Regulation of Gut Microflora by Lactobacillus casei Zhang Attenuates Liver Injury in Mice Caused by Anti-Tuberculosis Drugs
Abstract
1. Introduction
2. Results
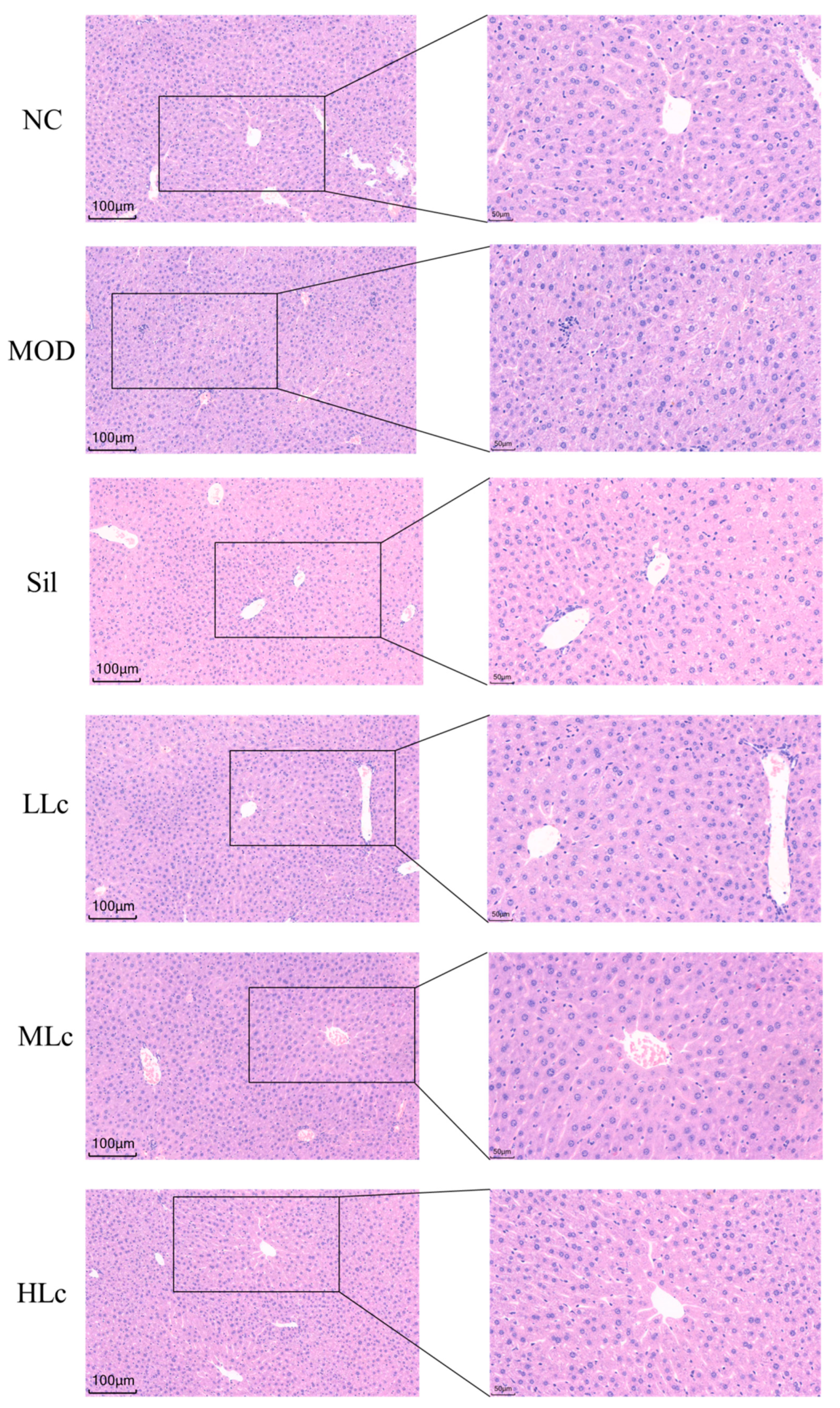
2.1. Lactobacillus casei Attenuated Liver Injury Induced by Anti-TB Drugs in Mice
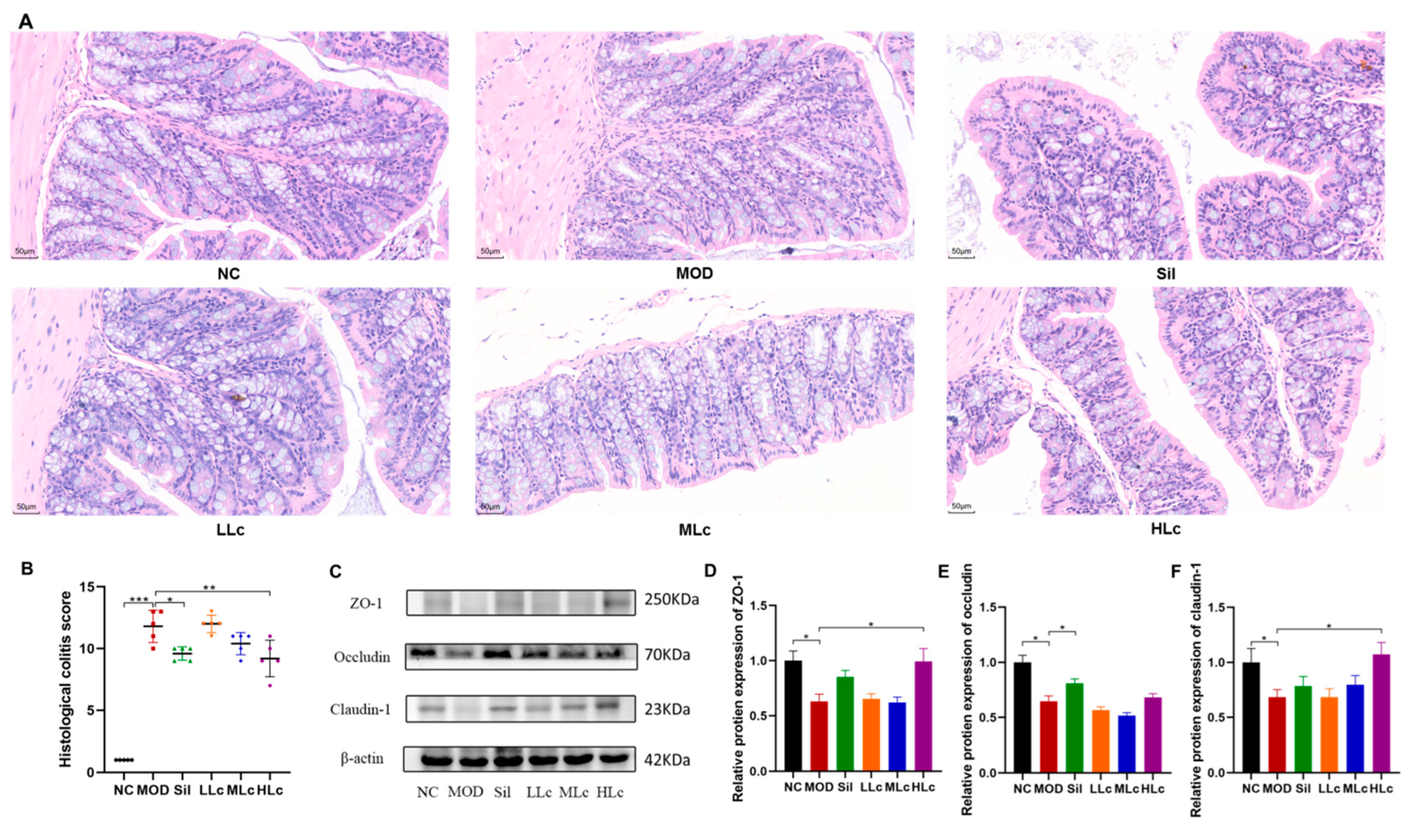
2.2. Lactobacillus casei Renovated the Intestinal Barrier in Mice with Anti-TB Drugs
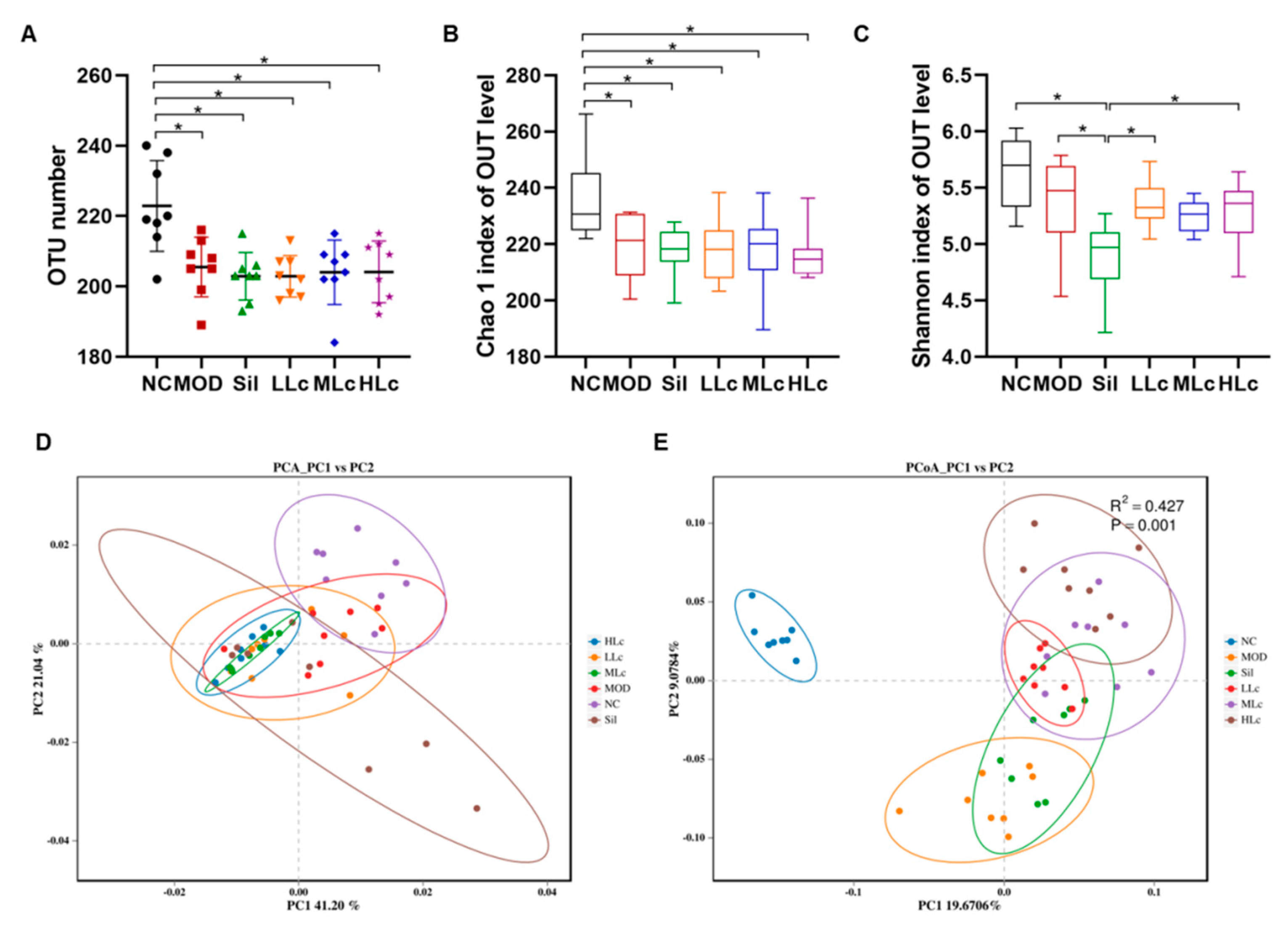
2.3. Lactobacillus casei Modulated the Diversity of Gut Microbiota in Mice with Anti-TB Drugs
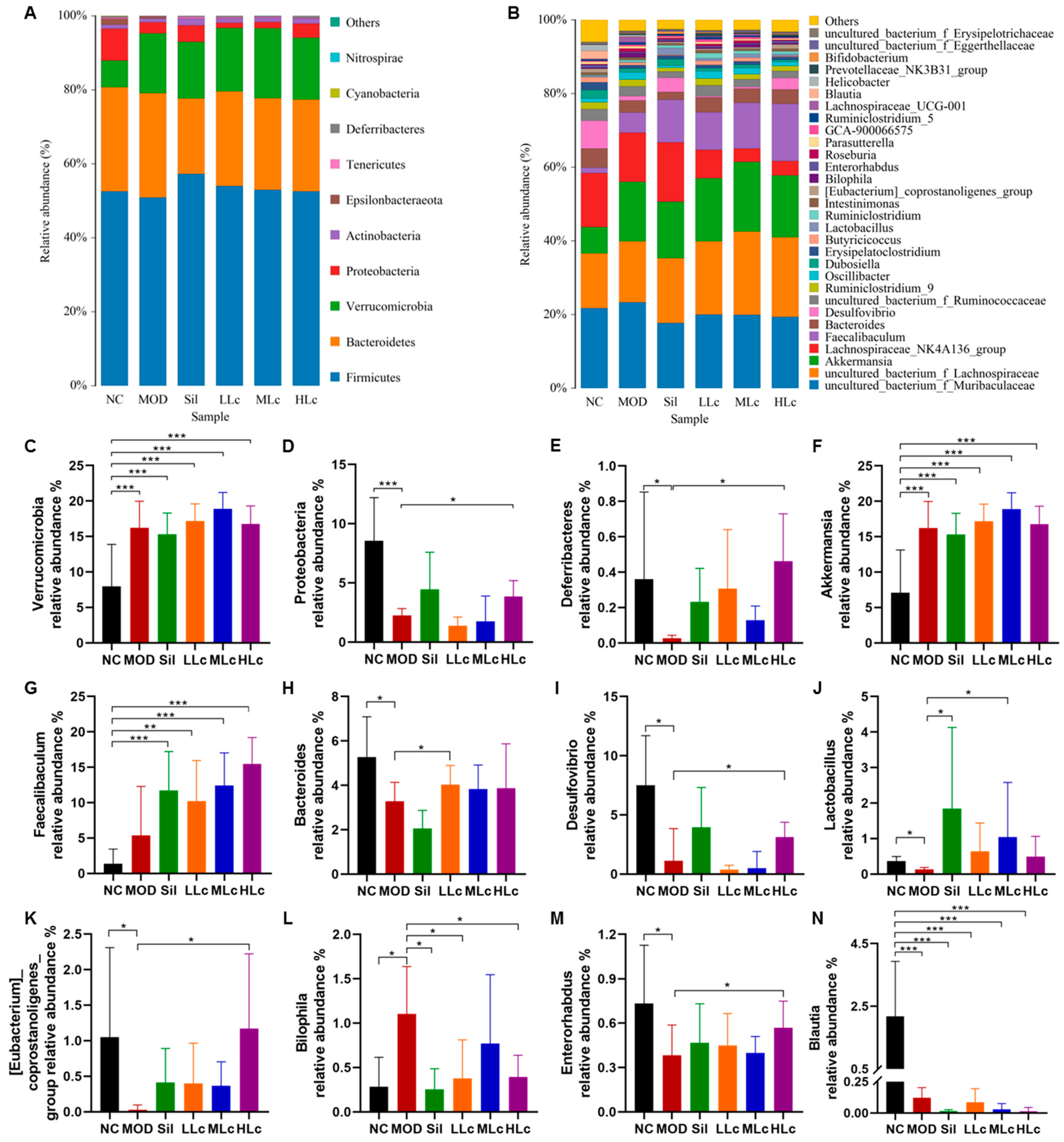
2.4. Lactobacillus casei Modulated Microbial Taxonomic Profiles in Mice with Anti-TB Drugs
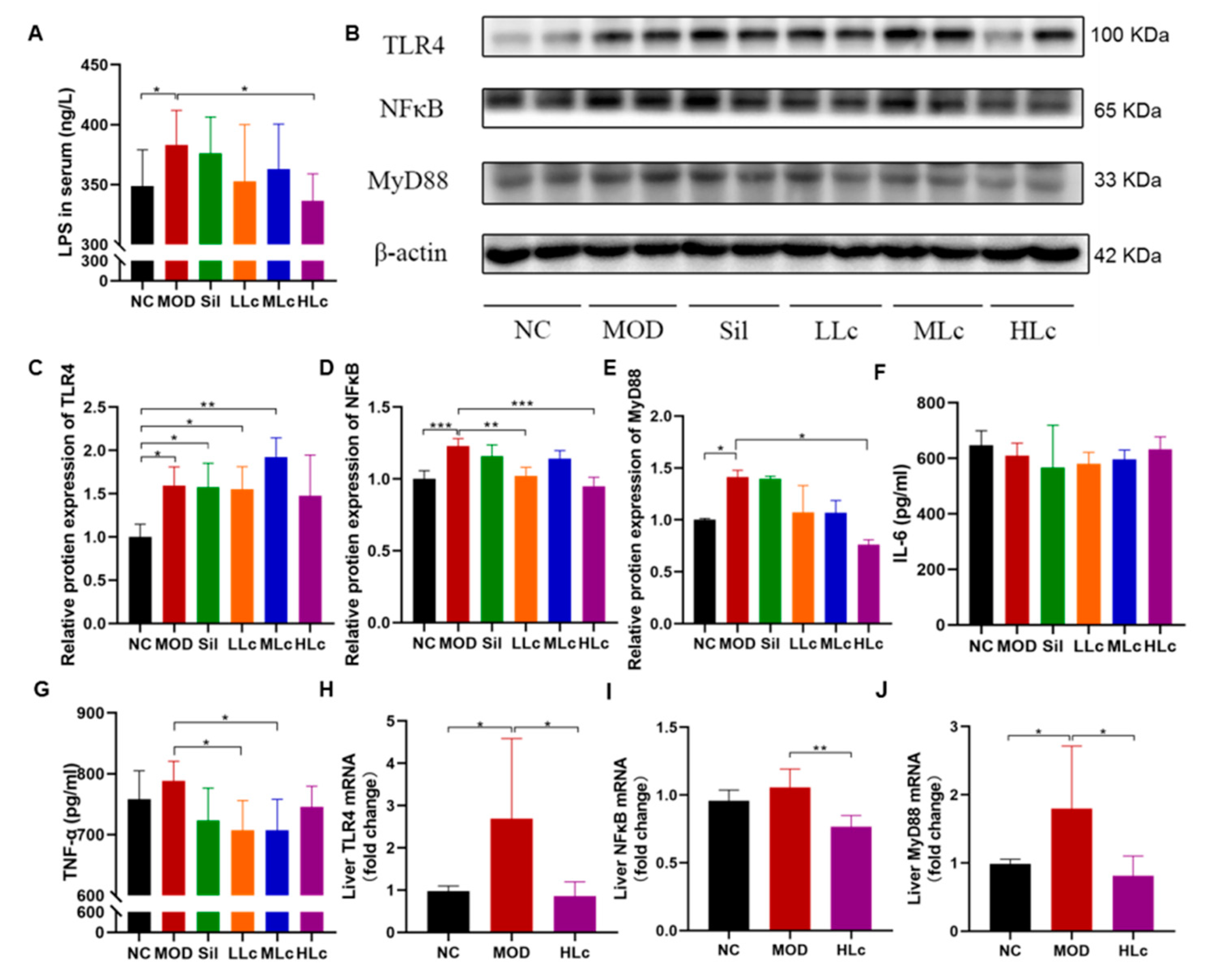
2.5. Lactobacillus casei Mediated Regulation of Intestinal Inflammation though the TLR4–NF-κB–MyD88 Pathway
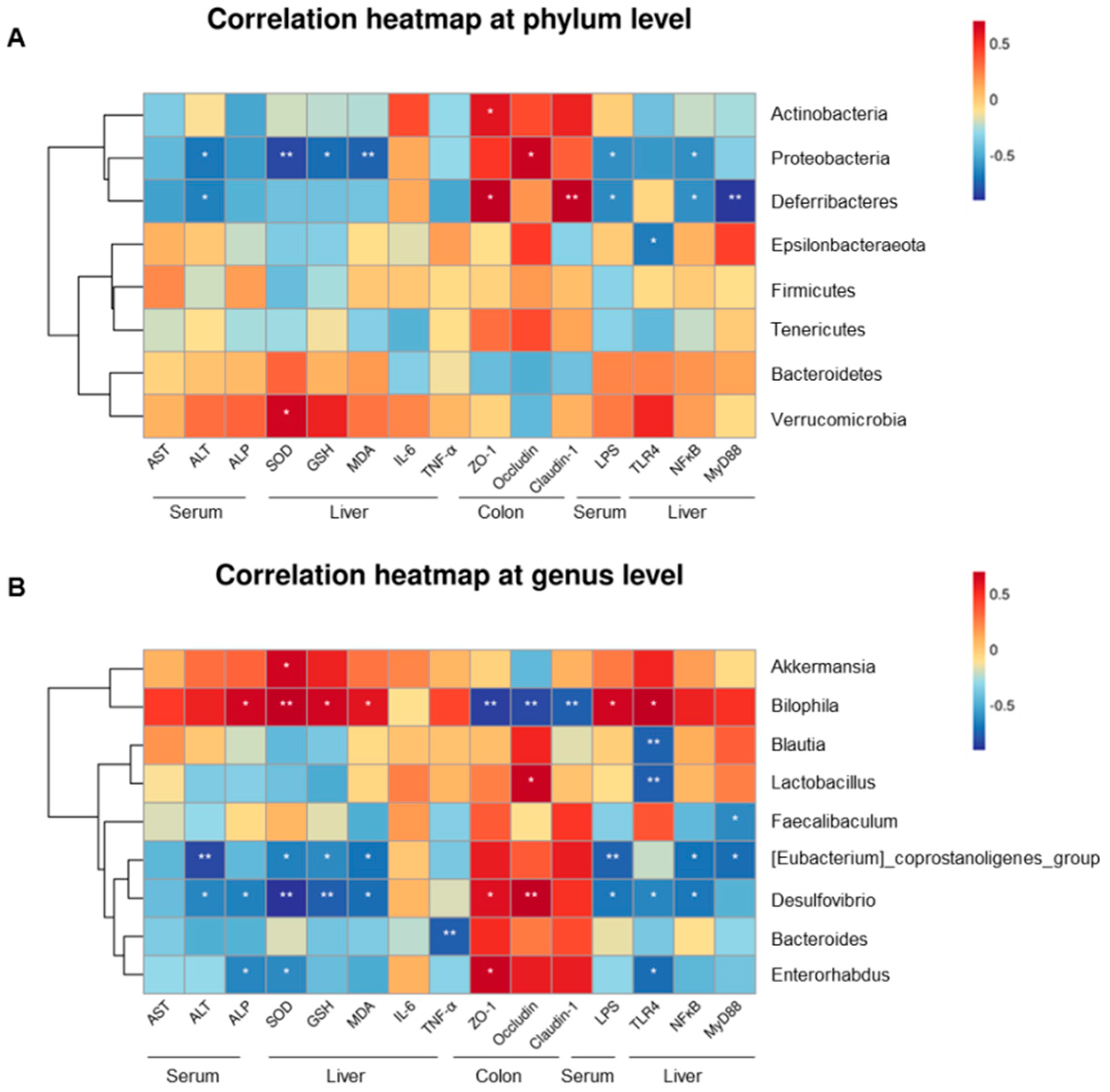
2.6. Gut Microbiota-Associated Indicators of Hepatotoxicity, Degree of LPS, and Protein Expression Concentration
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animal and Ethics Statement
4.3. Experimental Design
4.4. Hematological Tests of Hepatic Function and Inflammatory Cytokines
4.5. Serum LPS Level
4.6. Histological Examination
4.7. 16S rRNA Amplicon Sequencing Analyses
4.8. Western Blot
4.9. Real-Time Quantitative PCR (qRT-PCR)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furin, J.; Cox, H.; Pai, M. Tuberculosis. Lancet 2019, 393, 1642–1656. [Google Scholar] [CrossRef] [PubMed]
- Yew, W.W.; Chang, K.C.; Chan, D.P. Oxidative Stress and First-Line Antituberculosis Drug-Induced Hepatotoxicity. Antimicrob. Agents Chemother. 2018, 62, e02637-17. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, M.J.; Alcobia, C.; Oliveiros, B.; Mesquita, L.A.; Carvalho, A.; Matos, F.; Carvalho, J.M.; Villar, M.; Duarte, R.; Mendes, J.; et al. Clinical and Genetic Risk Factors for Drug-Induced Liver Injury Associated with Anti-Tuberculosis Treatment—A Study from Patients of Portuguese Health Centers. J. Pers. Med. 2022, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Low, E.X.S.; Zheng, Q.; Chan, E.; Lim, S.G. Drug induced liver injury: East versus West—A systematic review and meta-analysis. Clin. Mol. Hepatol. 2020, 26, 142–154. [Google Scholar] [CrossRef]
- Xu, Z.W.; Li, Y.; Xu, J.M. Clinical characteristics of the adaptive phenomenon of antituberculosis drug-induced liver injury. Chin. J. Hepatol. 2013, 21, 697–700. [Google Scholar]
- Zhang, S.; Pan, H.; Peng, X.; Lu, H.; Fan, H.; Zheng, X.; Xu, G.; Wang, M.; Wang, J. Preventive use of a hepatoprotectant against anti-tuberculosis drug-induced liver injury: A randomized controlled trial. J. Gastroenterol. Hepatol. 2016, 31, 409–416. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Angelico, R. Formulation Strategies for Enhancing the Bioavailability of Silymarin: The State of the Art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef]
- Stickel, F.; Schuppan, D. Herbal medicine in the treatment of liver diseases. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2007, 39, 293–304. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Bai, H.; Wang, M.; Jiao, L.; Peng, W.; Wu, T.; Liu, T.; Chen, H.; Song, X.; et al. Genetic polymorphisms in PXR and NF-κB1 influence susceptibility to anti-tuberculosis drug-induced liver injury. PLoS ONE 2019, 14, e0222033. [Google Scholar] [CrossRef]
- Schneider, K.M.; Bieghs, V.; Heymann, F.; Hu, W.; Dreymueller, D.; Liao, L.; Frissen, M.; Ludwig, A.; Gassler, N.; Pabst, O.; et al. CX3CR1 is a gatekeeper for intestinal barrier integrity in mice: Limiting steatohepatitis by maintaining intestinal homeostasis. Hepatology 2015, 62, 1405–1416. [Google Scholar] [CrossRef]
