Cross-Dressing of Multiple Myeloma Cells Mediated by Extracellular Vesicles Conveying MIC and ULBP Ligands Promotes NK Cell Killing
Abstract
1. Introduction
2. Results
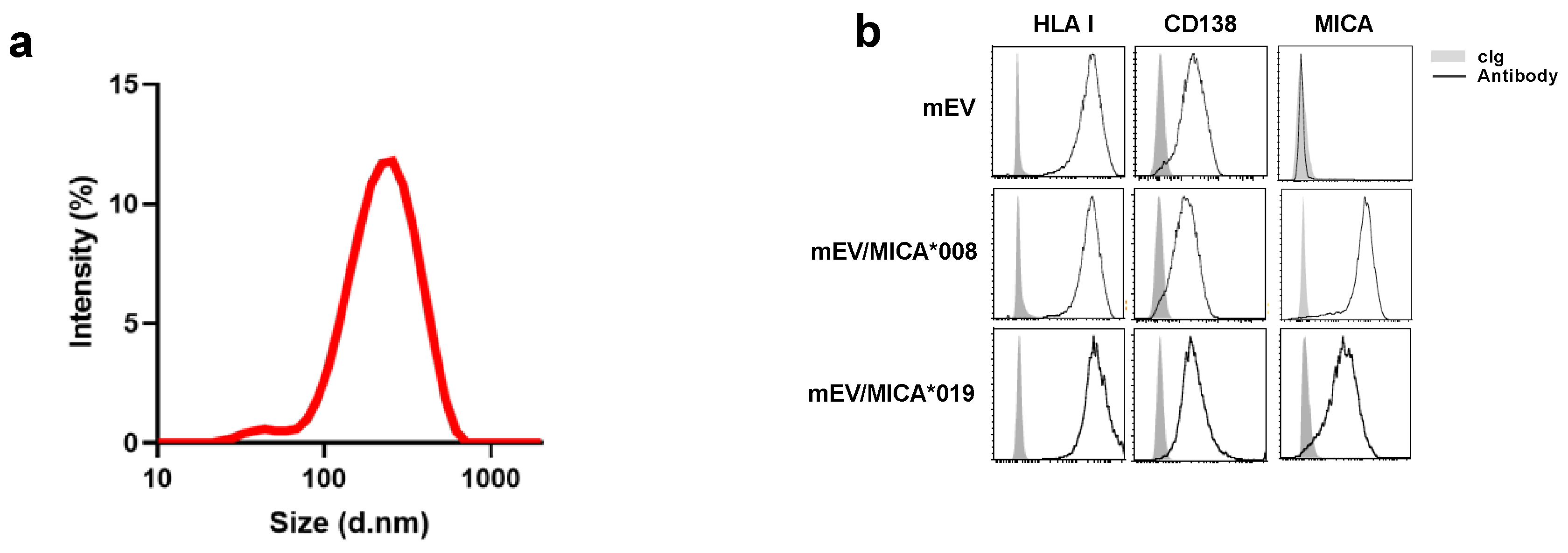
2.1. Surface Expression of MICA on mEVs Derived from Both MICA*008 and MICA*019 Expressing Cells
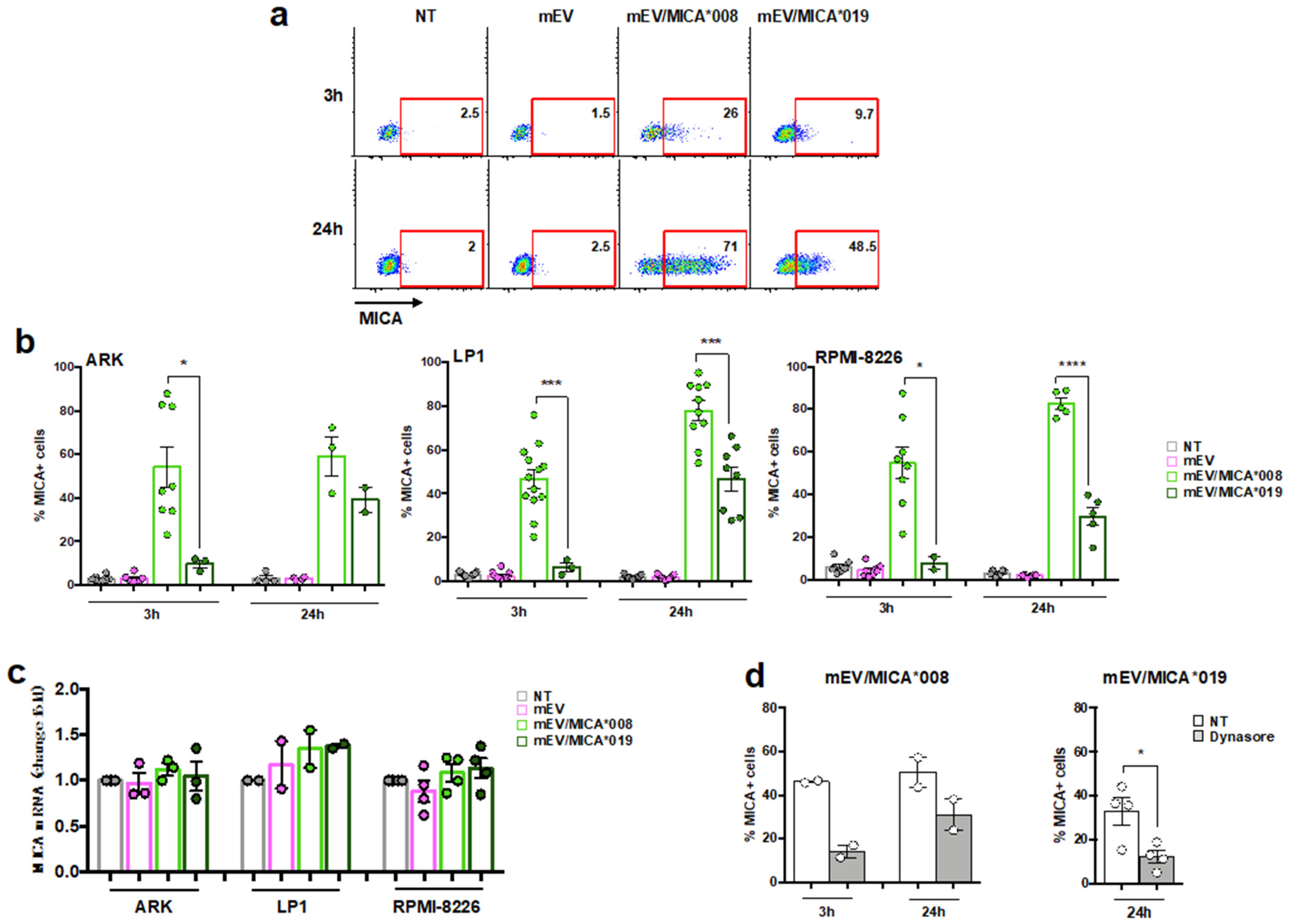
2.2. Both MICA*008 and MICA*019 Allelic Variants Can Be Transferred from mEV to MM Cells
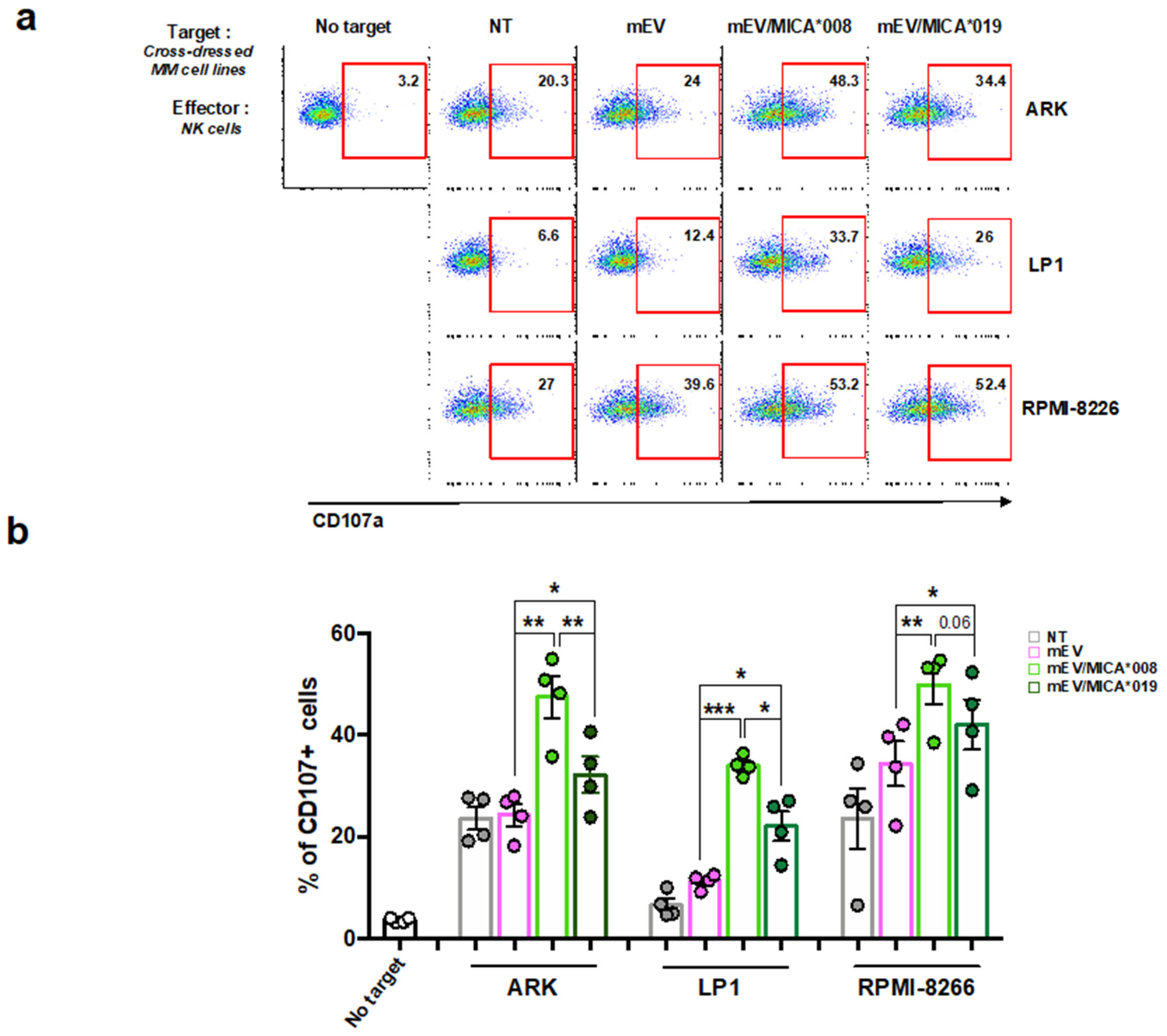
2.3. MICA Transferred on MM Cells Increases Their Susceptibility to NK Cell Lysis
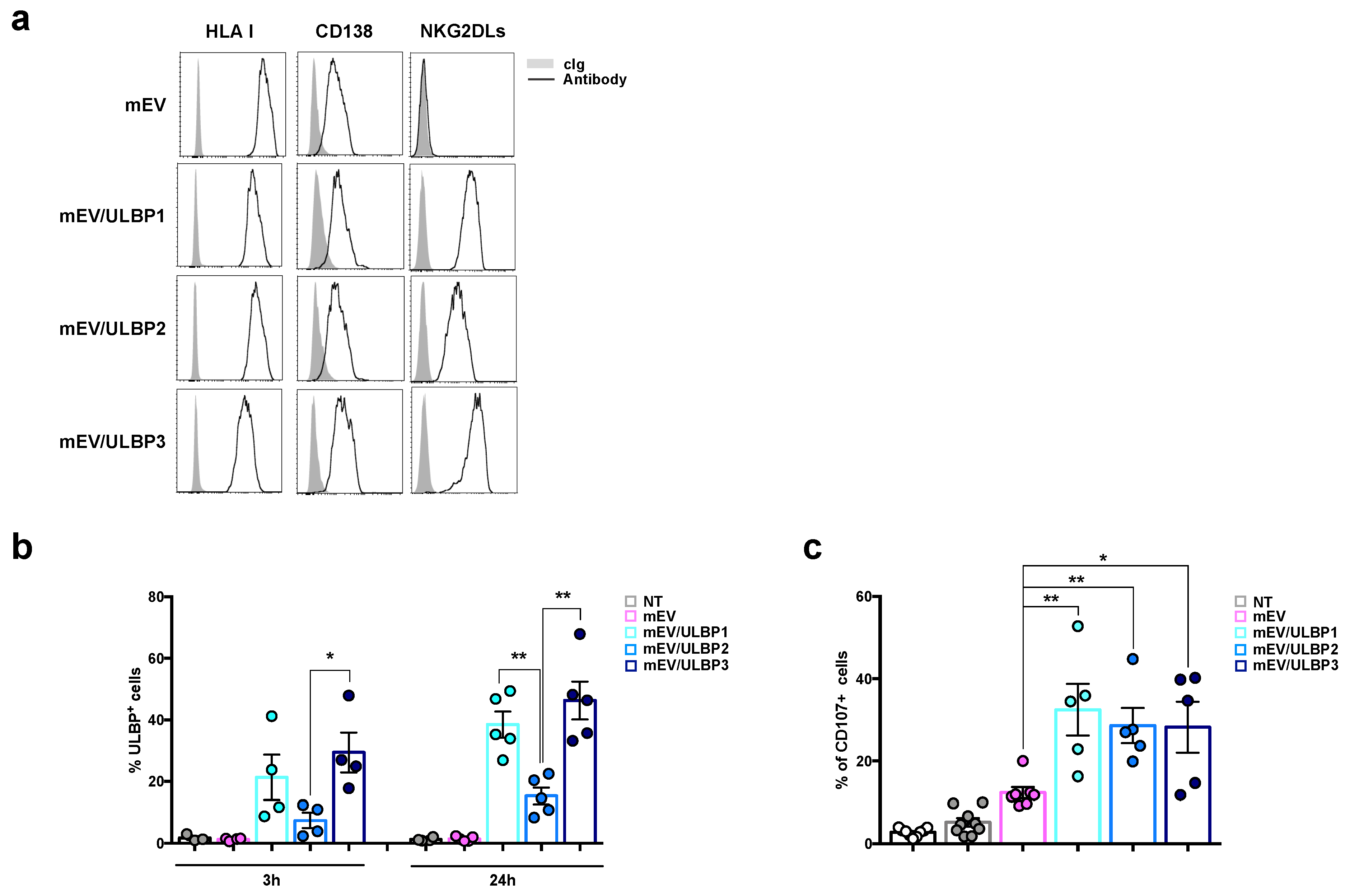
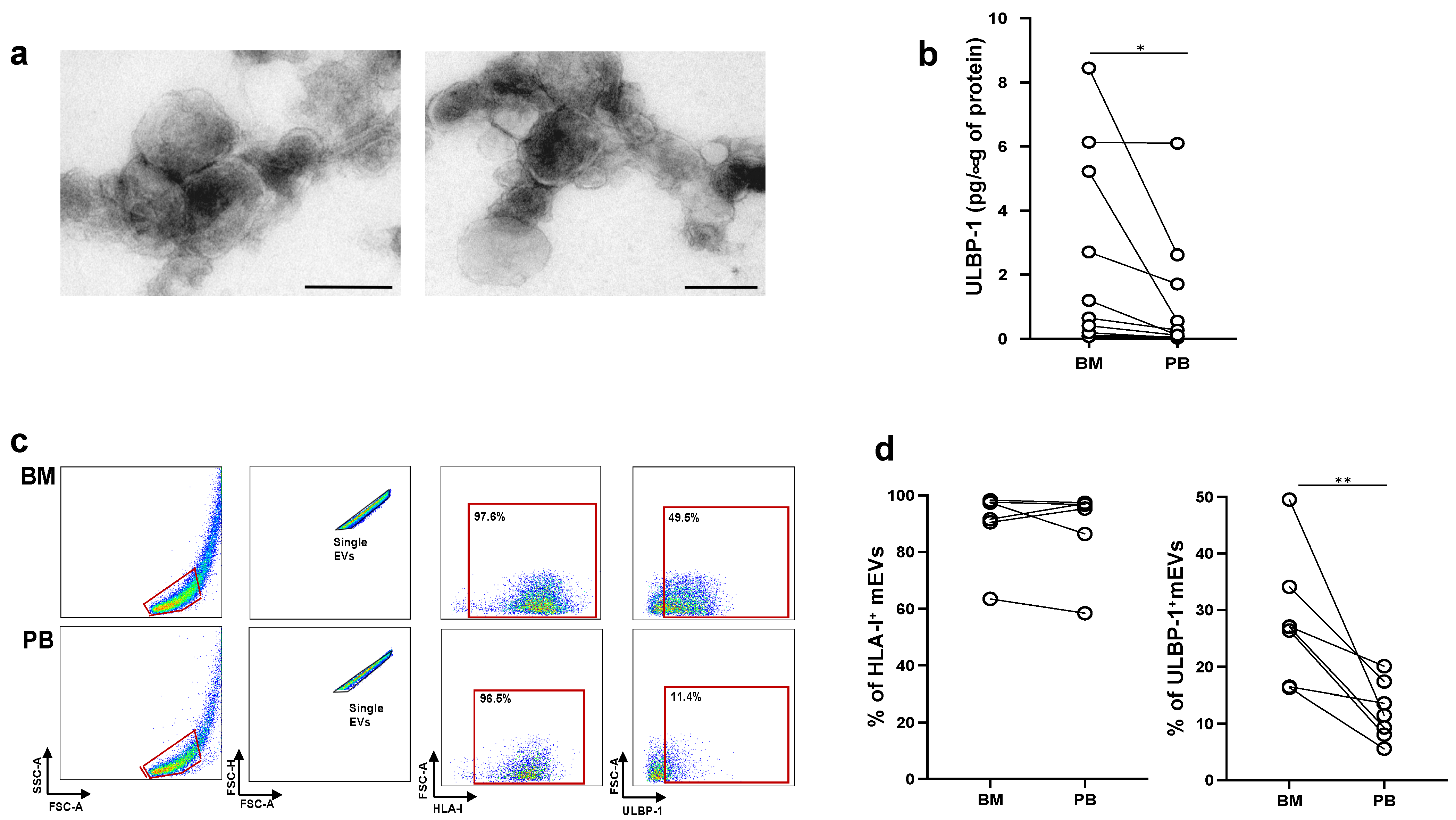
2.4. MM Cells Acquire ULBP Molecules from mEVs and Become Sensitive to NK Cell Attack
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Cell Lines and Human Polyclonal NK Cell Preparations
4.3. Virus Production and In Vitro Transduction
4.4. Extracellular Vesicle Purification
4.5. Ultrastructural Analysis
4.6. Size Experiments
4.7. RNA Isolation, RT-PCR, and Real-Time PCR
4.8. ELISA
4.9. Flow Cytometry of Cells and EVs
4.10. Extracellular Vesicles Uptake
4.11. NK Cell Stimulation and Degranulation
4.12. FACS Analysis
4.13. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groh, V.; Smythe, K.; Dai, Z.; Spies, T. Fas-ligand-mediated paracrine T cell regulation by the receptor NKG2D in tumor immunity. Nat. Immunol. 2006, 7, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol. Res. 2015, 3, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Jelenčić, V.; Lenartić, M.; Wensveen, F.M.; Polić, B. NKG2D: A versatile player in the immune system. Immunol. Lett. 2017, 189, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Zingoni, A.; Molfetta, R.; Fionda, C.; Soriani, A.; Paolini, R.; Cippitelli, M.; Cerboni, C.; Santoni, A. NKG2D and Its Ligands: “One for All, All for One”. Front. Immunol. 2018, 9, 476. [Google Scholar] [CrossRef]
