Helicobacter pylori Antibiotic Resistance: Molecular Basis and Diagnostic Methods
Abstract
1. Introduction
2. Characteristics of H. pylori Antibiotic Resistance
3. Methods for Detection of H. pylori Antibiotic Resistance
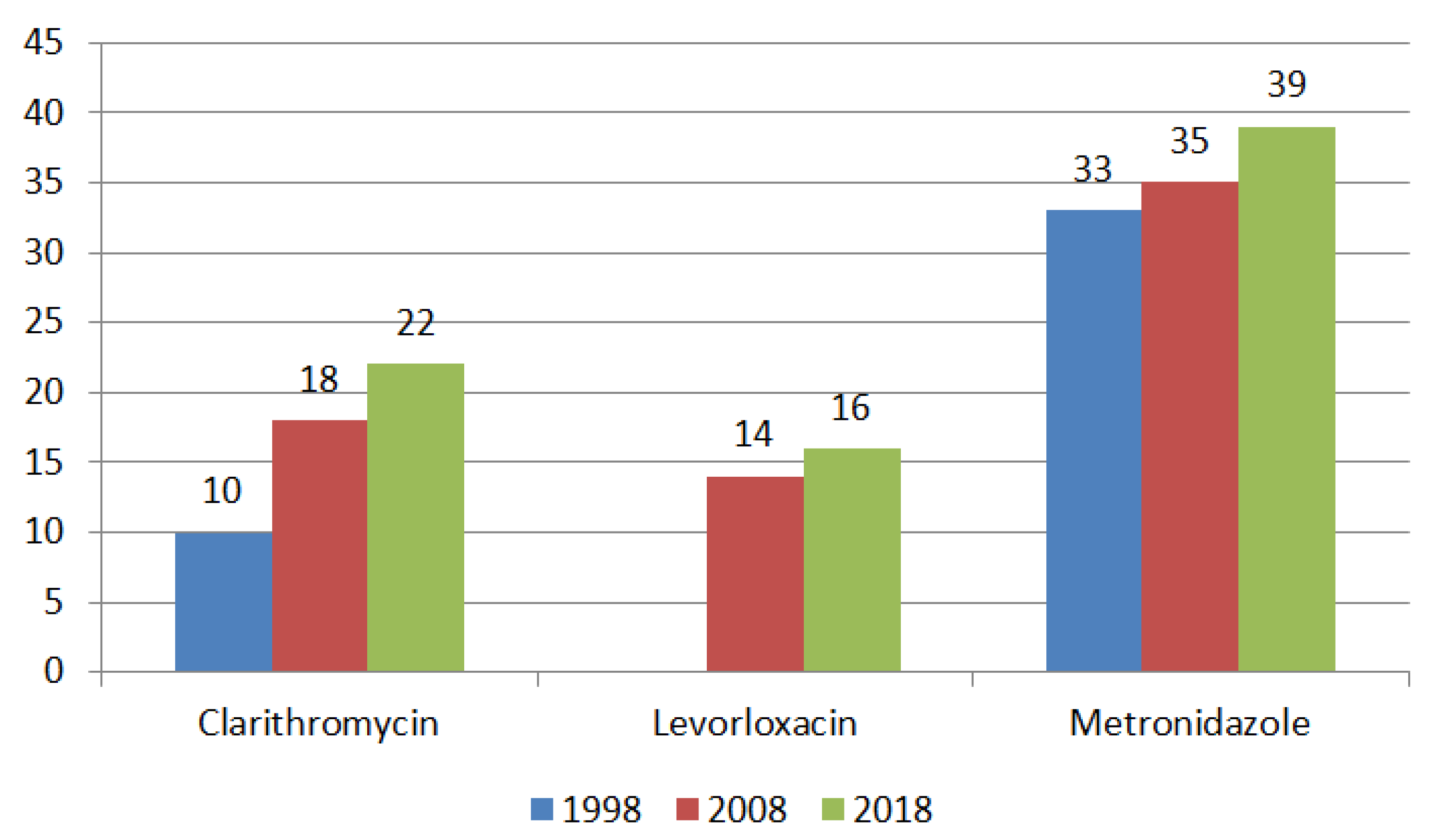
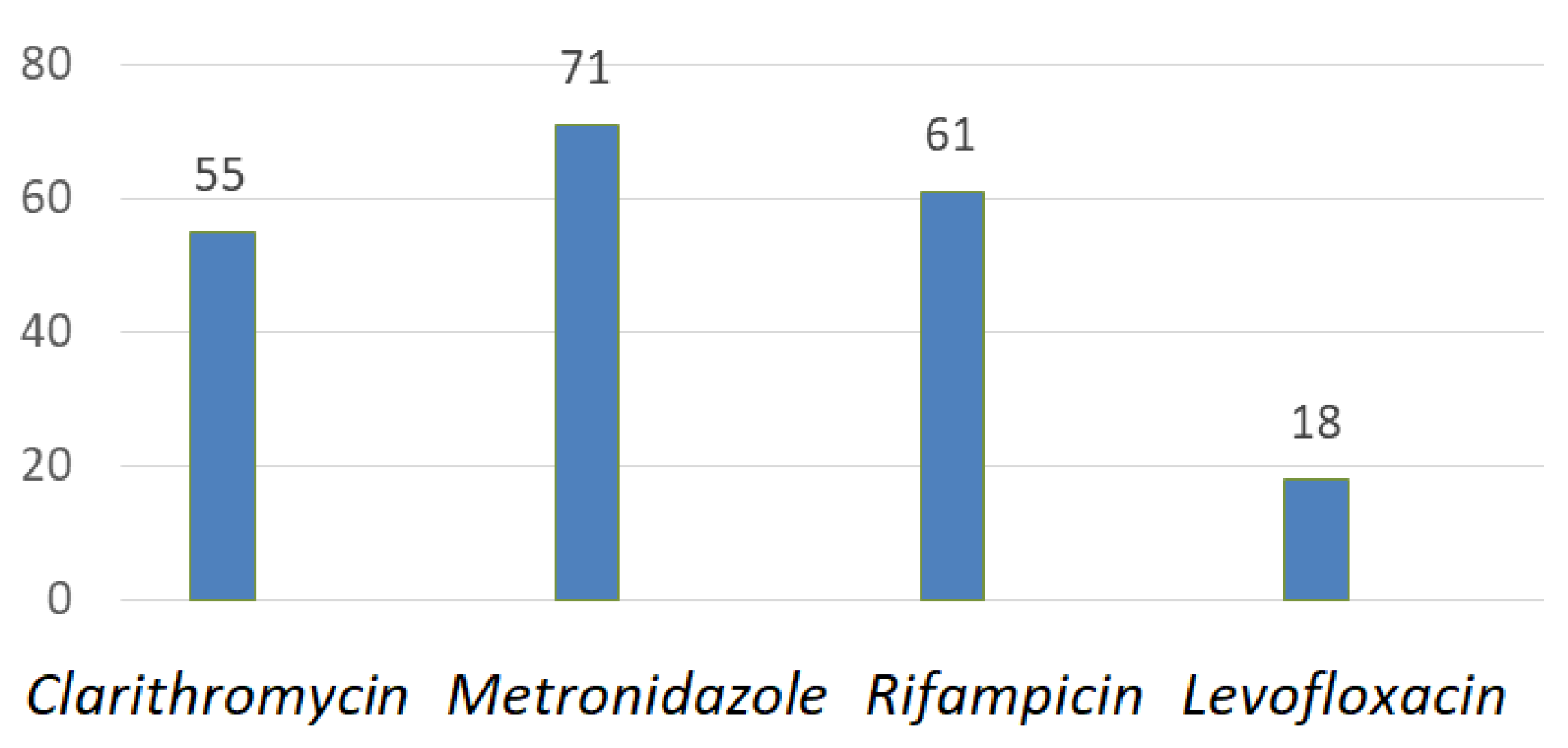
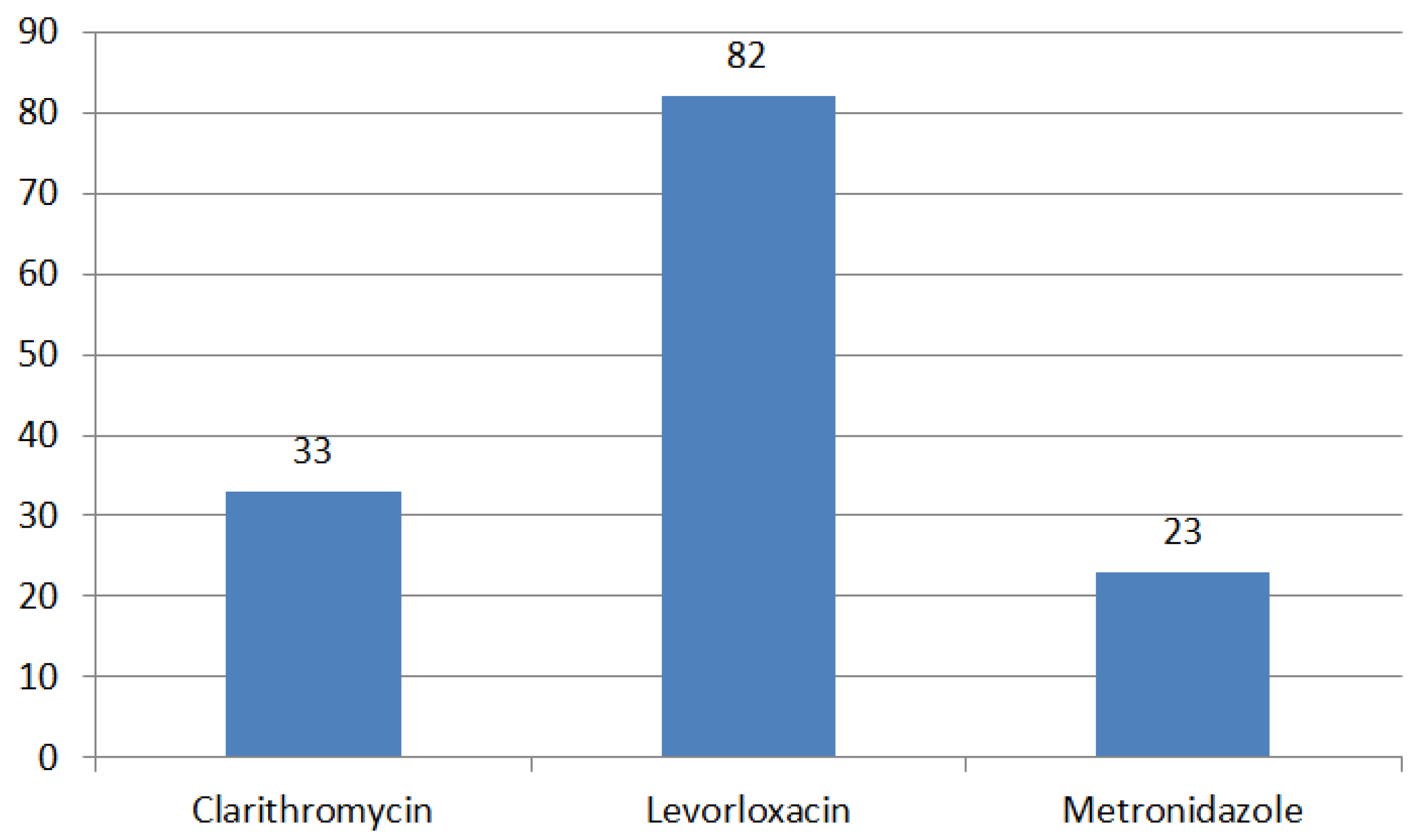
4. Regional Characteristics of H. pylori Resistance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maev, I.V. Helicobacter Pylori Infection: Monograph; GEOTAR-Mediayu: Moscow, Russia, 2016; p. 256. [Google Scholar]
- Mezmale, L.; Coelho, L.G.; Bordin, D.; Leja, M. Epidemiology of Helicobacter pylori. Helicobacter 2020, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- De Brito, B.B.; Da Silva, F.A.F.; Soares, A.S.; Pereira, V.A.; Santos, M.L.C.; Sampaio, M.M.; Neves, P.H.M.; De Melo, F.F. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J. Gastroenterol. 2019, 25, 5578–5589. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastrix VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Saad, T.; Tesfaye, M.; Walelign, S.; Wordofa, M.; Abera, D.; Desta, K.; Tsegaye, A.; Ay, A.; Taye, B. Analysis of risk factors of Helicobacter pylori (H. pylori) and prediction of prevalence: Machine learning approach: Infectious diseases. Gut 2022, 22, 655. [Google Scholar] [CrossRef]
- Noto, J.M.; Rose, K.L.; Hachey, A.J.; Delgado, A.G.; Romero-Gallo, J.; Wroblewski, L.E.; Schneider, B.G.; Shah, S.C.; Cover, T.L.; Wilson, K.T.; et al. Carcinogenic strains of Helicobacter pylori selectively dysregulate the stomach proteome In Vivo, which may be associated with the progression of gastric cancer. Mol. Cell Proteom. 2019, 18, 352–371. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Aldubaib, M.; Abalkhail, F.; Anagreyyah, S.; Anajirih, N.; Almuzaini, A.M.; Rawway, M.; Alfadhel, A.; Draz, A.; et al. Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges. Antibiotics 2023, 12, 191. [Google Scholar] [CrossRef]
- Kotilea, K.; Bontems, P.; Touati, E. Epidemiology, diagnosis and risk factors of Helicobacter pylori infection. Adv. Exp. Med. Biol. 2019, 11, 17–33. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y. Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2019, 8, 420–429. [Google Scholar] [CrossRef]
- Bordin, D.; Morozov, S.; Plavnik, R.; Bakulina, N.; Voynovan, I.; Skibo, I.; Isakov, V.; Bakulin, I.; Andreev, D.; Maev, I. Helicobacter pylori infection prevalence in ambulatory settings in 2017–2019 in Russia: The data of real-world national multicenter trial. Helicobacter 2022, 27, 5. [Google Scholar] [CrossRef]