- Yan, A.W.; Fouts, D.E.; Brandl, J.; Stärkel, P.; Torralba, M.; Schott, E.; Tsukamoto, H.; Nelson, K.E.; Brenner, D.A.; Schnabl, B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011, 53, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Han, Y.; Zhao, H.; Zhang, H.; Yan, B.; Pei, S.; He, X.; Li, Y.; Meng, X.; Chen, L.; et al. Punicalagin alleviates renal injury via the gut-kidney axis in high-fat diet-induced diabetic mice. Food Funct. 2022, 13, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tian, H.; Kang, Y.; Tian, Y.; Li, L.; Kang, X.; Yang, H.; Wang, Y.; Tian, J.; Zhang, F.; et al. Probiotics alleviate autoimmune hepatitis in mice through modulation of gut microbiota and intestinal permeability. J. Nutr. Biochem. 2021, 98, 108863. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Q.; Liu, J.; Yang, X.; Zhang, Y.; Huang, F. Sinomenine protects against E. coli-induced acute lung injury in mice through Nrf2-NF-κB pathway. Biomed. Pharmacother. 2018, 107, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Liu, B.; He, H.; Gu, Z.; Liu, Y.; Su, Y.; Zhu, D.; Cang, J.; Luo, Z. MicroRNA-27a alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis through modulating TLR4/MyD88/NF-κB pathway. Cell Cycle 2018, 17, 2001–2018. [Google Scholar] [CrossRef]
- Hu, N.; Wang, C.; Dai, X.; Zhou, M.; Gong, L.; Yu, L.; Peng, C.; Li, Y. Phillygenin inhibits LPS-induced activation and inflammation of LX2 cells by TLR4/MyD88/NF-κB signaling pathway. J. Ethnopharmacol. 2020, 248, 112361. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef]
- Raftar, S.K.A.; Ashrafian, F.; Abdollahiyan, S.; Yadegar, A.; Moradi, H.R.; Masoumi, M.; Vaziri, F.; Moshiri, A.; Siadat, S.D.; Zali, M.R. The anti-inflammatory effects of Akkermansia muciniphila and its derivates in HFD/CCL4-induced murine model of liver injury. Sci. Rep. 2022, 12, 2453. [Google Scholar] [CrossRef]