- Ota, M.; Katsuyama, Y.; Mizuki, N.; Ando, H.; Furihata, K.; Ono, S.; Pivetti-Pezzi, P.; Tabbara, K.F.; Palimeris, G.D.; Nikbin, B.; et al. Trinucleotide repeat polymorphism within exon 5 of the MICA gene (MHC class I chain-related gene A): Allele frequency data in the nine population groups Japanese, Northern Han, Hui, Uygur, Kazakhstan, Iranian, Saudi Arabian, Greek and Italian. Tissue Antigens 1997, 49, 448–454. [Google Scholar] [CrossRef]
- López-Cobo, S.; Campos-Silva, C.; Valés-Gómez, M. Glycosyl-Phosphatidyl-Inositol (GPI)-Anchors and Metalloproteases: Their Roles in the Regulation of Exosome Composition and NKG2D-Mediated Immune Recognition. Front. Cell Dev. Biol. 2016, 4, 97. [Google Scholar] [CrossRef]
- Ashiru, O.; López-Cobo, S.; Fernández-Messina, L.; Pontes-Quero, S.; Pandolfi, R.; Reyburn, H.T.; Valés-Gómez, M. A GPI anchor explains the unique biological features of the common NKG2D-ligand allele MICA*008. Biochem. J. 2013, 454, 295–302. [Google Scholar] [CrossRef]
- Fuertes, M.B.; Domaica, C.I.; Zwirner, N.W. Leveraging NKG2D Ligands in Immuno-Oncology. Front. Immunol. 2021, 12, 713158. [Google Scholar] [CrossRef]
- Dhar, P.; Wu, J.D. NKG2D and its ligands in cancer. Curr. Opin. Immunol. 2018, 51, 55–61. [Google Scholar] [CrossRef]
- Chitadze, G.; Bhat, J.; Lettau, M.; Janssen, O.; Kabelitz, D. Generation of soluble NKG2D ligands: Proteolytic cleavage, exosome secretion and functional implications. Scand. J. Immunol. 2013, 78, 120–129. [Google Scholar] [CrossRef]
- Chitadze, G.; Lettau, M.; Bhat, J.; Wesch, D.; Steinle, A.; Fürst, D.; Mytilineos, J.; Kalthoff, H.; Janssen, O.; Oberg, H.-H.; et al. Shedding of endogenous MHC class I-related chain molecules A and B from different human tumor entities: Heterogeneous involvement of the “a disintegrin and metalloproteases” 10 and 17. Int. J. Cancer 2013, 133, 1557–1566. [Google Scholar] [CrossRef]