- Puah, S.M.; Goh, K.L.; Ng, H.K.; Chua, K.H. The current state of resistance of Helicobacter pylori to clarithromycin and levofloxacin in Malaysia—Results of a molecular study. PeerJ 2021, 1, 12. [Google Scholar] [CrossRef]
- Li, Y.; Lv, T.; He, C.; Wang, H.; Cram, D.S.; Zhou, L.; Zhang, J.; Jiang, W. Evaluation of multiplex ARMS-PCR for detection of Helicobacter pylori mutations conferring resistance to clarithromycin and levofloxacin. Gut Pathog. 2020, 7, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Ziver-Sarp, T.; Yuksel-Mayda, P.; Saribas, S.; Demiryas, S.; Gareayaghi, N.; Ergin, S.; Tasci, I.; Ozbey, D.; Bal, K.; Erzin, Y. Point mutations at gyrA and gyrB genes of levofloxacin resistant Helicobacter pylori strains and dual resistance with clarithromycin. Clin. Lab. 2021, 67, 10. [Google Scholar] [CrossRef]
- Gong, M.; Han, Y.; Wang, X.; Tao, H.; Meng, F.; Hou, B.; Sun, B.B.; Wang, G. Effect of temperature on metronidazole resistance in Helicobacter pylori. Front. Microbiol. 2021, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Marais, A.; Bilardi, C.; Cantet, F.; Mendz, G.L.; Mégraud, F. Characterization of the genes rdxA and frxA involved in metronidazole resistance in Helicobacter pylori. Res. Microbiol. 2003, 154, 137–144. [Google Scholar] [CrossRef]
- Tran, T.T.; Nguyen, A.T.; Quach, D.T.; Pham, D.T.-H.; Cao, N.M.; Nguyen, U.T.-H.; Dang, A.N.-T.; Tran, M.A.; Quach, L.H.; Tran, K.T. Emergence of amoxicillin resistance and identification of novel mutations of the pbp1A gene in Helicobacter pylori in Vietnam. BMC Microbiol. 2022, 2, 41. [Google Scholar] [CrossRef]
- Tseng, Y.-S.; Wu, D.-C.; Chang, C.-Y.; C-H Kuo, C.-H.; Yang, Y.-C.; Jan, C.-M.; Su, Y.-C.; Kuo, F.-C.; Chang, L.-L. Amoxicillin resistance with β-lactamase production in Helicobacter pylori. Eur. J. Clin. Investig. 2009, 9, 807–812. [Google Scholar] [CrossRef]
- Qureshi, N.N.; Gallaher, B.; Schiller, N.L. Evolution of amoxicillin resistance of Helicobacter pylori In Vitro: Characterization of resistance mechanisms. Microb Drug Resist. 2014, 12, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-G.; Ke, J.-N.; Kuo, T.; Lin, C.-Y.; Hsieh, S.-Y.; Chiu, Y.-F.; Wu, H.-Y.; Huang, M.-Z.; Bui, N.-N.; Chiu, C.-H. Multiple amino acid substitutions in penicillin-binding protein-1A confer amoxicillin resistance in refractory Helicobacter pylori infection. J. Microbiol. Immunol. Infect. 2023, 56, 40–47. [Google Scholar] [CrossRef]
- Contreras, M.; Benejat, L.; Mujica, H.; Peña, L.; García-Amado, M.-A.; Michelangeli, F.; Lehours, P. Real-time PCR detection of 16S rRNA-a single mutation of Helicobacter pylori isolates associated with a decrease in susceptibility and resistance to tetracycline in the mucous membrane of gastroesophageal individual hosts. J. Med. Microbiol. 2019, 68, 1287–1291. [Google Scholar] [CrossRef]