- Hong, Y.; Sheng, L.; Zhong, J.; Tao, X.; Zhu, W.; Ma, J.; Yan, J.; Zhao, A.; Zheng, X.; Wu, G.; et al. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microbes 2021, 13, 1930874. [Google Scholar] [CrossRef]
- Yan, R.; Wang, K.; Wang, Q.; Jiang, H.; Lu, Y.; Chen, X.; Zhang, H.; Su, X.; Du, Y.; Chen, L.; et al. Probiotic Lactobacillus casei Shirota prevents acute liver injury by reshaping the gut microbiota to alleviate excessive inflammation and metabolic disorders. Microb. Biotechnol. 2022, 15, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cao, C.; Wu, Z.; Zhang, H.; Sun, Z.; Wang, M.; Xu, H.; Zhao, Z.; Wang, Y.; Pei, G.; et al. The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. 2021, 33, 1926–1942.e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, J.; Li, Y.; Dong, S.; Liu, H.; Chen, J.; Wang, Y.; Zhao, S.; Zhang, Y.; Zhang, H. Probiotic Lactobacillus casei Zhang reduces pro-inflammatory cytokine production and hepatic inflammation in a rat model of acute liver failure. Eur. J. Nutr. 2016, 55, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Cao, C.; Zhang, M.; Kwok, L.Y.; Zhang, H.; Zhang, W. Lactobacillus casei Zhang exerts probiotic effects to antibiotic-treated rats. Comput. Struct. Biotechnol. J. 2021, 19, 5888–5897. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Xie, J.; Zhang, Y.; Wang, J.; Sun, X.; Zhang, H. Protective effects of probiotic Lactobacillus casei Zhang against endotoxin- and d-galactosamine-induced liver injury in rats via anti-oxidative and anti-inflammatory capacities. Int. Immunopharmacol. 2013, 15, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Society of Tuberculosis, C.M.A. Guidelines for diagnosis and treatment of anti-tuberculosis drug-induced liver injury (2019 edition). Chin. J. Tuberc. Respir. Dis. 2019, 42, 343–356. [Google Scholar]
