subregional Immunohistochemical Distribution of BDNF and trkB in the Dorsal and Ventral Hippocampus
3.3. Insights into the Possible Role of Stress Hormones in the Effects of Tail Pinch on BDNF/trkB Signaling in the Dorsal and Ventral Hippocampus
4. Materials and Methods
4.1. Animals
4.2. TP and Behavioral Measurements
4.3. Sampling
4.4. Western Blot
4.5. Immunohistochemistry
4.6. Image Densitometry
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | Avidin–Biotin–peroxidase Complex |
| BDNF | Brain Derived Neurotrophic Factor |
| DG | Dentate Gyrus |
| dHC | dorsal Hippocampus |
| FS | Forced Swimming |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| HPA | Hypothalamus–Pituitary–Adrenal |
| IHC | Immunohistochemistry |
| O.D. | Relative optical density |
| PBS | Phosphate Buffered Saline |
| PFC | Prefrontal cortex |
| RHA | Roman High-Avoidance |
| RLA | Roman Low-Avoidance |
| SDS-PAGE | Sodium dodecyl sulphate-polyacrylamide gel electrophoresis |
| TBS-T | Tris base, Sodium chloride, Tween 2 |
| TP | Tail Pinch |
| trkB | tyrosine receptor kinase B |
| vHC | ventral Hippocampus |
| VTA | Ventral Tegmental Area |
| WB | Western Blot |
References
- Broadhurst, P.L.; Bignami, G. Correlative effects of psychogenetic selection: A study of the Roman high and low avoidance strains of rats. Behav. Res. Ther. 1965, 3, 273–280. [Google Scholar] [CrossRef]
- Driscoll, P.; Bättig, K. Behavioral, emotional and neurochemical profiles of rats selected for extreme differences in active, two-way avoidance performance. In Genetics of the Brain, 1st ed.; Lieblich, I., Ed.; Elsevier Biomedical Press: Amsterdam, NL, USA, 1982; pp. 95–123. [Google Scholar]
- Fernández-Teruel, A.; Escorihuela, R.M.; Gray, J.A.; Aguilar, R.; Gil, L.; Giménez-Llort, L.; Tobeña, A.; Bhomra, A.; Nicod, A.; Mott, R.; et al. A quantitative trait locus influencing anxiety in the laboratory rat. Genome Res. 2002, 12, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, O.; Lecca, D.; Piras, G.; Driscoll, P.; Corda, M.G. Dissociation between mesocortical dopamine release and fear-related behaviours in two psychogenetically selected lines of rats that differ in coping strategies to aversive conditions. Eur. J. Neurosci. 2003, 17, 2716–2726. [Google Scholar] [CrossRef] [PubMed]
- Piras, G.; Giorgi, O.; Corda, M.G. Effects of antidepressants on the performance in the forced swim test of two psychogenetically selected lines of rats that differ in coping strategies to aversive conditions. Psychopharmacology 2010, 211, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Piras, G.; Piludu, M.A.; Giorgi, O.; Corda, M.G. Effects of chronic antidepressant treatments in a putative genetic model of vulnerability (Roman low-avoidance rats) and resistance (Roman high-avoidance rats) to stress-induced depression. Psychopharmacology 2014, 231, 43–53. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 15, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Heninger, G.R.; Nestler, E.J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry 1997, 54, 597–606. [Google Scholar] [CrossRef]
- Gold, P.W.; Chrousos, G.P. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs. low CRH/NE states. Mol. Psychiatry 2002, 7, 254–275. [Google Scholar] [CrossRef]
- Grønli, J.; Bramham, C.; Murison, R.; Kanhema, T.; Fiske, E.; Bjorvatn, B.; Ursin, R.; Portas, C.M. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol. Biochem. Behav. 2006, 85, 842–849. [Google Scholar] [CrossRef]
- Thompson Ray, M.; Weickert, C.S.; Wyatt, E.; Webster, M.J. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J. Psychiatry Neurosci. 2011, 36, 195–203. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Z. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol. Sin. 2011, 32, 3–11. [Google Scholar] [CrossRef]