- Resina, E.; Gisbert, J.P. Rescue therapy with furazolidone in patients with at least five failures of eradication treatment and multi-resistant H. pylori infection. Antibiotics 2021, 10, 1028. [Google Scholar] [CrossRef]
- Srisuphanunt, M.; Wilairatana, P.; Kooltheat, N.; Duangchan, T.; Katzenmeier, G.; Rose, J.B. Molecular Mechanisms of Antibiotic Resistance and Novel Treatment Strategies for Helicobacter pylori Infections. Trop. Med. Infect. Dis. 2023, 8, 163. [Google Scholar] [CrossRef]
- Fauzia, K.A.; Miftahussurur, M.; Syam, A.F.; Waskito, L.A.; Doohan, D.; Rezkitha, Y.A.A.; Matsumoto, T.; Tuan, V.P.; Akada, J.; Yonezawa, H. Biofilm formation and antibiotic resistance phenotype of Helicobacter pylori clinical isolates. Toxins 2020, 7, 473. [Google Scholar] [CrossRef] [PubMed]
- Miftahussurur, M.; Fauzia, K.A.; Nusi, I.A.; Setiawan, P.B.; Syam, A.F.; Waskito, L.A.; Doohan, D.; Ratnasari, N.; Khomsan, A.; I Ketut Adnyana, I.K.; et al. E-test versus agar dilution for antibiotic susceptibility testing of Helicobacter pylori: A comparison study. BMC Res. Notes 2020, 9, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Vilaichone, R.K.; Aumpan, N.; Ratanachu-Ek, T.; Uchida, T.; Tshering, L.; Mahachai, V.; Yamaoka, Y. Population-based study of Helicobacter pylori infection and antibiotic resistance in Bhutan. Int. J. Infect. Dis. 2022, 97, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Raro, O.H.F.; Collar, G.S.; Da Silva, R.M.C.; Vezzaro, P.; Mott, M.P.; Da Cunha, G.R.; Riche, C.V.W.; Dias, C.; Caierão, J. Performance of polymyxin B agar-based tests among carbapenem-resistant Enterobacterales. Lett. Appl. Microbiol. 2021, 72, 767–773. [Google Scholar] [CrossRef]
- Patel, J.B. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, MI, USA, 2017; pp. 1–42. [Google Scholar]
- Siavoshi, F.; Saniee, P.; Latifi-Navid, S.; Massarrat, S.; Sheykholeslami, A. Increased resistance of H. pylori isolates to metronidazole and tetracycline—Comparison of three 3-year studies. Arch. Iran. Med. 2021, 4, 1–5. [Google Scholar]
- Rebrikov, D.V.; Samatov, G.A.; Trofimov, D.Y.; Semenov, P.A.; Savilova, A.M.; Kofiadi, I.A.; Abramov, I.A. Real-time PCR-M; Knowledge Laboratory: Stanford, CA, USA, 2019; 223p. [Google Scholar]
- Pichon, M.; Freche, B.; Christophe Burucoa, C. New Strategy for the Detection and Treatment of Helicobacter pylori Infections in Primary Care Guided by a Non-Invasive PCR in Stool: Protocol of the French HepyPrim Study. J. Clin. Med. 2022, 3, 11. [Google Scholar] [CrossRef]
- Borodinov, A.G.; Manoilov, V.V.; Zarutsky, I.V.; Petrov, A.I.; Kurochkin, V.E. Generations of DNA sequencing methods. Sci. Instrum. 2020, 4, 3–20. [Google Scholar]
