What Other Than Acridinium Esters? Computational Search for New Acridinium-Based Chemiluminogens
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of Investigated Objects
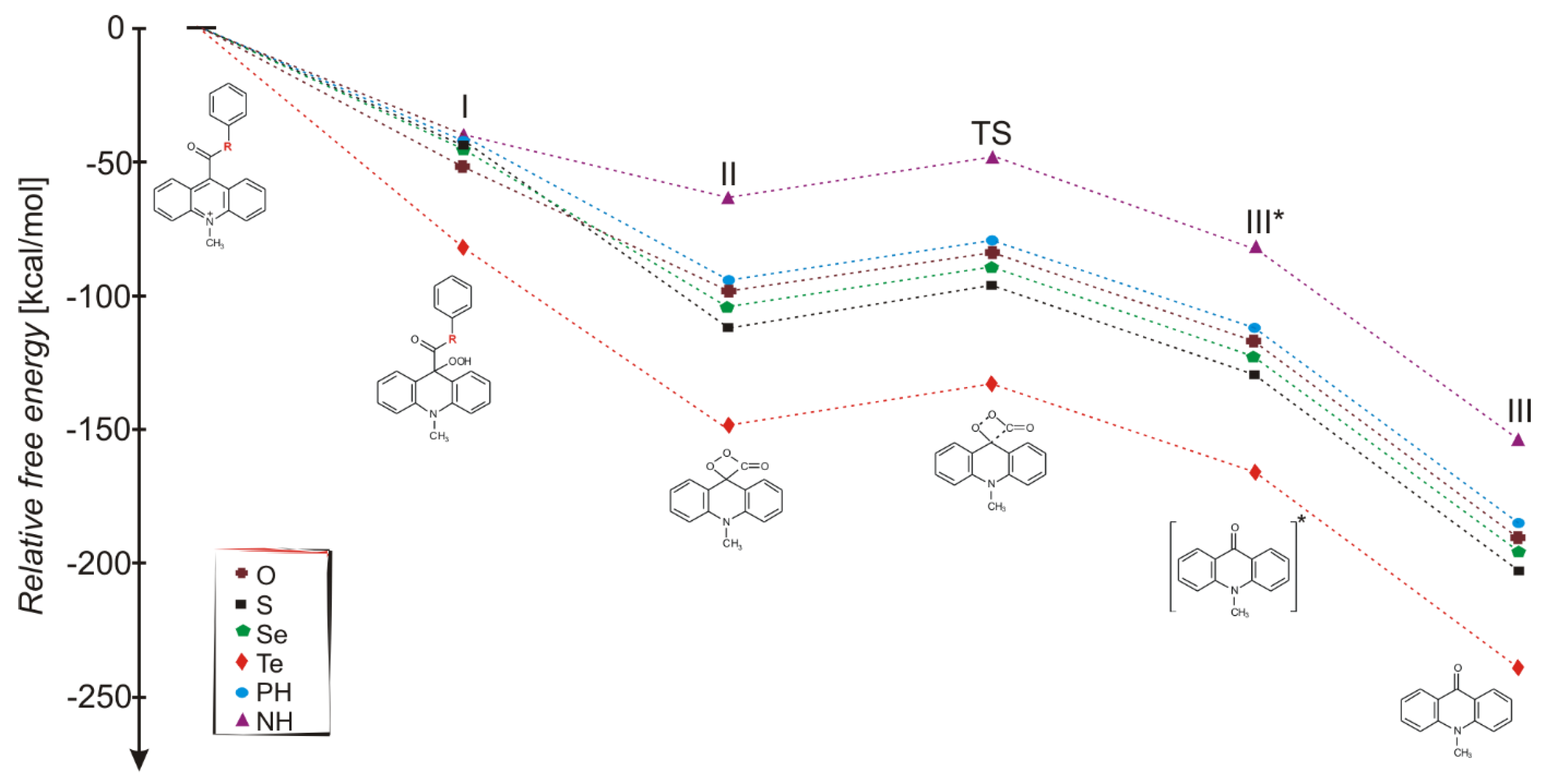
2.2. Susceptibility to Nucleophilic Attack
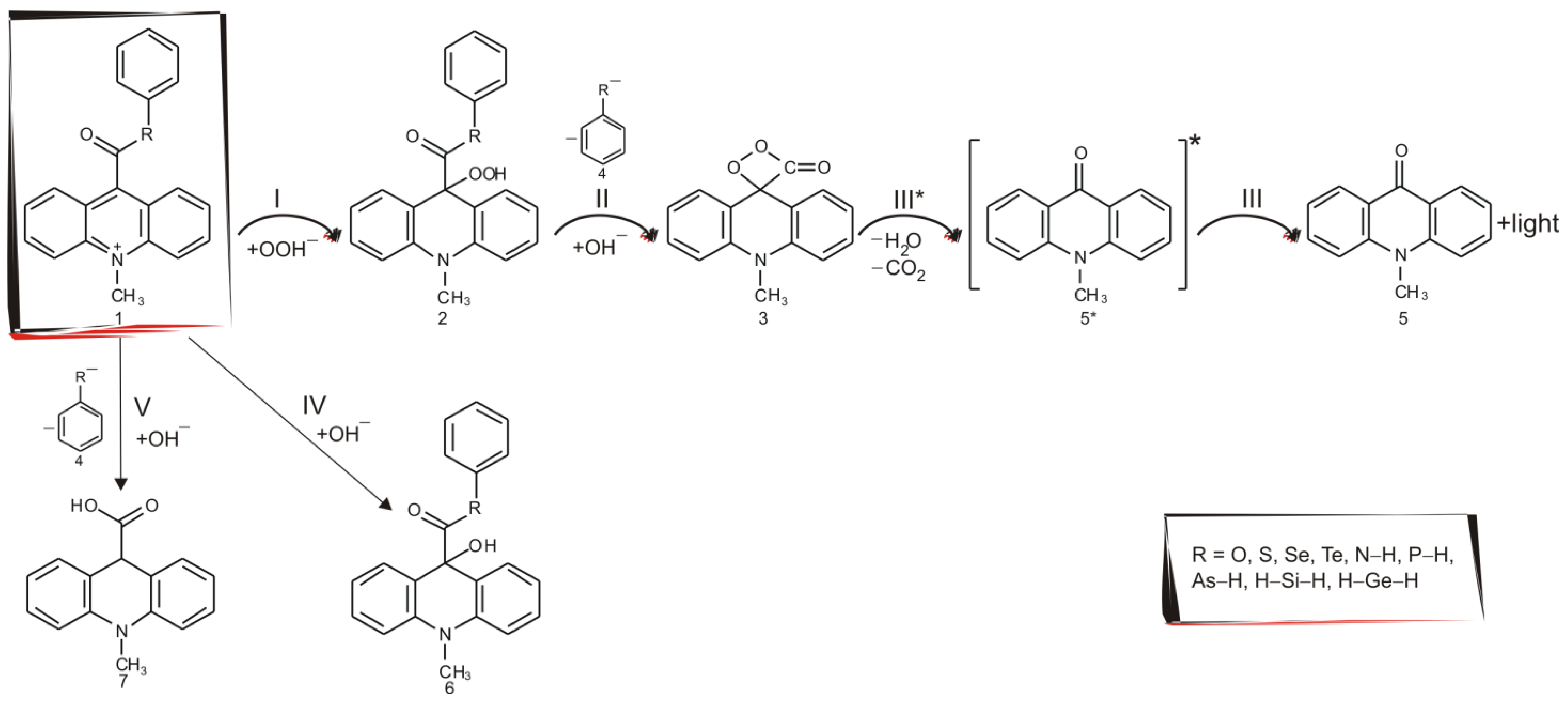
2.3. Light Pathway of Chemiluminescence Reaction and Competitive Pathways
- 1.
- step I—nucleophilic attack of anionic form of oxidant (e.g., OOH−) at the C9 atom on acridinium moiety and formation of molecule 2;
- 2.
- step II—reaction of the addition product 2 with hydroxide ions to form cyclic intermediate 3 after elimination of R-phenyl anion (molecule 4);
- 3.
- steps III* and III—a unimolecular decomposition of the cyclic entity 3 after elimination of carbon dioxide and formation of the electronically excited 10-methyl-9-acridinone (molecule 5*), and then returned to the ground state (molecule 5), by applying the approach:
- the thermally accessible dioxetanone to reach an electronically excited state of 10-methyl-9-acridinone molecule in S0;
- nonadiabatic transition through spin-orbit coupling between S0 and S1;
- the final decomposition to reach 10-methyl-9-acridinone in S1.