- Su, Q.; Liu, Q.; Liu, J.; Fu, L.; Liu, T.; Liang, J.; Peng, H.; Pan, X. Study on the associations between liver damage and antituberculosis drug rifampicin and relative metabolic enzyme gene polymorphisms. Bioengineered 2021, 12, 11700–11708. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Wan, F.; Wu, S.; Jiang, X.; Tang, Z.; Zhang, X.; Huang, R.; Hu, L. Arsenic or/and antimony induced mitophagy and apoptosis associated with metabolic abnormalities and oxidative stress in the liver of mice. Sci. Total Environ. 2021, 777, 146082. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, D.; Han, S.; Zhou, S.; Jia, G. Hepatotoxicity and the role of the gut-liver axis in rats after oral administration of titanium dioxide nanoparticles. Part. Fibre Toxicol. 2019, 16, 48. [Google Scholar] [CrossRef]
- McCord, J.M.; Edeas, M.A. SOD, oxidative stress and human pathologies: A brief history and a future vision. Biomed. Pharmacother. 2005, 59, 139–142. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Ge, J.; Lv, M.W.; Talukder, M.; Guo, K.; Li, Y.H.; Li, J.L. Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated Mitophagy. Food Funct. 2020, 11, 1856–1868. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Hou, M.; Gao, T.; Sun, J.; Luo, H.; Wang, F.; Zhong, F.; Ma, A.; Cai, J. Lactobacillus casei Improve Anti-Tuberculosis Drugs-Induced Intestinal Adverse Reactions in Rat by Modulating Gut Microbiota and Short-Chain Fatty Acids. Nutrients 2022, 14, 1668. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ma, X.; Wang, H.; Li, R.; Cui, J.; Yan, P.; Wang, Y.; Yu, X.; Du, P.; Yu, H.; et al. Prebiotic-like cyclodextrin assisted silybin on NAFLD through restoring liver and gut homeostasis. J. Control. Release Off. J. Control. Release Soc. 2022, 348, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Li, X.; Ji, H. Regulation of gut microbiota in Alzheimer’s disease mice by silibinin and silymarin and their pharmacological implications. Appl. Microbiol. Biotechnol. 