- Ishibashi, F.; Suzuki, S.; Nagai, n.; Mochida, K.; Morishita, T. Optimizing Helicobacter pylori Treatment: An Updated Review of Empirical and Susceptibility Test-Based Treatments. Gut Liver. 2023. ahead of print. [Google Scholar] [CrossRef]
- Hulten, K.G.; Genta, R.M.; Kalfus, I.N.; Zhou, Y.; Zhang, H.; Graham, D.Y. Comparison of Culture With Antibiogram to Next-Generation Sequencing Using Bacterial Isolates and Formalin-Fixed, Paraffin-Embedded Gastric Biopsies. Gastroenterology 2021, 161, 1433–1442. [Google Scholar] [CrossRef]
- Moss, S.F.; Dang, L.P.; Chua, D.; Sobrado, J.; Zhou, Y.; Graham, D.Y. Comparable Results of Helicobacter pylori Antibiotic Resistance Testing of Stools vs Gastric Biopsies Using Next-Generation Sequencing. Gastroenterology 2022, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Celiberto, F.; Losurdo, G.; Pricci, M.; Girardi, B.; Marotti, A.; Leo, A.D.; Ierardi, E. The State of the Art of Molecular Fecal Investigations for Helicobacter pylori (H. pylori) Antibiotic Resistances. Int. J. Mol. Sci. 2023, 24, 4361. [Google Scholar] [CrossRef] [PubMed]
- Megraud, F.; Bruyndonckx, R.; Coenen, S.; Wittkop, L.; Huang, T.-D.; Hoebeke, M.; Bénéjat, L.; Lehours, F.; Goossens, H.; Glupczynski, Y. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 2021, 70, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology 2018, 155, 1372–1382. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Yang, F.; Chi, W.; Ding, L.; Liu, T.; Zhu, F.; Ji, D.; Zhou, J.; Fang, Y.; et al. Antimicrobial resistance patterns and genetic elements associated with the antibiotic resistance of Helicobacter pylori strains from Shanghai. Gut Pathog. 2022, 14, 14. [Google Scholar] [CrossRef]
- Cho, J.H.; Jin, S.Y. Current guidelines for Helicobacter pylori treatment in East Asia 2022: Differences am ong China, Japan, and South Korea. World J. Clin. Cases. 2022, 10, 6349–6359. [Google Scholar] [CrossRef]
- Li, J.; Deng, J.; Wang, Z.; Li, H.; Wan, C. Antibiotic resistance of Helicobacter pylori strains isolated from pediatric patients in Southwest China. Front Microbiol. 2021, 11, 621791. [Google Scholar] [CrossRef]
- Shu, X.; Ye, D.; Hu, C.; Peng, K.; Zhao, H.; Li, H.; Jiang, M. Alarming antibiotics resistance of Helicobacter pylori from children in Southeast China over 6 years. Sci. Rep. 2022, 21, 66. [Google Scholar] [CrossRef]
- Andreev, D.A.; Maev, I.V.; Kucheryavyy, Y.A. Helicobacter pylori resistance in the Russian Federation: A meta-analysis of studies over the past 10 years. Ther. Arch. 2020, 92, 24–30. [Google Scholar]