- 4.
- step IV—nucleophilic attack of hydroxide ions at the C9 atom on acridinium moiety and formation of so-called pseudobase (molecule 6);
- 5.
- step V—nucleophilic attack of hydroxide ions at the C15 atom of the carbonyl group and formation of 10-methyl-9-carboxyacridinium acid (molecule 7).
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CL | Chemiluminescence |
| DFT | Density functional theory |
| HOMO | Highest Occupied Molecular Orbital |
| LCAO | Linear combination of atomic orbitals |
| LUMO | Lowest Unoccupied Molecular Orbital |
| PCM | Polarizable Continuum Model |
| TD DFT | Time dependent density functional theory |
| TS | Transition State |
References
- Gundermann, K.D.; McCapra, F. Chemiluminescence in Organic Chemistry; Reactivity and Structure: Concepts in Organic Chemistry; Springer: Berlin/Heidelberg, Germany, 1987; pp. 109–118. [Google Scholar] [CrossRef]
- Nakazono, M.; Oshikawa, Y.; Nakamura, M.; Kubota, H.; Nanbu, S. Strongly Chemiluminescent Acridinium Esters under Neutral Conditions: Synthesis, Properties, Determination, and Theoretical Study. J. Org. Chem. 2017, 82, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Pasini, P.; Guardigli, M.; Baraldini, M.; Musiani, M.; Mirasoli, M. Bio- and Chemiluminescence in Bioanalysis. Fresenius J. Anal. Chem. 2000, 366, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Krzymiński, K.; Ożóg, A.; Malecha, P.; Roshal, A.D.; Wróblewska, A.; Zadykowicz, B.; Błażejowski, J. Chemiluminogenic Features of 10-Methyl-9-(Phenoxycarbonyl)Acridinium Trifluoromethanesulfonates Alkyl Substituted at the Benzene Ring in Aqueous Media. J. Org. Chem. 2011, 76, 1072–1085. [Google Scholar] [CrossRef]
- Zadykowicz, B.; Czechowska, J.; Ożóg, A.; Renkevich, A.; Krzymiński, K. Effective Chemiluminogenic Systems Based on Acridinium Esters Bearing Substituents of Various Electronic and Steric Properties. Org. Biomol. Chem. 2016, 14, 652–668. [Google Scholar] [CrossRef]
- Pieńkos, M.; Zadykowicz, B. Computational Insights on the Mechanism of the Chemiluminescence Reaction of New Group of Chemiluminogens—10-Methyl-9-thiophenoxycarbonylacridinium Cations. Int. J. Mol. Sci. 2020, 21, 4417. [Google Scholar] [CrossRef] [PubMed]
- Czechowska, J.; Kawecka, A.; Romanowska, A.; Marczak, M.; Wityk, P.; Krzymiński, K.; Zadykowicz, B. Chemiluminogenic Acridinium Salts: A Comparison Study. Detection of Intermediate Entities Appearing upon Light Generation. J. Lumin. 2017, 187, 102–112. [Google Scholar] [CrossRef]
- Nakazono, M.; Nanbu, S.; Akita, T.; Hamase, K. Synthesis, Chemiluminescence, and Application of 2,4-Disubstituted Phenyl 10-Methyl-10λ4-Acridine-9-Carboxylates. Dyes Pigment. 2019, 170, 107628. [Google Scholar] [CrossRef]