- Waldhauer, I.; Goehlsdorf, D.; Gieseke, F.; Weinschenk, T.; Wittenbrink, M.; Ludwig, A.; Stevanovic, S.; Rammensee, H.-G.; Steinle, A. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008, 68, 6368–6376. [Google Scholar] [CrossRef] [PubMed]
- Waldhauer, I.; Steinle, A. Proteolytic release of soluble UL16-binding protein 2 from tumor cells. Cancer Res. 2006, 66, 2520–2526. [Google Scholar] [CrossRef] [PubMed]
- Zingoni, A.; Cecere, F.; Vulpis, E.; Fionda, C.; Molfetta, R.; Soriani, A.; Petrucci, M.T.; Ricciardi, M.R.; Fuerst, D.; Amendola, M.G.; et al. Genotoxic Stress Induces Senescence-Associated ADAM10-Dependent Release of NKG2D MIC Ligands in Multiple Myeloma Cells. J. Immunol. 2015, 195, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Ashiru, O.; Boutet, P.; Fernández-Messina, L.; Agüera-González, S.; Skepper, J.N.; Valés-Gómez, M.; Reyburn, H.T. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010, 70, 481–489. [Google Scholar] [CrossRef]
- Fernández-Messina, L.; Ashiru, O.; Boutet, P.; Agüera-González, S.; Skepper, J.N.; Reyburn, H.T.; Valés-Gómez, M. Differential mechanisms of shedding of the glycosylphosphatidylinositol (GPI)-anchored NKG2D ligands. J. Biol. Chem. 2010, 285, 8543–8551. [Google Scholar] [CrossRef]
- Viaud, S.; Terme, M.; Flament, C.; Taieb, J.; André, F.; Novault, S.; Escudier, B.; Robert, C.; Caillat-Zucman, S.; Tursz, T.; et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: A role for NKG2D ligands and IL-15Ralpha. PLoS ONE 2009, 4, e4942. [Google Scholar] [CrossRef]
- Vulpis, E.; Loconte, L.; Peri, A.; Molfetta, R.; Caracciolo, G.; Masuelli, L.; Tomaipitinca, L.; Peruzzi, G.; Petillo, S.; Petrucci, M.T.; et al. Impact on NK cell functions of acute versus chronic exposure to extracellular vesicle-associated MICA: Dual role in cancer immunosurveillance. J. Extracell. Vesicles 2022, 11, e12176. [Google Scholar] [CrossRef] [PubMed]