- Serra, M.P.; Poddighe, L.; Boi, M.; Sanna, F.; Piludu, M.A.; Corda, M.G.; Giorgi, O.; Quartu, M. Expression of BDNF and trkB in the hippocampus of a rat genetic model of vulnerability (Roman low-avoidance) and resistance (Roman high-avoidance) to stress-induced depression. Brain Behav. 2017, 7, e008612017. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.P.; Sanna, F.; Boi, M.; Poddigher, L.; Secci, L.; Trucas, M.; Fernández-Teruel, A.; Corda, M.G.; Giorgi, O.; Quartu, M. Acute Stress Induces Different Changes on the Expression of BDNF and trkB in the Mesocorticolimbic System of Two Lines of Rats Differing in Their Response to Stressors. Int. J. Mol. Sci. 2022, 23, 14995. [Google Scholar] [CrossRef] [PubMed]
- Fanous, S.; Hammer, R.P., Jr.; Nikulina, E.M. Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience 2010, 167, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Shirayama, Y.; Chen, A.C.; Nakagawa, S.; Russell, D.S.; Duman, R.S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002, 22, 223251–223261. [Google Scholar] [CrossRef] [PubMed]
- Eisch, A.J.; Bolaños, C.A.; de Wit, J.; Simonak, R.D.; Pudiak, C.M.; Barrot, M.; Verhaagen, J.; Nestler, E.J. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: A role in depression. Biol. Psychiatry 2003, 54, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.P.; Poddighe, L.; Boi, M.; Sanna, F.; Piludu, M.A.; Sanna, F.; Corda, M.G.; Giorgi, O.; Quartu, M. Effect of Acute Stress on the Expression of BDNF, trkB, and PSA-NCAM in the Hippocampus of the Roman Rats: A Genetic Model of Vulnerability/Resistance to Stress-Induced Depression. Int. J. Mol. Sci. 2018, 19, 3745. [Google Scholar] [CrossRef]
- D’Angio, M.; Serrano, A.; Driscoll, P.; Scatton, B. Stressful environmental stimuli increase extracellular DOPAC levels in the prefrontal cortex of hypoemotional (Roman high-avoidance) but not hyperemotional (Roman low-avoidance) rats. An in vivo voltammetric study. Brain Res. 1988, 451, 237–247. [Google Scholar] [CrossRef]
- Brake, W.G.; Sullivan, R.M.; Gratton, A. Perinatal distress leads to lateralized medial prefrontal cortical dopamine hypofunction in adult rats. J. Neurosci. 2000, 20, 5538–5543. [Google Scholar] [CrossRef]
- Butts, K.A.; Phillips, A.G. Glucocorticoid receptors in the prefrontal cortex regulate dopamine efflux to stress via descending glutamatergic feedback to the ventral tegmental area. Int. J. Neuropsychopharmacol. 2013, 16, 1799–1807. [Google Scholar] [CrossRef]
- Naef, R.; Ward, R.; Mahrer-Imhof, R.; Grande, G. Characteristics of the bereavement experience of older persons after spousal loss: An integrative review. Int. J. Nurs. Stud. 2013, 50, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.; Goeddel, D.V.; Nguyen, T.; Martin, E.; Burton, L.E.; Shih, A.; Laramee, G.R.; Wurm, F.; Mason, A.; Nikolics, K.; et al. Primary structure and biological activity of human brain-derived neurotrophic factor. Endocrinology 1991, 129, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Parada, L.F.; Coulier, F.; Barbacid, M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 1989, 8, 3701–3709. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998; p. 237. ISBN 10: 0125476191. [Google Scholar]
- Steimer, T.; Python, A.; Schulz, P.E.; Aubry, J.M. Plasma corticosterone, dexamethasone (DEX) suppression and DEX/CRH tests in a rat model of genetic vulnerability to depression. Psychoneuroendocrinology 2007, 32, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Maggio, N.; Segal, M. Corticosteroid regulation of synaptic plasticity in the hippocampus. Sci. World J. 2010, 10, 462–469. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- Fernández-Teruel, A.; Tobeña, A. Revisiting the role of anxiety in the initial acquisition of two-way active avoidance: Pharmacological, behavioural and neuroanatomical convergence. Neurosci. Biobehav. Rev. 2020, 118, 739–758. [Google Scholar] [CrossRef]