- Nyssen, O.P.; Vaira, D.; Tepes, B.; Kupcinskas, L.; Bordin, D.; Pérez-Aisa, A.; Gasbarrini, A.; Castro-Fernández, M.; Bujanda, L.; Garre, A. Hp-EuReg Investigators. Room for Improvement in the Treatment of Helicobacter pylori Infection: Lessons from the European Registry on H. pylori Management (Hp-EuReg). J. Clin. Gastroenterol. 2022, 2, 98–108. [Google Scholar] [CrossRef]
- Bujanda, L.; Nyssen, O.P.; Vaira, D.; Saracino, L.M.; Fiorini, G.; Lerang, F.; Georgopoulos, S.; Tepes, B.; Heluwaert, F.; Antonio Gasbarrini, A. The Hp-EuReg Investigators. Antibiotic Resistance Prevalence and Trends in Patients Infected with Helicobacter pylori in the Period 2013-2020: Results of the European Registry on H. pylori Management (Hp-EuReg). Antibiotics 2021, 9, 1058. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Hadzhiyski, P.; Gergova, R.; Markovska, R. Evolution of Helicobacter pylori Resistance to Antibiotics: A Topic of Increasing Concern. Antibiotics 2023, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Mégraud, F.; Alix, C.; Charron, P.; Bénéjat, L.; Ducournau, A.; Bessède, E.; Lehours, F. Survey of the antimicrobial resistance of Helicobacter pylori in France in 2018 and evolution during the previous 5 years. Helicobacter 2021, 26, e12767. [Google Scholar] [CrossRef] [PubMed]
- Mosites, E.; Bruden, D.; Morris, J.; Reasonover, A.; Rudolph, K.; Hurlburt, D.; Hennessy, T.; McMahon, B.; Bruce, M. Antimicrobial resistance among Helicobacter pylori isolates in Alaska, 2000–2016. J. Glob. Antimicrob. Resist. 2018, 15, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.; Dzink-Fox, J.; Shen, Z.; Piazuelo, M.B.; Wilson, K.T.; Correa, P.; Peek, R.M., Jr.; Camargo, M.C.; Fox, J.G. Antimicrobial resistance of Helicobacter pylori and gene variants in populations with high and low risk of stomach cancer. J. Clin. Microbiol. 2021, 59, e03203-20. [Google Scholar] [CrossRef]
- Alavifard, H.; Mirzaei, N.; Yadegar, A.; Baghaei, K.; Smith, S.M.; Sadeghi, A.; Zali, M.R. Investigation of mutations associated with clarithromycin resistance and Helicobacter pylori virulence genotypes isolated from the Iranian population: A cross-sectional study. Curr. Microbiol. 2021, 78, 244–254. [Google Scholar] [CrossRef]
- Subsomwong, P.; Doohan, D.; Fauzia, K.A.; Akada, J.; Matsumoto, T.; Yee, T.T.; Htet, K.; Waskito, L.A.; Tuan, V.; Uchida, T. Next-Generation Sequencing-Based Study of Helicobacter pylori Isolates from Myanmar and Their Susceptibility to Antibiotics. Microorganosms 2022, 10, 196. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, J.G. Substitutions in penicillin-binding protein 1 in amoxicillin-resistant Helicoobacter pylori strains isolated from Korean patients. Gut Liver. 2013, 7, 655–660. [Google Scholar] [CrossRef]