- Ren, L.; Cui, H. Chemiluminescence Accompanied by the Reaction of Acridinium Ester and Manganese (II). Luminescence 2014, 29, 929–932. [Google Scholar] [CrossRef]
- Smith, K.; Ahmed, Z.; Woodhead, J.S.; El-Hiti, G.A. Syntheses of Hindered-Polymethylacridinium Esters with Potential for Biological Probe Nanoarchitectonics. J. Oleo Sci. 2023, 460, 447–460. [Google Scholar] [CrossRef]
- Smith, K.; Mu, X.; Li, Z.; Holland, A.M.; Woodhead, J.S.; El-Hiti, G.A. Synthesis, structure elucidation, and chemiluminescent activity of new 9-substituted 10-(ω-(succinimidyloxycarbonyl)alkyl)acridinium esters. Luminescence 2023, 38, 487–496. [Google Scholar] [CrossRef]
- Lan, Y.; Yuan, F.; Fereja, T.H.; Wang, C.; Lou, B.; Li, J.; Xu, G. Chemiluminescence of Lucigenin/Riboflavin and Its Application for Selective and Sensitive Dopamine Detection. Anal. Chem. 2019, 91, 2135–2139. [Google Scholar] [CrossRef]
- Montano, L.A.; Ingle, J.D. Investigation of the Lucigenin Chemiluminescence Reaction. Anal. Chem. 1979, 51, 919–926. [Google Scholar] [CrossRef]
- Wu, F.; He, Z.; Luo, Q.; Zeng, Y. HPLC determination of oxalic acid using tris(1,10-phenanthroline)ruthenium(II) chemiluminescence-application to the analysis of spinach. Food Chem. 1999, 65, 543–546. [Google Scholar] [CrossRef]
- He, Z.; Gao, H. Simultaneous determination of oxalic and tartaric acid with chemiluminescence detection. Analyst 1997, 122, 1343–1345. [Google Scholar] [CrossRef]
- Hamilton, P.A.; Murrells, T.P. Mechanism for the Chemiluminescence in Oxygen-Phosphorus Systems. J. Chem. Phys. 1986, 90, 182–185. [Google Scholar] [CrossRef]
- VanZee, R.J.; Khan, A.U. Transient emitting species in phosphorus chemiluminescence. J. Chem. Phys. 1976, 65, 1764–1772. [Google Scholar] [CrossRef]
- Hutte, R.S.; Sievers, R.E.; Birks, J.W. Gas chromatography detectors based on chemiluminescence. J. Chromatogr. Sci. 1986, 24, 499–505. [Google Scholar] [CrossRef]
- Wróblewska, A.; Huta, O.M.; Midyanyj, S.V.; Patsay, I.O.; Rak, J.; Błażejowski, J. Origin of Chemiluminescence Accompanying the Reaction of the 9-Cyano-10-methylacridinium Cation with Hydrogen Peroxide. J. Org. Chem. 2004, 69, 1607–1614. [Google Scholar] [CrossRef]
- Zomer, G.; Stavenuiter, J.F.C. Chemiluminogenic Labels, Old and New. Anal. Chim. Acta 1989, 227, 11–19. [Google Scholar] [CrossRef]
- Arakawa, H.; Tsuruoka, K.; Ohno, K.I.; Tajima, N.; Nagano, H. Development of a Highly Sensitive Chemiluminescent Assay for Hydrogen Peroxide under Neutral Conditions Using Acridinium Ester and Its Application to an Enzyme Immunoassay. Luminescence 2014, 29, 374–377. [Google Scholar] [CrossRef]