- Bryant, K.G.; Barker, J.M. Arbitration of Approach-Avoidance Conflict by Ventral Hippocampus. Front. Neurosci. 2020, 14, 615337. [Google Scholar] [CrossRef]
- Roelofs, K.; Dayan, P. Freezing revisited: Coordinated autonomic and central optimization of threat coping. Nat. Rev. Neurosci. 2022, 23, 568–580. [Google Scholar] [CrossRef]
- Fernández-Teruel, A.; McNaughton, N. Post-encounter freezing during approach-avoidance conflict: The role of the hippocampus. Nat. Rev. Neurosci. 2023. [Google Scholar] [CrossRef]
- Murakami, S.; Imbe, H.; Morikawa, Y.; Kubo, C.; Senba, E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci. Res. 2005, 53, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Vadodaria, K.C.; Banerjee, S.B.; Benekareddy, M.; Dias, B.G.; Duman, R.S.; Vaidya, V.A. Stressor-specific regulation of distinct brain-derived neurotrophic factor transcripts and cyclic AMP response element-binding protein expression in the postnatal and adult rat hippocampus. Neuropsychopharmacology 2007, 32, 1504–1519. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C.; Duman, R.S. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 2008, 33, 88–109. [Google Scholar] [CrossRef] [PubMed]
- Kozisek, M.E.; Middlemas, D.; Bylund, D.B. The differential regulation of BDNF and TrkB levels in juvenile rats after four days of escitalopram and desipramine treatment. Neuropharmacology 2008, 54, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Molteni, R.; Calabrese, F.; Cattaneo, A.; Mancini, M.; Gennarelli, M.; Racagni, G.; Riva, M.A. Acute stress responsiveness of the neurotrophin BDNF in the rat hippocampus is modulated by chronic treatment with the antidepressant duloxetine. Neuropsychopharmacology 2009, 34, 1523–1532. [Google Scholar] [CrossRef]
- Rendeiro, C.; Spencer, J.P.; Vauzour, D.; Butler, L.T.; Ellis, J.A.; Williams, C.M. The impact of flavonoids on spatial memory in rodents: From behaviour to underlying hippocampal mechanisms. Genes Nutr. 2009, 4, 251–270. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 239–245. [Google Scholar] [CrossRef]
- Marmigère, F.; Givalois, L.; Rage, F.; Arancibia, S.; Tapia-Arancibia, L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus 2003, 13, 646–655. [Google Scholar] [CrossRef]
- Shi, S.; Shao, S.; Yuan, B.; Pan, F.; Li, Z. Acute Stress and Chronic Stress Change Brain-Derived Neurotrophic Factor (BDNF) and Tyrosine Kinase-Coupled Receptor (TrkB) Expression in Both Young and Aged Rat Hippocampus. Yonsei. Med. J. 2010, 51, 661–671. [Google Scholar] [CrossRef]
- Uysal, N.; Sisman, A.R.; Dayi, A.; Ozbal, S.; Cetin, F.; Baykara, B.; Aksu, I.; Tas, A.; Cavus, S.A.; Gonenc-Arda, S.; et al. Acute footshock-stress increases spatial learning-memory and correlates to increased hippocampal BDNF and VEGF and cell numbers in adolescent male and female rats. Neurosci. Lett. 2012, 514, 141–146. [Google Scholar] [CrossRef]
- Dorey, R.; Piérard, C.; Chauveau, F.; David, V.; Béracochéa, D. Stress-induced memory retrieval impairments: Different time-course involvement of corticosterone and glucocorticoid receptors in dorsal and ventral hippocampus. Neuropsychopharmacology 2012, 37, 2870–2880. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Rutishauser, U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J. Neurosci. 1998, 18, 3757–3766. [Google Scholar] [CrossRef] [PubMed]
- Isgor, C.; Pare, C.; McDole, B.; Coombs, P.; Guthrie, K. Expansion of the dentate mossy fiber-CA3 projection in the brain-derived neurotrophic factor-enriched mouse hippocampus. Neuroscience 2015, 288, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Holsboer, F.; Barden, N. Antidepressants and HPA regulation. Endocrinol. Rev. 1996, 17, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Rybakowski, J.K.; Twardowska, K. The dexamethasone/corticotropin-releasing hormone test in depression in bipolar and unipolar affective illness. J. Psychiatric Res. 1999, 33, 363–370. [Google Scholar] [CrossRef]