- De Palma, G.Z.; Mendiondo, N.; Wonaga, A.; Viola, L.; Ibarra, D.; Campitelli, E.; Salim, N.; Corti, R.; Goldman, C.; Catalano, M. Occurrence of mutations in the antimicrobial target genesrelated to levofloxacin, clarithromycin, and amoxicillin resistance in Helicobacter pylori isolates from Buenos Aires city. Microb. Drug Resist. 2017, 23, 351–358. [Google Scholar] [CrossRef]
- Chtourou, L.; Moalla, M.; Mnif, B.; Smaoui, H.; Gdoura, H.; Boudabous, M.; Mnif, L.; Amouri, A.; Hammami, A.; Tahri, N. Prevalence of Helicobacter pylori resistance to clarithromycin in Tunisia. J. Med. Microbiol. 2022, 8, 71. [Google Scholar] [CrossRef]
- Albasha, A.M.; Elnosh, M.M.; Osman, E.H.; Zeinalabdin, D.M.; Fadl, A.A.M.; Ali, M.A.; Altayb, H.N. Helicobacter pylori 23S rRNA gene A2142G, A2143G, T2182C, and C2195T mutations associated with clarithromycin resistance detected in Sudanese patients. BMC Microbiol. 2021, 2, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.H.; Ha, T.M.T.; Le, P.T.Q.; Phan, T.N.; Tran, T.N.H. Characterisation of point mutations in domain V of the 23S rRNA gene of clinical Helicobacter pylori strains and clarithromycin-resistant phenotype in central Vietnam. J. Glob. Antimicrob. Resist. 2019, 3, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Tsapkova, L.A.; Polyakova, V.V.; Bodunova, N.A.; Baratova, I.V.; Voynovan, I.N.; Dekhnich, N.N.; Ivanchik, N.V.; Sabelnikova, E.A.; Bordin, D.S. Possibilities of a molecular genetic method for detecting resistance to clarithromycin and levofloxacin in Helicobacter pylori. Eff. Pharmacother. 2022, 42, 16–20. [Google Scholar]
- Iannone, A.; Giorgio, F.; Russo, F.; Riezzo, G.; Girardi, B.; Pricci, M.; Palmer, S.C.; Barone, M.; Principi, M.; Strippoli, G.F. New fecal test for non-invasive Helicobacter pylori detection: A diagnostic accuracy study. Clin. Trial 2018, 24, 3021–3029. [Google Scholar] [CrossRef]
- Gong, Y.; Zhai, R.; Sun, L.; He, L.; Wang, H.; Guo, Y.; Zhang, J. RdxA Diversity and Mutations Associated with Metronidazole Resistance of Helicobacter pylori. Microbiol. Spectr. 2023, 21, e0390322. [Google Scholar] [CrossRef]
- Fauzia, K.A.; Aftab, H.; Tshibangu-Kabamba, E.; Alfaray, R.I.; Saruuljavkhlan, B.; Cimuanga-Mukanya, A.; Matsumoto, T.; Subsomwong, P.; Akada, J.; Miftahussurur, M.; et al. Bangladesh—Mutations Related to Antibiotics Resistance in Helicobacter pylori Clinical Isolates from Bangladesh. Antibiotics 2023, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Domanovich-Asor, T.; Craddock, H.A.; Motro, Y.; Khalfin, B.; Peretz, A.; Moran-Gilad, J. Unraveling antimicrobial resistance in Helicobacter pylori: Global resistome meets global phylogeny. Helicobacter 2021, 5, 25. [Google Scholar] [CrossRef]