- Roda, A.; Guardigli, M. Analytical Chemiluminescence and Bioluminescence: Latest Achievements and New Horizons. Anal. Bioanal. Chem. 2012, 402, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Kishikawa, N.; Ohyama, K.; Ohba, Y.; Kohno, M.; Masuda, T.; Takadate, A.; Nakashima, K.; Kuroda, N. Evaluation of Chemiluminescence Reagents for Selective Detection of Reactive Oxygen Species. Anal. Chim. Acta 2010, 665, 74–78. [Google Scholar] [CrossRef]
- Giokas, D.L.; Vlessidis, A.G.; Tsogas, G.Z.; Evmiridis, N.P. Nanoparticle-Assisted Chemiluminescence and Its Applications in Analytical Chemistry. Trends Anal. Chem. 2010, 29, 1113–1126. [Google Scholar] [CrossRef]
- Jones, M.R.; Lee, K. Determination of Environmental H2O2 for Extended Periods by Chemiluminescence with Real-Time Inhibition of Iron Interferences. Microchem. J. 2019, 147, 1021–1027. [Google Scholar] [CrossRef]
- Natrajan, A.; Sharpe, D.; Wen, D. Chemiluminescence from Alkoxy-Substituted Acridinium Dimethylphenyl Ester Labels. Org. Biomol. Chem. 2012, 10, 3432–3447. [Google Scholar] [CrossRef] [PubMed]
- Best, Q.A.; Haack, R.A.; Swift, K.M.; Bax, B.M.; Tetin, S.Y.; Hershberger, S.J. A rainbow of acridinium chemiluminescence. Luminescence 2021, 36, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Chaichi, M.J.; Va’ezi, Z.; Hosseini, M.; Hosseinkhani, S.; Shamsipur, M. The study of chemiluminescence of acridinium ester in presence of rhodamin B as a fluorescer. Iran. J. Chem. Chem. Eng. 2011, 30, 89–96. [Google Scholar] [CrossRef]
- Dodeigne, C.; Thunus, L.; Lejeune, R. Chemiluminescence as a Diagnostic Tool. A Review. Talanta 2000, 51, 415–439. [Google Scholar] [CrossRef]
- Natrajan, A.; Sharpe, D. Synthesis and Properties of Differently Charged Chemiluminescent Acridinium Ester Labels. Org. Biomol. Chem. 2013, 11, 1026–1039. [Google Scholar] [CrossRef]
- Richardson, A.P.; Kim, J.B.; Barnard, G.J.; Collins, W.P.; McCapra, F. Chemiluminescence immunoassay of plasma progesterone, with progesterone-acridinium ester used as the labeled antigen. Clin. Chem. 1985, 31, 1664–1668. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, L.; Chu, X. Chemiluminescence Immunoassay. Trends Anal. Chem. 2009, 28, 404–415. [Google Scholar] [CrossRef]
- Goryacheva, I.Y.; Lenain, P.; De Saeger, S. Nanosized Labels for Rapid Immunotests. Trends Anal. Chem. 2013, 46, 30–43. [Google Scholar] [CrossRef]
- Brown, R.C.; Li, Z.; Rutter, A.J.; Mu, X.; Weeks, O.H.; Smith, K.; Weeks, I. Development and application of a novel acridinium ester for use as a chemiluminescent emitter in nucleic acid hybridisation assays using chemiluminescence quenching. Org. Biomol. Chem. 2009, 7, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Pasini, P.; Mirasoli, M.; Michelini, E.; Guardigli, M. Biotechnological applications of bioluminescence and chemiluminescence. Trends Biotechnol. 2004, 22, 295–303. [Google Scholar] [CrossRef]