- Vulpis, E.; Soriani, A.; Cerboni, C.; Santoni, A.; Zingoni, A. Cancer Exosomes as Conveyors of Stress-Induced Molecules: New Players in the Modulation of NK Cell Response. Int. J. Mol. Sci. 2019, 20, 611. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Israelsson, P.; Ottander, U.; Lundin, E.; Nagaev, I.; Nagaeva, O.; Dehlin, E.; Baranov, V.; Mincheva-Nilsson, L. Differential expression of ligands for NKG2D and DNAM-1 receptors by epithelial ovarian cancer-derived exosomes and its influence on NK cell cytotoxicity. Tumour. Biol. 2016, 37, 5455–5466. [Google Scholar] [CrossRef]
- Lundholm, M.; Schröder, M.; Nagaeva, O.; Baranov, V.; Widmark, A.; Mincheva-Nilsson, L.; Wikström, P. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: Mechanism of immune evasion. PLoS ONE 2014, 9, e108925. [Google Scholar] [CrossRef] [PubMed]
- López-Cobo, S.; Campos-Silva, C.; Moyano, A.; Oliveira-Rodríguez, M.; Paschen, A.; Yáñez-Mó, M.; Blanco-López, M.C.; Valés-Gómez, M. Immunoassays for scarce tumour-antigens in exosomes: Detection of the human NKG2D-Ligand, MICA, in tetraspanin-containing nanovesicles from melanoma. J. Nanobiotechnol. 2018, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Borrelli, C.; Ricci, B.; Vulpis, E.; Fionda, C.; Ricciardi, M.R.; Petrucci, M.T.; Masuelli, L.; Peri, A.; Cippitelli, M.; Zingoni, A.; et al. Drug-Induced Senescent Multiple Myeloma Cells Elicit NK Cell Proliferation by Direct or Exosome-Mediated IL15 Trans-Presentation. Cancer Immunol. Res. 2018, 6, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Vulpis, E.; Cecere, F.; Molfetta, R.; Soriani, A.; Fionda, C.; Peruzzi, G.; Caracciolo, G.; Palchetti, S.; Masuelli, L.; Simonelli, L.; et al. Genotoxic stress modulates the release of exosomes from multiple myeloma cells capable of activating NK cell cytokine production: Role of HSP70/TLR2/NF-kB axis. Oncoimmunology 2017, 6, e1279372. [Google Scholar] [CrossRef] [PubMed]
- Tabiasco, J.; Espinosa, E.; Hudrisier, D.; Joly, E.; Fournié, J.-J.; Vercellone, A. Active trans-synaptic capture of membrane fragments by natural killer cells. Eur. J. Immunol. 2002, 32, 1502–1508. [Google Scholar] [CrossRef]