- Domanovich-Asor, T.; Motro, Y.; Khalfin, B.; Craddock, H.A.; Peretz, A.; Moran-Gilad, J. Genomic Analysis of Antimicrobial Resistance Genotype-to-Phenotype Agreement in Helicobacter pylori. Microorganisms 2021, 9, 2. [Google Scholar] [CrossRef]
- Azrad, M.; Vazana, D.; On, A.; Paritski, M.; Rohana, H.; Roshrosh, H.; Agay-Shay, K.; Peretz, A. Antibiotic resistance patterns of Helicobacter pylori in North Israel—A six-year study. Helicobacter 2022, 12, 12932. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Z.; Shen, Y.; Song, X.; Benghezal, M.; Marshall, B.J.; Tang, H.; Hong Li, H. Antibiotic resistance patterns of Helicobacter pylori strains isolated from the Tibet Autonomous Region, China. Microbiology 2022, 22, 196. [Google Scholar] [CrossRef]
- Lopo, I.; Libânio, D.; Pita, I.; Dinis-Ribeiro, M.; Pimentel-Nunes, P. Helicobacter pylori antibiotic resistance in Portugal: Systematic review and meta-analysis. Helicobacter 2018, 8, 23. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Dore, M.P.; Graham, D.Y. Diagnosis and treatment of Helicobacter pylori infection. Annu. Rev. Med. 2022, 4, 183–195. [Google Scholar] [CrossRef] [PubMed]

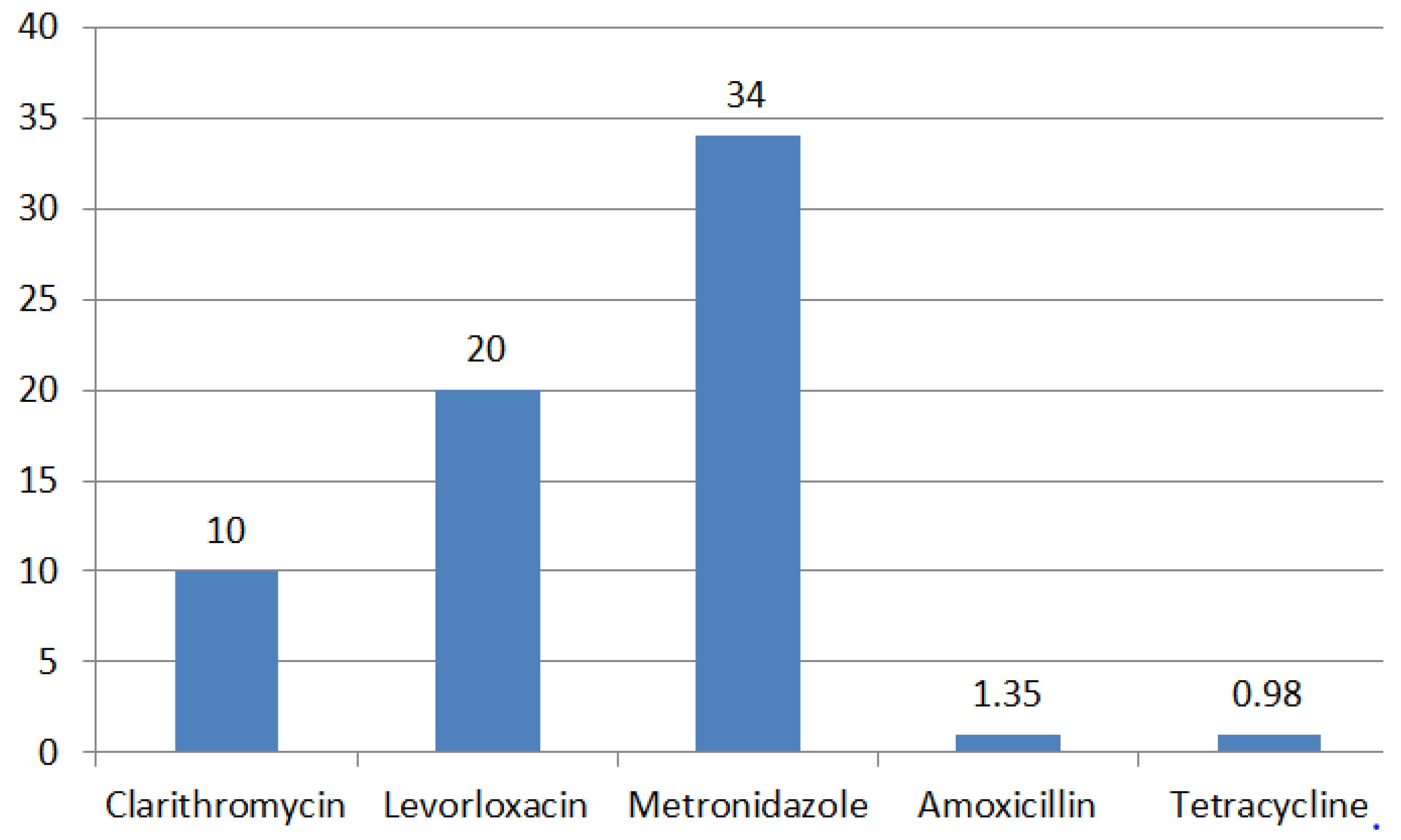
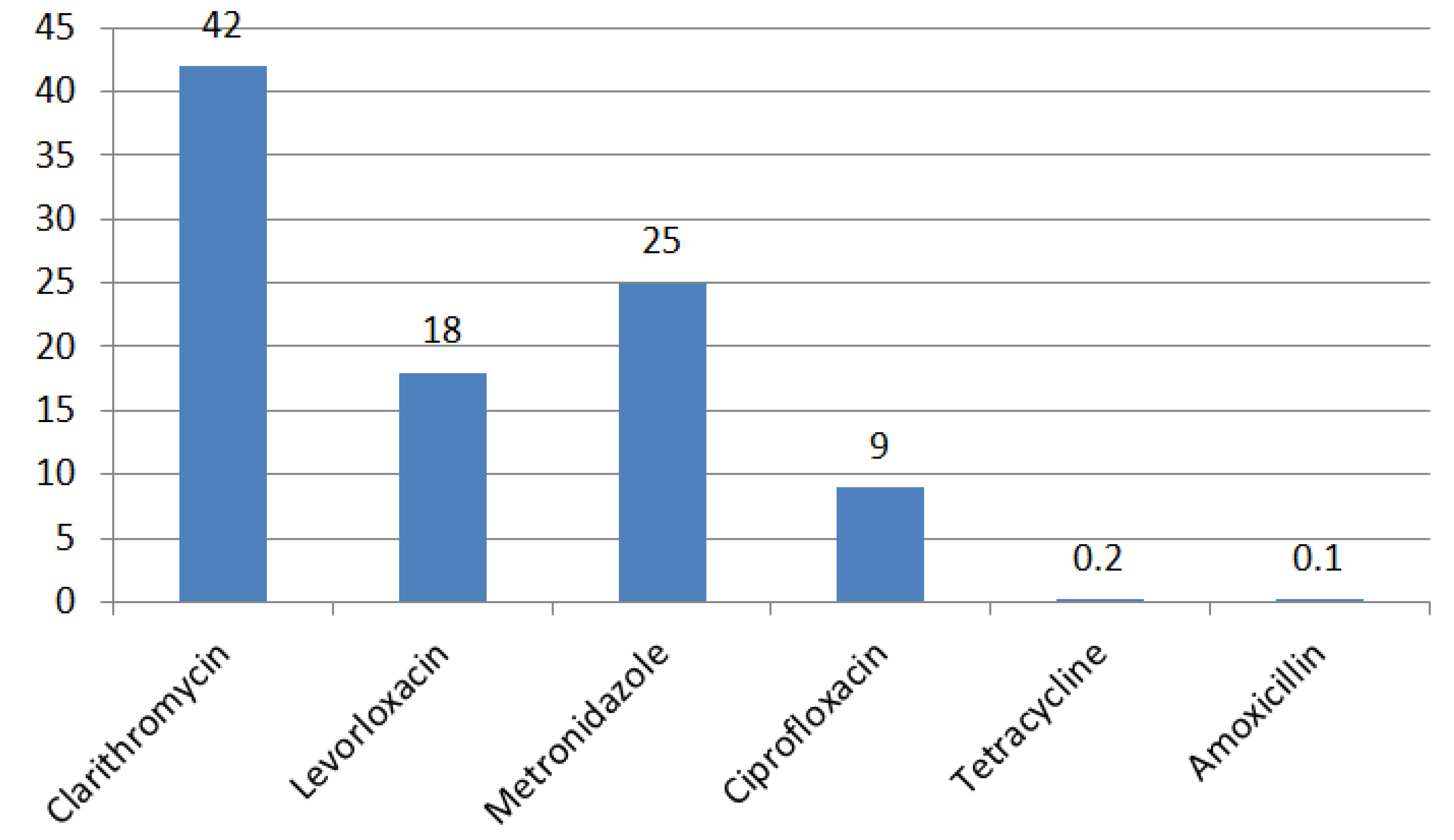
| Antibiotic | MIC, mg/L | |
|---|---|---|
| Sensitive, ≤ | Resistant, > | |
| Amoxicillin | 0.25 | 0.125 |
| Clarithromycin | 0.25 | 0.5 |
| Levofloxacin | 1.0 | 1.0 |
| Tetracycline | 1.0 | 1.0 |
| Metronidazole | 8.0 | 8.0 |
| Rifampicin | 1.0 | 1.0 |
| Method | Sensitivity/Specificity (%) | The Degree of Labor Intensity | Automation Degree | Time Cost |
|---|---|---|---|---|
| Diffusion method | 96/99 | High | Low | 18–48 h |
| Serial dilution method | High | High | Low | 16–48 h |
| E-test | 96/99 | High | Low | 16–48 h |
| PCR method | 96/99 | Low | High | 4–5 h |
| Direct sequencing | 98/98 | Medium | High | 8–9 h |
| NGS | 98/98 | High | Medium | up to 72 h |
| FISH | High | Medium | High | 14–20 h |
| Region | Number of Samples | Specimen | Methods Used | Antibiotic Type | Gen (Mutation) | Source |
|---|---|---|---|---|---|---|
| Columbia, S. America | n = 59 | biopsy | PCR, WGS | Amoxicillin Clarithromycin Rifampicin Tetracycline Levofloxacin Metronidazole | WT WT WT 16S rRNA (A926G) gyrA (N87I/K, 91G/Y) rdxA, frxA | [48] |
| Iran | n = 82 | biopsy | PCR, Sequencing | Clarithromycin | 23S rRNA (A2143G, A2142G) | [49] |
| Myanmar | n = 150 | biopsy | NGS | Metronidazole Amoxicillin Levofloxacin Clarithromycin | rdxA (V175I, S91P, R16H/C), frxA (L33M) pdp1-A (V45I, S414R, V414R, D465K/D, V471H, N564Y) gyrA (D91N/G/Y, D210N, K230Q, A524V, A661T) gyrB (A584V, N679H, M676V, V614I) 23S rRNA (T248C) | [50] |