- Pieńkos, M.; Zadykowicz, B. Solvent Effect on the Chemiluminescence of Acridinium Thioester: A Computational Study. ChemPhysChem 2022, 23, e202200166. [Google Scholar] [CrossRef]
- Krzymiński, K.; Roshal, A.D.; Zadykowicz, B.; Białk-Bielińska, A.; Sieradzan, A. Chemiluminogenic Properties of 10-Methyl-9- (phenoxycarbonyl)acridinium Cations in Organic Environments. J. Chem. Phys. A 2010, 114, 10550–10562. [Google Scholar] [CrossRef]
- Natrajan, A.; Wen, D. Effect of Branching in Remote Substituents on Light Emission and Stability of Chemiluminescent Acridinium Esters. RSC Adv. 2014, 4, 21852–21863. [Google Scholar] [CrossRef]
- Batmanghelich, S.; Woodhead, J.S.; Smith, K.; Weeks, I. Synthesis and chemiluminescent evaluation of a series of phenyl N-alkylacridinium-9-carboxylates. J. Photochem. Photobiol. A Chem. 1991, 56, 249–254. [Google Scholar] [CrossRef]
- Krzymiński, K.; Roshal, A.D.; Niziołek, A. Spectral features of substituted 9-(phenoxycarbonyl)-acridines and their protonated and methylated cation derivatives. Spectrochim. Acta A 2008, 70, 394–402. [Google Scholar] [CrossRef]
- White, E.H.; Bursey, M.M. Analogs of Luminol. Synthesis and Chemiluminescence of Two Methoxy-Substituted Aminophthalic Hydrazides. J. Org. Chem. 1966, 31, 1912–1917. [Google Scholar] [CrossRef]
- Gnaim, S.; Green, O.; Shabat, D. The emergence of aqueous chemiluminescence: New promising class of phenoxy 1,2-dioxetane luminophores. Chem. Commun. 2018, 54, 2073–2085. [Google Scholar] [CrossRef]
- Smith, K.; Yang, J.J.; Li, Z.; Weeks, I.; Woodhead, J.S. Synthesis and properties of novel chemiluminescent biological probes: 2- and 3-(2-Succinimidyloxycarbonylethyl)phenyl acridinium esters. J. Photochem. Photobiol. A Chem. 2009, 203, 72–79. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J.; Keeler, J. Atkins’ Physical Chemistry, 11th ed.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; John Wiley & Sons Ltd.: New York, NY, USA, 1976. [Google Scholar]
- Aihara, J.I. Reduced HOMO-LUMO Gap as an Index of Kinetic Stability for Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. A 1999, 103, 7487–7495. [Google Scholar] [CrossRef]
- Manolopoulos, D.E.; May, J.C.; Down, S.E. Theoretical studies of the fullerenes: C34 to C70. Chem. Phys. Lett. 1991, 181, 105–111. [Google Scholar] [CrossRef]
- Ruiz-Morales, Y. HOMO-LUMO gap as an index of molecular size and structure for polycyclic aromatic hydrocarbons (PAHs) and asphaltenes: A theoretical study. I. J. Phys. Chem. A 2002, 106, 11283–11308. [Google Scholar] [CrossRef]