- Hasim, M.S.; Marotel, M.; Hodgins, J.J.; Vulpis, E.; Makinson, O.J.; Asif, S.; Shih, H.-Y.; Scheer, A.K.; MacMillan, O.; Alonso, F.G.; et al. When killers become thieves: Trogocytosed PD-1 inhibits NK cells in cancer. Sci. Adv. 2022, 8, eabj3286. [Google Scholar] [CrossRef]
- López-Cobo, S.; Romera-Cárdenas, G.; García-Cuesta, E.M.; Reyburn, H.T.; Valés-Gómez, M. Transfer of the human NKG2D ligands UL16 binding proteins (ULBP) 1–3 is related to lytic granule release and leads to ligand retransfer and killing of ULBP-recipient natural killer cells. Immunology 2015, 146, 70–80. [Google Scholar] [CrossRef]
- McCann, F.E.; Eissmann, P.; Onfelt, B.; Leung, R.; Davis, D.M. The activating NKG2D ligand MHC class I-related chain A transfers from target cells to NK cells in a manner that allows functional consequences. J. Immunol. 2007, 178, 3418–3426. [Google Scholar] [CrossRef]
- Zeng, F.; Morelli, A.E. Extracellular vesicle-mediated MHC cross-dressing in immune homeostasis, transplantation, infectious diseases, and cancer. Semin. Immunopathol. 2018, 40, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.; Kleinschmidt, A.; Brühl, H.; Klier, C.; Nelson, P.J.; Cihak, J.; Plachý, J.; Stangassinger, M.; Erfle, V.; Schlöndorff, D. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: A mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 2000, 6, 769–775. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Federzoni, E.A.; Wang, X.; Dharmawan, A.; Hu, X.; Wang, H.; Hawley, R.J.; Stevens, S.; Sykes, M.; et al. CD47 cross-dressing by extracellular vesicles expressing CD47 inhibits phagocytosis without transmitting cell death signals. Elife 2022, 11, e73677. [Google Scholar] [CrossRef] [PubMed]