2019, 103, 7141–7149. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, Y.; Wang, Y.; Wang, P.; Song, H.; Wang, F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS ONE 2019, 14, e0218384. [Google Scholar] [CrossRef] [PubMed]
- Nobel, Y.R.; Cox, L.M.; Kirigin, F.F.; Bokulich, N.A.; Yamanishi, S.; Teitler, I.; Chung, J.; Sohn, J.; Barber, C.M.; Goldfarb, D.S.; et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat. Commun. 2015, 6, 7486. [Google Scholar] [CrossRef]
- Vrieze, A.; Out, C.; Fuentes, S.; Jonker, L.; Reuling, I.; Kootte, R.S.; van Nood, E.; Holleman, F.; Knaapen, M.; Romijn, J.A.; et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 2014, 60, 824–831. [Google Scholar] [CrossRef]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef]
- Calabrese, V.; Cighetti, R.; Peri, F. Molecular simplification of lipid A structure: TLR4-modulating cationic and anionic amphiphiles. Mol. Immunol. 2015, 63, 153–161. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Sutti, S.; Locatelli, I.; Bruzzì, S.; Jindal, A.; Vacchiano, M.; Bozzola, C.; Albano, E. CX3CR1-expressing inflammatory dendritic cells contribute to the progression of steatohepatitis. Clin. Sci. 2015, 129, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Mandal, S. Natural polysaccharides for controlled delivery of oral therapeutics: A recent update. Carbohydr. Polym. 2020, 230, 115617. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, M.; Mi, J.; Chen, H.; Sheng, L.; Li, Y. Protective Effect of Bicyclol on Anti-Tuberculosis Drug Induced Liver Injury in Rats. Molecules 2017, 22, 524. [Google Scholar] [CrossRef] [PubMed]