- Labanowski, J.K.; Andzelm, J.W. Density Functional Methods in Chemistry; Springer: New York, NY, USA, 1991. [Google Scholar]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Chiodo, S.; Russo, N.; Sicilia, E. LANL2DZ basis sets recontracted in the framework of density functional theory. J. Chem. Phys. 2006, 125, 104107. [Google Scholar] [CrossRef]
- Sundararajan, K.; Sankaran, K.; Kavitha, V. Reactions of laser-ablated tellurium atoms with oxygen molecules: Matrix isolation infrared and DFT studies. J. Mol. Struct. 2008, 876, 240–249. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Hehre, W.J.; Radom, L.; Schleyer, P.v.R.; People, J.A. Ab Initio Molecular Orbital Theory; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Boużyk, A.; Jóźwiak, L.; Wróblewska, A.; Rak, J.; Błażejowski, J. Structure, properties, thermodynamics, and isomerization ability of 9-acridinones. J. Phys. Chem. A 2002, 106, 3957–3963. [Google Scholar] [CrossRef]
- Fukui, K. The Path of Chemical Reactions—The IRC Approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. A New Definition of Cavities for the Computation of Solvation Free Energies by the Polarizable Continuum Model. J. Chem. Phys. 1997, 107, 3210–3221. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Persico, M. Molecular Interactions in Solution: An Overview of Methods Based on Continuous Distributions of the Solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and Properties of Excited States in the Gas Phase and in Solution: Theory and Application of a Time-Dependent Density Functional Theory Polarizable Continuum Model. J. Chem. Phys. 2006, 124, 094107. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Chemcraft (Version 1.8)—Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 17 March 2023).
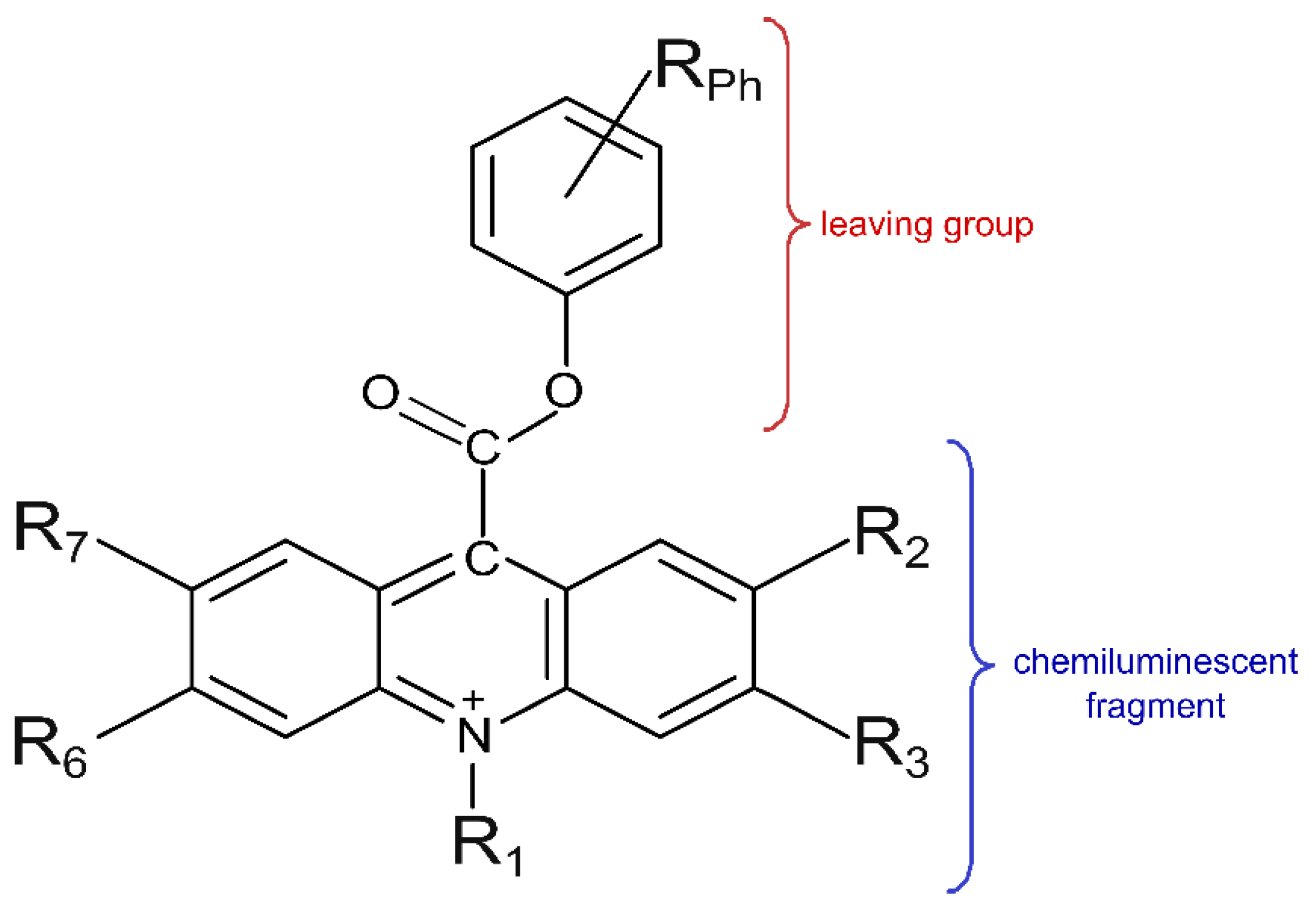
 | R | Electronegativity (χ) in the Pauling Scale [44] | C–R Bond Length (Å) | Ease of Synthesis/Availability of Substrates |
| O | 3.44 | 1.342 [6] | + | |
| S | 2.58 | 1.794 [6] | + | |
| Se | 2.55 | 1.923 | + | |
| Te | 2.10 | 2.199 | + | |
| N (N–H) | 3.04 | 1.359 | + | |
| P (P–H) | 2.19 | 1.887 | + | |
| As (As–H) | 2.18 | 2.018 | +/− | |
| Si (H–Si–H) | 1.90 | 1.958 | +/− | |
| Ge (H–Ge–H) | 2.01 | 2.013 | +/− |
| R | LCAO Coefficient of pZ LUMO Orbital at | HOMO–LUMO Gap (eV) | |
|---|---|---|---|
| C9 | C15 | ||
| O | 0.3145 | 0.0257 | 2.53 |
| S | 0.3154 | 0.0275 | 2.62 |
| Se | 0.3266 | 0.0098 | 2.78 |
| Te | 0.3405 | 0.0170 | 2.23 |
| N (N–H) | 0.3265 | 0.0001 | 2.14 |
| P (P–H) | 0.3121 | 0.0255 | 2.40 |
| As (As–H) | 0.3333 | 0.0027 | 2.44 |
| Si (H–Si–H) | 0.3180 | 0.0197 | 2.73 |
| Ge (H–Ge–H) | 0.3387 | 0.0030 | 2.63 |
| Thermodynamic Characteristic | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Step No. (Scheme 2) | R | Gaseous Phase | Aqueous Phase | Step No. (Scheme 2) | R | Gaseous Phase | Aqueous Phase | ||