- Fionda, C.; Stabile, H.; Molfetta, R.; Soriani, A.; Bernardini, G.; Zingoni, A.; Gismondi, A.; Paolini, R.; Cippitelli, M.; Santoni, A. Translating the anti-myeloma activity of Natural Killer cells into clinical applications. Cancer Treat. Rev. 2018, 70, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Soriani, A.; Vulpis, E.; Cuollo, L.; Santoni, A.; Zingoni, A. Cancer extracellular vesicles as novel regulators of NK cell response. Cytokine Growth Factor. Rev. 2020, 51, 19–26. [Google Scholar] [CrossRef]
- Duan, S.; Guo, W.; Xu, Z.; He, Y.; Liang, C.; Mo, Y.; Wang, Y.; Xiong, F.; Guo, C.; Li, Y.; et al. Natural killer group 2D receptor and its ligands in cancer immune escape. Mol. Cancer 2019, 18, 29. [Google Scholar] [CrossRef]
- Zingoni, A.; Vulpis, E.; Nardone, I.; Soriani, A.; Fionda, C.; Cippitelli, M.; Santoni, A. Targeting NKG2D and NKp30 Ligands Shedding to Improve NK Cell-Based Immunotherapy. Crit. Rev. Immunol. 2016, 36, 445–460. [Google Scholar] [CrossRef]
- Nückel, H.; Switala, M.; Sellmann, L.; Horn, P.A.; Dürig, J.; Dührsen, U.; Küppers, R.; Grosse-Wilde, H.; Rebmann, V. The prognostic significance of soluble NKG2D ligands in B-cell chronic lymphocytic leukemia. Leukemia 2010, 24, 1152–1159. [Google Scholar] [CrossRef]
- Paschen, A.; Sucker, A.; Hill, B.; Moll, I.; Zapatka, M.; Nguyen, X.D.; Sim, G.C.; Gutmann, I.; Hassel, J.; Becker, J.C.; et al. Differential clinical significance of individual NKG2D ligands in melanoma: Soluble ULBP2 as an indicator of poor prognosis superior to S100B. Clin. Cancer Res. 2009, 15, 5208–5215. [Google Scholar] [CrossRef] [PubMed]
- Rebmann, V.; Schütt, P.; Brandhorst, D.; Opalka, B.; Moritz, T.; Nowrousian, M.R.; Grosse-Wilde, H. Soluble MICA as an independent prognostic factor for the overall survival and progression-free survival of multiple myeloma patients. Clin. Immunol. 2007, 123, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Jinushi, M.; Vanneman, M.; Munshi, N.C.; Tai, Y.-T.; Prabhala, R.H.; Ritz, J.; Neuberg, D.; Anderson, K.C.; Carrasco, D.R.; Dranoff, G. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc. Natl. Acad. Sci. USA 2008, 105, 1285–1290. [Google Scholar] [CrossRef]