- de Bellis, M.D.; Gold, P.W.; Geracioti, T.D., Jr.; Listwak, S.J.; Kling, M.A. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin releasing hormone and arginine vasopressin in patients with major depression. Am. J. Psychiatry 1993, 150, 656–657. [Google Scholar] [CrossRef]
- Gentsch, C.; Lichtsteiner, M.; Driscoll, P.; Feer, H. Differential hormonal and physiological responses to stress in Roman high- and low-avoidance rats. Physiol. Behav. 1982, 28, 259–263. [Google Scholar] [CrossRef]
- Aubry, J.M.; Bartanusz, V.; Driscoll, P.; Schulz, P.; Steimer, T.; Kiss, J.Z. Corticotropin-releasing factor and vasopressin mRNAlevels in Roman high-and low-avoidance rats: Response to open field exposure. Neuroendocrinology 1995, 61, 89–97. [Google Scholar] [CrossRef]
- Steimer, T.; Driscoll, P. Divergent stress responses and coping styles in psychogenetically selected Roman high-(RHA) and low-(RLA) avoidance rats: Behavioural, neuroendocrine and developmental aspects. Stress 2003, 6, 87–100. [Google Scholar] [CrossRef]
- Castanon, N.; Dulluc, J.; Le Moal, M.; Mormède, P. Maturation of the behavioral and neuroendocrine differences between the Roman rat lines. Physiol. Behav. 1994, 55, 775–782. [Google Scholar] [CrossRef]
- Steimer, T.; Driscoll, P. Inter-individual vs. line/strain differences in psychogenetically selected Roman High-(RHA) and Low-(RLA) Avoidance rats: Neuroendocrine and behavioural aspects. Neurosci. Biobehav. Rev. 2005, 29, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Bajaj, T.; Klengel, C.; Chatzinakos, C.; Ebert, T.; Dedic, N.; McCullough, K.M.; Lardenoije, R.; Joëls, M.; Meijer, O.C.; et al. Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Rep. 2021, 35, 109185. [Google Scholar] [CrossRef]
- Walker, C.D.; Rivest, R.V.; Meaney, M.J.; Aubert, M.L. Differential activation of the pituitary-adrenocortical axis after stress in the rat: Use of two genetically selected lines (Roman low- and high-avoidance rats) as a model. J. Endocrinol. 1989, 123, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Maggio, N.; Segal, M. Differential corticosteroid modulation of inhibitory synaptic currents in the dorsal and ventral hippocampus. J. Neurosci. 2009, 29, 2857–2866. [Google Scholar] [CrossRef] [PubMed]
- Segal, M.; Vlachos, A.; Korkotian, E. The spine apparatus, synaptopodin, and dendritic spine plasticity. Neuroscientist 2010, 16, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Segal, M.; Richter-Levin, G.; Maggio, M. Stress-induced dynamic routing of hippocampal connectivity: A hypothesis. Hippocampus 2010, 20, 1332–1338. [Google Scholar] [CrossRef]
- Robertson, D.A.; Beattie, J.E.; Reid, I.C.; Balfour, D.J. Regulation of corticosteroid receptors in the rat brain: The role of serotonin and stress. Eur. J. Neurosci. 2005, 21, 1511–1520. [Google Scholar] [CrossRef]
- Caudal, D.; Jay, T.M.; Godsil, B.P. Behavioral stress induces regionally-distinct shifts of brain mineralocorticoid and glucocorticoid receptor levels. Front. Behav. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Schaaf, M.J.; Hoetelmans, R.W.; de Kloet, E.R.; Vreugdenhil, E. Corticosterone regulates expression of BDNF and trkB but not NT-3 and trkC mRNA in the rat hippocampus. J. Neurosci. Res. 1997, 48, 334–341. [Google Scholar] [CrossRef]
- Jeanneteau, F.; Garabedian, M.J.; Chao, M.V. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proc. Natl. Acad. Sci. USA 2008, 105, 4862–4867. [Google Scholar] [CrossRef]