| Δr,298H0 | Δr,298G0 | Δr,298G0 | Δr,298H0 | Δr,298G0 | Δr,298G0 | ||||
| I | O | −166.6 a | −153.6 a | −51.5 a | II | O | −60.5 b | −73.7 b | −47.6 b |
| S | −166.2 b | −153.0 b | −43.1 b | S | −80.1 b | −93.2 b | −69.0 b | ||
| Se | −166.3 | −153.1 | −43.8 | Se | −73.1 | −86.4 | −60.8 | ||
| Te | −165.8 | −198.8 | −82.7 | Te | −104.1 | −117.1 | −65.9 | ||
| N (N–H) | −167.1 | −153.7 | −42.6 | N (N–H) | −34.7 | −48.8 | −21.7 | ||
| P (P–H) | −166.4 | −153.1 | −42.8 | P (P–H) | −66.8 | −79.9 | −52.9 | ||
| As (As–H) | −163.9 | −150.5 | −42.6 | As (As–H) | −64.6 | −77.1 | −48.1 | ||
| Si (H–Si–H) | −167.3 | −153.8 | −36.3 | Si (H–Si–H) | −55.4 | −69.0 | −49.4 | ||
| Ge (H–Ge–H) | −168.4 | −155.4 | −45.8 | Ge (H–Ge–H) | −30.6 | −42.8 | −14.0 | ||
| III* | −15.5 b | −27.2 b | −33.2 b | III | −78.1 b | −77.6 b | −73.3 b | ||
| IV | O | −186.1 a | −175.6 a | −73.3 a | V | O | −72.5 b | −74.3 b | −49.1 b |
| S | −197.1 b | −187.5 b | −61.9 b | S | −92.0 b | −93.2 b | −68.9 b | ||
| Se | −192.0 | −181.0 | −63.5 | Se | −85.0 | −86.5 | −61.4 | ||
| Te | −191.5 | −179.2 | −103.2 | Te | −115.5 | −162.9 | −105.4 | ||
| N (N–H) | −194.3 | −182.3 | −63.4 | N (N–H) | −47.5 | −49.5 | −21.1 | ||
| P (P–H) | −192.5 | −181.3 | −63.4 | P (P–H) | −78.9 | −80.0 | −52.5 | ||
| As (As–H) | −195.1 | −183.0 | −64.9 | As (As–H) | −74.1 | −74.7 | −47.5 | ||
| Si (H–Si–H) | −194.0 | −182.3 | −64.6 | Si (H–Si–H) | −68.4 | −69.8 | −42.5 | ||
| Ge (H–Ge–H) | −194.6 | −183.0 | −66.5 | Ge (H–Ge–H) | −44.7 | −45.2 | −16.6 | ||
| Kinetic Characteristic | |||||||||
| Step No. (Scheme 2) | Gaseous Phase | Aqueous Phase | |||||||
| Δa,298H0 | Δa,298G0 | 298k0 (298τ99) | Δa,298G0 | ||||||
| TS | 12.9 b | 14.2 b | 2.5 × 102 (1.9 × 10−2) b | 15.6 b | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieńkos, M.; Zadykowicz, B. What Other Than Acridinium Esters? Computational Search for New Acridinium-Based Chemiluminogens. Int. J. Mol. Sci. 2023, 24, 9468. https://doi.org/10.3390/ijms24119468
Pieńkos M, Zadykowicz B. What Other Than Acridinium Esters? Computational Search for New Acridinium-Based Chemiluminogens. International Journal of Molecular Sciences. 2023; 24(11):9468. https://doi.org/10.3390/ijms24119468
Chicago/Turabian StylePieńkos, Milena, and Beata Zadykowicz. 2023. "What Other Than Acridinium Esters? Computational Search for New Acridinium-Based Chemiluminogens" International Journal of Molecular Sciences 24, no. 11: 9468. https://doi.org/10.3390/ijms24119468
APA StylePieńkos, M., & Zadykowicz, B. (2023). What Other Than Acridinium Esters? Computational Search for New Acridinium-Based Chemiluminogens. International Journal of Molecular Sciences, 24(11), 9468. https://doi.org/10.3390/ijms24119468