- Zingoni, A.; Vulpis, E.; Cecere, F.; Amendola, M.G.; Fuerst, D.; Saribekyan, T.; Achour, A.; Sandalova, T.; Nardone, I.; Peri, A.; et al. MICA-129 Dimorphism and Soluble MICA Are Associated With the Progression of Multiple Myeloma. Front. Immunol. 2018, 9, 926. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Zuo, J.; Mohammed, F.; Moss, P. The Biological Influence and Clinical Relevance of Polymorphism Within the NKG2D Ligands. Front. Immunol. 2018, 9, 1820. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakayama, M.; Kawano, M.; Amagai, R.; Ishii, T.; Harigae, H.; Ogasawara, K. Fratricide of natural killer cells dressed with tumor-derived NKG2D ligand. Proc. Natl. Acad. Sci. USA 2013, 110, 9421–9426. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Mastoridis, S.; Londoño, M.-C.; Kurt, A.; Kodela, E.; Crespo, E.; Mason, J.; Bestard, O.; Martínez-Llordella, M.; Sánchez-Fueyo, A. Impact of donor extracellular vesicle release on recipient cell “cross-dressing” following clinical liver and kidney transplantation. Am. J. Transplant. 2021, 21, 2387–2398. [Google Scholar] [CrossRef]
- Gonzalez-Nolasco, B.; Wang, M.; Prunevieille, A.; Benichou, G. Emerging role of exosomes in allorecognition and allograft rejection. Curr. Opin. Organ Transplant. 2018, 23, 22–27. [Google Scholar] [CrossRef]
- Molfetta, R.; Quatrini, L.; Capuano, C.; Gasparrini, F.; Zitti, B.; Zingoni, A.; Galandrini, R.; Santoni, A.; Paolini, R. c-Cbl regulates MICA- but not ULBP2-induced NKG2D down-modulation in human NK cells. Eur. J. Immunol. 2014, 44, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Zingoni, A.; Palmieri, G.; Morrone, S.; Carretero, M.; Lopez-Botel, M.; Piccoli, M.; Frati, L.; Santoni, A. CD69-triggered ERK activation and functions are negatively regulated by CD94 / NKG2-A inhibitory receptor. Eur. J. Immunol. 