- Knodell, R.G.; Ishak, K.G.; Black, W.C.; Chen, T.S.; Craig, R.; Kaplowitz, N.; Kiernan, T.W.; Wollman, J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981, 1, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Peña, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef]

| Category | Scores and Criteria | ||||||
|---|---|---|---|---|---|---|---|
| Periportal with or without bridging necrosis | 0: none | 1: mild piecemeal necrosis | 3: moderate piecemeal necrosis (less than involvement around the portal tracts) | 4: marked piecemeal necrosis (more than involvement around the portal tracts) | 5: moderate piecemeal necrosis plus bridging necrosis | 6: marked piecemeal necrosis plus bridging necrosis | 10: multilobular necrosis |
| Intralobular degeneration and focal necrosis | 0: none | 1: mild (acidophilic bodies, ballooning degeneration and/or scattered foci of hepatocellular necrosis in <1/3 of lobules or nodules) | 3: moderate (involvement of 1/3–2/3 of lobules or nodules) | 4: marked (involvement of >2/3 of lobules or nodules) | |||
| Portal inflammation | 0: no portal inflammation | 1: mild (sprinkling of inflammatory cells in <1/3 of portal tracts) | 3: moderate (increased inflammatory cells in 1/3–2/3 of portal tracts) | 4: marked (dense packing of inflammatory cells in >2/3 of portal tracts) | |||
| Fibrosis | 0: no fibrosis | 1: fibrous portal expansion | 3: bridging fibrosis (portal–portal or portal–central linkage) | 4: cirrhosis | |||
| Category | Scores and Criteria | ||||
|---|---|---|---|---|---|
| Inflammation | 0: none | 1: slight | 2: moderate | 3: severe | |
| Extent | 0: none | 1: mucosa | 2: mucosa and submucosa | 3: transmural | |
| Regeneration | 0: complete regeneration or normal tissue | 1: almost complete regeneration | 2: regeneration with crypt depletion | 3: surface epithelium not intact | 4: no tissue repair |