| South Korea | n = 144 | biopsy | PCR, Sequencing | Amoxicillin | pdp1 (Val16Ile, Val45Ile, Ser414Arg, Asn562Tyr, Thr593Ala, Gly595Ser, Ala599Thr) | [51] |
| USA | n = 262 | biopsy feces | NGS | Amoxicillin Clarithromycin Metronidazole Levofloxacin Tetracycline Rifabutin | pdp1 23S rRNA rdxA, frxA gyrA 16S rRNA rpoB | [33,34,52] |
| Tunisia | n = 124 | biopsy | PCR | Clarithromycin | 23S rRNA (2142G, 2143G) | [53] |
| Sudan | n = 288 | biopsy | PCR | Clarithromycin | 23S rRNA (A2142G, A2143G, T2182C, C2195T) | [54] |
| Vietnam | n = 185 n = 308 | biopsy | Sequencing | Clarithromycin Amoxicillin | 23S rRNA (A2142G, A2143G and other point mutations) pbp1A (366, 414,473, ins595–596) | [55] [16] |
| Russia | n = 15 | cultures from the biopsy | Sequencing | Clarithromycin Levofloxacin | 23S rRNA (2142G, 2143G) gyrA (N87I/K, 91G/Y) | [56] |
| Italy | n = 95 | feces | - | Clarithromycin Levofloxacin | 23S rRNA (A2142G, A2143G) gyrA (N87I/K, 91G/Y) | [57] |
| China | n = 511 | cultures from the biopsy | Sequencing | Metronidazole | rdxA (R16H/C, Y47C, A67V/T, A80T/S, V204I) | [58] |
| Bangladesh | n = 133 | cultures from the biopsy | WGS | Metronidazole Amoxicillin Levofloxacin Clarithromycin | ribF (D253E), frxA, rdxA, mdaB, omp11, pbp1a (N562Y), pbp2 pbp3, pbp4 gyrA (87, 91), gyrB (A343V) 23S rRNA, infB | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medakina, I.; Tsapkova, L.; Polyakova, V.; Nikolaev, S.; Yanova, T.; Dekhnich, N.; Khatkov, I.; Bordin, D.; Bodunova, N. Helicobacter pylori Antibiotic Resistance: Molecular Basis and Diagnostic Methods. Int. J. Mol. Sci. 2023, 24, 9433. https://doi.org/10.3390/ijms24119433
Medakina I, Tsapkova L, Polyakova V, Nikolaev S, Yanova T, Dekhnich N, Khatkov I, Bordin D, Bodunova N. Helicobacter pylori Antibiotic Resistance: Molecular Basis and Diagnostic Methods. International Journal of Molecular Sciences. 2023; 24(11):9433. https://doi.org/10.3390/ijms24119433
Chicago/Turabian StyleMedakina, Irina, Larisa Tsapkova, Vera Polyakova, Sergey Nikolaev, Tatyana Yanova, Natalia Dekhnich, Igor Khatkov, Dmitry Bordin, and Natalia Bodunova. 2023. "Helicobacter pylori Antibiotic Resistance: Molecular Basis and Diagnostic Methods" International Journal of Molecular Sciences 24, no. 11: 9433. https://doi.org/10.3390/ijms24119433
APA StyleMedakina, I., Tsapkova, L., Polyakova, V., Nikolaev, S., Yanova, T., Dekhnich, N., Khatkov, I., Bordin, D., & Bodunova, N. (2023). Helicobacter pylori Antibiotic Resistance: Molecular Basis and Diagnostic Methods. International Journal of Molecular Sciences, 24(11), 9433. https://doi.org/10.3390/ijms24119433