2000, 30, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Mekhloufi, A.; Kosta, A.; Stabile, H.; Molfetta, R.; Zingoni, A.; Soriani, A.; Cippitelli, M.; Paolini, R.; Gismondi, A.; Ricciardi, M.R.; et al. Bone Marrow Stromal Cell-Derived IL-8 Upregulates PVR Expression on Multiple Myeloma Cells via NF-kB Transcription Factor. Cancers 2020, 12, 440. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vulpis, E.; Loconte, L.; Cassone, C.; Antonangeli, F.; Caracciolo, G.; Masuelli, L.; Fazio, F.; Petrucci, M.T.; Fionda, C.; Soriani, A.; et al. Cross-Dressing of Multiple Myeloma Cells Mediated by Extracellular Vesicles Conveying MIC and ULBP Ligands Promotes NK Cell Killing. Int. J. Mol. Sci. 2023, 24, 9467. https://doi.org/10.3390/ijms24119467
Vulpis E, Loconte L, Cassone C, Antonangeli F, Caracciolo G, Masuelli L, Fazio F, Petrucci MT, Fionda C, Soriani A, et al. Cross-Dressing of Multiple Myeloma Cells Mediated by Extracellular Vesicles Conveying MIC and ULBP Ligands Promotes NK Cell Killing. International Journal of Molecular Sciences. 2023; 24(11):9467. https://doi.org/10.3390/ijms24119467
Chicago/Turabian StyleVulpis, Elisabetta, Luisa Loconte, Chiara Cassone, Fabrizio Antonangeli, Giulio Caracciolo, Laura Masuelli, Francesca Fazio, Maria Teresa Petrucci, Cinzia Fionda, Alessandra Soriani, and et al. 2023. "Cross-Dressing of Multiple Myeloma Cells Mediated by Extracellular Vesicles Conveying MIC and ULBP Ligands Promotes NK Cell Killing" International Journal of Molecular Sciences 24, no. 11: 9467. https://doi.org/10.3390/ijms24119467
APA StyleVulpis, E., Loconte, L., Cassone, C., Antonangeli, F., Caracciolo, G., Masuelli, L., Fazio, F., Petrucci, M. T., Fionda, C., Soriani, A., Cerboni, C., Cippitelli, M., Santoni, A., & Zingoni, A. (2023). Cross-Dressing of Multiple Myeloma Cells Mediated by Extracellular Vesicles Conveying MIC and ULBP Ligands Promotes NK Cell Killing. International Journal of Molecular Sciences, 24(11), 9467. https://doi.org/10.3390/ijms24119467