- Pandya, C.; Kutiyanawalla, A.; Turecki, G.; Pillai, A. Glucocorticoid regulates TrkB protein levels via c-Cbl dependent ubiquitination: A decrease in c-Cbl mRNA in the prefrontal cortex of suicide subjects. Psychoneuroendocrinology 2014, 45, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Maggio, N.; Segal, M. Striking variations in corticosteroid modulation of long-term potentiation along the septotemporal axis of the hippocampus. J. Neurosci. 2007, 27, 5757–5765. [Google Scholar] [CrossRef] [PubMed]
- Bray, B.; Clement, K.A.; Bachmeier, D.; Weber, M.A.; Forster, G.L. Corticosterone in the ventral hippocampus differentially alters accumbal dopamine output in drug-naïve and amphetamine-withdrawn rats. Neuropharmacology 2020, 165, 107924. [Google Scholar] [CrossRef]
- Arango-Lievano, M.; Lambert, W.M.; Bath, K.G.; Garabedian, M.J.; Chao, M.V.; Jeanneteau, F. Neurotrophic-priming of glucocorticoid receptor signaling is essential for neuronal plasticity to stress and antidepressant treatment. Proc. Natl. Acad. Sci. USA 2015, 112, 15737–15742. [Google Scholar] [CrossRef]
- Palkovits, M. Punch sampling biopsy technique. Methods Enzymol. 1983, 103, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurements with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–327. [Google Scholar] [CrossRef] [PubMed]
- Sanna, F.; Poddighe, L.; Serra, M.P.; Boi, M.; Bratzu, J.; Sanna, F.; Corda, M.G.; Giorgi, O.; Melis, M.R.; Argiolas, A.; et al. c-Fos, ΔFosB, BDNF, trkB and Arc expression in the limbic system of male Roman High and Low Avoidance rats that show differences in sexual behaviour: Effect of sexual activity. Neuroscience 2018, 396, 1–23. [Google Scholar] [CrossRef]
- Linz, R.; Puhlmann, L.M.C.; Apostolakou, F.; Mantzou, E.; Papassotiriou, I.; Chrousos, G.P.; Engert, V.; Singer, T. Acute psychosocial stress increases serum BDNF levels: An antagonistic relation to cortisol but no group differences after mental training. Neuropsychopharmacology 2019, 44, 1797–1804. [Google Scholar] [CrossRef]
- Hermann, R.; Schaller, A.; Lay, D.; Bloch, W.; Albus, C.; Petrowski, K. Effect of acute psychosocial stress on the brain-derived neurotrophic factor in humans—A randomized cross within trial. Stress 2021, 24, 442–449. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]


| Behavioral Measures (s) | RHA | RLA |
|---|---|---|
| Clamp biting | 789 ± 60 | 294 ± 51 **** |
| Tail licking | 161 ± 23 | 326 ± 34 *** |
| Freezing | 5 ± 2 | 134 ± 17 **** |
| Grooming | 43 ± 5 | 81 ± 11 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, M.P.; Sanna, F.; Boi, M.; Trucas, M.; Fernández-Teruel, A.; Corda, M.G.; Giorgi, O.; Quartu, M. Effects of Tail Pinch on BDNF and trkB Expression in the Hippocampus of Roman Low- (RLA) and High-Avoidance (RHA) Rats. Int. J. Mol. Sci. 2023, 24, 9498. https://doi.org/10.3390/ijms24119498
Serra MP, Sanna F, Boi M, Trucas M, Fernández-Teruel A, Corda MG, Giorgi O, Quartu M. Effects of Tail Pinch on BDNF and trkB Expression in the Hippocampus of Roman Low- (RLA) and High-Avoidance (RHA) Rats. International Journal of Molecular Sciences. 2023; 24(11):9498. https://doi.org/10.3390/ijms24119498
Chicago/Turabian StyleSerra, Maria Pina, Francesco Sanna, Marianna Boi, Marcello Trucas, Alberto Fernández-Teruel, Maria Giuseppa Corda, Osvaldo Giorgi, and Marina Quartu. 2023. "Effects of Tail Pinch on BDNF and trkB Expression in the Hippocampus of Roman Low- (RLA) and High-Avoidance (RHA) Rats" International Journal of Molecular Sciences 24, no. 11: 9498. https://doi.org/10.3390/ijms24119498
APA StyleSerra, M. P., Sanna, F., Boi, M., Trucas, M., Fernández-Teruel, A., Corda, M. G., Giorgi, O., & Quartu, M. (2023). Effects of Tail Pinch on BDNF and trkB Expression in the Hippocampus of Roman Low- (RLA) and High-Avoidance (RHA) Rats. International Journal of Molecular Sciences, 24(11), 9498. https://doi.org/10.3390/ijms24119498