| Crypt damage | 0: none | 1: basal 1/3 damaged | 2: basal 2/3 damaged | 3: only surface epithelium | 4: entire crypt and epithelium lost |
| Percent involvement | 1: 1–25% | 2: 26–50% | 3: 51–75% | 4: 76–100% | |
| Name | Sequence (5′–3′) |
|---|---|
| TLR4 | F: 5′–ACACCTACCTGGAATGGGAGG–3′ R: 5′–TCAGGTCCAAGTTGCCGTTTC–3′ |
| NF-κB | F: 5′–CCATTGTGTTCCGGACTCCTC–3′ R: 5′–GTGGCGATCATCTGTGTCTGG–3′ |
| MyD88 | F: 5′–TACAGGTGGCCAGAGTGGAA–3′ R: 5′–GCAGTAGCAGATAAAGGCATCGAA–3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhao, L.; Sun, C.; Yang, J.; Zhang, X.; Dou, S.; Hua, Q.; Ma, A.; Cai, J. Regulation of Gut Microflora by Lactobacillus casei Zhang Attenuates Liver Injury in Mice Caused by Anti-Tuberculosis Drugs. Int. J. Mol. Sci. 2023, 24, 9444. https://doi.org/10.3390/ijms24119444
Li Y, Zhao L, Sun C, Yang J, Zhang X, Dou S, Hua Q, Ma A, Cai J. Regulation of Gut Microflora by Lactobacillus casei Zhang Attenuates Liver Injury in Mice Caused by Anti-Tuberculosis Drugs. International Journal of Molecular Sciences. 2023; 24(11):9444. https://doi.org/10.3390/ijms24119444
Chicago/Turabian StyleLi, Yue, Liangjie Zhao, Changyu Sun, Jingyi Yang, Xinyue Zhang, Sheng Dou, Qinglian Hua, Aiguo Ma, and Jing Cai. 2023. "Regulation of Gut Microflora by Lactobacillus casei Zhang Attenuates Liver Injury in Mice Caused by Anti-Tuberculosis Drugs" International Journal of Molecular Sciences 24, no. 11: 9444. https://doi.org/10.3390/ijms24119444
APA StyleLi, Y., Zhao, L., Sun, C., Yang, J., Zhang, X., Dou, S., Hua, Q., Ma, A., & Cai, J. (2023). Regulation of Gut Microflora by Lactobacillus casei Zhang Attenuates Liver Injury in Mice Caused by Anti-Tuberculosis Drugs. International Journal of Molecular Sciences, 24(11), 9444. https://doi.org/10.3390/ijms24119444






